Abstract
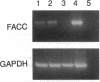
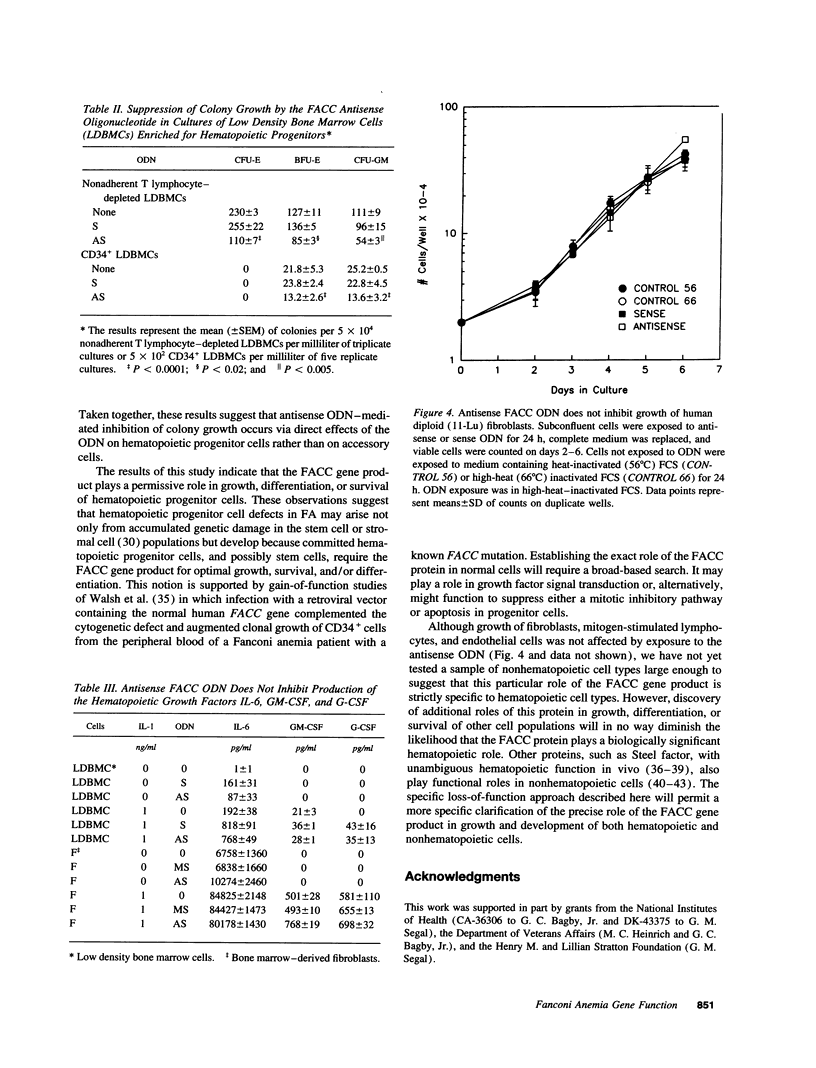
Bone marrow failure is a consistent feature of Fanconi anemia (FA) but it is not known whether the bone marrow failure is a direct and specific result of the inherited mutation or a consequence of accumulated stem cell losses resulting from nonspecific DNA damage. We tested the hypothesis that the protein encoded by the FA group C complementing gene (FACC) plays a regulatory role in hematopoiesis. We exposed normal human lymphocytes, bone marrow cells, endothelial cells, and fibroblasts to an antisense oligodeoxynucleotide (ODN) complementary to bases -4 to +14 of FACC mRNA. The mitomycin C assay demonstrated that the antisense ODN, but not missense or sense ODNs, repressed FACC gene expression in lymphocytes. Treatment with the antisense ODN substantially reduced, in a sequence-specific fashion, cytoplasmic levels of FACC mRNA in bone marrow cells and lymphocytes. Escalating doses of antisense ODN increasingly inhibited clonal growth of erythroid and granulocyte-macrophage progenitor cells but did not inhibit growth of fibroblasts or endothelial cells. The antisense ODN did not inhibit growth factor gene expression by low density bone marrow cells or marrow-derived fibroblasts. We conclude that, while the FACC gene product plays a role in defining cellular tolerance to cross-linking agents, it also functions to regulate growth, differentiation, and/or survival of normal hematopoietic progenitor cells.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alter B. P., Knobloch M. E., Weinberg R. S. Erythropoiesis in Fanconi's anemia. Blood. 1991 Aug 1;78(3):602–608. [PubMed] [Google Scholar]
- Alter B. P. Leukemia and preleukemia in Fanconi's anemia. Cancer Genet Cytogenet. 1992 Feb;58(2):206–209. doi: 10.1016/0165-4608(92)90116-p. [DOI] [PubMed] [Google Scholar]
- Auerbach A. D., Adler B., Chaganti R. S. Prenatal and postnatal diagnosis and carrier detection of Fanconi anemia by a cytogenetic method. Pediatrics. 1981 Jan;67(1):128–135. [PubMed] [Google Scholar]
- Auerbach A. D., Allen R. G. Leukemia and preleukemia in Fanconi anemia patients. A review of the literature and report of the International Fanconi Anemia Registry. Cancer Genet Cytogenet. 1991 Jan;51(1):1–12. doi: 10.1016/0165-4608(91)90002-c. [DOI] [PubMed] [Google Scholar]
- Auerbach A. D., Rogatko A., Schroeder-Kurth T. M. International Fanconi Anemia Registry: relation of clinical symptoms to diepoxybutane sensitivity. Blood. 1989 Feb;73(2):391–396. [PubMed] [Google Scholar]
- Bagby G. C., Jr, McCall E., Bergstrom K. A., Burger D. A monokine regulates colony-stimulating activity production by vascular endothelial cells. Blood. 1983 Sep;62(3):663–668. [PubMed] [Google Scholar]
