Abstract
The title compound, C27H19BrN4O, is a mono-anil Schiff base ligand. Three intramolecular O—H⋯N and N—H⋯N hydrogen bonds involving the hydroxy and amino groups generate S(6) and S(5) ring motifs, respectively. In the crystal structure, weak intermolecular N—H⋯O and C—H⋯O hydrogen bonds together with π–π interactions [centroid–centroid distances = 3.628 (3)–3.729 (3) Å] link neighboring molecules.
Related literature
For details of hydrogen-bond motifs, see: Bernstein et al. (1995 ▶). For bond-length data, see: Allen et al. (1987 ▶). For related structures see, for example: Corden et al. (1996 ▶); Govindasamy et al. (1999 ▶). For applications and bioactivities see, for example: Blower (1998 ▶); Cohen & Schmidt (1964 ▶); Granovski et al. (1993 ▶); Kia et al. (2004 ▶); Li & Chang (1991 ▶); Shahrokhian et al. (2000 ▶); Uhlenbrock et al. (1996 ▶); Unaleroglu & Hokelek (2002 ▶). For related literature, see: Anderson et al. (1997 ▶); Blower (1998 ▶).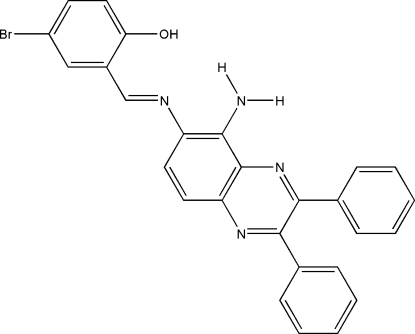
Experimental
Crystal data
C27H19BrN4O
M r = 495.37
Monoclinic,

a = 22.923 (5) Å
b = 7.344 (5) Å
c = 12.573 (5) Å
β = 92.070 (5)°
V = 2115.2 (17) Å3
Z = 4
Mo Kα radiation
μ = 1.97 mm−1
T = 100.0 (1) K
0.39 × 0.37 × 0.08 mm
Data collection
Bruker SMART APEXII CCD area-detector diffractometer
Absorption correction: multi-scan (SADABS; Bruker, 2005 ▶) T min = 0.510, T max = 0.853
22554 measured reflections
6205 independent reflections
3722 reflections with I > 2σ(I)
R int = 0.070
Refinement
R[F 2 > 2σ(F 2)] = 0.045
wR(F 2) = 0.142
S = 1.06
6205 reflections
305 parameters
H atoms treated by a mixture of independent and constrained refinement
Δρmax = 0.61 e Å−3
Δρmin = −1.07 e Å−3
Data collection: APEX2 (Bruker, 2005 ▶); cell refinement: APEX2; data reduction: SAINT (Bruker, 2005 ▶); program(s) used to solve structure: SHELXTL (Sheldrick, 2008 ▶); program(s) used to refine structure: SHELXTL; molecular graphics: SHELXTL; software used to prepare material for publication: SHELXTL and PLATON (Spek, 2003 ▶).
Supplementary Material
Crystal structure: contains datablocks global, I. DOI: 10.1107/S1600536808017716/sj2516sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536808017716/sj2516Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Table 1. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| O1—H1O1⋯N1 | 0.83 | 1.83 | 2.586 (4) | 151 |
| N2—H2N2⋯N4 | 0.88 (4) | 2.35 (4) | 2.740 (4) | 107 (3) |
| N2—H1N2⋯N1 | 0.88 (4) | 2.44 (4) | 2.756 (4) | 102 (3) |
Acknowledgments
HKF and RK thank the Malaysian Government and Universiti sains Malaysia for the Science Fund grant No. 305/PFIZIK/613312. RK thanks Universiti Sains Malaysia and the University of Bath for a post-doctoral research fellowship.
supplementary crystallographic information
Comment
Schiff bases are among the most prevalent mixed-donor ligands in coordination chemistry. Schiff bases and their biologically active complexes have been studied over several decades (Anderson et al., 1997; Blower 1998; Corden et al., 1996; Govindasamy et al., 1999; Granovski et al., 1993; Li & Chang, 1991; Shahrokhian et al., 2000). 2-hydroxy Schiff base ligands are of interest mainly due to the existence of O—H···N and O···H—N type hydrogen bonds and tautomerization between the phenol-imine and keto-amine forms (Unaleroglu & Hokelek, 2002; Kia et al., 2004). This type of tautomerism plays an important role for distinguishing their photochromic and thermochromic properties (Cohen & Schmidt, 1964). Knowing the structures of free Schiff bases in solution and in the solid state is important in view of the intramolecular hydrogen bonding and comparison with the structure of Schiff base complexes. In view of the importance of these organic ligands, the title compound (I) was synthesized and its crystal structure is reported here.
