Abstract
There are two independent molecules in the asymmetric unit of the title compound, C11H13N3O3. The interplanar angles for the two rings of the benzimidazole ring system is 2.21 (12)° in one molecule and 0.72 (12)° in the other. The nitro group is twisted in the same direction relative to the least-squares plane through its attached benzene ring in both molecules, with interplanar angles of 15.22 (9) and 18.02 (8)°. In the crystal structure, molecules are stacked along the a axis through π–π interactions (centroid–centroid distance 4.1954 Å). C—H⋯O hydrogen bonds are also present.
Related literature
For background to the biological applications of benzimidazole cores, see: Townsend & Revankar (1970 ▶); Kamal et al. (2006 ▶); Bentancor et al. (2004 ▶); Somsak et al. (2003 ▶); Scholz et al. (2003 ▶); Sachs et al. (1995 ▶); Shin et al. (1997 ▶); Chackalamannil et al. (2003 ▶); Nicolaou et al. (1998 ▶); Lanusse & Prichard (1993 ▶); Wang (1984 ▶); Banks (1984 ▶); Sharma & Abuzar (1983 ▶); Lopez-Rodriguez et al. (2002 ▶). For other related literature on benzimidazole cores, see: Elderfield et al. (1946 ▶); Grimmett (2002 ▶); Kumar et al. (1982 ▶); Mizzoni & Spoerri (1945 ▶); Reddy & Reddy (1979 ▶). For related literature, see: Flaherty et al. (2008 ▶).
Experimental
Crystal data
C11H13N3O3
M r = 235.24
Orthorhombic,

a = 7.63920 (10) Å
b = 16.0029 (3) Å
c = 17.9496 (3) Å
V = 2194.33 (6) Å3
Z = 8
Mo Kα radiation
μ = 0.11 mm−1
T = 150 (2) K
0.43 × 0.33 × 0.28 mm
Data collection
Bruker SMART APEXII diffractometer
Absorption correction: multi-scan (SADABS; Sheldrick, 2002 ▶) T min = 0.959, T max = 0.971
39389 measured reflections
3989 independent reflections
3649 reflections with I > 2σ(I)
R int = 0.026
Refinement
R[F 2 > 2σ(F 2)] = 0.034
wR(F 2) = 0.099
S = 1.09
3989 reflections
313 parameters
H-atom parameters constrained
Δρmax = 0.47 e Å−3
Δρmin = −0.20 e Å−3
Data collection: APEX2 and SMART (Bruker, 1998 ▶); cell refinement: SAINT (Bruker, 1998 ▶); data reduction: SAINT; program(s) used to solve structure: SHELXS97 (Sheldrick, 2008 ▶); program(s) used to refine structure: SHELXL97 (Sheldrick, 2008 ▶); molecular graphics: ORTEP-3 for Windows (Farrugia, 1997 ▶); software used to prepare material for publication: SHELXTL (Sheldrick, 2008 ▶) and PLATON (Spek, 2003 ▶).
Supplementary Material
Crystal structure: contains datablocks I, global. DOI: 10.1107/S160053680801859X/zl2123sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S160053680801859X/zl2123Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Table 1. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| C15—H1⋯O6i | 0.93 | 2.46 | 3.3670 (8) | 164 |
| C4—H15⋯O3ii | 0.93 | 2.49 | 3.3723 (8) | 159 |
Symmetry codes: (i)  ; (ii)
; (ii)  .
.
Acknowledgments
Financial support from Duquesne University (Mylan School of Pharmacy), an equipment grant from the National Science Foundation (NMR: CHE 0614785), and an NSF X-ray facility grant (CRIF 0234872) are gratefully acknowledged.
supplementary crystallographic information
Comment
The benzimidazole core has found application in multiple biologically active compounds including ATP mimics (Townsend & Revankar, 1970, Kamal et al., 2006, Bentancor et al., 2004, Somsak et al., 2003, Scholz et al., 2003), ATPase inhibitors (Sachs et al., 1995, Shin et al., 1997), peptide mimics (Chackalamannil et al., 2003, Nicolaou et al., 1998, antihelmentics (Lanusse & Prichard, 1993, Wang, 1984, Banks, 1984, Sharma & Abuzar, 1983), and serotonergic compounds (Lopez-Rodriguez et al., 2002). Although multiple strategies exist to synthesize the imidazole ring onto the parent phenyl ring, the most prevalent strategy utilizes an ortho-phenyldiamine precursor, for example, compound 2 (Grimmett, 2002). Subsequent N-alkylation on the benzimidazole core presents an ambident nucleophile (Kumar et al., 1982, Reddy & Reddy, 1979). Alkylation on the preformed benzimidazole has been shown to occur on the most nucleophilic nitrogen. However, this strategy often presents a mixture of products and fails completely with bulky, less-reactive electrophiles. A strategy of one-pot N-alkylation on the precursor phenyldiamine has been developed, followed by cyclization to afford 1-N-alkyl-4-nitro-6-methoxybenzimidazole (Flaherty et al., In preparation). Unequivocal characterization of the alkylation site was essential to determine the utility of this method.
