Abstract
The comparative drug dispositions, urinary pharmacokinetics, and effects on renal function of multilamellar liposomal nystatin (LNYS; Nyotran) and amphotericin B deoxycholate (DAMB; Fungizone) were studied in rabbits. Drug concentrations were determined by high-performance liquid chromatography as total concentrations of LNYS and DAMB. In comparison to a standard dose of 1 mg of DAMB/kg of body weight, therapeutic dosages of LNYS, i.e., 2, 4, and 6 mg/kg, resulted in escalating maximum concentrations (Cmax) (17 to 56 μg/ml for LNYS versus 3.36 μg/ml for DAMB; P < 0.001) and values for the area under the concentration-time curve from 0 to 24 h (AUC0-24) (17 to 77 μg · h/ml for LNYS versus 12 μg · h/ml for DAMB; P < 0.001) in plasma but a significantly faster total clearance from plasma (0.117 to 0.080 liter/h/kg for LNYS versus 0.055 liter/h/kg for DAMB; P = 0.013) and a ≤8-fold-smaller volume of distribution at steady state (P = 0.002). Urinary drug concentration data revealed a ≥10-fold-higher Cmax (16 to 10 μg/ml for LNYS versus 0.96 μg/ml for DAMB; P = 0.015) and a 4- to 7-fold-greater AUC0-24 (63 to 35 μg · h/ml for LNYS versus 8.9 μg · h/ml for DAMB; P = 0.015) following the administration of LNYS, with a dose-dependent decrease in the dose-normalized AUC0-24 in urine (P = 0.001) and a trend toward a dose-dependent decrease in renal clearance. Except for the kidneys, the mean concentrations of LNYS in liver, spleen, and lung 24 h after dosing were severalfold lower than those after administration of DAMB (P, <0.002 to <0.001). Less than 1% each of the total dose of LNYS was recovered from the kidneys, liver, spleen, and lungs; in contrast, a quarter of the total dose was recovered from the livers of DAMB-treated animals. LNYS had dose-dependent effects on glomerular filtration and distal, but not proximal, renal tubular function which did not exceed those of DAMB at the highest investigated dosage of 6 mg/kg. The results of this experimental study demonstrate fundamental differences in the dispositions of LNYS and DAMB. Based on its enhanced urinary exposure, LNYS may offer a therapeutic advantage in systemic fungal infections involving the upper and lower urinary tracts that require therapy with antifungal polyenes.
Nystatin (C47H75NO17; molecular weight, 926.13), which was discovered to be the first antifungal polyene antibiotic in the early 1950s (21), contains three biologically active components, A1, A2, and A3. Nystatin A1 is a macrocyclic lactone consisting of a hydroxylated tetraene diene backbone and a mycosamine residue. In a manner similar to that of amphotericin B, nystatin acts by selectively binding to ergosterol in the fungal cell membrane (25) and has potent and broad-spectrum fungicidal activity in vitro (1, 7, 23, 30). While nystatin has found wide therapeutic application for superficial mycoses of the skin and mucous membranes, early problems with solubilization and toxicity after parenteral administration precluded its use for systemic treatment of invasive fungal infections (12).
More recently, a multilamellar liposomal formulation consisting of dimyristoyl phosphatidylcholine (DMPC), dimyristoyl phosphatidylglycerol (DMPG), and nystatin A1 in a 7:3:1 molar ratio and with a particle size of 0.1 to 3 μm (28, 38) was launched for clinical development. This distinct liposomal polyene formulation has been shown to have reduced toxicity to mammalian cells (28; Nyotran investigator brochure, Aronex Pharmaceuticals, The Woodlands, Tex.) but preserved in vitro antifungal activity in comparison to the parent product (1, 7, 23, 30) and has demonstrated an encouraging level of efficacy in animal models of invasive opportunistic fungal infections (10, 13, 15, 29, 34). It was well tolerated without dose-limiting toxicity at dosages of up to 8 mg/kg of body weight/day in a formal phase I dose escalation study (E. I. Boutati, H. C. Maltezou, G. Lopez-Berestein, S. E. Vartivarian, and E. Anaissie, Abstr. 35th Intersci. Conf. Antimicrob. Agents Chemother., abstr. LM22, 1995) and has shown favorable efficacy in phase II clinical trials in nonneutropenic patients with candidemia (A. H. Williams and J. E. Moore, Abstr. 39th Intersci. Conf. Antimicrob. Agents Chemother., abstr. 1420, 1999) and in immunocompromised patients with invasive aspergillosis refractory to or intolerant of standard therapies (F. C. J. Offner, R. Herbrecht, D. Engelhard, H. F. L. Guiot, G. Samonis, A. Marinus, R. J. Roberts, and B. E. DePauw, Abstr. 40th Intersci. Conf. Antimicrob. Agents Chemother., abstr. 1102, 2000).
The pharmacokinetics of liposomal nystatin (LNYS) is characterized by achievement of high peak concentrations but a rapid elimination from the bloodstream, with a terminal half-life ranging from 1 to 1.5 and 2 to 6 h in rabbits and human patients, respectively (16; P. A. Cossum, J. Wyse, V. Simmons, T. L. Wallace, and A. Rios, Abstr. 36th Intersci. Conf. Antimicrob. Agents Chemother., abstr. A88, 1996; Nyotran investigator brochure, Aronex Pharmaceuticals). Little knowledge exists, however, about the disposition of this novel polyene formulation in the body and its differential renal effects. We therefore investigated the single-dose pharmacokinetics of LNYS in plasma and urine and its recovery from tissues and body fluids in rabbits and evaluated its effects on renal function in comparison to those of the standard antifungal polyene, amphotericin B deoxycholate (DAMB).
MATERIALS AND METHODS
Study design.
The study consisted of five experimental cohorts. Four cohorts of three animals each received a single intravenous bolus of either LNYS at 2, 4, or 6 mg/kg or DAMB at 1 mg/kg. Six untreated, healthy animals served as controls. Plasma samples were obtained from all animals prior to dosing and serially for up to 24 h after dosing, and urine was collected at 2-h intervals for a total of 24 h postdosing to determine drug concentrations and parameters of renal function. Treated animals were sacrificed 24 h postdosing, and samples from all parenchymatous organs, muscle, fat tissue, and bile fluid were obtained at autopsy for determination of drug concentrations. The primary end points of the study were the comparative analyses of plasma pharmacokinetics, urinary pharmacokinetics, drug disposition in tissues, and recovery of LNYS and DAMB from tissues, plasma, and urine after one single dose. A secondary end point of the study was the differential assessment by standard methods of the renal effects of a single dose of either LNYS or DAMB.
Animals.
Healthy female New Zealand White rabbits (Hazleton, Denver, Pa.) weighing 3.0 to 3.2 kg were used in all experiments. They were individually housed and given water and standard rabbit feed ad libitum according to the National Institutes of Health Guidelines for Laboratory Animal Care (8) and in fulfillment of American Association for Accreditation of Laboratory Animal Care criteria. Nontraumatic vascular access was established in each rabbit prior to experimentation by the surgical placement of a subcutaneous silastic central venous catheter as previously described (35). Sedation during manual expression of the urinary bladder was achieved by administering 0.3 ml of a 2:1 mixture (vol/vol) of 100 mg of ketamine (Fort Dodge Laboratories) per ml and 20 mg of xylazine (Mobay Corp., Shawney, Kans.) per ml intravenously.
Study drugs.
LNYS (Nyotran [Aronex Pharmaceuticals]; 50-mg vials; 50 mg of 95% pure nystatin A1 [USP] incorporated into a mixture of 350 mg of DMPC and 150 mg of DMPG at a DMPC/DMPG ratio of 7:3 [wt/wt] and a drug/lipid ratio of 1:10 [wt/wt]; particle size after reconstitution, 0.1 to 3.0 μm) was provided as a lyophilized powder and maintained at 4°C. The drug was freshly reconstituted prior to use with 50 ml of sterile normal saline to a 1-mg/ml solution as recommended by the manufacturer and administered intravenously over 10 min at either 2, 4, or 6 mg/kg. DAMB (Fungizone, 50-mg vials; Bristol-Myers Squibb, Princeton, N.J.) was reconstituted as recommended by the manufacturer in 5% dextrose in water to a 1-mg/ml solution and administered intravenously over 10 min at a dosage of 1 mg/kg.