In the title compound (Fig. 1), intramolecular O—H···N, and N—H···N hydrogen bonds form six and five-membered rings, producing S(6) and S(5) ring motifs, respectively (Bernstein et al., 1995). The two phenyl substituents on the quinoxaline unit are inclined at an angle of 17.87 (17)° to one another. They also form dihedral angles of 38.96 (15) and 44.46 (15)° with the ten–membered quinoxaline ring. In the crystal packing (Fig. 2), molecules are stacked along the b axis by π···π interactions with Cg2···Cg3 distances ranging from 3.628 (3) – 3.729 (3) Å: symmetry codes 1 - x, 1/2 + y, 3/2 - z and 1 - x, -1/2 + y, 3/2 - z; Cg2 and Cg3 are the centroids of the C1–C6 and C8/C9/C10/C11/C14/C15 phenyl rings, respectively. The crystal structure is stabilized by intramolecular O—H···N, and N—H···N hydrogen bonds, weak intermolecular N—H···O and C—H···O hydrogen bonds, and π···π interactions.
Experimental
A mixture of o-diaminoquinoxaline (313 mg, 1 mmol) and 5-bromo salicylaldehyde (210 mg, 1 mmol) was suspended in 30 ml absolute ethanol. The reaction mixture was stirred under reflux for 1 h. After cooling, the precipitate was filtered, washed with ethanol and ether and dried under vacuum. Single crystals suitable for X-ray diffraction were obtained by evaporation of a mixed chloroform-ethanol (3/1, v/v) solution at room temperature.
Refinement
The H-atoms attached to O1 were located from the difference Fourier map and refined as riding with the parent atom with an isotropic thermal parameter 1.2 times that of the parent atom. The H-atoms bound to N were located in a difference Fourier map and refined freely with the parent atom with an isotropic thermal parameter 1.2 times that of the parent atom. The rest of the hydrogen atoms were positioned geometrically [C—H = 0.93 Å] and refined using a riding model, with thermal parameters 1.2 times those of the parent atoms.
Figures
Fig. 1.
The molecular structure of the title compound, showing 50% probability displacement ellipsoids and the atomic numbering. Intramolecular interactions are drawn as dashed lines.
Fig. 2.
The crystal packing of (I), viewed along the b-axis with hydrogen bonds drawn as dashed lines.
Crystal data
| C27H19BrN4O | F000 = 1008 |
| Mr = 495.37 | Dx = 1.556 Mg m−3 |
| Monoclinic, P21/c | Mo Kα radiation λ = 0.71069 Å |
| Hall symbol: -P 2ybc | Cell parameters from 4509 reflections |
| a = 22.923 (5) Å | θ = 2.9–28.3º |
| b = 7.344 (5) Å | µ = 1.97 mm−1 |
| c = 12.573 (5) Å | T = 100.0 (1) K |
| β = 92.070 (5)º | Block, yellow |
| V = 2115.2 (17) Å3 | 0.39 × 0.37 × 0.08 mm |