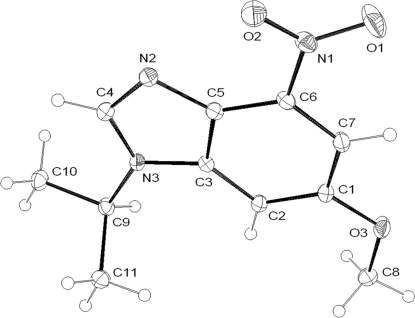
There are two molecules in the asymmetric unit of the title compound (Fig. 1). The nitro groups are twisted away from the least squares plane of the attached benzene rings, with the O2—N1—C6—C5 torsion angles being 17.24 (2)° and 14.72 (2)° for each molecule in the asymmetric unit, respectively, and the O1—N1—C6—C7 torsion angles being 18.51 (2)° and 15.10 (2)° for each molecule, respectively. Molecules in the crystal structure (Fig. 2) are stacked in parallel pairs along the a axis through π-π interactions, where the distance between C1 to C6 (centroid Cg2) and C12 to C18 (centroid Cg5) is 4.1954 Å [symmetry operation: x, y, z]. The crystal structure is further stabilized by weak C—H···O hydrogen bonds (Table 1). Additional stabilization arises from C—H···π interactions involving C22—H10A with C3—C4—C5—N2—N3 (centroid Cg1) and C10—H14C with C14—C15—C16—N5—N6 (centroid Cg4) imidazole rings having X—Cg···H distances of 3.6317 (17) Å and 3.6818 (17) Å, respectively.
Experimental
Known compound 1 (Fig. 3) could be prepared in one step from commercially available starting material according to the procedure of Elderfield (Elderfield et al., 1946). Desymmetrization by Zinin mono reduction (Mizzoni & Spoerri, 1945) to 5-methoxy-3-nitrobenzene-1,2-diamine, 2 (Fig. 3), proceeded well. Reductive alkylation between 2 (Fig.3) and acetone generated the 2-isopropyl intermediate which was directly cyclized to 3 (Fig. 3) in formic acid and concentrated hydrochloric acid.
All compounds were obtained from Aldrich or Acros and used as supplied unless otherwise indicated. All reactions were conducted under an atmosphere of N2 unless otherwise indicated. Melting points were determined on a MelTemp apparatus and are uncorrected. 1H NMR analyses were determined on a 400 MHz Brucker NMR, and elemental analyses were preformed by Atlantic Microlabs.
CAUTION: Although the nitration to generate 1 (Fig. 3) proceeded in a controlled manner consistent with the literature (Elderfield et al., 1946) and the differential scanning calorimetry of solid 1 (Fig. 3) did not indicate an exothermic event upon melting, these compounds should be handled with great care and proper precaution.
5-Methoxy-3-nitrobenzene-1,2-diamine (2, Fig. 3): A solution of 4-methoxy-2,6-dinitroaniline (Elderfield et al., 1946) (1, Fig.3) (1.07 g, 5.0 mmole) in 50 ml of 100% EtOH was added to a 10% aqueous solution of (NH4)2S. This mixture was warmed to 333 K (60 °C). and stirred at 333 K (60 °C) for 1.5 h. This mixture was cooled to 273 K (0 °C) and the solid was collected on a #1 Whatman filter paper. The collected solids were washed with 5 ml of ice-cold CS2 then 5.0 ml of ice cold water to afford 0.65 g red needles after drying (70%). MP: 456–457.9 K (183–184.9 °C). 1H NMR (CDCl3) δ 3.53 (br s, 2H), 3.78 (s, 3H), 5.70 (s, 3H), 6.63 (s, 1H), 7.17 (s, 1H). Anal. Calcd for C7H9N3O3: C, 45.90; H, 4.95; N, 22.94. Found: C, 45.97; H, 4.99; N, 22.82.