Processing of samples.
Blood samples for analytical and biochemical purposes were collected in heparinized syringes, and plasma was immediately separated by centrifugation and stored at −80°C. Urine was collected and separated from feces at 2-h intervals. For accurate quantitative collection, the urinary bladder was expressed under intravenous sedation prior to and at the end of the experiment. The volume of excreted urine was measured and recorded. The urine was filtered with sterile 0.45-μm-pore-size filter units (Millipore, Bedford, Mass.) to remove particulate matter. A urinalysis was performed (Chemstrip; Boehringer Mannheim, Indianapolis, Ind.), and an aliquot from each sampling interval was transferred into 1.5-ml polypropylene tubes and stored at −80°C until determination of drug concentrations. The remainder of the urine was cumulatively collected in equal portions either without preservative or with sulfamic acid and was maintained at 4°C until submission to biochemical analysis at the end of the 24-h collection period. Tissues and bile fluid were sampled at autopsy; parenchymatous organs were weighed, and aliquots of tissues and bile fluid were stored at −80°C until assay.
Determination of drug concentrations. (i) Sample preparation.
Nystatin and amphotericin B were extracted from plasma, body fluids, and tissues by liquid-liquid extraction. Before assay, tissues were thawed and an aliquot of approximately 1 g was weighed out for each sample on a precision balance (model AE 163; Mettler Instrument Corp., Hightstown, N.J.). The specimens were thoroughly rinsed with phosphate-buffered saline (pH 7.4) (Quality Biological, Inc., Gaithersburg, Md.). The remaining buffer solution on the tissue surface was blotted with Micro Wipes (Scott Paper Company, Philadelphia, Pa.). Specimens were then reweighed and homogenized in ice-cold high-performance liquid chromatography (HPLC)-grade methanol (1:2 [wt/wt]; Fisher Scientific, Fair Lawn, N.J.) in a high-speed tissue homogenizer (Tissumizer; Tekmar, Cincinnati, Ohio) twice for 30 s each. Homogenized samples were incubated for 30 min at 4°C and centrifuged at 2,000 × g for 10 min. The methanolic supernatant was transferred to 1.5-ml polypropylene tubes and centrifuged at 10,000 × g for 4 min. Four hundred microliters of the resulting supernatant was transferred to 0.22-μm-pore-size Durapore filter tubes (Ultra-Free MC; Millipore), centrifuged at 4,000 × g for 4 min, and submitted to assay. Standards and quality control samples were similarly prepared by adding known amounts of either bulk nystatin (Gist-Procades, Wilmington, Del.) or amphotericin B (Sigma, St. Louis, Mo.) to drug-free tissue homogenates. Blank samples of tissue homogenates were also submitted to assay to ensure the absence of interfering peaks. Tissue drug concentrations were calculated to 1 g of tissue.
Extraction of the drug from plasma and body fluids, including urine, involved the addition of 1 ml of HPLC-grade methanol to 500 μl of sample (2:1 [vol/vol]) and incubation at 4°C for 30 min. This was followed by centrifugation at 2,000 × g for 10 min, transfer of the supernatant to 1.5-ml polypropylene tubes, and a second centrifugation at 10,000 × g for 4 min. Four hundred microliters of the resulting supernatant was transferred to 0.22-μm-pore-size Durapore filter tubes (Ultra-Free MC; Millipore), centrifuged at 4,000 × g for 4 min, and submitted to assay. Standards and quality control samples were similarly prepared by adding known amounts of either bulk nystatin (Gist-Procades) or amphotericin B (Sigma) to normal rabbit serum (Gibco Laboratories, Grand Island, N.Y.) or normal rabbit bile or urine, followed by precipitation with HPLC-grade methanol (1:2 [wt/wt]). Blank samples of all matrices also were extracted to ensure the absence of interfering peaks.
(ii) Analytical methods.
Concentrations of nystatin and amphotericin B were determined as total drug concentrations by reversed-phase HPLC and by using bulk nystatin (Gist-Procades) and amphotericin B (Sigma) as reference standards as previously described (14, 17).
In brief, for nystatin, the mobile phase consisted of 10 mM sodium phosphate, 1 mM Na EDTA, 30% HPLC-grade methanol, and 30% HPLC-grade acetonitrile (Fisher Scientific), adjusted to pH 6. Samples were kept at 4°C by a cooling module attached to the autosampler. The injection volume was 200 μl, and the flow rate was 2.0 ml/min. The two major isomers of nystatin eluted at 7.5 to 8.5 and 9.5 to 10.5 min with a C18 analytical column (μBondapak C18 [Waters Corp., Milford, Mass.]; 300 by 3.9 mm inside diameter, 100 Å, 10-μm particle size) maintained at 30°C in conjunction with an in-line precolumn filter (NewGuard RP-18; PerkinElmer, Norwalk, Conn.) and UV detection at 305 nm.
For amphotericin B, the mobile phase consisted of methanol-acetonitrile-0.0025 M Na EDTA (500:350:200 [vol/vol/vol]; Fisher Scientific), delivered at 1.6 ml/min. Samples were kept at 4°C by a cooling module attached to the autosampler. The injection volume was 100 μl. Amphotericin B eluted at 3.4 to 4.5 min with a C18 analytical column (μBondapak C18; Waters Corp.) maintained at room temperature in conjunction with an in-line precolumn filter (NewGuard RP-18; PerkinElmer) and UV detection at 382 nm.
Quantification was based on the sum of the peak height of the two major isomers of nystatin and the peak height of amphotericin B and the nonweighted concentration response of the external calibration standards. Six- to 10-point standard curves were linear with goodness-of-fit (r2) values of >0.990. The lower limit of quantification (LLQ) in plasma was 0.050 μg/ml for nystatin and 0.040 μg/ml for amphotericin B. Accuracies for the nystatin assay were within ±8% (plasma) and ±12% (remaining body fluids and tissues), and intra- and interday variabilities (precision) were <6% for all matrices (±11 and ≤7.5%, respectively, for all matrices in the amphotericin B assay).
Pharmacokinetic data analysis. (i) Pharmacokinetic modeling.
Pharmacokinetic parameters of nystatin and amphotericin B in plasma were determined by using sparse sampling and compartmental analysis.
The time points for sparse plasma sampling were determined by using an optimal sampling theory implemented by the ADAPT II computer program (D. Z. D'Argenio and A. Schumitzky, Biomedical Simulations Resource, University of Southern California, Los Angeles) and full concentration-versus-time plasma drug profiles derived from prior pharmacokinetic studies for the same species (16, 27). Weighting was by maximum a posteriori probability, and model selection was guided by Akaike's information criterion (41). In these studies, plasma profiles of nystatin after administration of the multilamellar liposomal formulation fitted best to a two-compartment pharmacokinetic model, and those of DAMB were best described by a three-compartment pharmacokinetic model with intravenous bolus input and elimination from the central compartment. The selected time points for plasma sampling were immediately after administration of the dose (at the time of maximum concentration of drug in serum [Cmax]) and 0.5, 1, 3, 6, 12, and 24 h postdosing for nystatin and at the Cmax and 0.25, 2, 3, 8, 12, and 24 h postdosing for amphotericin B.
Estimation of pharmacokinetic parameters was based on Bayesian estimation using the observed plasma drug concentration data at the selected sampling time points and microconstants derived from full profiles from the above-referenced studies as prior estimates. The r2 value between model-estimated and observed concentration data ranged from 0.996 to 1.000 for nystatin and 0.971 to 0.974 for amphotericin B. The regression lines through the plot of observed versus estimated concentrations did not differ from the line of identity, and no bias was observed.
(ii) Urinary pharmacokinetics and recovery.
