Abstract
In search of treatment alternatives against vancomycin-resistant S. aureus (VRSA), an in vitro pharmacodynamic model with simulated endocardial vegetations incorporating protein and a high inoculum was used to simulate daptomycin, linezolid, quinupristin-dalfopristin, and vancomycin against the Michigan VRSA strain. Daptomycin and quinupristin-dalfopristin exhibited the greatest bacterial reductions, and all tested agents except vancomycin exhibited bactericidal activity against the VRSA.
Grave concerns regarding gram-positive resistance were recently amplified with the first two reports of infections due to vancomycin-resistant Staphylococcus aureus (VRSA) (8, 9). The possibility of further identification of infections due to VRSA and the difficult complications associated with this pathogen (i.e., endocarditis) emphasize the need for evaluation of antimicrobial agents that possess bactericidal activity in the presence of high inoculum, protein, and antibiotic penetration barriers.
Daptomycin, a novel cyclic lipopeptide, represents a potential alternative for resistant gram-positive pathogens (2, 3, 14, 16, 25-27; N. Safdar, D. R. Andes, and W. A. Craig, Abstr. 39th Intersci. Conf. Antimicrob. Agents Chemother., abstr. 1769, 1999). Other potential options for drug-resistant gram-positive pathogens, including methicillin-resistant staphylococci, include quinupristin-dalfopristin and linezolid (4, 7, 10, 13, 18, 21, 23, 24). We investigated the pharmacodynamics of daptomycin, quinupristin-dalfopristin, linezolid, and vancomycin against the recent Michigan VRSA strain in an in vitro pharmacodynamic model with simulated endocardial vegetations.
Two clinical strains of S. aureus, including the reported VRSA isolate (DMC83006A) and an earlier vancomycin-sensitive, methicillin-resistant S. aureus (MRSA) isolate (DMC82991; the presumptive VRSA parent) from the same patient were evaluated at the Department of Microbiology, DMC University Laboratories, Wayne State University, Detroit, Mich. (9).
Microdilutional MICs and minimum bactericidal concentrations (MBCs) were determined pre- and postexposure according to NCCLS guidelines, and E-test methods were employed for confirmation of results (22). In addition, daptomycin MIC and MBC analyses were performed in the presence of human albumin (American Red Cross, Detroit, Mich.) at 4 g/dl (20, 25).
Mueller-Hinton broth (Becton-Dickinson, St. Louis, Mo.) supplemented with 25 mg of calcium per liter and 12.5 mg of magnesium per liter was used for experiments with vancomycin, quinupristin, dalfopristin, and linezolid. Mueller-Hinton broth supplemented with 75 mg of calcium per liter and 12.5 mg of magnesium per liter was used for daptomycin experiments due to its dependence on calcium for activity (16, 19). E-test MICs for quinupristin-dalfopristin, vancomycin, and linezolid were determined by using tryptic soy agar (TSA; Becton-Dickinson) plates. IsoSensitest agar (Oxoid, Inc., Ogdensburg, N.Y.) was used for daptomycin E tests.
As previously described, an in vitro pharmacodynamic model with simulated endocardial vegetations was utilized (1, 17). The following regimens were simulated: daptomycin, 6 mg/kg of body weight every 24 h (peak, 98.6 μg/ml; average half-life, 8 h); quinupristin-dalfopristin, 7.5 mg/kg every 8 h (quinupristin [peak, 3 μg/ml; average half-life, 1 h] and dalfopristin [peak, 8 μg/ml; average half-life, 0.7 h]); linezolid, 600 mg every 12 h(peak, 18 μg/ml; average half-life, 5 h), and vancomycin, 1 g every 12 h (peak, 40 μg/ml; average half-life, 6 h). For quinupristin-dalfopristin simulations, each component was administered separately in order to facilitate the simulation of their respective half-lives by setting the elimination at the shorter half-life and supplementing the agent with the longer half-life (6). All in vitro pharmacodynamic experiments were performed in triplicate. In addition, growth control conditions were tested.