1-Isopropyl-6-methoxy-4-nitro-1H-benzo[d]imidazole (3, Fig. 3): A solution of NaHB(O2CH)3 was prepared by slowly adding 99% formic acid (10.0 ml, 290 mmole) to a suspension of NaBH4 (1.83 g, 48.4 mmole) in THF (50 ml) at 273 K (0 °C) followed by stirring for an additional 5 min at 273 K (0 °C). To this solution was added a solution of 5-methoxy-3-nitrobenzene-1,2-diamine (2, Fig. 3) (2.1609 g, 11.8 mmole) in ACS grade acetone (20 ml) and THF (50.0 ml). The ice bath was removed and the mixture was permitted to stir for an additional 2 h at 296 K (23 °C). The solvent was removed under reduced pressure and the residue was dissolved into ice-cold 200 ml formic acid and then 60.0 ml concentrated HCl was added at 273 K (0 °C). This was brought directly to reflux for 15 min. The reaction was cooled to ambient temperature and the solvent was removed under reduced pressure. The residue was taken up into 15 ml ice-cold water and with cooling with an ice bath was made basic with a slight excess of 6 N NaOH. The precipitated dark solid was collected on #1 Whatman filter paper to 2.8 g. This solid was separated on SiO2 with 1:1 hexanes/ethyl acetate to afford 1.7 g of a bright yellow solid (74%). This material was recrystallized from CHCl3 to produce the material for crystallographic analysis. MP: 399–400 K (126–127 °C). 1H (CDCl3): δ 1.67 (2 t, 30 Hz, 6H), 3.96 (s, 1H), 4.69 (m, 1H), 7.24 (s, 1H), 7.82 (s, 1H), 8.26 (s, 1H). Anal. Calcd for C11H13N3O3: C, 56.16; H, 5.57; N, 17.86. C, 56.45; H,5.62; N, 17.55.
Refinement
H atoms were positioned geometrically (aromatic C—H = 0.93 Å, methyl C—H = 0.96 Å, and methylene C–H = 0.98 Å) and treated with a riding model in subsequent refinement cycles. The isotropic displacement parameters were set to 1.2 Ueq (C) or 1.5 Ueq (methyl C) of the carrier atom.
Figures
Fig. 1.
The molecular structure of the title compound, shown with 30% probability displacement ellipsoids (arbitrary spheres for H atoms).
Fig. 2.
A packing diagram of (I) showing the π-π stacking interactions along the a axis between the benzimidazole rings (dashed line).
Fig. 3.
The molecular synthesis scheme used to produce the title compound (3). The following were also used: a: (NH4)2S,EtOH; b: formic acid, acetone, THF; c: conc. HCl, formic acid.
Crystal data
| C11H13N3O3 | F000 = 992.0 |
| Mr = 235.24 | Dx = 1.424 Mg m−3 |
| Orthorhombic, P212121 | Mo Kα radiation λ = 0.71073 Å |
| Hall symbol: P 2ac 2ab | Cell parameters from 12882 reflections |
| a = 7.63920 (10) Å | θ = 2.3–30.9º |
| b = 16.0029 (3) Å | µ = 0.11 mm−1 |
| c = 17.9496 (3) Å | T = 150 (2) K |
| V = 2194.33 (6) Å3 | Rhomboid, yellow |
| Z = 8 | 0.43 × 0.33 × 0.28 mm |
Data collection
| Bruker SMART APEXII diffractometer | 3989 independent reflections |
| Radiation source: fine-focus sealed tube | 3649 reflections with I > 2σ(I) |
| Monochromator: graphite | Rint = 0.026 |
| T = 296(2) K | θmax = 31.2º |
| φ and ω scans | θmin = 1.7º |
| Absorption correction: multi-scan(SADABS; Sheldrick, 2002) | h = −11→11 |
