Abstract
The title compound, C14H18N2O4S, contains hydrogen-bonded dimeric pairs of molecules arranged around inversion centers, forming 14-membered rings with an R 2 2(14) motif. The structure is stabilized by extensive intramolecular interactions. The thiazine ring adopts a half-chair conformation, with the S and N atoms displaced by −0.485 (3) and 0.296 (3) Å, respectively, from the plane formed by the remaining atoms of the ring.
Related literature
For related literature, see: Ahmad, Siddiqui, Ahmad et al. (2008 ▶); Ahmad, Siddiqui, Zia-ur-Rehman et al. (2008 ▶); Bernstein et al. (1994 ▶); Gupta et al. (1993 ▶, 2002 ▶); Kojić-Prodić & Rużić-Toroš (1982 ▶); Lombardino (1971 ▶); Lombardino & Wiseman (1972 ▶); Rehman et al. (2005 ▶, 2006 ▶); Sianesi et al. (1973 ▶); Siddiqui et al. (2008 ▶); Zinnes et al. (1982 ▶); Drebushchak et al. (2006 ▶).
Experimental
Crystal data
C14H18N2O4S
M r = 310.36
Monoclinic,

a = 10.233 (2) Å
b = 14.780 (4) Å
c = 10.365 (5) Å
β = 108.79 (2)°
V = 1484.1 (9) Å3
Z = 4
Mo Kα radiation
μ = 0.23 mm−1
T = 173 (2) K
0.14 × 0.12 × 0.06 mm
Data collection
Nonius KappaCCD diffractometer
Absorption correction: multi-scan (SORTAV; Blessing, 1997 ▶) T min = 0.968, T max = 0.986
12380 measured reflections
3405 independent reflections
2646 reflections with I > 2σ(I)
R int = 0.041
Refinement
R[F 2 > 2σ(F 2)] = 0.039
wR(F 2) = 0.100
S = 1.03
3405 reflections
198 parameters
H atoms treated by a mixture of independent and constrained refinement
Δρmax = 0.30 e Å−3
Δρmin = −0.43 e Å−3
Data collection: COLLECT (Hooft, 1998 ▶); cell refinement: HKL DENZO (Otwinowski & Minor, 1997 ▶); data reduction: SCALEPACK (Otwinowski & Minor, 1997 ▶); program(s) used to solve structure: SHELXS97 (Sheldrick, 2008 ▶); program(s) used to refine structure: SHELXL97 (Sheldrick, 2008 ▶); molecular graphics: ORTEP-3 for Windows (Farrugia, 1997 ▶); software used to prepare material for publication: SHELXL97.
Supplementary Material
Crystal structure: contains datablocks Global, I. DOI: 10.1107/S160053680801670X/bh2176sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S160053680801670X/bh2176Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Table 1. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| O3—H3O⋯O4 | 0.88 (2) | 1.76 (2) | 2.572 (2) | 153 (2) |
| N2—H2N⋯O2i | 0.87 (2) | 2.21 (2) | 3.052 (2) | 161 (2) |
| N2—H2N⋯N1 | 0.87 (2) | 2.34 (2) | 2.753 (2) | 109 (2) |
| C9—H9B⋯O2 | 0.98 | 2.49 | 2.864 (2) | 102 |
Symmetry code: (i)  .
.
supplementary crystallographic information
Comment
Several benzothiazine derivatives like piroxicam, sudoxicam (Lombardino & Wiseman, 1972; Rehman et al., 2005) and isoxicam (Zinnes et al., 1982) have been reported in the literature to be potential anti-inflammatory agents. Some of the derivatives of benzothiazines are found to be analgesic (Gupta et al., 2002), anti-cancer (Gupta et al., 1993) and exhibitors of central nervous system activity (Sianesi et al., 1973). We have reported anti-bacterial activities (Rehman et al., 2006) of a series of 1,2-benzothiazines. In continuation of our work on 1,2-benzothiazines 1,1-dioxides (Ahmad, Siddiqui, Ahmad, Irfan Ashiq & Tizzard, 2008; Ahmad, Siddiqui, Zia-ur-Rehman, Ashiq & Tizzard, 2008), we report in this paper the crystal structure of the title compound, (I), which was patented for Pfizer Inc. (Lombardino, 1971).