| Z = 4 |
Data collection
| Bruker SMART APEXII CCD area-detector diffractometer | 6205 independent reflections |
| Radiation source: fine-focus sealed tube | 3722 reflections with I > 2σ(I) |
| Monochromator: graphite | Rint = 0.070 |
| T = 100.0(1) K | θmax = 30.1º |
| φ and ω scans | θmin = 0.9º |
| Absorption correction: multi-scan(SADABS; Bruker, 2005) | h = −32→32 |
| Tmin = 0.510, Tmax = 0.853 | k = −10→8 |
| 22554 measured reflections | l = −16→17 |
Refinement
| Refinement on F2 | Secondary atom site location: difference Fourier map |
| Least-squares matrix: full | Hydrogen site location: inferred from neighbouring sites |
| R[F2 > 2σ(F2)] = 0.045 | H atoms treated by a mixture of independent and constrained refinement |
| wR(F2) = 0.142 | w = 1/[σ2(Fo2) + (0.0698P)2] where P = (Fo2 + 2Fc2)/3 |
| S = 1.07 | (Δ/σ)max = 0.001 |
| 6205 reflections | Δρmax = 0.62 e Å−3 |
| 305 parameters | Δρmin = −1.07 e Å−3 |
| Primary atom site location: structure-invariant direct methods | Extinction correction: none |
Special details
| Experimental. The low-temperature data was collected with the Oxford Cyrosystem Cobra low-temperature attachment. |
| Geometry. All e.s.d.'s (except the e.s.d. in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell e.s.d.'s are taken into account individually in the estimation of e.s.d.'s in distances, angles and torsion angles; correlations between e.s.d.'s in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell e.s.d.'s is used for estimating e.s.d.'s involving l.s. planes. |
| Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F2, conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > σ(F2) is used only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2 are statistically about twice as large as those based on F, and R- factors based on ALL data will be even larger. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| Br1 | 0.259515 (13) | 0.53134 (5) | 0.59180 (3) | 0.02170 (12) | |
| N1 | 0.52495 (11) | 0.6363 (4) | 0.7764 (2) | 0.0171 (6) | |
| N2 | 0.60814 (13) | 0.5339 (4) | 0.9285 (2) | 0.0186 (6) | |
| N4 | 0.72399 (11) | 0.5294 (4) | 0.8819 (2) | 0.0154 (6) | |
| N3 | 0.76064 (11) | 0.6677 (4) | 0.6873 (2) | 0.0169 (6) | |
| O1 | 0.44969 (9) | 0.7345 (3) | 0.91363 (18) | 0.0206 (5) | |
| H1O1 | 0.4813 | 0.7101 | 0.8877 | 0.025* | |
| C1 | 0.40804 (13) | 0.6934 (4) | 0.8389 (3) | 0.0177 (7) | |
| C2 | 0.34965 (14) | 0.7191 (4) | 0.8608 (3) | 0.0188 (7) | |
| H2 | 0.3398 | 0.7694 | 0.9256 | 0.023* | |
| C3 | 0.30643 (13) | 0.6715 (5) | 0.7882 (3) | 0.0205 (8) | |