| Tmin = 0.959, Tmax = 0.971 | k = −23→23 |
| 39389 measured reflections | l = −25→26 |
Refinement
| Refinement on F2 | Secondary atom site location: difference Fourier map |
| Least-squares matrix: full | Hydrogen site location: inferred from neighbouring sites |
| R[F2 > 2σ(F2)] = 0.034 | H-atom parameters constrained |
| wR(F2) = 0.099 | w = 1/[σ2(Fo2) + (0.0626P)2 + 0.1952P] where P = (Fo2 + 2Fc2)/3 |
| S = 1.09 | (Δ/σ)max = 0.006 |
| 3989 reflections | Δρmax = 0.47 e Å−3 |
| 313 parameters | Δρmin = −0.20 e Å−3 |
| Primary atom site location: structure-invariant direct methods | Extinction correction: none |
Special details
| Geometry. All e.s.d.'s (except the e.s.d. in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell e.s.d.'s are taken into account individually in the estimation of e.s.d.'s in distances, angles and torsion angles; correlations between e.s.d.'s in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell e.s.d.'s is used for estimating e.s.d.'s involving l.s. planes. |
| Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F2, conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > σ(F2) is used only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2 are statistically about twice as large as those based on F, and R- factors based on ALL data will be even larger. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| O6 | 0.21889 (17) | 0.44035 (7) | 0.15295 (6) | 0.0298 (2) | |
| N4 | 0.04946 (18) | 0.68089 (7) | 0.29007 (7) | 0.0257 (2) | |
| C14 | 0.24765 (18) | 0.47797 (8) | 0.35375 (7) | 0.0192 (2) | |
| N6 | 0.28545 (16) | 0.45465 (7) | 0.42604 (6) | 0.0205 (2) | |
| C16 | 0.17746 (18) | 0.55944 (8) | 0.35923 (7) | 0.0201 (2) | |
| C18 | 0.13756 (19) | 0.55466 (9) | 0.22533 (8) | 0.0233 (3) | |
| H5 | 0.0985 | 0.5799 | 0.1817 | 0.028* | |
| C13 | 0.26894 (18) | 0.43410 (8) | 0.28697 (7) | 0.0209 (2) | |
| H7 | 0.3191 | 0.3812 | 0.2856 | 0.025* | |
| O5 | −0.0279 (2) | 0.70366 (8) | 0.23365 (7) | 0.0401 (3) | |
| C17 | 0.12331 (19) | 0.59655 (8) | 0.29232 (8) | 0.0213 (2) | |
| C12 | 0.2109 (2) | 0.47402 (9) | 0.22273 (7) | 0.0222 (3) | |
| N5 | 0.17167 (17) | 0.58463 (7) | 0.43318 (7) | 0.0236 (2) | |
| O4 | 0.0687 (2) | 0.72566 (7) | 0.34467 (7) | 0.0436 (3) | |
| C21 | 0.2167 (2) | 0.30465 (8) | 0.43829 (8) | 0.0258 (3) | |
| H9A | 0.1150 | 0.3176 | 0.4675 | 0.039* | |
| H9B | 0.2632 | 0.2517 | 0.4537 | 0.039* | |
| H9C | 0.1850 | 0.3020 | 0.3866 | 0.039* | |
| C19 | 0.2891 (2) | 0.35769 (9) | 0.14715 (8) | 0.0290 (3) | |
| H11A | 0.4060 | 0.3569 | 0.1668 | 0.043* | |
| H11B | 0.2913 | 0.3410 | 0.0958 | 0.043* | |
| H11C | 0.2172 | 0.3197 | 0.1750 | 0.043* | |
| C20 | 0.35454 (19) | 0.37233 (8) | 0.44953 (8) | 0.0222 (2) | |
| H8 | 0.4568 | 0.3588 | 0.4189 | 0.027* | |
| C15 | 0.2357 (2) | 0.52027 (8) | 0.46970 (8) | 0.0235 (3) | |
| H1 | 0.2463 | 0.5195 | 0.5213 | 0.028* | |
| C22 | 0.4111 (2) | 0.37599 (10) | 0.53068 (8) | 0.0300 (3) | |
| H10A | 0.4943 | 0.4204 | 0.5372 | 0.045* | |
| H10B | 0.4640 | 0.3238 | 0.5445 | 0.045* | |