Calculation of the area under the concentration-time curve from 0 to 24 h for urine (AUC0-24U) was based on drug concentrations measured in 2-h urine collections and performed by the trapezoidal rule. Mean Cmax achieved in urine (CmaxU) and time to occurrence of CmaxU (TmaxU) were calculated from the parameters for individual animals. Renal clearance (CLR) was determined by the ratio of the total amount of drug excreted into urine within the 24-h period to the estimated AUC0-24U (11). The urinary excretion rates were determined at 2-h intervals over the entire dosing interval and normalized for body weight.
The percent recovery of drug from body fluids and tissues at 24 h postdosing was calculated from the total doses administered, drug concentrations, organ weights, blood volumes, and the volumes of 24-h urine collections, respectively. The percent recovery of compound from plasma was based on the assumption of a total blood volume of 80 ml/kg in each rabbit (9) and on individually determined hematocrit values by the formula C24hP × [(80 × kgBW) × (100 − HCT/100)], where C24hP is the drug concentration in plasma at 24 h after dosing, kgBW is the body weight in kilograms, and HCT is the hematocrit. The calculation of the percent recovery from skeletal muscle and adipose tissue was based on an estimated relative muscle mass of 50% and an estimated relative fat mass of 5% of the total body weight in each rabbit (9).
Assessment of renal effects.
Blood samples for biochemical tests were collected at 0 h (prior to dosing or urine collection) and thereafter at 12 and 24 h in all animals and immediately submitted to analysis. Blood urea nitrogen (BUN), serum creatinine, potassium, phosphate, calcium, and magnesium values were determined by standard nephelometric methods. Levels of β2 microglobulin, phosphate, potassium, magnesium, calcium, sodium, BUN, and creatinine in urine were determined from 24-h urine collections maintained at 4°C as required during the collection period and immediately thereafter using a solid-phase enzyme-linked immunosorbent assay and nephelometric methods, respectively.
The 24-h creatinine clearance normalized to body weight was determined by the formula [(UCR × Uvol)/(SCR × 1,440)]/body weight, where UCR and SCR are the creatinine levels in urine and serum, respectively, and Uval is the volume of urine collected within 24 h, with the 12-h serum creatinine level used for calculation.The urinary output was determined at 2-h intervals over the entire dosing interval and normalized for body weight. Urinary excretion of β2 microglobulin was assessed as excretion during the 24-h collection interval and normalized for creatinine excretion; urinary excretion of electrolytes was assessed as weight-normalized excretion during the 24-h collection interval; the fractional electrolyte excretion was calculated from 24-h urine and 12-h serum collection values by the formula (Ue/Pe) × (PCR/UCR) × 100%, where e is the electrolyte of interest, U is the concentration in urine, and P is the concentration in plasma (22). The fractional tubular phosphate reabsorption was determined by the formula Pphos − [(Uphos × PCR)/UCR], where phos is phosphate (5).
Statistical analysis.
All values are presented as means ± standard errors of the means (SEM). Differences between means of continuous parameters across cohorts were evaluated by Kruskal-Wallis analysis of variance (ANOVA) with Dunn's correction for multiple comparisons. For comparison of baseline and 24-h values in the assessment of parameters of renal function of drug in plasma, Student's t test or Welch's t test was used, as appropriate. A two-tailed P value of <0.05 was considered statistically significant.
RESULTS
Plasma pharmacokinetics.
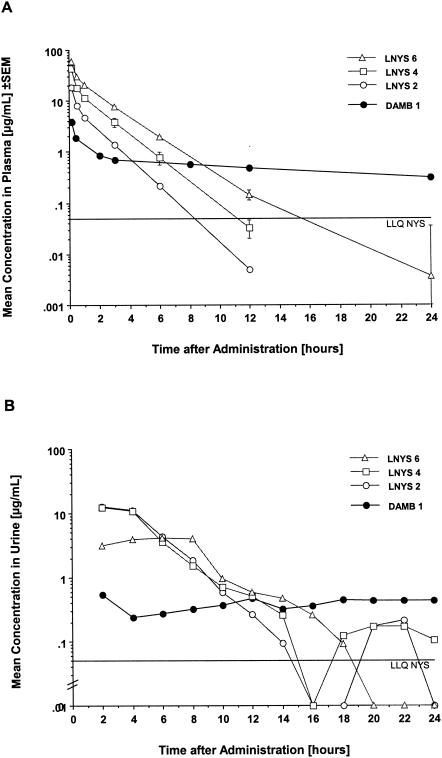
Estimated pharmacokinetic parameters of LNYS and DAMB in plasma are given in Table 1, and the corresponding plasma drug concentration profilesare depicted in Fig. 1A.
TABLE 1.
| Cohort and dose (mg/kg) | Cmax (μg/ml) | Cmin (μg/ml) | AUC0-24 (μg·h/ml) | V1 (liter/kg) | V2 (liter/kg) | V3 (liter/kg) | Vss (liters/kg) | CL2 (liter/h/kg) | CL3 (liter/h/kg) | CLt (liter/h/kg) | t1/2α (h) | t1/2β (h) | t1/2γ (h) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LNYS | |||||||||||||
| 2 | 17.00 ± 0.62 | ND | 17.08 ± 0.48 | 0.093 ± 0.003 | 0.063 ± 0.001 | NA | 0.155 ± 0.003 | 0.1260 ± 0.019 | NA | 0.1173 ± 0.003 | 0.17 ± 0.01 | 1.10 ± 0.00 | NA |
| 4 | 39.27 ± 0.79 | ND | 42.74 ± 5.25 | 0.075 ± 0.001 | 0.063 ± 0.004 | NA | 0.138 ± 0.003 | 0.1233 ± 0.010 | NA | 0.0928 ± 0.012 | 0.16 ± 0.00 | 1.26 ± 0.09 | NA |
| 6 | 56.01 ± 0.42 | ND | 77.12 ± 9.65 | 0.085 ± 0.000 | 0.059 ± 0.002 | NA | 0.145 ± 0.002 | 0.1365 ± 0.006 | NA | 0.0805 ± 0.010 | 0.16 ± 0.01 | 1.44 ± 0.18 | NA |
| DAMB, 1 | 3.36 ± 0.42 | 0.25 ± 0.03 | 12.23 ± 0.95 | 0.241 ± 0.040 | 0.631 ± 0.002 | 0.3306 ± 0.027 | 1.200 ± 0.043 | 0.2016 ± 0.015 | 0.4906 ± 0.040 | 0.0552 ± 0.007 | 0.16 ± 0.01 | 1.15 ± 0.07 | 16.82 ± 1.60 |
All values represent the means ± SEM of results for three rabbits each. Cmin, concentration in plasma at the end of the recommended dosing interval (24 h); V1, V2, V3, volume of distribution of the first, second, and third compartment, respectively; CL2, CL3, distribution clearances; t1/2α, distributional half-life; t1/2β, apparent elimination half-life; t1/2γ, terminal elimination half-life; ND, not detectable; NA, not applicable.
P values (based on Kruskal-Wallis nonparametric ANOVA): for Cmax, <0.0001; for AUC0-24, <0.0001; for V1, <0.0001; for V2, <0.0382; for Vss, 0.0028; for CL2, 0.0599; for CLt, 0.0137; for t1/2α, 0.9006; for t1/2β, 0.2775.
FIG. 1.
(A) Observed concentration-time plasma profiles after single dosages of 2, 4, and 6 mg of LNYS/kg or 1 mg of DAMB/kg. Each point represents the mean concentration for three rabbits each at that time ± SEM. Note that the error bars for LNYS at 2 mg/kg (LNYS 2) and DAMB at 1 mg/kg are included but are too small to be perceptible. Concentration-time points below LLQ were not used for pharmacokinetic modeling but are depicted for clarity of the plot. (B) Concentration-time urine profiles after administration of the same dosages described for panel A. Each point plots the mean concentration for three rabbits each for the respective 2-h urine fraction. The standard errors are omitted for clarity of the plot but can be appreciated from the assessment of pharmacokinetic parameters shown in Table 2. Note that concentration-time points below LLQ were set at 0 for pharmacokinetic calculations but are depicted for clarity of the plot.