| H3 | 0.2675 | 0.6883 | 0.8044 | 0.025* | |
| C4 | 0.32022 (13) | 0.5985 (5) | 0.6910 (3) | 0.0183 (7) | |
| C5 | 0.37775 (13) | 0.5769 (4) | 0.6645 (3) | 0.0176 (7) | |
| H5 | 0.3866 | 0.5305 | 0.5982 | 0.021* | |
| C6 | 0.42273 (13) | 0.6246 (4) | 0.7373 (3) | 0.0162 (7) | |
| C7 | 0.48319 (13) | 0.6048 (5) | 0.7087 (3) | 0.0166 (7) | |
| H7 | 0.4917 | 0.5688 | 0.6400 | 0.010 (8)* | |
| C8 | 0.58402 (12) | 0.6335 (4) | 0.7490 (3) | 0.0146 (7) | |
| C9 | 0.60282 (13) | 0.6891 (4) | 0.6473 (3) | 0.0174 (7) | |
| H9 | 0.5752 | 0.7203 | 0.5945 | 0.021* | |
| C10 | 0.66057 (13) | 0.6973 (4) | 0.6258 (3) | 0.0170 (7) | |
| H10 | 0.6721 | 0.7344 | 0.5591 | 0.020* | |
| C11 | 0.70293 (13) | 0.6494 (4) | 0.7053 (3) | 0.0159 (7) | |
| C14 | 0.68498 (13) | 0.5891 (4) | 0.8062 (3) | 0.0135 (6) | |
| C15 | 0.62412 (13) | 0.5842 (4) | 0.8283 (3) | 0.0157 (7) | |
| C12 | 0.79874 (13) | 0.6218 (5) | 0.7641 (3) | 0.0165 (7) | |
| C13 | 0.78033 (13) | 0.5390 (4) | 0.8603 (3) | 0.0161 (7) | |
| C16 | 0.82122 (13) | 0.4561 (5) | 0.9406 (3) | 0.0174 (7) | |
| C17 | 0.81113 (14) | 0.4750 (5) | 1.0488 (3) | 0.0185 (7) | |
| H17 | 0.7793 | 0.5428 | 1.0702 | 0.022* | |
| C18 | 0.84782 (13) | 0.3944 (5) | 1.1246 (3) | 0.0199 (7) | |
| H18 | 0.8408 | 0.4086 | 1.1965 | 0.024* | |
| C19 | 0.89531 (14) | 0.2918 (5) | 1.0935 (3) | 0.0223 (8) | |
| H19 | 0.9205 | 0.2393 | 1.1444 | 0.027* | |
| C20 | 0.90483 (14) | 0.2686 (5) | 0.9858 (3) | 0.0224 (8) | |
| H20 | 0.9358 | 0.1970 | 0.9645 | 0.027* | |
| C21 | 0.86863 (13) | 0.3509 (5) | 0.9104 (3) | 0.0196 (7) | |
| H21 | 0.8758 | 0.3364 | 0.8386 | 0.024* | |
| C22 | 0.86036 (13) | 0.6776 (4) | 0.7467 (3) | 0.0166 (7) | |
| C23 | 0.89337 (13) | 0.7640 (5) | 0.8263 (3) | 0.0198 (7) | |
| H23 | 0.8786 | 0.7758 | 0.8939 | 0.024* | |
| C24 | 0.94824 (14) | 0.8330 (5) | 0.8060 (3) | 0.0220 (8) | |
| H24 | 0.9701 | 0.8916 | 0.8595 | 0.026* | |
| C25 | 0.97015 (14) | 0.8138 (5) | 0.7048 (3) | 0.0215 (8) | |
| H25 | 1.0068 | 0.8603 | 0.6906 | 0.026* | |
| C26 | 0.93829 (14) | 0.7271 (5) | 0.6262 (3) | 0.0215 (8) | |
| H26 | 0.9535 | 0.7137 | 0.5590 | 0.026* | |
| C27 | 0.88320 (13) | 0.6588 (5) | 0.6463 (3) | 0.0198 (7) | |
| H27 | 0.8615 | 0.6004 | 0.5925 | 0.024* | |
| H2N2 | 0.6362 (17) | 0.487 (5) | 0.970 (3) | 0.024* | |
| H1N2 | 0.5740 (17) | 0.479 (5) | 0.925 (3) | 0.024* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| Br1 | 0.01741 (16) | 0.02067 (18) | 0.0269 (2) | −0.00067 (13) | −0.00060 (13) | 0.00133 (17) |
| N1 | 0.0177 (13) | 0.0150 (15) | 0.0187 (16) | 0.0014 (10) | 0.0023 (11) | 0.0015 (12) |