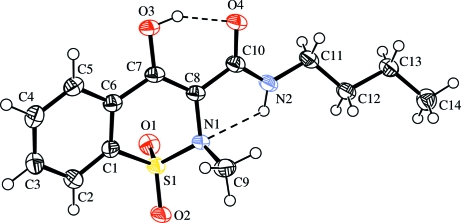
The structure of (I), (Fig. 1), contains dimeric pairs of molecules lying about inversion centers resulting from N2—H2N···O2 hydrogen bonds (Fig. 2). The 14-membered rings thus formed represent R22(14) motif in the graph set notation (Bernstein et al., 1994). Similar hydrogen-bonded dimers have been reported in structures related to the title compound (Siddiqui et al., 2008; Drebushchak et al., 2006; Kojić-Prodić & Rużić-Toroš, 1982). The structure is stabilized by extensive intramolecular interactions (Fig. 1 and Table 1). The thiazine ring in (I) adopts a half-chair conformation with atoms S1 and N1 displaced by -0.485 (3) and 0.296 (3) Å, respectively, from the plane formed by the remaining atoms of the ring.
Experimental
Methyl-4-hydroxy-2-methyl-2H-1,2-benzothiazine-3-carboxylate-1,1-dioxide (1.0 g, 3.72 mmoles) was dissolved in n-butyl amine (5 ml) in a test tube. The mixture was placed at room temperature for 7 days. Crystals of (I) suitable for crystallographic analysis were found, which were washed with MeOH.
Refinement
Though all the H atoms could be found in a difference map, the H atoms bonded to C atoms were included at geometrically idealized positions and refined in riding-model approximation with the following constraints: C—H distances were set to 0.95, 0.98 and 0.99 Å for aryl, methyl and methylene H atoms, respectively, and Uiso(H) = 1.2Ueq(C). H atoms bonded to N2 and O3 were taken from a difference map and were allowed to refine with Uiso = 1.2 times Ueq of the parent atom. The final difference map was free of any chemically significant features.
Figures
Fig. 1.
ORTEP-3 (Farrugia, 1997) drawing of (I) with displacement ellipsoids plotted at 50% probability level; intramolecular interactions have been indicated by dashed lines.
Fig. 2.
Unit cell packing of (I) showing hydrogen bonds with dashed lines; H atoms not involved in hydrogen bonds have been omitted.
Crystal data
| C14H18N2O4S | F000 = 656 |
| Mr = 310.36 | Dx = 1.389 Mg m−3 |
| Monoclinic, P21/c | Mo Kα radiation λ = 0.71073 Å |
| Hall symbol: -P 2ybc | Cell parameters from 12380 reflections |
| a = 10.233 (2) Å | θ = 3.4–27.6º |
| b = 14.780 (4) Å | µ = 0.24 mm−1 |
| c = 10.365 (5) Å | T = 173 (2) K |
| β = 108.79 (2)º | Prism, colorless |
| V = 1484.1 (9) Å3 | 0.14 × 0.12 × 0.06 mm |