In comparison to the results with the standard dosage of 1 mg of DAMB/kg, LNYS displayed a markedly different disposition in plasma after single-dose administration of therapeutic levels of 2 to 6 mg/kg, as evidenced by a faster clearance from plasma, a ≤10-fold-shorter relevant elimination half-life, and a ≤8-fold-smaller V. The administration of ≤6-fold-higher dosages was reflected in escalating Cmaxs and AUC0-24 values that ranged from 27 to 56 μg/ml and 17 to 77 μg · h/ml, respectively, and were ≤16- and ≤6-fold higher than corresponding values achieved with DAMB. As evidenced by the concentration-time profiles, nystatin achieved comparatively high peak levels in plasma but was rapidly eliminated from plasma within 6 to 12 h; in contrast, amphotericin B achieved lower peak levels in plasma and showed a rapid distributive phase but was eliminated only slowly from plasma and maintained detectable concentrations throughout the dosing interval.
Urinary pharmacokinetics.
Peak drug concentrations in urine (CmaxU) collected at 2-h intervals, TmaxU values, AUC0-24U values, and CLR values are summarized in Table 2, and the corresponding concentration-time profiles for drug in urine are depicted in Fig. 1B.
TABLE 2.
| Group and dose (mg/kg) | CmaxU (μg/ml) | TmaxU (h) | AUC0-24U (μg · h/ml) | CLR (liter/kg/h) |
|---|---|---|---|---|
| LNYS | ||||
| 2 | 16.83 ± 3.54 | 3.33 ± 1.33 | 63.12 ± 18.84 | 0.0081 ± 0.001 |
| 4 | 18.52 ± 4.85 | 2.67 ± 0.66 | 60.71 ± 8.68 | 0.0036 ± 0.000 |
| 6 | 10.00 ± 0.89 | 6.00 ± 1.15 | 35.27 ± 6.89 | 0.0028 ± 0.000 |
| DAMB, 1 | 0.962 ± 0.352 | 12.67 ± 6.36 | 8.95 ± 3.14 | 0.0039 ± 0.001 |
All values represent the means ± SEM of results for three rabbits each.
bComparison among dosage groups was done by Kruskal-Wallis nonparametric ANOVA. P values: for CmaxU, 0.0152; TmaxU, 0.2541; AUC0-24U, 0.0152; CLR, 0.0681.
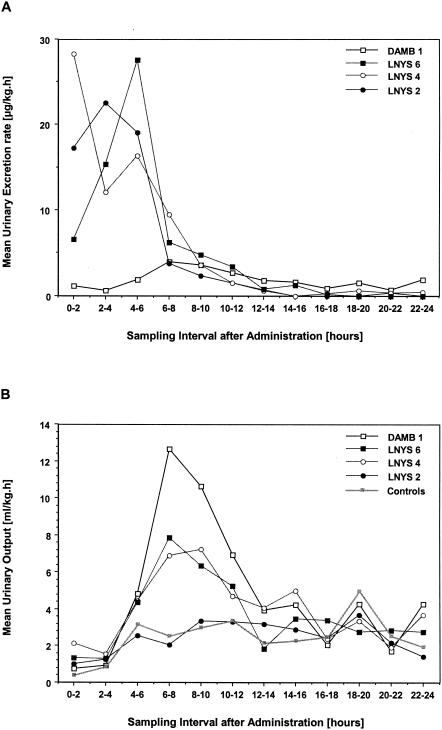
In comparison to the results with DAMB, mean CmaxU values after administration of LNYS were ≥10-fold higher and were observed earlier after dosing. Similarly, AUC0-24U values were four- to sevenfold greater after administration of LNYS. CmaxU and AUC0-24U values after administration of 4 mg of LNYS/kg were not different from those after administration of 2 mg of LNYS/kg; following administration of 6 mg/kg, both parameters were lower than after administration of 2 or 4 mg/kg. This result coincided with an apparent decrease in the mean CLR of LNYS across the investigated dosage range (by ANOVA, P = 0.0714). Notably, there were no significant differences in CLR between LNYS and DAMB. The urinary excretion rates of nystatin peaked during the first 8 h postadministration, with a marked decrease thereafter. In contrast, excretion rates of amphotericin were generally lower and showed a more constant pattern (Fig. 2A). When calculations were done for the entire 24-h dosing interval, the urinary excretion rates of rabbits treated with 2, 4, or 6 mg of LNYS/kg or with 1 mg of DAMB/kg were 5.66 ± 0.64, 6.17 ± 0.34, 9.07 ± 1.71, and 1.90 ± 0.38 μg/kg · h, respectively (mean ± SEM; by ANOVA, P < 0.0001).
FIG. 2.
(A) Excretion rates into urine in micrograms of nystatin or amphotericin B per kilogram per hour after single dosing with 2, 4, or 6 mg of LNYS/kg or 1 mg of DAMB/kg. Each point plots the mean value for three rabbits during the respective sampling interval. The standard errors are omitted for clarity of the plot but are incorporated in the panel's description in Results. (B) Urinary output in milliliters per kilogram per hour after single dosing with 2, 4, or 6 mg of LNYS/kg or 1 mg of DAMB/kg. Each point plots the mean value for three rabbits during the respective sampling interval. The standard errors are omitted for clarity of the plot but are incorporated in the panel's description in Results.
Concentrations in tissues and body fluids.
The concentrations of LNYS and DAMB in tissues and body fluids 24 h after single-dose administration are given in Table 3.
TABLE 3.
| Group and dose (mg/kg) | Drug concn
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Liver (μg/g) | Spleen (μg/g) | Kidney (μg/g) | Lung (μg/g) | Muscle (μg/g) | Fat (μg/g) | Brain (μg/g) | Bile (μg/ml) | Urine (μg/ml) | Plasma (μg/ml) | |
| LNYS | ||||||||||
| 2 | 0.16 ± 0.00 | 0.39 ± 0.08 | 0.39 ± 0.00 | 0.18 ± 0.00 | ND | ND | ND | 1.63 ± 0.41 | ND | ND |
| 4 | 0.26 ± 0.03 | 0.81 ± 0.12 | 1.15 ± 0.14 | 0.33 ± 0.01 | ND | ND | ND | 1.67 ± 0.26 | 0.10 ± 0.10 | ND |
| 6 | 0.28 ± 0.10 | 0.86 ± 0.10 | 1.59 ± 0.24 | 0.44 ± 0.03 | ND | ND | ND | 2.10 ± 1.08 | ND | ND |
| DAMB, 1 | 7.04 ± 1.06 | 8.57 ± 0.06 | 1.19 ± 0.10 | 1.10 ± 0.22 | 0.024 ± 0.00 | ND | 0.027 ± 0.00 | 1.85 ± 0.43 | 0.43 ± 0.06 | 0.25 ± 0.03 |
P values were calculated by Kruskal-Wallis nonparametric ANOVA. P value: for liver, 0.0026; for spleen, 0.0014; for kidney, 0.0137; for lung, 0.0001; for bile, 0.9753.
ND, not detectable.
Corresponding to the comparatively small volume of distribution at steady state (Vss), the concentrations of LNYS in parenchymatous organs were low. The highest concentrations were measured in the kidney. In muscle, fat, and brain tissues, nystatin was undetectable at all investigated dosages. While nystatin was detectable in urine in only one animal 24 h after dosing, the compound was detectable in bile fluid in all animals. With the exception of the kidney, mean concentrations of amphotericin B in tissue were severalfold higher despite the lower dosage; similarly, while nystatin was undetectable in plasma at 24 h, all animals treated with DAMB had detectable levels of amphotericin B at the end of the dosing interval.
Recoveries from tissues and urine.
