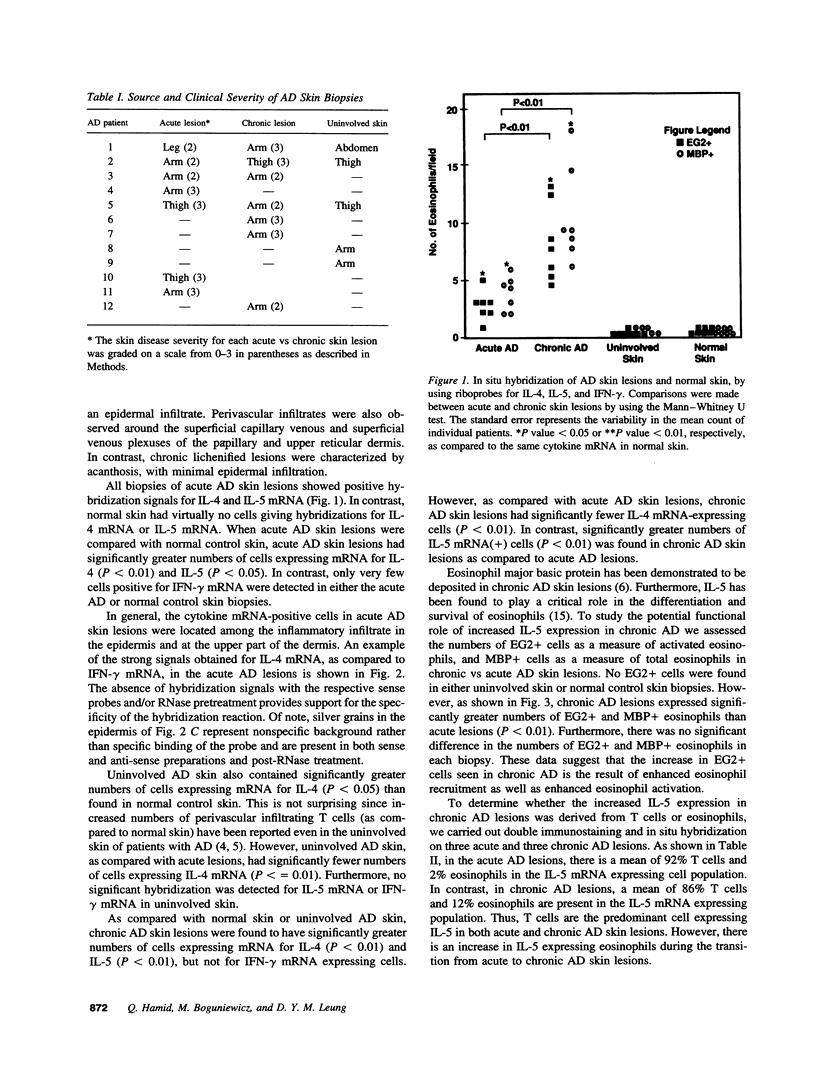
Abstract
The mechanisms involved in the initiation and maintenance of skin inflammation in atopic dermatitis (AD) are poorly understood. Recent data suggest that the pattern of cytokines expressed locally plays a critical role in modulating the nature of tissue inflammation. In this study, we used in situ hybridization to investigate the expression of interleukin 4 (IL-4), IL-5, and interferon-gamma (IFN-gamma) messenger RNA (mRNA) in skin biopsies from acute and chronic skin lesions of patients with AD. As compared with normal control skin or uninvolved skin of patients with AD, acute and chronic skin lesions had significantly greater numbers of cells that were positive for mRNA, IL-4 (P < 0.01), and IL-5 (P < 0.01), but not for IFN-gamma mRNA expressing cells. However, as compared with acute AD skin lesions, chronic AD skin lesions had significantly fewer IL-4 mRNA-expressing cells (P < 0.01), but significantly greater IL-5 mRNA (P < 0.01). T cells constituted the majority of IL-5-expressing cells in acute and chronic AD lesions. Chronic lesions also expressed significantly greater numbers of activated EG2+ eosinophils than acute lesions (P < 0.01). These data indicate that although acute and chronic AD lesions are associated with increased activation of IL-4 and IL-5 genes, initiation of acute skin inflammation in AD is associated with a predominance of IL-4 expression whereas maintenance of chronic inflammation is predominantly associated with increased IL-5 expression and eosinophil infiltration.