| N2 | 0.0185 (13) | 0.0240 (15) | 0.0134 (15) | −0.0013 (12) | 0.0037 (11) | 0.0011 (13) |
| N4 | 0.0168 (12) | 0.0141 (13) | 0.0152 (15) | −0.0001 (10) | 0.0007 (10) | −0.0019 (12) |
| N3 | 0.0178 (13) | 0.0166 (15) | 0.0163 (15) | −0.0007 (10) | 0.0011 (11) | −0.0018 (12) |
| O1 | 0.0200 (11) | 0.0251 (14) | 0.0169 (13) | −0.0002 (9) | 0.0033 (10) | −0.0002 (11) |
| C1 | 0.0213 (16) | 0.0129 (17) | 0.0190 (19) | −0.0012 (12) | 0.0019 (14) | 0.0034 (14) |
| C2 | 0.0228 (16) | 0.0151 (17) | 0.0189 (19) | 0.0015 (12) | 0.0044 (14) | 0.0051 (14) |
| C3 | 0.0166 (15) | 0.0174 (18) | 0.028 (2) | 0.0021 (12) | 0.0043 (14) | 0.0079 (15) |
| C4 | 0.0191 (15) | 0.0133 (16) | 0.022 (2) | −0.0001 (12) | 0.0016 (13) | 0.0046 (15) |
| C5 | 0.0197 (15) | 0.0155 (17) | 0.0178 (19) | 0.0018 (12) | 0.0028 (13) | 0.0011 (13) |
| C6 | 0.0186 (15) | 0.0131 (17) | 0.0168 (18) | −0.0003 (12) | 0.0026 (13) | 0.0018 (14) |
| C7 | 0.0198 (15) | 0.0130 (16) | 0.0171 (19) | 0.0009 (12) | 0.0010 (13) | 0.0022 (14) |
| C8 | 0.0155 (14) | 0.0128 (16) | 0.0156 (18) | −0.0012 (11) | 0.0012 (12) | −0.0013 (13) |
| C9 | 0.0194 (15) | 0.0153 (17) | 0.0174 (19) | −0.0003 (12) | 0.0001 (13) | 0.0022 (14) |
| C10 | 0.0190 (15) | 0.0168 (17) | 0.0155 (18) | −0.0007 (12) | 0.0027 (13) | 0.0025 (14) |
| C11 | 0.0179 (15) | 0.0135 (16) | 0.0163 (18) | −0.0003 (11) | 0.0034 (13) | −0.0002 (13) |
| C14 | 0.0181 (15) | 0.0085 (14) | 0.0137 (17) | 0.0002 (11) | −0.0012 (12) | −0.0002 (13) |
| C15 | 0.0216 (15) | 0.0112 (15) | 0.0145 (18) | −0.0016 (12) | 0.0027 (13) | −0.0036 (13) |
| C12 | 0.0154 (14) | 0.0182 (17) | 0.0161 (18) | 0.0002 (12) | 0.0028 (13) | −0.0020 (14) |
| C13 | 0.0164 (14) | 0.0151 (16) | 0.0169 (18) | 0.0011 (12) | 0.0021 (12) | −0.0046 (15) |
| C16 | 0.0155 (14) | 0.0176 (17) | 0.0192 (19) | −0.0021 (12) | 0.0018 (12) | 0.0008 (15) |
| C17 | 0.0195 (15) | 0.0181 (16) | 0.0180 (19) | −0.0017 (13) | 0.0024 (13) | −0.0013 (15) |
| C18 | 0.0201 (16) | 0.0218 (18) | 0.0179 (19) | −0.0010 (13) | 0.0003 (13) | 0.0030 (15) |
| C19 | 0.0174 (16) | 0.025 (2) | 0.024 (2) | 0.0000 (13) | −0.0026 (14) | 0.0043 (16) |
| C20 | 0.0173 (15) | 0.0227 (19) | 0.028 (2) | 0.0054 (13) | 0.0036 (14) | 0.0021 (16) |
| C21 | 0.0201 (16) | 0.0191 (18) | 0.0198 (19) | 0.0015 (12) | 0.0037 (14) | 0.0013 (15) |
| C22 | 0.0160 (14) | 0.0162 (17) | 0.0180 (18) | 0.0006 (12) | 0.0034 (13) | −0.0004 (14) |
| C23 | 0.0188 (15) | 0.0250 (19) | 0.0158 (19) | −0.0009 (13) | 0.0034 (13) | −0.0012 (15) |
| C24 | 0.0202 (16) | 0.0253 (19) | 0.020 (2) | −0.0019 (13) | 0.0001 (14) | 0.0012 (16) |