| Z = 4 |
Data collection
| Nonius KappaCCD diffractometer | 3405 independent reflections |
| Radiation source: fine-focus sealed tube | 2646 reflections with I > 2σ(I) |
| Monochromator: graphite | Rint = 0.041 |
| T = 173(2) K | θmax = 27.6º |
| ω and φ scans | θmin = 3.4º |
| Absorption correction: Multi-scan(SORTAV; Blessing, 1997) | h = −13→13 |
| Tmin = 0.968, Tmax = 0.986 | k = −19→19 |
| 12380 measured reflections | l = −13→13 |
Refinement
| Refinement on F2 | Secondary atom site location: difference Fourier map |
| Least-squares matrix: full | Hydrogen site location: inferred from neighbouring sites |
| R[F2 > 2σ(F2)] = 0.039 | H atoms treated by a mixture of independent and constrained refinement |
| wR(F2) = 0.100 | w = 1/[σ2(Fo2) + (0.045P)2 + 0.606P] where P = (Fo2 + 2Fc2)/3 |
| S = 1.03 | (Δ/σ)max = 0.001 |
| 3405 reflections | Δρmax = 0.30 e Å−3 |
| 198 parameters | Δρmin = −0.43 e Å−3 |
| Primary atom site location: structure-invariant direct methods | Extinction correction: none |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| S1 | 0.53051 (4) | 0.13868 (3) | 0.96872 (5) | 0.03033 (13) | |
| O1 | 0.45310 (12) | 0.12905 (9) | 0.82760 (14) | 0.0384 (3) | |
| O2 | 0.46724 (13) | 0.11547 (9) | 1.06916 (15) | 0.0400 (3) | |
| O3 | 0.86081 (13) | 0.22923 (9) | 0.83438 (13) | 0.0336 (3) | |
| H3O | 0.899 (2) | 0.1799 (16) | 0.816 (2) | 0.050* | |
| O4 | 0.92527 (12) | 0.06200 (9) | 0.82242 (13) | 0.0356 (3) | |
| N1 | 0.67274 (13) | 0.07980 (9) | 0.99855 (14) | 0.0265 (3) | |
| N2 | 0.78434 (15) | −0.04424 (10) | 0.86553 (16) | 0.0327 (3) | |
| H2N | 0.714 (2) | −0.0525 (14) | 0.894 (2) | 0.039* | |
| C1 | 0.59359 (17) | 0.24980 (11) | 0.99677 (17) | 0.0283 (4) | |
| C2 | 0.53309 (18) | 0.31325 (12) | 1.05849 (19) | 0.0342 (4) | |
| H2 | 0.4587 | 0.2967 | 1.0897 | 0.041* | |
| C3 | 0.58309 (19) | 0.40116 (12) | 1.07376 (19) | 0.0359 (4) | |
| H3 | 0.5430 | 0.4455 | 1.1159 | 0.043* | |
| C4 | 0.69144 (19) | 0.42436 (12) | 1.02763 (18) | 0.0349 (4) | |
| H4 | 0.7235 | 0.4851 | 1.0363 | 0.042* | |
| C5 | 0.75381 (17) | 0.36033 (12) | 0.96906 (18) | 0.0309 (4) | |
| H5 | 0.8289 | 0.3772 | 0.9392 | 0.037* | |
| C6 | 0.70664 (17) | 0.27103 (11) | 0.95385 (17) | 0.0276 (4) | |
| C7 | 0.77716 (16) | 0.19970 (12) | 0.90287 (17) | 0.0272 (4) | |
| C8 | 0.76003 (16) | 0.11021 (12) | 0.92283 (17) | 0.0269 (4) | |