As depicted in Table 4, less than 0.5% of the dose was recovered from the liver, spleen, kidneys, and lungs at 24 h after single-dose administration of LNYS. There were no significant differences in these recoveries for the three selected dosages of the compound. A quantitively important disposition in muscle and adipose tissue was not detectable, even with the lower limit of the analytical assay taken into consideration. In contrast, a quarter of the total dose was recovered from the livers of DAMB-treated animals. The recoveries from other organs and in the plasma pool were markedly lower and ranged from 0.35 to 1.23%.
TABLE 4.
Recoveries of LNYS and DAMB from tissues and fluids 24 h after administration of a single dosea
| Tissue or fluid | % Recovery of drug after administration of dose indicated
|
P valueb | |||
|---|---|---|---|---|---|
| LNYS
|
DAMB, 1 μg/ml | ||||
| 2 μg/ml | 4 μg/ml | 6 μg/ml | |||
| Liver | 0.285 ± 0.005 | 0.248 ± 0.031 | 0.180 ± 0.021 | 25.87 ± 3.57 | 0.0028 |
| Spleen | 0.125 ± 0.064 | 0.085 ± 0.014 | 0.069 ± 0.008 | 0.351 ± 0.06 | 0.1364 |
| Kidney | 0.128 ± 0.008 | 0.206 ± 0.024 | 0.207 ± 0.033 | 0.988 ± 0.090 | 0.0020 |
| Lung | 0.065 ± 0.015 | 0.036 ± 0.001 | 0.035 ± 0.003 | 0.643 ± 0.150 | 0.0234 |
| Muscle | 0.000 ± 0.000 | 0.000 ± 0.000 | 0.000 ± 0.000 | 1.230 ± 0.272 | NA |
| Fat | 0.000 ± 0.000 | 0.000 ± 0.000 | 0.000 ± 0.000 | 0.000 ± 0.000 | NA |
| Brain | 0.000 ± 0.000 | 0.000 ± 0.000 | 0.000 ± 0.000 | 0.006 ± 0.001 | NA |
| Plasma | 0.000 ± 0.000 | 0.000 ± 0.000 | 0.000 ± 0.000 | 1.130 ± 0.837 | NA |
| Urine | 6.772 ± 0.772 | 3.730 ± 0.184 | 3.628 ± 0.683 | 4.697 ± 0.910 | 0.0788 |
Values represent means ± SEM of results for three treated and six untreated animals each.
P values are for the comparison among dosage groups by Kruskal-Wallis nonparametric ANOVA. NA, not applicable.
Following one single dose of LNYS, the mean recovery of nystatin from 24-h urine samples accounted for 6.77, 3.73, and 3.62% of the total dosage of 2, 4, and 6 mg/kg (by ANOVA, P = 0.05). The mean recovery of DAMB from urine was 4.69% and not significantly different from that after administration of LNYS at 2 to 6 mg/kg.
Effects on renal function. (i) Plasma parameters.
Relevant parameters of renal function of drugs in plasma before and 24 h after administration of LNYS and DAMB are given in Table 5. In all treated cohorts, mean serum creatinine and mean BUN values were higher at 24 h after administration than at the baseline, and there was an apparent dose-dependent effect of LNYS on these parameters. An increase of the serum creatinine level to 1.5 times the baseline value was found in zero, one, two, and one of three animals each treated with LNYS at 2, 4, or 6 mg/kg or DAMB at 1 mg/kg, respectively. An increase to 2 times the baseline value or higher was not observed. There was no effect on the mean potassium values, but in all cohorts, there was a slight increase in the mean serum magnesium value 24 h after dosing.
TABLE 5.
Effects of single doses of LNYS and DAMB on renal function parameters in plasmaa
| Cohort and dosage (mg/kg) | Parameter level
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Creatinine (mg/dl)
|
BUN (mg/dl)
|
Potassium (mmol/liter)
|
Magnesium (mmol/liter)
|
|||||
| Baseline | 24 h | Baseline | 24 h | Baseline | 24 h | Baseline | 24 h | |
| Controls | 0.68 ± 0.02 | 0.68 ± 0.01 | 16 ± 0.66 | 15 ± 0.66 | 4.42 ± 0.10 | 4.45 ± 0.16 | 1.04 ± 0.05 | 0.98 ± 0.06 |
| LNYS | ||||||||
| 2 | 0.66 ± 0.06 | 0.76 ± 0.06 | 15 ± 0.33 | 16 ± 0.57 | 4.27 ± 0.03 | 4.27 ± 0.12 | 0.95 ± 0.04 | 1.00 ± 0.01 |
| 4 | 0.70 ± 0.05 | 1.00 ± 0.15 | 19 ± 1.53 | 23 ± 1.20 | 4.30 ± 0.17 | 3.97 ± 0.20 | 0.92 ± 0.00 | 1.08 ± 0.08 |
| 6 | 0.66 ± 0.03 | 1.07 ± 0.08b | 19 ± 0.33 | 23 ± 0.33b | 4.50 ± 0.47 | 4.57 ± 0.37 | 0.93 ± 0.01 | 1.08 ± 0.04 |
| DAMB, 1 | 0.60 ± 0.05 | 0.83 ± 0.06 | 18 ± 3.93 | 24 ± 2.73 | 4.27 ± 0.17 | 4.13 ± 0.23 | 0.92 ± 0.01 | 1.03 ± 0.02b |
All values represent the means ± SEM of results for three treated and six untreated animals each.
Significantly different from results for controls at a P of <0.05 (t test).
(ii) Urinary output and creatinine clearance.
The effects of one single dose of LNYS or DAMB on urinary output are graphically depicted in Fig. 2B. During the first 2 to 4 h postdosing, the mean urinary output was ≤2 ml/h/kg for all cohorts. While the mean urinary output of animals treated with LNYS at 2 mg/kg was not different from that of untreated control animals during the remainder of the dosing interval and was between 2 and 4 ml/h/kg, a polyuric phase of 6- to 8-h duration was noted in animals treated with LNYS at 4 and 6 mg/kg and was even more pronounced in animals treated with DAMB. When calculations were done for the entire dosing interval, the urinary output of untreated animals was 2.50 ± 0.30 ml/h/kg (mean ± SEM); this parameter increased from 2.46 ± 0.42 after administration of 2 mg of LNYS/kg to 3.96 ± 0.74 and 3.63 ± 0.20 ml/h/kg in animals treated with 4 and 6 mg of LNYS/kg, respectively. For comparison, the mean urinary output of animals receiving DAMB was 4.78 ± 0.65 ml/h/kg (by ANOVA, P = 0.0378). The normalized mean creatinine clearance (±SEM) for untreated controls was 2.11 ± 0.33 ml/min/kg and accounted for 2.47 ± 0.18, 2.72 ± 0.29, and 2.28 ± 0.10 ml/min/kg in animals treated with LNYS at 2, 4, and 6 mg/kg, respectively. The mean creatinine clearance following single-dose administration of DAMB was 3.43 ± 0.60 ml/min/kg (by ANOVA, P = 0.3691).
(iii) β2 Microglobulin and electrolyte excretion.
There were no significant differences among all five cohorts with respect to the excretion of β2 microglobulin in 24-h urine samples. The fractional phosphate excretion was less than 2% for all cohorts and, similar to the fractional tubular phosphate reabsorption values, was not different among cohorts. The minimal β2 microglobulin excretion level and the almost complete tubular phosphate reabsorption level suggest the absence of an acute toxic effect of polyene formulation on the proximal tubule. Significant differences among the five cohorts were noted with respect to the weight-normalized total excretion of sodium and calcium in 24-h urine samples (Table 6). No differences were found for the weight-normalized excretion of potassium and magnesium, although treated animals appeared to exhibit higher excretion levels of both electrolytes in comparison to those of untreated controls. Analysis of the fractional electrolyte excretion revealed similar trends. The fractional potassium excretion rate in rabbits receiving 6 mg of LNYS/kg or 1 mg of DAMB/kg exceeded 100%, which may be indicative of renal damage.
TABLE 6.