Three simulated endocardial vegetations were removed from each model (total, nine for each drug regimen experiment at each time point) at 0, 8, 24, 32, 48, and 72 h. Simulated endocardial vegetations were then homogenized and diluted as necessary (10- to 100,000-fold) into sterilized cold 0.9% sodium chloride solution. Aliquots of all dilutions were then plated onto TSA in triplicate and incubated at 35°C for 24 h. This method results in a lower limit of detection of 2.0 log10 CFU/g. Antimicrobial carryover was minimized by serial dilution of plated samples in conjunction with gravity filtration. Pharmacodynamic profiles were constructed by plotting time-kill curves in log10 CFU/g over 72 h. Bactericidal activity (99.9% kill) was defined as a ≥3-log10 CFU/g reduction in colony count from the initial inoculum. Bacteriostatic activity was defined as a 0.5- to 2.99-log10 CFU/g reduction in colony count from the initial inoculum, while inactivity was defined as exhibiting no observed reductions. The time to achieve a 99.9% (T99) bacterial load reduction was determined by subtracting density of samples from the initial inoculum and identifying the first time point for which a 99.9% kill occurred.
Pharmacokinetic samples from the central compartment were obtained at peak (2 min after the end of antimicrobial administration) and at 1, 2, 4, 8, 24, 32, 48, and 72 h for verification of target concentrations (17). Vancomycin concentrations were determined by fluorescence polarization immunoassay (Abbott Diagnostics TDx). Concentrations of daptomycin were determined by a previously described microbioassay (1). Quinupristin and dalfopristin concentrations were determined separately by a previously reported microbioassay (15). Linezolid concentrations were determined by a previously described validated high-pressure liquid chromatography assay (4). A one-compartment model with bolus intravenous input and first-order elimination was applied to concentration data with PK Analyst software (Micromath Research, St. Louis, Mo.) to determine elimination rates and free peak and trough concentrations.
Development of resistance was evaluated at 24, 48, and 72 h by plating 100 μl of each sample onto TSA plates containing four and eight times the MIC of the respective antimicrobial agent. Plates were then examined for growth after 48 h of incubation at 35°C.
Changes in the number of CFU per gram at 24, 48, and 72 h were compared by one-way analysis of variance with Tukey's post hoc test. A P value of ≤0.05 was considered significant. All statistical analyses were performed using SPSS statistical software (version 10.07; SPSS, Inc., Chicago, Ill.).
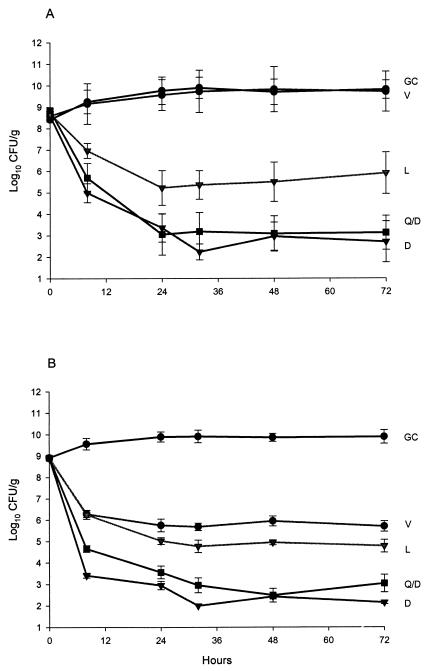
Susceptibility results are presented in Table1. Pharmacodynamic results (change in log CFU per gram over 72 h) are presented in Fig. 1. For all simulations, pH ranged between 6.98 and 7.14 and the temperature was maintained at 37°C. Initial inocula for all regimen simulations were within 0.5 log10 CFU/g of the target inoculum. All simulations achieved peak concentrations, and half-lives were within 10% of targeted values. Daptomycin and quinupristin-dalfopristin achieved rapid bactericidal activity, with a T99 of 8 h against both tested organisms. Furthermore, bactericidal activity was maintained by both antimicrobials for the study duration. Linezolid achieved bactericidal activity, with a T99 of 24 h against both tested isolates. The bactericidal activity of linezolid was maintained for the study duration against the presumptive parent MRSA but not against the VRSA. Vancomycin exhibited no activity against the VRSA isolate. Against the vancomycin-sensitive MRSA isolate, vancomycin exhibited bactericidal activity with a T99 of 24 h. Maximal bacterial reductions of 6, 5.8, and 3.4 log10 CFU/g were noted for daptomycin, quinupristin-dalfopristin, and linezolid, respectively. Daptomycin and quinupristin-dalfopristin demonstrated greater bacterial reductions than that achieved with vancomycin against both isolates at 24, 48, and 72 h (P < 0.05), while linezolid demonstrated greater activity than vancomycin only against the presumptive parent MRSA. There were no significant (>1 dilution) changes in postexposure MICs, and there was no observable resistance to daptomycin, quinupristin-dalfopristin, and linezolid.