| C25 | 0.0162 (15) | 0.027 (2) | 0.022 (2) | 0.0006 (13) | 0.0034 (14) | 0.0050 (16) |
| C26 | 0.0203 (16) | 0.028 (2) | 0.0165 (19) | 0.0026 (13) | 0.0049 (14) | 0.0002 (16) |
| C27 | 0.0210 (16) | 0.0221 (19) | 0.0164 (19) | 0.0029 (13) | 0.0015 (13) | −0.0019 (15) |
Geometric parameters (Å, °)
| Br1—C4 | 1.900 (4) | C10—H10 | 0.9300 |
| N1—C7 | 1.279 (4) | C11—C14 | 1.418 (4) |
| N1—C8 | 1.409 (3) | C14—C15 | 1.433 (4) |
| N2—C15 | 1.376 (4) | C12—C13 | 1.431 (4) |
| N2—H2N2 | 0.88 (4) | C12—C22 | 1.495 (4) |
| N2—H1N2 | 0.88 (4) | C13—C16 | 1.483 (5) |
| N4—C13 | 1.331 (4) | C16—C17 | 1.396 (5) |
| N4—C14 | 1.355 (4) | C16—C21 | 1.397 (4) |
| N3—C12 | 1.322 (4) | C17—C18 | 1.382 (5) |
| N3—C11 | 1.357 (4) | C17—H17 | 0.9300 |
| O1—C1 | 1.350 (4) | C18—C19 | 1.391 (4) |
| O1—H1O1 | 0.8254 | C18—H18 | 0.9300 |
| C1—C2 | 1.389 (4) | C19—C20 | 1.390 (5) |
| C1—C6 | 1.425 (5) | C19—H19 | 0.9300 |
| C2—C3 | 1.368 (5) | C20—C21 | 1.377 (5) |
| C2—H2 | 0.9300 | C20—H20 | 0.9300 |
| C3—C4 | 1.382 (5) | C21—H21 | 0.9300 |
| C3—H3 | 0.9300 | C22—C23 | 1.386 (5) |
| C4—C5 | 1.381 (4) | C22—C27 | 1.391 (4) |
| C5—C6 | 1.398 (5) | C23—C24 | 1.388 (4) |
| C5—H5 | 0.9300 | C23—H23 | 0.9300 |
| C6—C7 | 1.452 (4) | C24—C25 | 1.392 (5) |
| C7—H7 | 0.9300 | C24—H24 | 0.9300 |
| C8—C15 | 1.380 (5) | C25—C26 | 1.365 (5) |
| C8—C9 | 1.424 (4) | C25—H25 | 0.9300 |
| C9—C10 | 1.362 (4) | C26—C27 | 1.390 (4) |
| C9—H9 | 0.9300 | C26—H26 | 0.9300 |
| C10—C11 | 1.413 (5) | C27—H27 | 0.9300 |
| C7—N1—C8 | 122.5 (3) | N2—C15—C14 | 118.5 (3) |
| C15—N2—H2N2 | 116 (3) | C8—C15—C14 | 118.8 (3) |
| C15—N2—H1N2 | 110 (3) | N3—C12—C13 | 121.2 (3) |
| H2N2—N2—H1N2 | 118 (4) | N3—C12—C22 | 115.2 (3) |
| C13—N4—C14 | 117.4 (3) | C13—C12—C22 | 123.3 (3) |
| C12—N3—C11 | 118.3 (3) | N4—C13—C12 | 120.8 (3) |
| C1—O1—H1O1 | 106.6 | N4—C13—C16 | 115.7 (3) |
| O1—C1—C2 | 119.6 (3) | C12—C13—C16 | 123.4 (3) |
| O1—C1—C6 | 121.3 (3) | C17—C16—C21 | 118.5 (3) |
| C2—C1—C6 | 119.1 (3) | C17—C16—C13 | 120.0 (3) |
| C3—C2—C1 | 120.9 (3) | C21—C16—C13 | 121.4 (3) |
| C3—C2—H2 | 119.6 | C18—C17—C16 | 120.8 (3) |
| C1—C2—H2 | 119.6 | C18—C17—H17 | 119.6 |
| C2—C3—C4 | 120.4 (3) | C16—C17—H17 | 119.6 |
| C2—C3—H3 | 119.8 | C17—C18—C19 | 120.0 (3) |
| C4—C3—H3 | 119.8 | C17—C18—H18 | 120.0 |
| C5—C4—C3 | 120.6 (3) | C19—C18—H18 | 120.0 |
| C5—C4—Br1 | 119.7 (3) | C20—C19—C18 | 119.5 (3) |
| C3—C4—Br1 | 119.7 (2) | C20—C19—H19 | 120.2 |
| C4—C5—C6 | 120.1 (3) | C18—C19—H19 | 120.2 |
| C4—C5—H5 | 119.9 | C21—C20—C19 | 120.3 (3) |