Effects of single doses of LNYS and DAMB on urinary electrolyte excretion
| Cohort and dosage (mg/kg) | Urinary electrolyte excretion rate (mmol/24 h/kg)a
|
|||
|---|---|---|---|---|
| Sodium | Calcium | Potassium | Magnesium | |
| Controls | 0.96 ± 0.17 | 0.112 ± 0.01 | 9.17 ± 0.18 | 0.554 ± 0.12 |
| LNYS | ||||
| 2 | 1.99 ± 0.48 | 0.072 ± 0.01 | 14.18 ± 2.20 | 0.834 ± 0.07 |
| 4 | 2.57 ± 0.45 | 0.178 ± 0.04 | 15.62 ± 1.89 | 1.040 ± 0.08 |
| 6 | 3.06 ± 0.27 | 0.116 ± 0.00 | 12.52 ± 1.16 | 0.862 ± 0.21 |
| DAMB, 1 | 3.81 ± 0.08 | 0.245 ± 0.03 | 15.27 ± 2.44 | 1.030 ± 0.26 |
All values represent the means ± SEM of results for three treated and six untreated animals each. Comparisons among dosage groups were done by Kruskal-Wallis nonparametric ANOVA. P values: for sodium results, 0.0065; for calcium, 0.0373; for potassium, 0.2580; for magnesium, 0.1356.
DISCUSSION
The results of this study demonstrate fundamental differences in the dispositions of multilamellar LNYS and DAMB. In comparison to the standard 1-mg/kg dosage of DAMB, single-dose administration of LNYS at therapeutic levels of 2 to 6 mg/kg resulted in escalating peak concentrations in plasma but a significantly faster clearance, with rapid elimination from plasma and a ≤8-fold-smaller V. Urinary drug concentration data revealed ≤10-fold-higher peak concentrations and 4- to 7-fold-greater AUC values following administration of LNYS but no difference in CLR. Corresponding to the smaller V, concentrations of LNYS in tissues at the end of the 24-h dosing interval were severalfold lower than those after administration of DAMB, despite the higher dosages. Less than 1% of the total dose of LNYS was recovered from the liver, spleen, kidneys, and lungs each; in contrast, a quarter of the total dose was recovered from the liver in DAMB-treated animals. CLR and recovery from urine of LNYS were not significantly different from those of DAMB, suggesting differences in biliary excretion and/or hepatic or extrahepatic metabolism as the cause of the marked differences in the dispositions of the two polyene formulations. LNYS had dose-dependent effects on glomerular filtration and distal renal tubular function as assessed by serum creatinine level, urinary output, and electrolyte excretion. However, at the highest investigated dosage of 6 mg/kg, the observed changes appeared not to exceed those following administration of DAMB.
Across species, the achievement of high peak drug levels in plasma, the rapid elimination from plasma, and the small V following administration of LNYS are markedly different from the pharmacokinetic profile of DAMB in plasma and the small unilamellar liposomal formulation of amphotericin B (LAMB; AmBisome) (2, 16, 27; Nyotran investigator brochure, Aronex Pharmaceuticals). Whereas DAMB achieves approximately similar dose-normalized peak levels in plasma but is more slowly eliminated from the bloodstream and has a much higher V, LAMB achieves and maintains very high concentrations of amphotericin B in plasma over prolonged periods of time, is eliminated much more slowly, and has an intermediate V (2, 27).
The pharmacological basis of the differences in dispositions between DAMB and LAMB have recently been elucidated in a phase IV study of healthy subjects (2, 3). In plasma, amphotericin B is highly (>95%) protein bound, and the percentage of protein bound increases with increasing drug concentrations. In comparison to a single dose of DAMB (0.6 mg/kg), following administration of a single dose of LAMB (2 mg/kg), exposure to both unbound and nonliposomal (i.e., protein-bound) drug was lower, and most of the amphotericin B remained liposome associated (i.e., 97% at 4 h and 55% at 168 h). While LAMB markedly reduced the total and fecal recoveries of amphotericin B, urinary and fecal clearances based on unbound amphotericin B were similar for both formulations; the urinary clearances of the unbound drug were equal to the glomerular filtration rate, and tubular transit rates were <16% of the urinary excretion rate (3). Greater than 90% of DAMB was accounted for in mass balance calculations at 1 week, with two-thirds excreted unchanged in the urine (20.6%) and feces (42.5%). In contrast, <10% of LAMB was excreted unchanged (2). Taken together, LAMB increases total amphotericin B concentrations in plasma while decreasing unbound amphotericin B concentrations and excretion of the unbound drug in urine and feces through sequestration of amphotericin B in long-circulating liposomes. Independent of its formulation, elimination of amphotericin B from the body occurs via the pool of the unbound drug, and glomerular filtration of the unbound drug is the mechanism of CLR (3).
Following intravenous administration of LNYS, nystatin may circulate while simultaneously bound to its original liposomal carrier, while protein bound, or as a free drug. The plasma profile of LNYS is characteristic for a multilamellar liposomal formulation that is rapidly cleared by the mononuclear phagocytic system (33); however, in vitro experiments have also demonstrated a rapid dissociation of nystatin from its liposomal carrier and opsonization by and distribution with lipoproteins and aqueous plasma proteins (37, 38). Both processes would be consistent with yet unpublished tissue drug concentration profiles for rabbits that showed escalating concentrations, particularly in the liver and spleen at 2 h after dosing, but they appear inconsistent with the lack of accumulation in these organs at 24 h after dosing and the mass balance deficit of >90% of the unchanged drug at this time point. In the present study, less than 0.5% of the total dosage of nystatin was recovered from the liver, spleen, kidney, and lung tissues, and there was negligible disposition in muscle and adipose tissue. In contrast, a quarter of the total dose of amphotericin B was found in the liver after a single dose of DAMB. The mean recovery of unchanged nystatin in urine ranged from 6.77 to 3.62% of the total dose and was not different from that of amphotericin B (4.6%), suggesting that the differential dispositions of these two polyene formulations cannot be explained by differences in renal elimination.
The disposition of LNYS in rabbits is consistent with unpublished findings for rats (Nyotran investigator brochure, Aronex Pharmaceuticals) that revealed a similar rapid elimination of nystatin from the bloodstream and tissues and a recovery from urine of 5%; more than 50% of compound was recovered from the feces. It thus appears likely that hepatic elimination accounted for much of the mass balance deficit in our study and possibly also for the differences in the dispositions of the two compounds. Indeed, while both compounds undergo negligible intestinal reabsorption (12, 19), the subtle differences in the molecular structures of nystatin and amphotericin B may be responsible for a different rate of biliary excretion. However, on the same basis, the rapid disappearance of nystatin from the bloodstream and tissues may also be a reflection of metabolite formation or physicochemical instability of the compound in physiological environments. Recent studies with human volunteers suggest that metabolism plays at most a minor role in the elimination of amphotericin B (2). Nevertheless, formal studies of metabolism and stability of nystatin following intravenous administration of the multilamellar liposomal formulation or nonliposomally administered nystatin have not been performed to date but would be highly desirable.
The urinary disposition of LNYS requires particular notice. In comparison to a standard dose of DAMB, LNYS achieved ≥10-fold-higher peak concentrations and 4- to 7-fold-greater AUC values. Levels of nystatin in urine exceeded the MICs reported for yeast-like fungal pathogens (1, 7, 23, 30) and remained above these values for several hours. Since time-kill studies have demonstrated a concentration-dependent and rapidly fungicidal activity of nystatin against Candida spp. in vitro (18), the achievement of high concentrations in urine over a relatively short time through administration of LNYS may represent a therapeutic advantage for treatment of fungal infections of the urinary tract that require therapy with polyenes.