| H10C | 0.3108 | 0.3861 | 0.5616 | 0.045* | |
| C5 | 0.63745 (18) | 0.55518 (8) | 0.10934 (7) | 0.0200 (2) | |
| N1 | 0.51798 (17) | 0.67741 (7) | 0.17962 (7) | 0.0257 (2) | |
| O3 | 0.74288 (17) | 0.44725 (7) | 0.31636 (5) | 0.0290 (2) | |
| C6 | 0.60053 (18) | 0.59506 (8) | 0.17718 (8) | 0.0209 (2) | |
| N3 | 0.74022 (16) | 0.45062 (7) | 0.04131 (6) | 0.0197 (2) | |
| C3 | 0.71541 (17) | 0.47535 (8) | 0.11420 (7) | 0.0185 (2) | |
| C1 | 0.71502 (19) | 0.47734 (9) | 0.24614 (7) | 0.0215 (2) | |
| C2 | 0.75604 (18) | 0.43486 (8) | 0.18085 (7) | 0.0205 (2) | |
| H18 | 0.8078 | 0.3823 | 0.1816 | 0.025* | |
| N2 | 0.61682 (17) | 0.57845 (7) | 0.03556 (6) | 0.0233 (2) | |
| C7 | 0.63817 (19) | 0.55723 (9) | 0.24427 (8) | 0.0229 (3) | |
| H20 | 0.6126 | 0.5847 | 0.2886 | 0.027* | |
| O1 | 0.5265 (2) | 0.71814 (9) | 0.23746 (8) | 0.0493 (4) | |
| O2 | 0.44371 (17) | 0.70267 (7) | 0.12361 (7) | 0.0342 (3) | |
| C11 | 0.6778 (2) | 0.29985 (8) | 0.03307 (8) | 0.0252 (3) | |
| H13A | 0.6454 | 0.3014 | 0.0847 | 0.038* | |
| H13B | 0.7288 | 0.2465 | 0.0217 | 0.038* | |
| H13C | 0.5757 | 0.3082 | 0.0028 | 0.038* | |
| C4 | 0.67924 (19) | 0.51421 (8) | −0.00193 (8) | 0.0231 (3) | |
| H15 | 0.6815 | 0.5125 | −0.0537 | 0.028* | |
| C9 | 0.80998 (18) | 0.36854 (8) | 0.01721 (7) | 0.0207 (2) | |
| H12 | 0.9166 | 0.3568 | 0.0457 | 0.025* | |
| C10 | 0.8569 (2) | 0.37134 (10) | −0.06505 (8) | 0.0268 (3) | |
| H14A | 0.7523 | 0.3781 | −0.0940 | 0.040* | |
| H14B | 0.9138 | 0.3202 | −0.0789 | 0.040* | |
| H14C | 0.9343 | 0.4175 | −0.0742 | 0.040* | |
| C8 | 0.8120 (2) | 0.36434 (9) | 0.32175 (8) | 0.0270 (3) | |
| H22A | 0.7336 | 0.3259 | 0.2978 | 0.041* | |
| H22B | 0.8245 | 0.3494 | 0.3733 | 0.041* | |
| H22C | 0.9242 | 0.3621 | 0.2978 | 0.041* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| O6 | 0.0465 (6) | 0.0262 (5) | 0.0168 (4) | 0.0047 (5) | 0.0021 (4) | −0.0026 (4) |
| N4 | 0.0284 (6) | 0.0188 (5) | 0.0301 (6) | −0.0002 (5) | −0.0001 (5) | 0.0018 (4) |
| C14 | 0.0220 (6) | 0.0178 (5) | 0.0179 (5) | −0.0014 (5) | 0.0023 (4) | −0.0004 (4) |
| N6 | 0.0256 (5) | 0.0189 (5) | 0.0169 (5) | 0.0000 (4) | 0.0010 (4) | −0.0004 (4) |
| C16 | 0.0227 (5) | 0.0177 (5) | 0.0201 (5) | −0.0023 (5) | 0.0020 (5) | −0.0006 (4) |
| C18 | 0.0282 (6) | 0.0230 (6) | 0.0187 (6) | −0.0020 (5) | 0.0006 (5) | 0.0012 (5) |
| C13 | 0.0250 (6) | 0.0192 (5) | 0.0185 (5) | −0.0004 (5) | 0.0031 (5) | −0.0010 (4) |
| O5 | 0.0549 (8) | 0.0277 (6) | 0.0377 (7) | 0.0083 (6) | −0.0124 (6) | 0.0042 (5) |
| C17 | 0.0233 (6) | 0.0171 (5) | 0.0236 (6) | −0.0016 (5) | 0.0014 (5) | 0.0007 (5) |
| C12 | 0.0277 (6) | 0.0225 (6) | 0.0165 (5) | −0.0024 (5) | 0.0026 (5) | −0.0004 (5) |
| N5 | 0.0296 (6) | 0.0204 (5) | 0.0207 (5) | −0.0006 (4) | 0.0031 (4) | −0.0032 (4) |
| O4 | 0.0650 (9) | 0.0257 (5) | 0.0402 (7) | 0.0130 (6) | −0.0125 (7) | −0.0092 (5) |
| C21 | 0.0304 (7) | 0.0203 (6) | 0.0266 (6) | −0.0002 (5) | 0.0000 (5) | −0.0001 (5) |
| C19 | 0.0358 (8) | 0.0259 (7) | 0.0252 (6) | −0.0001 (6) | 0.0027 (6) | −0.0064 (5) |