Full text
PDF

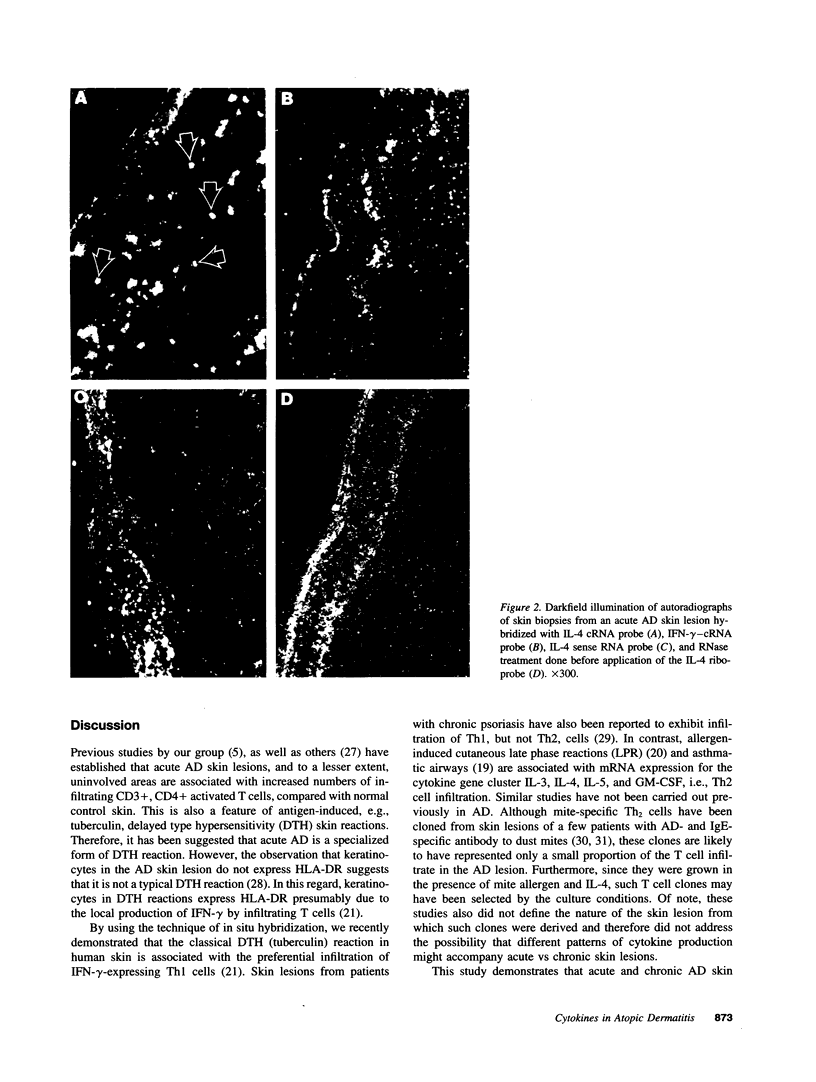



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barker J. N., MacDonald D. M. Epidermal class II human lymphocyte antigen expression in atopic dermatitis: a comparison with experimental allergic contact dermatitis. J Am Acad Dermatol. 1987 Jun;16(6):1175–1179. doi: 10.1016/s0190-9622(87)70153-4. [DOI] [PubMed] [Google Scholar]
- Berg E. L., Yoshino T., Rott L. S., Robinson M. K., Warnock R. A., Kishimoto T. K., Picker L. J., Butcher E. C. The cutaneous lymphocyte antigen is a skin lymphocyte homing receptor for the vascular lectin endothelial cell-leukocyte adhesion molecule 1. J Exp Med. 1991 Dec 1;174(6):1461–1466. doi: 10.1084/jem.174.6.1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bos J. D., Hagenaars C., Das P. K., Krieg S. R., Voorn W. J., Kapsenberg M. L. Predominance of "memory" T cells (CD4+, CDw29+) over "naive" T cells (CD4+, CD45R+) in both normal and diseased human skin. Arch Dermatol Res. 1989;281(1):24–30. doi: 10.1007/BF00424268. [DOI] [PubMed] [Google Scholar]
- Colver G. B., Symons J. A., Duff G. W. Soluble interleukin 2 receptor in atopic eczema. BMJ. 1989 May 27;298(6685):1426–1428. doi: 10.1136/bmj.298.6685.1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czech W., Krutmann J., Schöpf E., Kapp A. Serum eosinophil cationic protein (ECP) is a sensitive measure for disease activity in atopic dermatitis. Br J Dermatol. 1992 Apr;126(4):351–355. doi: 10.1111/j.1365-2133.1992.tb00677.x. [DOI] [PubMed] [Google Scholar]
- Gajewski T. F., Fitch F. W. Anti-proliferative effect of IFN-gamma in immune regulation. I. IFN-gamma inhibits the proliferation of Th2 but not Th1 murine helper T lymphocyte clones. J Immunol. 1988 Jun 15;140(12):4245–4252. [PubMed] [Google Scholar]
- Gajewski T. F., Schell S. R., Fitch F. W. Evidence implicating utilization of different T cell receptor-associated signaling pathways by TH1 and TH2 clones. J Immunol. 1990 Jun 1;144(11):4110–4120. [PubMed] [Google Scholar]
- Geha R. S. Regulation of IgE synthesis in humans. J Allergy Clin Immunol. 1992 Aug;90(2):143–150. doi: 10.1016/0091-6749(92)90064-9. [DOI] [PubMed] [Google Scholar]
- Gundel R. H., Gerritsen M. E., Gleich G. J., Wegner C. D. Repeated antigen inhalation results in a prolonged airway eosinophilia and airway hyperresponsiveness in primates. J Appl Physiol (1985) 1990 Feb;68(2):779–786. doi: 10.1152/jappl.1990.68.2.779. [DOI] [PubMed] [Google Scholar]
- Hamid Q., Wharton J., Terenghi G., Hassall C. J., Aimi J., Taylor K. M., Nakazato H., Dixon J. E., Burnstock G., Polak J. M. Localization of atrial natriuretic peptide mRNA and immunoreactivity in the rat heart and human atrial appendage. Proc Natl Acad Sci U S A. 1987 Oct;84(19):6760–6764. doi: 10.1073/pnas.84.19.6760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jujo K., Renz H., Abe J., Gelfand E. W., Leung D. Y. Decreased interferon gamma and increased interleukin-4 production in atopic dermatitis promotes IgE synthesis. J Allergy Clin Immunol. 1992 Sep;90(3 Pt 1):323–331. doi: 10.1016/s0091-6749(05)80010-7. [DOI] [PubMed] [Google Scholar]