- Bagby G. C., Jr, Segal G. M., Auerbach A. D., Onega T., Keeble W., Heinrich M. C. Constitutive and induced expression of hematopoietic growth factor genes by fibroblasts from children with Fanconi anemia. Exp Hematol. 1993 Oct;21(11):1419–1426. [PubMed] [Google Scholar]
- Bennett R. M. As nature intended? The uptake of DNA and oligonucleotides by eukaryotic cells. Antisense Res Dev. 1993 Fall;3(3):235–241. doi: 10.1089/ard.1993.3.235. [DOI] [PubMed] [Google Scholar]
- Berger R., Le Coniat M., Schaison G. Chromosome abnormalities in bone marrow of Fanconi anemia patients. Cancer Genet Cytogenet. 1993 Jan;65(1):47–50. doi: 10.1016/0165-4608(93)90057-s. [DOI] [PubMed] [Google Scholar]
- Brannan C. I., Lyman S. D., Williams D. E., Eisenman J., Anderson D. M., Cosman D., Bedell M. A., Jenkins N. A., Copeland N. G. Steel-Dickie mutation encodes a c-kit ligand lacking transmembrane and cytoplasmic domains. Proc Natl Acad Sci U S A. 1991 Jun 1;88(11):4671–4674. doi: 10.1073/pnas.88.11.4671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cervenka J., Hirsch B. A. Cytogenetic differentiation of Fanconi anemia, "idiopathic" aplastic anemia, and Fanconi anemia heterozygotes. Am J Med Genet. 1983 Jun;15(2):211–223. doi: 10.1002/ajmg.1320150205. [DOI] [PubMed] [Google Scholar]
- Dash P., Lotan I., Knapp M., Kandel E. R., Goelet P. Selective elimination of mRNAs in vivo: complementary oligodeoxynucleotides promote RNA degradation by an RNase H-like activity. Proc Natl Acad Sci U S A. 1987 Nov;84(22):7896–7900. doi: 10.1073/pnas.84.22.7896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flanagan J. G., Chan D. C., Leder P. Transmembrane form of the kit ligand growth factor is determined by alternative splicing and is missing in the Sld mutant. Cell. 1991 Mar 8;64(5):1025–1035. doi: 10.1016/0092-8674(91)90326-t. [DOI] [PubMed] [Google Scholar]
- Gavish H., dos Santos C. C., Buchwald M. A Leu554-to-Pro substitution completely abolishes the functional complementing activity of the Fanconi anemia (FACC) protein. Hum Mol Genet. 1993 Feb;2(2):123–126. doi: 10.1093/hmg/2.2.123. [DOI] [PubMed] [Google Scholar]
- Gille J. J., Wortelboer H. M., Joenje H. Antioxidant status of Fanconi anemia fibroblasts. Hum Genet. 1987 Sep;77(1):28–31. doi: 10.1007/BF00284708. [DOI] [PubMed] [Google Scholar]
- Hungerford D. A. Leukocytes cultured from small inocula of whole blood and the preparation of metaphase chromosomes by treatment with hypotonic KCl. Stain Technol. 1965 Nov;40(6):333–338. doi: 10.3109/10520296509116440. [DOI] [PubMed] [Google Scholar]
- Krieg A. M., Gmelig-Meyling F., Gourley M. F., Kisch W. J., Chrisey L. A., Steinberg A. D. Uptake of oligodeoxyribonucleotides by lymphoid cells is heterogeneous and inducible. Antisense Res Dev. 1991 Summer;1(2):161–171. doi: 10.1089/ard.1991.1.161. [DOI] [PubMed] [Google Scholar]