| C9 | 0.74832 (19) | 0.05636 (13) | 1.14249 (18) | 0.0355 (4) | |
| H9A | 0.8206 | 0.0121 | 1.1457 | 0.043* | |
| H9B | 0.6839 | 0.0305 | 1.1848 | 0.043* | |
| H9C | 0.7905 | 0.1110 | 1.1921 | 0.043* | |
| C10 | 0.82934 (17) | 0.04054 (12) | 0.86719 (17) | 0.0288 (4) | |
| C11 | 0.8470 (2) | −0.12047 (12) | 0.8176 (2) | 0.0385 (4) | |
| H11A | 0.9478 | −0.1107 | 0.8430 | 0.046* | |
| H11B | 0.8091 | −0.1238 | 0.7170 | 0.046* | |
| C12 | 0.82004 (18) | −0.20820 (12) | 0.8775 (2) | 0.0335 (4) | |
| H12A | 0.7192 | −0.2189 | 0.8486 | 0.040* | |
| H12B | 0.8537 | −0.2034 | 0.9781 | 0.040* | |
| C13 | 0.88952 (19) | −0.28900 (12) | 0.83487 (19) | 0.0339 (4) | |
| H13A | 0.8496 | −0.2975 | 0.7351 | 0.041* | |
| H13B | 0.9892 | −0.2761 | 0.8562 | 0.041* | |
| C14 | 0.8724 (2) | −0.37560 (13) | 0.9058 (2) | 0.0405 (5) | |
| H14A | 0.9237 | −0.4245 | 0.8799 | 0.049* | |
| H14B | 0.7743 | −0.3916 | 0.8787 | 0.049* | |
| H14C | 0.9079 | −0.3668 | 1.0048 | 0.049* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| S1 | 0.0252 (2) | 0.0286 (2) | 0.0417 (3) | −0.00243 (16) | 0.01704 (18) | −0.00165 (18) |
| O1 | 0.0263 (6) | 0.0414 (8) | 0.0450 (8) | −0.0039 (5) | 0.0078 (5) | −0.0038 (6) |
| O2 | 0.0400 (7) | 0.0340 (7) | 0.0597 (9) | −0.0031 (5) | 0.0352 (7) | −0.0005 (6) |
| O3 | 0.0347 (7) | 0.0328 (7) | 0.0411 (7) | −0.0041 (5) | 0.0232 (6) | 0.0015 (6) |
| O4 | 0.0316 (6) | 0.0379 (7) | 0.0455 (8) | −0.0004 (5) | 0.0239 (6) | 0.0008 (6) |
| N1 | 0.0267 (7) | 0.0273 (7) | 0.0306 (8) | −0.0003 (5) | 0.0160 (6) | 0.0018 (6) |
| N2 | 0.0306 (8) | 0.0311 (8) | 0.0433 (9) | −0.0001 (6) | 0.0215 (7) | −0.0031 (7) |
| C1 | 0.0269 (8) | 0.0267 (8) | 0.0330 (9) | 0.0003 (6) | 0.0121 (7) | 0.0023 (7) |
| C2 | 0.0317 (9) | 0.0342 (10) | 0.0404 (10) | 0.0042 (7) | 0.0167 (8) | 0.0028 (8) |
| C3 | 0.0400 (10) | 0.0296 (9) | 0.0393 (10) | 0.0073 (8) | 0.0142 (8) | 0.0019 (8) |
| C4 | 0.0407 (10) | 0.0269 (9) | 0.0353 (10) | −0.0007 (7) | 0.0097 (8) | 0.0024 (7) |
| C5 | 0.0306 (9) | 0.0309 (9) | 0.0315 (9) | −0.0027 (7) | 0.0104 (7) | 0.0040 (7) |
| C6 | 0.0255 (8) | 0.0307 (9) | 0.0267 (8) | −0.0011 (7) | 0.0087 (7) | 0.0019 (7) |
| C7 | 0.0229 (8) | 0.0338 (9) | 0.0264 (8) | −0.0028 (6) | 0.0098 (7) | 0.0014 (7) |
| C8 | 0.0234 (8) | 0.0318 (9) | 0.0282 (9) | −0.0018 (6) | 0.0122 (7) | 0.0008 (7) |