- Kay A. B., Ying S., Varney V., Gaga M., Durham S. R., Moqbel R., Wardlaw A. J., Hamid Q. Messenger RNA expression of the cytokine gene cluster, interleukin 3 (IL-3), IL-4, IL-5, and granulocyte/macrophage colony-stimulating factor, in allergen-induced late-phase cutaneous reactions in atopic subjects. J Exp Med. 1991 Mar 1;173(3):775–778. doi: 10.1084/jem.173.3.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leiferman K. M., Ackerman S. J., Sampson H. A., Haugen H. S., Venencie P. Y., Gleich G. J. Dermal deposition of eosinophil-granule major basic protein in atopic dermatitis. Comparison with onchocerciasis. N Engl J Med. 1985 Aug 1;313(5):282–285. doi: 10.1056/NEJM198508013130502. [DOI] [PubMed] [Google Scholar]
- Leiferman K. M. Eosinophils in atopic dermatitis. Allergy. 1989;44 (Suppl 9):20–26. [PubMed] [Google Scholar]
- Leung D. Y., Bhan A. K., Schneeberger E. E., Geha R. S. Characterization of the mononuclear cell infiltrate in atopic dermatitis using monoclonal antibodies. J Allergy Clin Immunol. 1983 Jan;71(1 Pt 1):47–56. doi: 10.1016/0091-6749(83)90546-8. [DOI] [PubMed] [Google Scholar]
- Leung D. Y. Immunopathology of atopic dermatitis. Springer Semin Immunopathol. 1992;13(3-4):427–440. doi: 10.1007/BF00200539. [DOI] [PubMed] [Google Scholar]
- Mihm M. C., Jr, Soter N. A., Dvorak H. F., Austen K. F. The structure of normal skin and the morphology of atopic eczema. J Invest Dermatol. 1976 Sep;67(3):305–312. doi: 10.1111/1523-1747.ep12514346. [DOI] [PubMed] [Google Scholar]
- Montefort S., Roche W. R., Howarth P. H., Djukanovic R., Gratziou C., Carroll M., Smith L., Britten K. M., Haskard D., Lee T. H. Intercellular adhesion molecule-1 (ICAM-1) and endothelial leucocyte adhesion molecule-1 (ELAM-1) expression in the bronchial mucosa of normal and asthmatic subjects. Eur Respir J. 1992 Jul;5(7):815–823. [PubMed] [Google Scholar]
- Moqbel R., Barkans J., Bradley B. L., Durham S. R., Kay A. B. Application of monoclonal antibodies against major basic protein (BMK-13) and eosinophil cationic protein (EG1 and EG2) for quantifying eosinophils in bronchial biopsies from atopic asthma. Clin Exp Allergy. 1992 Feb;22(2):265–273. doi: 10.1111/j.1365-2222.1992.tb03082.x. [DOI] [PubMed] [Google Scholar]
- Mosmann T. R., Cherwinski H., Bond M. W., Giedlin M. A., Coffman R. L. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol. 1986 Apr 1;136(7):2348–2357. [PubMed] [Google Scholar]