| C20 | 0.0237 (6) | 0.0210 (6) | 0.0218 (6) | 0.0029 (5) | 0.0009 (5) | 0.0013 (5) |
| C15 | 0.0294 (7) | 0.0218 (6) | 0.0194 (6) | −0.0021 (5) | 0.0019 (5) | −0.0031 (5) |
| C22 | 0.0319 (7) | 0.0327 (7) | 0.0254 (7) | 0.0010 (6) | −0.0068 (6) | 0.0043 (6) |
| C5 | 0.0221 (5) | 0.0175 (5) | 0.0204 (6) | −0.0012 (5) | −0.0016 (5) | −0.0002 (4) |
| N1 | 0.0285 (6) | 0.0191 (5) | 0.0296 (6) | 0.0024 (4) | −0.0017 (5) | −0.0039 (5) |
| O3 | 0.0439 (6) | 0.0263 (5) | 0.0167 (4) | 0.0065 (5) | −0.0040 (4) | 0.0010 (4) |
| C6 | 0.0228 (6) | 0.0167 (5) | 0.0230 (6) | 0.0002 (5) | −0.0020 (5) | −0.0028 (5) |
| N3 | 0.0256 (5) | 0.0179 (5) | 0.0157 (4) | 0.0001 (4) | −0.0005 (4) | −0.0007 (4) |
| C3 | 0.0215 (5) | 0.0170 (5) | 0.0170 (5) | −0.0018 (5) | −0.0001 (4) | −0.0016 (4) |
| C1 | 0.0257 (6) | 0.0225 (6) | 0.0162 (5) | −0.0012 (5) | −0.0023 (5) | −0.0003 (5) |
| C2 | 0.0247 (6) | 0.0187 (5) | 0.0181 (5) | 0.0000 (5) | −0.0021 (5) | 0.0001 (4) |
| N2 | 0.0291 (6) | 0.0197 (5) | 0.0210 (5) | 0.0005 (4) | −0.0031 (4) | 0.0008 (4) |
| C7 | 0.0275 (6) | 0.0226 (6) | 0.0185 (5) | 0.0001 (5) | −0.0022 (5) | −0.0032 (5) |
| O1 | 0.0723 (10) | 0.0350 (7) | 0.0405 (8) | 0.0216 (7) | −0.0183 (7) | −0.0192 (6) |
| O2 | 0.0425 (7) | 0.0281 (5) | 0.0320 (6) | 0.0112 (5) | −0.0047 (5) | 0.0008 (4) |
| C11 | 0.0312 (7) | 0.0197 (6) | 0.0247 (6) | −0.0003 (5) | 0.0022 (5) | −0.0016 (5) |
| C4 | 0.0292 (6) | 0.0214 (6) | 0.0187 (6) | −0.0005 (5) | −0.0022 (5) | 0.0017 (5) |
| C9 | 0.0237 (6) | 0.0195 (5) | 0.0189 (5) | 0.0024 (5) | 0.0000 (5) | −0.0020 (4) |
| C10 | 0.0306 (7) | 0.0297 (7) | 0.0201 (6) | 0.0021 (6) | 0.0036 (5) | −0.0017 (5) |
| C8 | 0.0335 (7) | 0.0232 (6) | 0.0243 (6) | −0.0003 (6) | −0.0019 (6) | 0.0051 (5) |
Geometric parameters (Å, °)
| O6—C12 | 1.3649 (16) | C5—N2 | 1.3846 (17) |
| O6—C19 | 1.4312 (18) | C5—C6 | 1.4035 (18) |
| N4—O4 | 1.2229 (17) | C5—C3 | 1.4122 (18) |
| N4—O5 | 1.2279 (18) | N1—O2 | 1.2231 (17) |
| N4—C17 | 1.4635 (17) | N1—O1 | 1.2277 (17) |
| C14—N6 | 1.3807 (16) | N1—C6 | 1.4615 (17) |
| C14—C13 | 1.3986 (17) | O3—C1 | 1.3659 (16) |
| C14—C16 | 1.4131 (18) | O3—C8 | 1.4314 (17) |
| N6—C15 | 1.3643 (17) | C6—C7 | 1.3781 (19) |
| N6—C20 | 1.4805 (16) | N3—C4 | 1.3620 (17) |
| C16—N5 | 1.3879 (17) | N3—C3 | 1.3800 (16) |
| C16—C17 | 1.4022 (18) | N3—C9 | 1.4820 (16) |
| C18—C17 | 1.3810 (19) | C3—C2 | 1.3955 (17) |
| C18—C12 | 1.408 (2) | C1—C2 | 1.3907 (18) |
| C18—H5 | 0.9300 | C1—C7 | 1.4073 (19) |
| C13—C12 | 1.3908 (18) | C2—H18 | 0.9300 |
| C13—H7 | 0.9300 | N2—C4 | 1.3179 (18) |
| N5—C15 | 1.3153 (18) | C7—H20 | 0.9300 |
| C21—C20 | 1.524 (2) | C11—C9 | 1.5195 (19) |
| C21—H9A | 0.9600 | C11—H13A | 0.9600 |
| C21—H9B | 0.9600 | C11—H13B | 0.9600 |
| C21—H9C | 0.9600 | C11—H13C | 0.9600 |
| C19—H11A | 0.9600 | C4—H15 | 0.9300 |
| C19—H11B | 0.9600 | C9—C10 | 1.5202 (19) |
| C19—H11C | 0.9600 | C9—H12 | 0.9800 |
| C20—C22 | 1.520 (2) | C10—H14A | 0.9600 |
| C20—H8 | 0.9800 | C10—H14B | 0.9600 |
| C15—H1 | 0.9300 | C10—H14C | 0.9600 |