- Lee M., Segal G. M., Bagby G. C. Interleukin-1 induces human bone marrow-derived fibroblasts to produce multilineage hematopoietic growth factors. Exp Hematol. 1987 Oct;15(9):983–988. [PubMed] [Google Scholar]
- Maciag T., Mehlman T., Friesel R., Schreiber A. B. Heparin binds endothelial cell growth factor, the principal endothelial cell mitogen in bovine brain. Science. 1984 Aug 31;225(4665):932–935. doi: 10.1126/science.6382607. [DOI] [PubMed] [Google Scholar]
- McCall E., Bagby G. C., Jr Monocyte-derived recruiting activity: kinetics of production and effects of endotoxin. Blood. 1985 Mar;65(3):689–695. [PubMed] [Google Scholar]
- McNiece I. K., Langley K. E., Zsebo K. M. Recombinant human stem cell factor synergises with GM-CSF, G-CSF, IL-3 and epo to stimulate human progenitor cells of the myeloid and erythroid lineages. Exp Hematol. 1991 Mar;19(3):226–231. [PubMed] [Google Scholar]
- Migliaccio A. R., Migliaccio G., Durand B., Mancini G. C., Adamson J. W. The generation of colony-forming cells (CFC) and the expansion of hematopoiesis in cultures of human cord blood cells is dependent on the presence of stem cell factor (SCF). Cytotechnology. 1993;11(2):107–113. doi: 10.1007/BF00748999. [DOI] [PubMed] [Google Scholar]
- Murphy M., Reid K., Williams D. E., Lyman S. D., Bartlett P. F. Steel factor is required for maintenance, but not differentiation, of melanocyte precursors in the neural crest. Dev Biol. 1992 Oct;153(2):396–401. doi: 10.1016/0012-1606(92)90124-y. [DOI] [PubMed] [Google Scholar]
- Okumura N., Tsuji K., Nakahata T. Changes in cell surface antigen expressions during proliferation and differentiation of human erythroid progenitors. Blood. 1992 Aug 1;80(3):642–650. [PubMed] [Google Scholar]
- Pesce M., Farrace M. G., Piacentini M., Dolci S., De Felici M. Stem cell factor and leukemia inhibitory factor promote primordial germ cell survival by suppressing programmed cell death (apoptosis). Development. 1993 Aug;118(4):1089–1094. doi: 10.1242/dev.118.4.1089. [DOI] [PubMed] [Google Scholar]
- Rosselli F., Sanceau J., Wietzerbin J., Moustacchi E. Abnormal lymphokine production: a novel feature of the genetic disease Fanconi anemia. I. Involvement of interleukin-6. Hum Genet. 1992 Apr;89(1):42–48. doi: 10.1007/BF00207040. [DOI] [PubMed] [Google Scholar]
- Rossi P., Albanesi C., Grimaldi P., Geremia R. Expression of the mRNA for the ligand of c-kit in mouse Sertoli cells. Biochem Biophys Res Commun. 1991 Apr 30;176(2):910–914. doi: 10.1016/s0006-291x(05)80272-4. [DOI] [PubMed] [Google Scholar]
- Schindler D., Hoehn H. Fanconi anemia mutation causes cellular susceptibility to ambient oxygen. Am J Hum Genet. 1988 Oct;43(4):429–435. [PMC free article] [PubMed] [Google Scholar]