- Müller K. M., Jaunin F., Masouyé I., Saurat J. H., Hauser C. Th2 cells mediate IL-4-dependent local tissue inflammation. J Immunol. 1993 Jun 15;150(12):5576–5584. [PubMed] [Google Scholar]
- Picker L. J., Butcher E. C. Physiological and molecular mechanisms of lymphocyte homing. Annu Rev Immunol. 1992;10:561–591. doi: 10.1146/annurev.iy.10.040192.003021. [DOI] [PubMed] [Google Scholar]
- Plaut M., Pierce J. H., Watson C. J., Hanley-Hyde J., Nordan R. P., Paul W. E. Mast cell lines produce lymphokines in response to cross-linkage of Fc epsilon RI or to calcium ionophores. Nature. 1989 May 4;339(6219):64–67. doi: 10.1038/339064a0. [DOI] [PubMed] [Google Scholar]
- Raghow B., Irish P., Kang A. H. Coordinate regulation of transforming growth factor beta gene expression and cell proliferation in hamster lungs undergoing bleomycin-induced pulmonary fibrosis. J Clin Invest. 1989 Dec;84(6):1836–1842. doi: 10.1172/JCI114369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renz H., Jujo K., Bradley K. L., Domenico J., Gelfand E. W., Leung D. Y. Enhanced IL-4 production and IL-4 receptor expression in atopic dermatitis and their modulation by interferon-gamma. J Invest Dermatol. 1992 Oct;99(4):403–408. doi: 10.1111/1523-1747.ep12616114. [DOI] [PubMed] [Google Scholar]
- Robinson D. S., Hamid Q., Ying S., Tsicopoulos A., Barkans J., Bentley A. M., Corrigan C., Durham S. R., Kay A. B. Predominant TH2-like bronchoalveolar T-lymphocyte population in atopic asthma. N Engl J Med. 1992 Jan 30;326(5):298–304. doi: 10.1056/NEJM199201303260504. [DOI] [PubMed] [Google Scholar]
- Saito H., Hatake K., Dvorak A. M., Leiferman K. M., Donnenberg A. D., Arai N., Ishizaka K., Ishizaka T. Selective differentiation and proliferation of hematopoietic cells induced by recombinant human interleukins. Proc Natl Acad Sci U S A. 1988 Apr;85(7):2288–2292. doi: 10.1073/pnas.85.7.2288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlaak J. F., Buslau M., Jochum W., Hermann E., Girndt M., Gallati H., Meyer zum Büschenfelde K. H., Fleischer B. T cells involved in psoriasis vulgaris belong to the Th1 subset. J Invest Dermatol. 1994 Feb;102(2):145–149. doi: 10.1111/1523-1747.ep12371752. [DOI] [PubMed] [Google Scholar]
- Schleimer R. P., Sterbinsky S. A., Kaiser J., Bickel C. A., Klunk D. A., Tomioka K., Newman W., Luscinskas F. W., Gimbrone M. A., Jr, McIntyre B. W. IL-4 induces adherence of human eosinophils and basophils but not neutrophils to endothelium. Association with expression of VCAM-1. J Immunol. 1992 Feb 15;148(4):1086–1092. [PubMed] [Google Scholar]
- Shah M., Foreman D. M., Ferguson M. W. Control of scarring in adult wounds by neutralising antibody to transforming growth factor beta. Lancet. 1992 Jan 25;339(8787):213–214. doi: 10.1016/0140-6736(92)90009-r. [DOI] [PubMed] [Google Scholar]