Following the administration of 2 or 4 mg of LNYS/kg, there were no differences in either urinary Cmaxs or AUC values. When 6 mg of LNYS/kg was administered, these values were even lower and there was a trend toward a dosage-dependent decrease in CLR and urinary recovery. It is conceivable that this dosage-dependent decrease in CLR contributes to the reported dose-dependent disposition of LNYS in plasma (16). Plausible explanations for this behavior include dosage-dependent decreases in renal perfusion and glomerular filtration rate as well as a concentration-dependent protein binding (3) resulting in a concentration-dependent reduction of the percentage of unbound nystatin available for glomerular filtration. Studies of amphotericin B in human volunteers indicate that independent of its formulation, glomerular filtration of the unbound drug is the mechanism of CLR (3). The similarities in CLR and urinary recovery between amphotericin B and nystatin would indeed suggest this is also the case for nystatin.
Administration of antifungal polyenes is limited by dose-dependent nephrotoxicity, particularly in conjunction with other nephrotoxic drugs (6, 20, 36, 40). While its exact mechanisms are still not completely clear, this renal toxicity is vascular and tubular and ultimately the result of the binding of free compound to membrane-associated cholesterol (31, 32). The administration of a single dose of LNYS resulted in an apparently dose-dependent increase in the mean serum creatinine and blood urea nitrogen values; an apparently dose-dependent, delayed polyuric phase of 6- to 8-h duration; increased urinary excretion of sodium, potassium, and magnesium; but unchanged β2 microglobulin excretion and normal tubular phosphate reabsorption, indicative of acute vascular and distal tubular toxicity but not of proximal tubular toxicity. While the quality of these toxicities was not different from those following administration of a standard dose of DAMB, all acute effects seemed to be of lesser extent following the administration of 2 or 4 mg/kg and partially following the administration of 6 mg of LNYS/kg. The question of whether this reduced toxicity is due to a smaller fraction of nystatin reaching the kidney as free drug (3, 4) or only minor binding to low-density lipoproteins (37, 38, 39) cannot be answered by our data. However, although nystatin has similar to slightly lower toxicity than amphotericin B against yeast cells, nystatin is much less toxic against mammalian cells than the latter (24, 26, 42). This may be due to a differential effect of the reduced unspecific (van der Waals) binding capacity of the diene-tetraene structure on the interaction with cholesterol (the weaker partner in mammalian cell membranes) compared to that of ergosterol (the stronger partner in fungal cell membranes). This reduced cytotoxicity would explain the apparently lower tubular toxicity of nystatin despite higher exposure to free drug in the renal collection system.
In summary, in comparison to a standard dose of 1 mg of DAMB/kg, the administration of LNYS at therapeutic dosages of 2 to 6 mg/kg to healthy rabbits resulted in a significantly faster clearance from plasma and a ≤8-fold-smaller V. Despite higher dosages, concentrations of LNYS in tissues at the end of the 24-h dosing interval were severalfold lower than those from earlier times, resulting in a mass balance deficit of >90%. In urine, LNYS produced ≥10-fold-higher peak concentrations and 4- to 7-fold-greater AUC values. However, CLR and recovery rates in urine were not significantly different from those of DAMB, suggesting differences in rate of biliary excretion and/or hepatic or extrahepatic metabolism as possible causes of the differences in the dispositions of the two polyenes. LNYS had dose-dependent effects on glomerular filtration and distal renal tubular function which did not exceed those of DAMB at the highest investigated dosage. Independent of further elucidation of its fate in the body, LNYS may offer a therapeutic advantage in systemic fungal infections involving the urinary tract that require treatment with antifungal polyenes.
Acknowledgments
We thank our colleagues Myrna Candelario and Aida Field-Ridley and the staff of the Laboratory Animal Science Branch and of the Veterinary Resources Program of the Division of Research Resources, National Institutes of Health, Bethesda, Md., for expert technical assistance.
REFERENCES
- 1.Arikan, S., L. Ostrosky-Zeichner, M. Lozano-Chiu, V. Paetznick, D. Gordon, T. Wallace, and J. H. Rex. 2002. In vitro activity of nystatin compared with those of liposomal nystatin, amphotericin B, and fluconazole against clinical Candida isolates. J. Clin. Microbiol. 40:1406-1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bekersky, I., R. M. Fielding, D. E. Dressler, J. W. Lee, D. N. Buell, and T. J. Walsh. 2002. Pharmacokinetics, excretion, and mass balance of liposomal amphotericin B (AmBisome) and amphotericin B deoxycholate in humans. Antimicrob. Agents Chemother. 46:828-833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bekersky, I., R. M. Fielding, D. E. Dressler, J. W. Lee, D. N. Buell, and T. J. Walsh. 2002. Plasma protein binding of amphotericin B and pharmacokinetics of bound versus unbound amphotericin B after administration of intravenous liposomal amphotericin B (AmBisome) and amphotericin B deoxycholate. Antimicrob. Agents Chemother. 46:834-840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brajtburg, J., and J. Bolard. 1996. Carrier effects on biological activity of amphotericin B. Clin. Microbiol. Rev. 9:512-531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brodehl, J., A. Krause, and P. F. Hoyer. 1988. Assessment of maximal tubular phosphate reabsorption: comparison of direct measurement with the nomogram of Bijvoet. Pediatr. Nephrol. 2:183-189. [DOI] [PubMed] [Google Scholar]
- 6.Butler, W. T., J. E. Bennett, and D. W. Alling. 1964. Nephrotoxicity of amphotericin B: early and late effects in 81 patients. Ann. Intern. Med. 62:175-187. [DOI] [PubMed] [Google Scholar]
- 7.Carrillo-Munoz, A. J., G. Quindos, C. Tur, M. T. Ruesga, Y. Miranda, O. del Valle, P. A. Cossum, and T. L. Wallace. 1999. In-vitro antifungal activity of liposomal nystatin in comparison with nystatin, amphotericin B cholesteryl sulphate, liposomal amphotericin B, amphotericin B lipid complex, amphotericin B desoxycholate, fluconazole and itraconazole. J. Antimicrob. Chemother. 44:397-401. [DOI] [PubMed] [Google Scholar]
- 8.Committee on the Care and Use of Laboratory Animals of the Institute of Laboratory Animal Resources, Commission on Life Sciences, National Research Council. 1996. Guide for the care and use of laboratory animals. National Academy Press, Washington, D.C.
- 9.Davies, B., and T. Morris. 1993. Physiological parameters in laboratory animals and humans. Pharm. Res. 10:1093-1095. [DOI] [PubMed] [Google Scholar]
- 10.Denning, D. W., and P. Warn. 1999. Dose range evaluation of liposomal nystatin and comparisons with amphotericin B and amphotericin B lipid complex in temporarily neutropenic mice infected with an isolate of Aspergillus fumigatus with reduced susceptibility to amphotericin B. Antimicrob. Agents Chemother. 43:2592-2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gibaldi, M., and D. Perrier. 1982. Pharmacokinetics, 2nd ed., p. 455-459. Marcel Dekker, New York, N.Y.