| C9 | 0.0385 (10) | 0.0390 (10) | 0.0331 (10) | −0.0019 (8) | 0.0171 (8) | 0.0021 (8) |
| C10 | 0.0245 (8) | 0.0345 (9) | 0.0290 (9) | 0.0006 (7) | 0.0107 (7) | 0.0003 (7) |
| C11 | 0.0432 (10) | 0.0326 (10) | 0.0502 (12) | 0.0037 (8) | 0.0294 (9) | −0.0028 (8) |
| C12 | 0.0324 (9) | 0.0340 (10) | 0.0398 (10) | 0.0013 (7) | 0.0194 (8) | −0.0026 (8) |
| C13 | 0.0329 (9) | 0.0348 (10) | 0.0367 (10) | 0.0015 (7) | 0.0152 (8) | −0.0036 (8) |
| C14 | 0.0365 (10) | 0.0356 (10) | 0.0486 (12) | −0.0003 (8) | 0.0128 (9) | −0.0003 (9) |
Geometric parameters (Å, °)
| S1—O1 | 1.4286 (15) | C5—C6 | 1.397 (2) |
| S1—O2 | 1.4335 (14) | C5—H5 | 0.9500 |
| S1—N1 | 1.6375 (14) | C6—C7 | 1.469 (2) |
| S1—C1 | 1.7543 (18) | C7—C8 | 1.359 (2) |
| O3—C7 | 1.349 (2) | C8—C10 | 1.470 (2) |
| O3—H3O | 0.88 (2) | C9—H9A | 0.9800 |
| O4—C10 | 1.254 (2) | C9—H9B | 0.9800 |
| N1—C8 | 1.438 (2) | C9—H9C | 0.9800 |
| N1—C9 | 1.484 (2) | C11—C12 | 1.501 (3) |
| N2—C10 | 1.333 (2) | C11—H11A | 0.9900 |
| N2—C11 | 1.460 (2) | C11—H11B | 0.9900 |
| N2—H2N | 0.87 (2) | C12—C13 | 1.526 (2) |
| C1—C2 | 1.388 (2) | C12—H12A | 0.9900 |
| C1—C6 | 1.402 (2) | C12—H12B | 0.9900 |
| C2—C3 | 1.387 (3) | C13—C14 | 1.514 (3) |
| C2—H2 | 0.9500 | C13—H13A | 0.9900 |
| C3—C4 | 1.385 (3) | C13—H13B | 0.9900 |
| C3—H3 | 0.9500 | C14—H14A | 0.9800 |
| C4—C5 | 1.386 (3) | C14—H14B | 0.9800 |
| C4—H4 | 0.9500 | C14—H14C | 0.9800 |
| O1—S1—O2 | 119.25 (9) | C7—C8—C10 | 121.34 (15) |
| O1—S1—N1 | 107.84 (8) | N1—C8—C10 | 117.28 (14) |
| O2—S1—N1 | 108.51 (8) | N1—C9—H9A | 109.5 |
| O1—S1—C1 | 108.42 (8) | N1—C9—H9B | 109.5 |
| O2—S1—C1 | 109.38 (8) | H9A—C9—H9B | 109.5 |
| N1—S1—C1 | 102.07 (8) | N1—C9—H9C | 109.5 |
| C7—O3—H3O | 104.4 (15) | H9A—C9—H9C | 109.5 |
| C8—N1—C9 | 114.06 (13) | H9B—C9—H9C | 109.5 |
| C8—N1—S1 | 113.67 (11) | O4—C10—N2 | 122.76 (16) |
| C9—N1—S1 | 117.24 (11) | O4—C10—C8 | 120.15 (15) |
| C10—N2—C11 | 122.83 (15) | N2—C10—C8 | 117.08 (15) |
| C10—N2—H2N | 116.6 (14) | N2—C11—C12 | 111.55 (15) |
| C11—N2—H2N | 120.6 (14) | N2—C11—H11A | 109.3 |
| C2—C1—C6 | 122.08 (16) | C12—C11—H11A | 109.3 |
| C2—C1—S1 | 120.92 (13) | N2—C11—H11B | 109.3 |
| C6—C1—S1 | 117.00 (13) | C12—C11—H11B | 109.3 |
| C3—C2—C1 | 118.85 (16) | H11A—C11—H11B | 108.0 |
| C3—C2—H2 | 120.6 | C11—C12—C13 | 113.08 (14) |
| C1—C2—H2 | 120.6 | C11—C12—H12A | 109.0 |