TABLE 1.
Susceptibility results (MIC/MBC in mg/liter)
| Antimicrobial agent | VRSA (DMC83006A) | MRSA (DMC82991) |
|---|---|---|
| Daptomycin | 0.25/0.50 | 0.125/0.25 |
| Daptomycin (albumin) | 1.0/4.0 | 1.0/2.0 |
| Linezolid | 2.0/32 | 2.0/16 |
| Quinupristin-dalfopristin | 0.25/0.50 | 0.25/0.50 |
| Vancomycin | 1,024/≥2,048 | 1.0/2.0 |
FIG. 1.
Pharmacodynamic profiles of daptomycin, linezolid, quinupristin-dalfopristin, and vancomycin against VRSA (A) and its presumptive methicillin-resistant parent (B). GC, growth control; D, daptomycin; L, linezolid; Q/D, quinupristin-dalfopristin; V, vancomycin.The lower limit of bacterial quantification is 2.0 log10 CFU/g. Plots represent mean values, and error bars represent standard deviations of 27 quantification determinations (nine samples from three model simulations plated in triplicate).
The need for optimal agents that exhibit pronounced bactericidal activity in difficult infections that consist of high inocula, protein, and difficult penetration barriers is apparent to promote not only clinical cure but also eradication of resistant organisms. Pharmacodynamic observations in this study for daptomycin and quinupristin-dalfopristin, including rapid and sustained bactericidal activity, are consistent with previous reports of endocarditis simulations against staphylococci (1, 5, 15). Furthermore, the high protein binding affinity of daptomycin does not appear to hamper its activity in simulated endocardial vegetations (1). As expected, vancomycin exhibited no kill activity against the vancomycin-resistant isolate. Overall, linezolid achieved rates and extent of bacterial kill activity against both staphylococcal isolates that were similar to those of vancomycin against the vancomycin-sensitive isolate. These observations with linezolid are consistent with previous staphylococcus-related endocarditis experiments performed with rabbits (11). It appears that development of vancomycin resistance does not hamper the utility of newer antimicrobial options with different mechanisms of action. The utility of novel alternatives and conventional agents that exhibit favorable susceptibilities should be further evaluated against VRSA with larger and longer in vivo endocarditis studies. Now, with the recent identification of S. aureus strains that are resistant to linezolid or quinupristin-dalfopristin, the search for optimal alternatives for vancomycin-resistant staphylococci is imperative (12, 28).
Acknowledgments
We thank Charles Peloquin from the Division of Infectious Diseases at the National Jewish Medical and Research Center (Denver, Colo.) for his analysis of linezolid samples.
A research grant for this investigation was generously provided by Cubist Pharmaceuticals.