| C22—H10A | 0.9600 | C8—H22A | 0.9600 |
| C22—H10B | 0.9600 | C8—H22B | 0.9600 |
| C22—H10C | 0.9600 | C8—H22C | 0.9600 |
| C12—O6—C19 | 116.63 (11) | N2—C5—C6 | 133.21 (12) |
| O4—N4—O5 | 123.03 (13) | N2—C5—C3 | 110.51 (11) |
| O4—N4—C17 | 118.16 (12) | C6—C5—C3 | 116.26 (11) |
| O5—N4—C17 | 118.82 (13) | O2—N1—O1 | 122.99 (13) |
| N6—C14—C13 | 130.20 (12) | O2—N1—C6 | 118.27 (12) |
| N6—C14—C16 | 105.27 (11) | O1—N1—C6 | 118.75 (13) |
| C13—C14—C16 | 124.53 (12) | C1—O3—C8 | 116.52 (11) |
| C15—N6—C14 | 105.88 (11) | C7—C6—C5 | 121.09 (12) |
| C15—N6—C20 | 128.35 (11) | C7—C6—N1 | 117.39 (12) |
| C14—N6—C20 | 125.65 (11) | C5—C6—N1 | 121.51 (12) |
| N5—C16—C17 | 133.37 (12) | C4—N3—C3 | 106.19 (11) |
| N5—C16—C14 | 110.28 (11) | C4—N3—C9 | 128.25 (11) |
| C17—C16—C14 | 116.30 (11) | C3—N3—C9 | 125.47 (11) |
| C17—C18—C12 | 120.35 (12) | N3—C3—C2 | 130.47 (12) |
| C17—C18—H5 | 119.8 | N3—C3—C5 | 105.00 (11) |
| C12—C18—H5 | 119.8 | C2—C3—C5 | 124.53 (12) |
| C12—C13—C14 | 116.29 (12) | O3—C1—C2 | 124.77 (12) |
| C12—C13—H7 | 121.9 | O3—C1—C7 | 114.03 (11) |
| C14—C13—H7 | 121.9 | C2—C1—C7 | 121.20 (12) |
| C18—C17—C16 | 121.13 (12) | C1—C2—C3 | 116.45 (12) |
| C18—C17—N4 | 117.00 (12) | C1—C2—H18 | 121.8 |
| C16—C17—N4 | 121.87 (12) | C3—C2—H18 | 121.8 |
| O6—C12—C13 | 124.43 (13) | C4—N2—C5 | 103.73 (11) |
| O6—C12—C18 | 114.21 (12) | C6—C7—C1 | 120.47 (12) |
| C13—C12—C18 | 121.36 (12) | C6—C7—H20 | 119.8 |
| C15—N5—C16 | 103.74 (11) | C1—C7—H20 | 119.8 |
| C20—C21—H9A | 109.5 | C9—C11—H13A | 109.5 |
| C20—C21—H9B | 109.5 | C9—C11—H13B | 109.5 |
| H9A—C21—H9B | 109.5 | H13A—C11—H13B | 109.5 |
| C20—C21—H9C | 109.5 | C9—C11—H13C | 109.5 |
| H9A—C21—H9C | 109.5 | H13A—C11—H13C | 109.5 |
| H9B—C21—H9C | 109.5 | H13B—C11—H13C | 109.5 |
| O6—C19—H11A | 109.5 | N2—C4—N3 | 114.57 (12) |
| O6—C19—H11B | 109.5 | N2—C4—H15 | 122.7 |
| H11A—C19—H11B | 109.5 | N3—C4—H15 | 122.7 |
| O6—C19—H11C | 109.5 | N3—C9—C10 | 110.01 (11) |
| H11A—C19—H11C | 109.5 | N3—C9—C11 | 110.34 (11) |
| H11B—C19—H11C | 109.5 | C10—C9—C11 | 111.10 (12) |
| N6—C20—C22 | 109.87 (11) | N3—C9—H12 | 108.4 |
| N6—C20—C21 | 110.38 (11) | C10—C9—H12 | 108.4 |
| C22—C20—C21 | 110.53 (12) | C11—C9—H12 | 108.4 |
| N6—C20—H8 | 108.7 | C9—C10—H14A | 109.5 |
| C22—C20—H8 | 108.7 | C9—C10—H14B | 109.5 |
| C21—C20—H8 | 108.7 | H14A—C10—H14B | 109.5 |
| N5—C15—N6 | 114.83 (12) | C9—C10—H14C | 109.5 |
| N5—C15—H1 | 122.6 | H14A—C10—H14C | 109.5 |
| N6—C15—H1 | 122.6 | H14B—C10—H14C | 109.5 |
| C20—C22—H10A | 109.5 | O3—C8—H22A | 109.5 |
| C20—C22—H10B | 109.5 | O3—C8—H22B | 109.5 |
| H10A—C22—H10B | 109.5 | H22A—C8—H22B | 109.5 |
| C20—C22—H10C | 109.5 | O3—C8—H22C | 109.5 |
| H10A—C22—H10C | 109.5 | H22A—C8—H22C | 109.5 |
| H10B—C22—H10C | 109.5 | H22B—C8—H22C | 109.5 |
Hydrogen-bond geometry (Å, °)
| D—H···A | D—H | H···A | D···A | D—H···A |
| C15—H1···O6i | 0.93 | 2.46 | 3.3670 (8) | 164 |
| C4—H15···O3ii | 0.93 | 2.49 | 3.3723 (8) | 159 |
Symmetry codes: (i) −x+1/2, −y+1, z+1/2; (ii) −x+3/2, −y+1, z−1/2.