| C4—C3—C2 | 119.98 (17) | C13—C12—H12A | 109.0 |
| C4—C3—H3 | 120.0 | C11—C12—H12B | 109.0 |
| C2—C3—H3 | 120.0 | C13—C12—H12B | 109.0 |
| C3—C4—C5 | 121.00 (17) | H12A—C12—H12B | 107.8 |
| C3—C4—H4 | 119.5 | C14—C13—C12 | 112.53 (15) |
| C5—C4—H4 | 119.5 | C14—C13—H13A | 109.1 |
| C4—C5—C6 | 120.23 (16) | C12—C13—H13A | 109.1 |
| C4—C5—H5 | 119.9 | C14—C13—H13B | 109.1 |
| C6—C5—H5 | 119.9 | C12—C13—H13B | 109.1 |
| C5—C6—C1 | 117.78 (16) | H13A—C13—H13B | 107.8 |
| C5—C6—C7 | 121.80 (15) | C13—C14—H14A | 109.5 |
| C1—C6—C7 | 120.31 (15) | C13—C14—H14B | 109.5 |
| O3—C7—C8 | 122.01 (15) | H14A—C14—H14B | 109.5 |
| O3—C7—C6 | 115.20 (15) | C13—C14—H14C | 109.5 |
| C8—C7—C6 | 122.79 (15) | H14A—C14—H14C | 109.5 |
| C7—C8—N1 | 121.38 (14) | H14B—C14—H14C | 109.5 |
| O1—S1—N1—C8 | 61.67 (13) | S1—C1—C6—C7 | −6.9 (2) |
| O2—S1—N1—C8 | −167.86 (11) | C5—C6—C7—O3 | −19.3 (2) |
| C1—S1—N1—C8 | −52.42 (13) | C1—C6—C7—O3 | 164.56 (15) |
| O1—S1—N1—C9 | −161.75 (12) | C5—C6—C7—C8 | 160.65 (16) |
| O2—S1—N1—C9 | −31.28 (14) | C1—C6—C7—C8 | −15.5 (2) |
| C1—S1—N1—C9 | 84.16 (13) | O3—C7—C8—N1 | 178.67 (14) |
| O1—S1—C1—C2 | 103.81 (15) | C6—C7—C8—N1 | −1.3 (2) |
| O2—S1—C1—C2 | −27.73 (17) | O3—C7—C8—C10 | −1.7 (3) |
| N1—S1—C1—C2 | −142.53 (15) | C6—C7—C8—C10 | 178.33 (15) |
| O1—S1—C1—C6 | −76.03 (15) | C9—N1—C8—C7 | −98.65 (19) |
| O2—S1—C1—C6 | 152.43 (13) | S1—N1—C8—C7 | 39.3 (2) |
| N1—S1—C1—C6 | 37.63 (15) | C9—N1—C8—C10 | 81.71 (18) |
| C6—C1—C2—C3 | 2.3 (3) | S1—N1—C8—C10 | −140.30 (13) |
| S1—C1—C2—C3 | −177.53 (14) | C11—N2—C10—O4 | 3.1 (3) |
| C1—C2—C3—C4 | 0.1 (3) | C11—N2—C10—C8 | −177.77 (16) |
| C2—C3—C4—C5 | −1.7 (3) | C7—C8—C10—O4 | 13.4 (3) |
| C3—C4—C5—C6 | 0.9 (3) | N1—C8—C10—O4 | −166.94 (15) |
| C4—C5—C6—C1 | 1.4 (2) | C7—C8—C10—N2 | −165.76 (16) |
| C4—C5—C6—C7 | −174.82 (16) | N1—C8—C10—N2 | 13.9 (2) |
| C2—C1—C6—C5 | −3.0 (3) | C10—N2—C11—C12 | 156.43 (17) |
| S1—C1—C6—C5 | 176.82 (13) | N2—C11—C12—C13 | −177.35 (16) |
| C2—C1—C6—C7 | 173.25 (16) | C11—C12—C13—C14 | 174.83 (17) |
Hydrogen-bond geometry (Å, °)
| D—H···A | D—H | H···A | D···A | D—H···A |
| O3—H3O···O4 | 0.88 (2) | 1.76 (2) | 2.572 (2) | 153 (2) |
| N2—H2N···O2i | 0.87 (2) | 2.21 (2) | 3.052 (2) | 161 (2) |
| N2—H2N···N1 | 0.87 (2) | 2.34 (2) | 2.753 (2) | 109 (2) |
| C9—H9B···O2 | 0.98 | 2.49 | 2.864 (2) | 102 |
Symmetry codes: (i) −x+1, −y, −z+2.