- Sowden J. M., Berth-Jones J., Ross J. S., Motley R. J., Marks R., Finlay A. Y., Salek M. S., Graham-Brown R. A., Allen B. R., Camp R. D. Double-blind, controlled, crossover study of cyclosporin in adults with severe refractory atopic dermatitis. Lancet. 1991 Jul 20;338(8760):137–140. doi: 10.1016/0140-6736(91)90134-b. [DOI] [PubMed] [Google Scholar]
- Tai P. C., Spry C. J., Peterson C., Venge P., Olsson I. Monoclonal antibodies distinguish between storage and secreted forms of eosinophil cationic protein. Nature. 1984 May 10;309(5964):182–184. doi: 10.1038/309182a0. [DOI] [PubMed] [Google Scholar]
- Tsicopoulos A., Hamid Q., Varney V., Ying S., Moqbel R., Durham S. R., Kay A. B. Preferential messenger RNA expression of Th1-type cells (IFN-gamma+, IL-2+) in classical delayed-type (tuberculin) hypersensitivity reactions in human skin. J Immunol. 1992 Apr 1;148(7):2058–2061. [PubMed] [Google Scholar]
- Weller P. F. Roles of eosinophils in allergy. Curr Opin Immunol. 1992 Dec;4(6):782–787. doi: 10.1016/0952-7915(92)90062-j. [DOI] [PubMed] [Google Scholar]
- Willemze R., de Graaff-Reitsma C. B., Cnossen J., Van Vloten W. A., Meijer C. J. Characterization of T-cell subpopulations in skin and peripheral blood of patients with cutaneous T-cell lymphomas and benign inflammatory dermatoses. J Invest Dermatol. 1983 Jan;80(1):60–66. doi: 10.1111/1523-1747.ep12531102. [DOI] [PubMed] [Google Scholar]
- Wong D. T., Elovic A., Matossian K., Nagura N., McBride J., Chou M. Y., Gordon J. R., Rand T. H., Galli S. J., Weller P. F. Eosinophils from patients with blood eosinophilia express transforming growth factor beta 1. Blood. 1991 Nov 15;78(10):2702–2707. [PubMed] [Google Scholar]
- Ying S., Durham S. R., Barkans J., Masuyama K., Jacobson M., Rak S., Löwhagen O., Moqbel R., Kay A. B., Hamid Q. A. T cells are the principal source of interleukin-5 mRNA in allergen-induced rhinitis. Am J Respir Cell Mol Biol. 1993 Oct;9(4):356–360. doi: 10.1165/ajrcmb/9.4.356. [DOI] [PubMed] [Google Scholar]
- van Joost T., Kozel M. M., Tank B., Troost R., Prens E. P. Cyclosporine in atopic dermatitis. Modulation in the expression of immunologic markers in lesional skin. J Am Acad Dermatol. 1992 Dec;27(6 Pt 1):922–928. [PubMed] [Google Scholar]
- van Reijsen F. C., Bruijnzeel-Koomen C. A., Kalthoff F. S., Maggi E., Romagnani S., Westland J. K., Mudde G. C. Skin-derived aeroallergen-specific T-cell clones of Th2 phenotype in patients with atopic dermatitis. J Allergy Clin Immunol. 1992 Aug;90(2):184–193. doi: 10.1016/0091-6749(92)90070-i. [DOI] [PubMed] [Google Scholar]
- van der Heijden F. L., Wierenga E. A., Bos J. D., Kapsenberg M. L. High frequency of IL-4-producing CD4+ allergen-specific T lymphocytes in atopic dermatitis lesional skin. J Invest Dermatol. 1991 Sep;97(3):389–394. doi: 10.1111/1523-1747.ep12480966. [DOI] [PubMed] [Google Scholar]