REFERENCES
- 1.Akins, R. L., and M. J. Rybak. 2001. Bactericidal activities of two daptomycin regimens against clinical strains of glycopeptide intermediate-resistant Staphylococcus aureus, vancomycin-resistant Enterococcus faecium, and methicillin-resistant Staphylococcus aureus isolates in an in vitro pharmacodynamic model with simulated endocardial vegetations. Antimicrob. Agents Chemother. 45:454-459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akins, R. L., and M. J. Rybak. 2000. In vitro activities of daptomycin, arbekacin, vancomycin, and gentamicin alone and/or in combination against glycopeptide intermediate-resistant Staphylococcus aureus in an infection model. Antimicrob. Agents Chemother. 44:1925-1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alborn, W. E., N. E. Allen, and D. A. Preston. 1990. Daptomycin disrupts membrane potential in growing Staphylococcus aureus. Antimicrob. Agents Chemother. 35:2282-2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Allen, G. P., R. Cha, and M. J. Rybak. 2002. In vitro activities of quinupristin-dalfopristin and cefepime, alone and in combination with various antimicrobials, against multidrug-resistant staphylococci and enterococci in an in vitro pharmacodynamic model. Antimicrob. Agents Chemother. 46:2606-2612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berthaud, N., Y. Huet, N. Diallo, and J. F. Desnottes. 1997. Antistaphylococcal activities of quinupristin/dalfopristin in vitro across platelet-fibrin matrices and in experimental endocarditis. J. Antimicrob. Chemother. 39(Suppl. A):93-98. [DOI] [PubMed] [Google Scholar]
- 6.Blaser, J. 1985. In-vitro model for simultaneous simulations of the serum kinetics of two drugs with different half-lives. J. Antimicrob. Chemother. 15(Suppl. A):125-130. [DOI] [PubMed] [Google Scholar]
- 7.Bouanchaud, D. H. 1997. In-vitro and in-vivo antibacterial activity of quinupristin/dalfopristin. J. Antimicrob. Chemother. 39(Suppl. A):15-21. [DOI] [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention. 2002. Public health dispatch: vancomycin-resistant Staphylococcus aureus—Pennsylvania, 2002. Morb. Mortal. Wkly. Rep. 51:902. [PubMed] [Google Scholar]
- 9.Centers for Disease Control and Prevention. 2002. Staphylococcus aureus resistant to vancomycin—United States, 2002. Morb. Mortal. Wkly. Rep. 51:565-567. [PubMed] [Google Scholar]
- 10.Chant, C., and M. J. Rybak. 1995. Quinupristin/dalfopristin (RP 59500): a new streptogramin antibiotic. Ann. Pharmacother. 29:1022-1027. [DOI] [PubMed] [Google Scholar]
- 11.Dailey, C. F., C. L. Dileto-Fang, L. V. Buchanan, M. P. Oramas-Shirey, D. H. Batts, C. W. Ford, and J. K. Gibson. 2001. Efficacy of linezolid in treatment of experimental endocarditis caused by methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 45:2304-2308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dowzicky, M., G. H. Talbot, C. Feger, P. Prokocimer, J. Etienne, and R. Leclerq. 2000. Characterization of isolates associated with emerging resistance to quinupristin/dalfopristin (Synercid) during a worldwide clinical program. Diagn. Microbiol. Infect. Dis. 37:57-62. [DOI] [PubMed] [Google Scholar]
- 13.Dresser, L. D., and M. J. Rybak. 1998. The pharmacologic and bacteriologic properties of oxazolidinones, a new class of synthetic antimicrobials. Pharmacotherapy 18:456-462. [PubMed] [Google Scholar]