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: BH2176).
References
- Ahmad, M., Siddiqui, H. L., Ahmad, S., Irfan Ashiq, M. & Tizzard, G. J. (2008). Acta Cryst. E64, o594. [DOI] [PMC free article] [PubMed]
- Ahmad, M., Siddiqui, H. L., Zia-ur-Rehman, M., Ashiq, M. I. & Tizzard, G. J. (2008). Acta Cryst. E64, o788. [DOI] [PMC free article] [PubMed]
- Bernstein, J., Etter, M. C. & Leiserowitz, L. (1994). Structure Correlation, edited by H.-B. Bürgi & J. D. Dunitz, Vol. 2, pp. 431–507. New York: VCH.
- Blessing, R. H. (1997). J. Appl. Cryst.30, 421–426.
- Drebushchak, T. N., Pankrushina, N. N., Shakhtshneider, T. P. & Apenina, S. A. (2006). Acta Cryst. C62, o429–o431. [DOI] [PubMed]
- Farrugia, L. J. (1997). J. Appl. Cryst.30, 565.
- Gupta, S. K., Bansal, P., Bhardwaj, R. K., Jaiswal, J. & Velpandian, T. (2002). Skin Pharmacol. Appl. Skin Physiol.15, 105–111. [DOI] [PubMed]
- Gupta, R. R., Dev, P. K., Sharma, M. L., Rajoria, C. M., Gupta, A. & Nyati, M. (1993). Anticancer Drugs, 4, 589–592. [DOI] [PubMed]
- Hooft, R. (1998). COLLECT Nonius BV, Delft, The Netherlands.
- Kojić-Prodić, B. & Rużić-Toroš, Ž. (1982). Acta Cryst. B38, 2948–2951.
- Lombardino, J. G. (1971). US Patent No. 3 591 584.
- Lombardino, J. G. & Wiseman, E. H. (1972). J. Med. Chem.15, 848–849. [DOI] [PubMed]
- Otwinowski, Z. & Minor, W. (1997). Methods in Enzymology, Vol. 276, Macromolecular Crystallography, Part A, edited by C. W. Carter Jr & R. M. Sweet, pp. 307–326. New York: Academic Press.
- Rehman, M. Z., Choudary, J. A. & Ahmad, S. (2005). Bull. Korean Chem. Soc.54, 1171–1175.
- Rehman, M. Z., Choudary, J. A., Ahmad, S. & Siddiqui, H. L. (2006). Chem. Pharm. Bull.54, 1175–1178. [DOI] [PubMed]
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Sianesi, E., Redaelli, R., Magistretti, M. J. & Massarani, E. (1973). J. Med. Chem.16, 1133–1137. [DOI] [PubMed]
- Siddiqui, W. A., Ahmad, S., Tariq, M. I., Siddiqui, H. L. & Parvez, M. (2008). Acta Cryst. C64, o4–o6. [DOI] [PubMed]
- Zinnes, H., Sircar, J. C., Lindo, N., Schwartz, M. L., Fabian, A. C., Shavel, J. Jr, Kasulanis, C. F., Genzer, J. D., Lutomski, C. & DiPasquale, G. (1982). J. Med. Chem.25, 12–18. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablocks Global, I. DOI: 10.1107/S160053680801670X/bh2176sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S160053680801670X/bh2176Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report