- Schroeder T. M., Tilgen D., Krüger J., Vogel F. Formal genetics of Fanconi's anemia. Hum Genet. 1976 Jun 29;32(3):257–288. doi: 10.1007/BF00295817. [DOI] [PubMed] [Google Scholar]
- Segal G. M., McCall E., Stueve T., Bagby G. C., Jr Interleukin 1 stimulates endothelial cells to release multilineage human colony-stimulating activity. J Immunol. 1987 Mar 15;138(6):1772–1778. [PubMed] [Google Scholar]
- Segal G. M., Smith T. D., Heinrich M. C., Ey F. S., Bagby G. C., Jr Specific repression of granulocyte-macrophage and granulocyte colony-stimulating factor gene expression in interleukin-1-stimulated endothelial cells with antisense oligodeoxynucleotides. Blood. 1992 Aug 1;80(3):609–616. [PubMed] [Google Scholar]
- Stark R., Thierry D., Richard P., Gluckman E. Long-term bone marrow culture in Fanconi's anaemia. Br J Haematol. 1993 Apr;83(4):554–559. doi: 10.1111/j.1365-2141.1993.tb04690.x. [DOI] [PubMed] [Google Scholar]
- Strathdee C. A., Duncan A. M., Buchwald M. Evidence for at least four Fanconi anaemia genes including FACC on chromosome 9. Nat Genet. 1992 Jun;1(3):196–198. doi: 10.1038/ng0692-196. [DOI] [PubMed] [Google Scholar]
- Strathdee C. A., Gavish H., Shannon W. R., Buchwald M. Cloning of cDNAs for Fanconi's anaemia by functional complementation. Nature. 1992 Apr 30;356(6372):763–767. doi: 10.1038/356763a0. [DOI] [PubMed] [Google Scholar]
- Strathdee C. A., Gavish H., Shannon W. R., Buchwald M. Cloning of cDNAs for Fanconi's anaemia by functional complementation. Nature. 1992 Jul 30;358(6385):434–434. doi: 10.1038/358434a0. [DOI] [PubMed] [Google Scholar]
- Sun Y., Moses R. E. Reactivation of psoralen-reacted plasmid DNA in Fanconi anemia, xeroderma pigmentosum, and normal human fibroblast cells. Somat Cell Mol Genet. 1991 May;17(3):229–238. doi: 10.1007/BF01232819. [DOI] [PubMed] [Google Scholar]
- Tso J. Y., Sun X. H., Kao T. H., Reece K. S., Wu R. Isolation and characterization of rat and human glyceraldehyde-3-phosphate dehydrogenase cDNAs: genomic complexity and molecular evolution of the gene. Nucleic Acids Res. 1985 Apr 11;13(7):2485–2502. doi: 10.1093/nar/13.7.2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walder R. Y., Walder J. A. Role of RNase H in hybrid-arrested translation by antisense oligonucleotides. Proc Natl Acad Sci U S A. 1988 Jul;85(14):5011–5015. doi: 10.1073/pnas.85.14.5011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams D. E., de Vries P., Namen A. E., Widmer M. B., Lyman S. D. The Steel factor. Dev Biol. 1992 Jun;151(2):368–376. doi: 10.1016/0012-1606(92)90176-h. [DOI] [PubMed] [Google Scholar]
- Zsebo K. M., Yuschenkoff V. N., Schiffer S., Chang D., McCall E., Dinarello C. A., Brown M. A., Altrock B., Bagby G. C., Jr Vascular endothelial cells and granulopoiesis: interleukin-1 stimulates release of G-CSF and GM-CSF. Blood. 1988 Jan;71(1):99–103. [PubMed] [Google Scholar]