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: ZL2123).
References
- Banks, B. J. (1984). Annu. Rep. Med. Chem.19, 147–156.
- Bentancor, L., Trelles, J. A., Nobile, M., Lewkowicz, E. S. & Iribarren, A. M. (2004). J. Mol. Catal. B: Enzym.29, 3–7.
- Bruker (1998). APEX2, SMART and SAINT Bruker AXS Inc., Madison, Wisconsin, USA.
- Chackalamannil, S., Ahn, H.-S., Xia, Y., Doller, D. & Foster, C. (2003). Curr. Med. Chem. Cardio. Hemato. Agent, 1, 37–45. [DOI] [PubMed]
- Elderfield, R. C., Gensler, W. J. & Birstein, O. (1946). J. Org. Chem., 8, 812–822. [DOI] [PubMed]
- Farrugia, L. J. (1997). J. Appl. Cryst. 837–838.
- Flaherty, P. T., Jain, P., Lipay, J., Chopra, I. & Yi, S. (2008). In preparation. For submission to Org. Lett
- Grimmett, M. R. (2002). Sci. Synth 12, 529–612.
- Kamal, A., Reddy, K. L., Devaiah, V., Shankaraiah, N. & Rao, M. V. (2006). Mini Rev. Med. Chem.6, 71–89. [DOI] [PubMed]
- Kumar, B. V., Rathore, H. G. S. & Reddy, V. M. (1982). Indian J. Chem. Sect. B, 21, 1126–1127.
- Lanusse, C. E. & Prichard, R. K. (1993). Drug Metab. Rev.25, 235–279. [DOI] [PubMed]
- Lopez-Rodriguez, M. L., Benhamu, B., Morcillo, M. J., Murcia, M., Viso, A., Campillo, M. & Pardo, L. (2002). Current Topics in Medicinal Chemistry, Vol 2, pp. 625–641. The Netherlands: Hilversum. [DOI] [PubMed]
- Mizzoni, R. H. & Spoerri, P. E. (1945). J. Chem. Soc 67, 1652–1654.
- Nicolaou, K. C., Trujillo, J. I., Jandeleit, B., Chibale, K., Rosenfeld, M., Diefenbach, B., Cheresh, D. A. & Goodman, S. L. (1998). Bioorg. Med. Chem.6, 1185–1208. [DOI] [PubMed]
- Reddy, V. M. & Reddy, K. K. (1979). Indian J. Chem. Sect. B, 17, 353–356.
- Sachs, G., Shin, J. M., Briving, C., Wallmark, B. & Hersey, S. (1995). Annu. Rev. Pharmacol. Toxicol.35, 277–305. [DOI] [PubMed]
- Scholz, B., Rechter, S., Drach, J. C., Townsend, L. B. & Bogner, E. (2003). Nucleic Acids Res.31, 1426–1433. [DOI] [PMC free article] [PubMed]
- Sharma, S. & Abuzar, S. (1983). Prog. Drug Res.27, 85–161. [DOI] [PubMed]
- Sheldrick, G. M. (2002). SADABS University of Göttingen, Germany.
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Shin, J. M., Besancon, M., Bamberg, K. & Sachs, G. (1997). Ann. N. Y. Acad. Sci.834, 65–76. [DOI] [PubMed]
- Somsak, L., Nagy, V., Hadady, Z., Docsa, T. & Gergely, P. (2003). Curr. Pharm. Des., 9, 1177–1189. [DOI] [PubMed]
- Spek, A. L. (2003). J. Appl. Cryst.36, 7–13.
- Townsend, L. B. & Revankar, G. R. (1970). Chem. Rev.70, 389–438. [DOI] [PubMed]
- Wang, C. C. (1984). J. Med. Chem.27, 1–9. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablocks I, global. DOI: 10.1107/S160053680801859X/zl2123sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S160053680801859X/zl2123Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report