- 12.Groll, A. H., S. C. Piscitelli, and T. J. Walsh. 1998. Clinical pharmacology of systemic antifungal agents: a comprehensive review of agents in clinical use, current investigational compounds, and putative targets for antifungal drug development. Adv. Pharmacol. 44:343-500. [DOI] [PubMed] [Google Scholar]
- 13.Groll, A. H., C. E. Gonzalez, N. Giri, K. Kligys, W. Love, J. Peter, E. Feuerstein, J. Bacher, S. C. Piscitelli, and T. J. Walsh. 1999. Liposomal nystatin against experimental pulmonary aspergillosis in persistently neutropenic rabbits: efficacy, safety, and non-compartmental pharmacokinetics. J. Antimicrob. Chemother. 43:95-103. [DOI] [PubMed] [Google Scholar]
- 14.Groll, A. H., D. Mickiene, K. Werner, S. C. Piscitelli, and T. J. Walsh. 1999. High performance liquid chromatographic determination of liposomal nystatin in plasma and tissues for pharmacokinetic and tissue distribution studies. J. Chromatogr. B 735:51-62. [DOI] [PubMed] [Google Scholar]
- 15.Groll, A. H., V. Petraitis, R. Petraitiene, A. Field-Ridley, M. Candelario, J. Bacher, S. C. Piscitelli, and T. J. Walsh. 1999. Safety and efficacy of multilamellar liposomal nystatin against disseminated candidiasis in persistently neutropenic rabbits. Antimicrob. Agents Chemother. 43:2463-2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Groll, A. H., D. Mickiene, K. Werner, R. Petraitiene, V. Petraitis, M. Calendario, A. Field-Ridley, J. Crisp, S. C. Piscitelli, and T. J. Walsh. 2000. Compartmental pharmacokinetics and tissue distribution of multilamellar liposomal nystatin in rabbits. Antimicrob. Agents Chemother. 44:950-957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Groll, A. H., N. Giri, V. Petraitis, R. Petraitiene, M. Candelario, J. S. Bacher, S. C. Piscitelli, and T. J. Walsh. 2000. Comparative efficacy and distribution of lipid formulations of amphotericin B in experimental Candida albicans infection of the central nervous system. J. Infect. Dis. 182:274-282. [DOI] [PubMed] [Google Scholar]
- 18.Gunderson, S. M., H. Hoffman, E. J. Ernst, M. A. Pfaller, and M. E. Klepser. 2000. In vitro pharmacodynamic characteristics of nystatin including time-kill and postantifungal effect. Antimicrob. Agents Chemother. 44:2887-2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hamilton-Miller, J. M. T. 1973. Chemistry and biology of the polyene macrolide antibiotics. Bacteriol. Rev. 37:166-196. [PMC free article] [PubMed] [Google Scholar]
- 20.Harbarth, S., S. L. Pestotnik, J. F. Lloyd, J. P. Burke, and M. H. Samore. 2001. The epidemiology of nephrotoxicity associated with conventional amphotericin B therapy. Am. J. Med. 111:528-534. [DOI] [PubMed] [Google Scholar]
- 21.Hazen, E. L., and R. Brown. 1950. Two antifungal agents produced by a soil actinomycete. Science 112:423. [PubMed] [Google Scholar]
- 22.Ho, P. T., K. Zimmerman, L. H. Wexler, S. Blaney, P. Jarosinski, L. Weaver-McClure, S. Izraeli, and F. M. Balis. 1995. A prospective evaluation of ifosfamide-related nephrotoxicity in children and young adults. Cancer 76:2557-2564. [DOI] [PubMed] [Google Scholar]
- 23.Johnson, E. M., J. O. Ojwang, A. Szekely, T. L. Wallace, and D. W. Warnock. 1998. Comparison of in vitro antifungal activities of free and liposome-encapsulated nystatin with those of four amphotericin B formulations. Antimicrob. Agents Chemother. 42:1412-1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kinsky, S. C. 1963. Comparative responses of mammalian erythrocytes and microbial protoplasts to polyene antibiotics and vitamin A. Arch. Biochem. 102:180-188. [DOI] [PubMed] [Google Scholar]
- 25.Kleinberg, M. E., and A. Finkelstein. 1984. Single-length and double-length channels formed by nystatin in lipid bilayer membranes. J. Membr. Biol. 80:257-269. [DOI] [PubMed] [Google Scholar]
- 26.Kotler-Brajtburg, J., G. Medoff, G. S. Kobayashi, S. Boggs, D. Schlessinger, R. C. Pandy, and K. L. Rinehart, Jr. 1979. Classification of polyene antibiotics according to chemical structure and biological effects. Antimicrob. Agents Chemother. 15:716-722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee, J. W., M. A. Amantea, P. A. Francis, E. E. Navarro, J. Bacher, P. A. Pizzo, and T. J. Walsh. 1994. Pharmacokinetics and safety of a unilamellar liposomal formulation of amphotericin B (AmBisome) in rabbits. Antimicrob. Agents Chemother. 38:713-718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mehta, R. T., R. L. Hopfer, L. A. Gunner, R. L. Juliano, and G. Lopez-Berestein. 1987. Formulation, toxicity, and antifungal activity in vitro of liposome-encapsulated nystatin as therapeutic agent for systemic candidiasis. Antimicrob. Agents Chemother. 31:1897-1900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mehta, R. T., R. L. Hopfer, T. McQueen, R. L. Juliano, and G. Lopez-Berestein. 1987. Toxicity and therapeutic effects in mice of liposome-encapsulated nystatin for systemic fungal infections. Antimicrob. Agents Chemother. 31:1901-1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oakley, K. L., C. B. Moore, and D. W. Denning. 1999. Comparison of in vitro activity of liposomal nystatin against Aspergillus species with those of nystatin, amphotericin B (AB) deoxycholate, AB colloidal dispersion, liposomal AB, AB lipid complex, and itraconazole. Antimicrob. Agents Chemother. 43:1264-1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sabra, R., and R. A. Branch. 1990. Amphotericin B nephrotoxicity. Drug Saf. 5:94-108. [DOI] [PubMed] [Google Scholar]
- 32.Sawaya, B. P., J. P. Briggs, and J. Schnermann. 1995. Amphotericin B nephrotoxicity: the adverse consequences of altered membrane properties. J. Am. Soc. Nephrol. 6:154-164. [DOI] [PubMed] [Google Scholar]
- 33.Schneider, M. 1985. Liposomes as drug carriers: 10 years of research, p. 119-134. In P. Buri and A. Gumma (ed.), Drug targeting. Elsevier Science Publishers, New York, N.Y.
- 34.Wallace, T. L., V. Paetznick, P. A. Cossum, G. Lopez-Berestein, J. H. Rex, and E. Anaissie. 1997. Activity of liposomal nystatin against disseminated Aspergillus fumigatus infection in neutropenic mice. Antimicrob. Agents Chemother. 41:2238-2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Walsh, T. J., P. Bacher, and P. A. Pizzo. 1988. Chronic silastic central venous catheterization for induction, maintenance, and support of persistent granulocytopenia in rabbits. Lab. Anim. Med. 38:467-470. [PubMed] [Google Scholar]
- 36.Walsh, T. J., R. W. Finberg, C. Arndt, J. Hiemenz, C. Schwartz, D. Bodensteiner, P. Pappas, N. Seibel, R. N. Greenberg, S. Dummer, M. Schuster, J. S. Holcenberg, et al. 1999. Liposomal amphotericin B for empirical therapy in patients with persistent fever and neutropenia. N. Engl. J. Med. 340:764-771. [DOI] [PubMed] [Google Scholar]
- 37.Wasan, K. M., and G. Lopez-Berestein. 1997. Diversity of lipid-based polyene formulations and their behavior in biological systems. Eur. J. Clin. Microbiol. Infect. Dis. 16:81-92. [DOI] [PubMed] [Google Scholar]
- 38.Wasan, K. M., M. Ramaswami, S. M. Cassidy, M. Kazemi, F. W. Strobel, and R. L. Thies. 1997. Physical characteristics and lipoprotein distribution of liposomal nystatin in human plasma. Antimicrob. Agents Chemother. 41:1871-1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wasan, K. M., M. G. Rosenblum, L. Cheung, and G. Lopez-Berestein. 1994. Influence of lipoproteins on renal cytotoxicity and antifungal activity of amphotericin B. Antimicrob. Agents Chemother. 38:223-227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wingard, J. R., P. Kubilis, L. Lee, G. Yee, M. White, L. Walshe, R. Bowden, E. Anaissie, J. Hiemenz, and J. Lister. 1999. Clinical significance of nephrotoxicity in patients treated with amphotericin B for suspected or proven aspergillosis. Clin. Infect. Dis. 29:1402-1407. [DOI] [PubMed] [Google Scholar]
- 41.Yamaoka, K., T. Nakagawa, and T. Uno. 1978. Application of Akaike's information criterion in the evaluation of linear pharmacokinetic equations. J. Pharmacokinet. Biopharm. 6:165-175. [DOI] [PubMed] [Google Scholar]
- 42.Zager, R. A. 2000. Polyene antibiotics: relative degrees of in vitro cytotoxicity and potential effects on tubule phospholipid and ceramide content. Am. J. Kidney Dis. 36:238-249. [DOI] [PubMed] [Google Scholar]