- 14.Eliopoulos, G. M., S. Wiley, E. Reiszner, P. G. Spitzer, G. Caputo, and R. C. Moellering. 1986. In vitro and in vivo activity of LY146032, a new cyclic lipopeptide antibiotic. Antimicrob. Agents Chemother. 30:532-535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Entenza, J. M., H. Drugeon, M. P. Glauser, and P. Moreillon. 1995. Treatment of experimental endocarditis due to erythromycin-susceptible or -resistant methicillin-resistant Staphylococcus aureus with RP 59500. Antimicrob. Agents Chemother. 39:1419-1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hanberger, H., L. E. Nilsson, R. Maller, and B. Isaksson. 1991. Pharmacodynamics of daptomycin and vancomycin on Enterococcus faecalis and Staphylococcus aureus demonstrated by studies of initial killing and postantibiotic effect and influence of Ca2+ and albumin on these drugs. Antimicrob. Agents Chemother. 35:1710-1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Houlihan, H. H., D. P. Stokes, and M. J. Rybak. 2000. Pharmacodynamics of vancomycin and ampicillin alone and in combination with gentamicin once-daily or thrice-daily against Enterococcus faecalis in an in vitro infection model. J. Antimicrob. Chemother. 46:79-86. [DOI] [PubMed] [Google Scholar]
- 18.Kang, S. L., and M. J. Rybak. 1997. In-vitro bactericidal activity of quinupristin/dalfopristin alone and in combination against resistant strains of Enterococcus species and Staphylococcus aureus. J. Antimicrob. Chemother. 39(Suppl. A):33-39. [DOI] [PubMed] [Google Scholar]
- 19.Lamp, K. C., M. J. Rybak, E. M. Bailey, and G. W. Kaatz. 1992. In vitro pharmacodynamic effects of concentration, pH, and growth phase on serum bactericidal activities of daptomycin and vancomycin. Antimicrob. Agents Chemother. 36:2709-2714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee, B. L., M. Sachdeva, and H. F. Chambers. 1991. Effect of protein binding of daptomycin on MIC and antibacterial activity. Antimicrob. Agents Chemother. 35:2505-2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Low, D. E., and H. L. Nadler. 1997. A review of in-vitro antibacterial activity of quinupristin/dalfopristin against methicillin-susceptible and -resistant Staphylococcus aureus. J. Antimicrob. Chemother. 39(Suppl. A):53-58. [DOI] [PubMed] [Google Scholar]
- 22.National Committee for Clinical Laboratory Standards. 2000. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, 5th ed. Approved standard M100-S10/M7. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 23.Patel, R., M. S. Rouse, K. E. Piper, and J. M. Steckelberg. 1999. In vitro activity of linezolid against vancomycin-resistant enterococci, methicillin-resistant Staphylococcus aureus and penicillin-resistant Streptococcus pneumoniae. Diagn. Microb. Infect. Dis. 34:119-122. [DOI] [PubMed] [Google Scholar]
- 24.Rybak, M. J., D. M. Cappelletty, T. Moldovan, J. R. Aeschlimann, and G. W. Kaatz. 1998. Comparative in vitro activities and postantibiotic effects of the oxazolidinone compounds eperezolid (PNU-100592) and linezolid (PNU-100766) versus vancomycin against Staphylococcus aureus, coagulase-negative staphylococci, Enterococcus faecalis, and Enterococcus faecium. Antimicrob. Agents Chemother. 42:721-724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rybak, M. J., E. M. Bailey, K. C. Lamp, and G. W. Kaatz. 1992. Pharmacokinetics and bactericidal rates of daptomycin and vancomycin in intravenous drug abusers being treated for gram-positive endocarditis and bacteremia. Antimicrob. Agents Chemother. 36:1109-1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rybak, M. J., E. Hershberger, T. Moldovan, and R. G. Grucz. 2000. In vitro activities of daptomycin, vancomycin, linezolid, and quinupristin-dalfopristin against staphylococci and enterococci, including vancomycin-intermediate and -resistant strains. Antimicrob. Agents Chemother. 44:1062-1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tally, F. P., and M. F. Debruin. 2000. Development of daptomycin for gram-positive infections. J. Antimicrob. Chemother. 46:523-526. [DOI] [PubMed] [Google Scholar]
- 28.Tsiodras, S., G. Sakoulas, C. Wennersten, G. M. Eliopoulos, R. C. Moellering, Jr., M. J. Ferraro, and H. S. Gold. 2001. Linezolid resistance in a clinical isolate of Staphylococcus aureus. Lancet 358:207-208. [DOI] [PubMed] [Google Scholar]