| C6—C5—H5 | 119.9 | C21—C20—H20 | 119.8 |
| C5—C6—C1 | 118.9 (3) | C19—C20—H20 | 119.8 |
| C5—C6—C7 | 120.0 (3) | C20—C21—C16 | 120.8 (3) |
| C1—C6—C7 | 121.1 (3) | C20—C21—H21 | 119.6 |
| N1—C7—C6 | 121.0 (3) | C16—C21—H21 | 119.6 |
| N1—C7—H7 | 119.5 | C23—C22—C27 | 119.1 (3) |
| C6—C7—H7 | 119.5 | C23—C22—C12 | 121.0 (3) |
| C15—C8—N1 | 116.6 (3) | C27—C22—C12 | 119.6 (3) |
| C15—C8—C9 | 120.6 (3) | C22—C23—C24 | 120.6 (3) |
| N1—C8—C9 | 122.7 (3) | C22—C23—H23 | 119.7 |
| C10—C9—C8 | 121.2 (3) | C24—C23—H23 | 119.7 |
| C10—C9—H9 | 119.4 | C23—C24—C25 | 119.3 (3) |
| C8—C9—H9 | 119.4 | C23—C24—H24 | 120.3 |
| C9—C10—C11 | 119.8 (3) | C25—C24—H24 | 120.3 |
| C9—C10—H10 | 120.1 | C26—C25—C24 | 120.5 (3) |
| C11—C10—H10 | 120.1 | C26—C25—H25 | 119.7 |
| N3—C11—C10 | 120.4 (3) | C24—C25—H25 | 119.7 |
| N3—C11—C14 | 119.8 (3) | C25—C26—C27 | 120.2 (3) |
| C10—C11—C14 | 119.8 (3) | C25—C26—H26 | 119.9 |
| N4—C14—C11 | 121.6 (3) | C27—C26—H26 | 119.9 |
| N4—C14—C15 | 118.6 (3) | C26—C27—C22 | 120.2 (3) |
| C11—C14—C15 | 119.8 (3) | C26—C27—H27 | 119.9 |
| N2—C15—C8 | 122.7 (3) | C22—C27—H27 | 119.9 |
| O1—C1—C2—C3 | −177.3 (3) | C11—C14—C15—N2 | −176.8 (3) |
| C6—C1—C2—C3 | 3.1 (5) | N4—C14—C15—C8 | −175.8 (3) |
| C1—C2—C3—C4 | −0.7 (5) | C11—C14—C15—C8 | 2.0 (5) |
| C2—C3—C4—C5 | −1.7 (5) | C11—N3—C12—C13 | 5.7 (5) |
| C2—C3—C4—Br1 | 179.5 (2) | C11—N3—C12—C22 | −169.0 (3) |
| C3—C4—C5—C6 | 1.6 (5) | C14—N4—C13—C12 | 4.5 (4) |
| Br1—C4—C5—C6 | −179.5 (2) | C14—N4—C13—C16 | −174.3 (3) |
| C4—C5—C6—C1 | 0.7 (5) | N3—C12—C13—N4 | −9.6 (5) |
| C4—C5—C6—C7 | −178.9 (3) | C22—C12—C13—N4 | 164.7 (3) |
| O1—C1—C6—C5 | 177.3 (3) | N3—C12—C13—C16 | 169.1 (3) |
| C2—C1—C6—C5 | −3.1 (5) | C22—C12—C13—C16 | −16.6 (5) |
| O1—C1—C6—C7 | −3.1 (5) | N4—C13—C16—C17 | −39.1 (4) |
| C2—C1—C6—C7 | 176.6 (3) | C12—C13—C16—C17 | 142.2 (3) |
| C8—N1—C7—C6 | −174.5 (3) | N4—C13—C16—C21 | 137.8 (3) |
| C5—C6—C7—N1 | −175.3 (3) | C12—C13—C16—C21 | −40.9 (5) |
| C1—C6—C7—N1 | 5.0 (5) | C21—C16—C17—C18 | 1.2 (5) |
| C7—N1—C8—C15 | −150.5 (3) | C13—C16—C17—C18 | 178.1 (3) |
| C7—N1—C8—C9 | 33.4 (5) | C16—C17—C18—C19 | −0.4 (5) |
| C15—C8—C9—C10 | −0.8 (5) | C17—C18—C19—C20 | −1.2 (5) |
| N1—C8—C9—C10 | 175.2 (3) | C18—C19—C20—C21 | 2.0 (5) |
| C8—C9—C10—C11 | 0.3 (5) | C19—C20—C21—C16 | −1.2 (5) |
| C12—N3—C11—C10 | 179.8 (3) | C17—C16—C21—C20 | −0.4 (5) |
| C12—N3—C11—C14 | 2.5 (4) | C13—C16—C21—C20 | −177.3 (3) |
| C9—C10—C11—N3 | −175.9 (3) | N3—C12—C22—C23 | 132.1 (3) |
| C9—C10—C11—C14 | 1.4 (5) | C13—C12—C22—C23 | −42.5 (5) |
| C13—N4—C14—C11 | 3.7 (5) | N3—C12—C22—C27 | −41.7 (4) |
| C13—N4—C14—C15 | −178.6 (3) | C13—C12—C22—C27 | 143.7 (3) |
| N3—C11—C14—N4 | −7.5 (5) | C27—C22—C23—C24 | 0.8 (5) |
| C10—C11—C14—N4 | 175.2 (3) | C12—C22—C23—C24 | −173.0 (3) |
| N3—C11—C14—C15 | 174.8 (3) | C22—C23—C24—C25 | −0.4 (5) |
| C10—C11—C14—C15 | −2.5 (5) | C23—C24—C25—C26 | −0.4 (5) |
| N1—C8—C15—N2 | 2.1 (5) | C24—C25—C26—C27 | 0.7 (5) |
| C9—C8—C15—N2 | 178.4 (3) | C25—C26—C27—C22 | −0.3 (5) |
| N1—C8—C15—C14 | −176.6 (3) | C23—C22—C27—C26 | −0.4 (5) |
| C9—C8—C15—C14 | −0.3 (5) | C12—C22—C27—C26 | 173.4 (3) |
| N4—C14—C15—N2 | 5.5 (4) |
Hydrogen-bond geometry (Å, °)
| D—H···A | D—H | H···A | D···A | D—H···A |
| O1—H1O1···N1 | 0.83 | 1.83 | 2.586 (4) | 151 |
| N2—H2N2···N4 | 0.88 (4) | 2.35 (4) | 2.740 (4) | 107 (3) |
| N2—H1N2···N1 | 0.88 (4) | 2.44 (4) | 2.756 (4) | 102 (3) |
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: SJ2516).
References
- Allen, F. H., Kennard, O., Watson, D. G., Brammer, L., Orpen, A. G. & Taylor, R. (1987). J. Chem. Soc. Perkin Trans. 2, pp. S1–S19.
- Anderson, O. P., Cour, A. L., Findeisen, M., Hennig, L., Simonsen, O., Taylor, L. & Toftland, H. L. (1997). J. Chem. Soc. Dalton Trans. pp. 111–120.
- Bernstein, J., Davis, R. E., Shimoni, L. & Chang, N.-L. (1995). Angew. Chem. Int. Ed. Eng1.34, 1555–1573.
- Blower, P. J. (1998). Transition Met. Chem.23, 109–112.
- Bruker (2005). APEX2, SAINT and SADABS Bruker AXS Inc., Madison, Wisconsin, USA.
- Cohen, M. D. & Schmidt, G. M. J. (1964). J. Chem. Soc. pp. 2041–2051.
- Corden, J. P., Bishop, P. R., Errington, W. & Wallbridge, M. G. H. (1996). Acta Cryst. C52, 2777–2779.
- Govindasamy, L., Velmurugan, D. & Rajendran, T. M. (1999). Acta Cryst. C55, 1368–1369.
- Granovski, A. D., Nivorozhkin, A. L. & Minkin, V. I. (1993). Coord. Chem. Rev.126, 1–69.
- Kia, R., Esmaeilbeig, A. & Harkema, S. (2004). Acta Cryst. A60, s267.
- Li, C. H. & Chang, T. C. (1991). Eur. Polym. J.27, 35–39.
- Shahrokhian, S., Amini, M. K., Kia, R. & Tangestaninejad, S. (2000). Anal. Chem.72, 956–962. [DOI] [PubMed]
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Spek, A. L. (2003). J. Appl. Cryst.36, 7–13.
- Uhlenbrock, S., Wegner, R. & Krebs, B. J. (1996). J. Chem. Soc. Dalton Trans. pp. 3731–3736.
- Unaleroglu, C. & Hokelek, T. (2002). Spectrosc. Lett.32, 317–326.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablocks global, I. DOI: 10.1107/S1600536808017716/sj2516sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536808017716/sj2516Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report




