Abstract
Pseudomonas aeruginosa harbors a chromosomal aminoglycoside phosphotransferase gene, aph(3′)-IIb, which confers P. aeruginosa resistance to several important aminoglycoside antibiotics, including kanamycin A and B, neomycin B and C, butirosin, and seldomycin F5. The aph(3′)-IIb gene has been found to be regulated by an AraC-type transcriptional regulator (HpaA) encoded by a gene located upstream of the aph(3′)-IIb gene. In the presence of 4-hydroxyphenylacetic acid (4-HPA), HpaA activates the expression of aph(3′)-IIb as well as that of the hpa regulon which encodes metabolic enzymes for the utilization of 4-HPA. hpaA and aph(3′)-IIb form an operon, and in response to the presence of 4-HPA, the wild-type P. aeruginosa strain PAK (but not its hpaA mutant strain) displays increased resistance to neomycin. A survey of 39 clinical and 19 environmental isolates of P. aeruginosa demonstrated in all of them the presence of an hpaA-aph gene cluster, while 56 out of the 58 isolates are able to utilize the 4-HPA as a sole carbon source, suggesting a feature common to P. aeruginosa strains. Interestingly, a larger portion of clinical isolates than environmental isolates showed 4-HPA-induced resistance to neomycin. The aph(3′)-IIb gene product is likely to function as a metabolic enzyme which has a cross-reactivity with aminoglycosides. These findings provide new insight into the possible mechanism of P. aeruginosa antibiotic resistance.
Pseudomonas aeruginosa, a gram-negative bacterium, is an opportunistic human pathogen. It is the major causative pathogen for morbidity and mortality in cystic fibrosis (CF) and burn patients as well as in immunocompromised patients (1, 8). This pathogen can survive in most environmental niches and infects a variety of hosts, demonstrating a tremendous capacity for adaptation and complex regulatory machinery (11, 12, 14).
P. aeruginosa is able to utilize 4-hydroxyphenylacetic acid (4-HPA) and 3,4-dihydroxyphenylacetic acid (3,4-DHPA) (5, 6) and catabolize some of the aromatic biogenic amines (such as tyramine and dopamine) found in mammalian nervous systems. The genetics of this particular metabolic pathway for Escherichia coli have been well described previously (3, 4). E. coli strains B, C, and W are able to utilize 3-hydroxyphenylacetic acid (3-HPA), 4-HPA, and 3,4-DHPA as alternative carbon sources through an hpa pathway consisting of the hydroxylation of 3-HPA or 4-HPA and the subsequent meta cleavage of 3,4-DHPA, which are encoded by the hydroxylase and meta operons, respectively (4). The hydroxylase operon is positively regulated by HpaA, an AraC family regulator, while the meta operon is repressed by HpaR, a negative regulator. Both HpaA and HpaR respond to the hpa substrate molecules (including 3-HPA and 4-HPA) to activate hpa regulon expression (19, 20). P. aeruginosa harbors homologues of the E. coli hpa pathway genes (29); however, there is no report on the function and regulation of these genes.
P. aeruginosa also harbors an array of aminoglycoside-modifying genes, enabling enzymatic inactivation of aminoglycosides by acetylation (7, 24), adenylation (25), or phosphorylation (APH) (9). These genes are either plasmid borne or chromosomally localized; in the latter case, a transposon-mediated mechanism has been suggested to be responsible for spreading the genes into this species (7, 18, 24). There has been no report of a study suggesting a possible correlation (either genetic and physiological) between an aminoglycoside-modifying gene and the HPA metabolism pathway.
In this report, aph(3′)-IIb, an aminoglycoside-phosphotransferase gene of P. aeruginosa (9), is shown to form an operon structure with its upstream hpaA homologue. The operon is activated by the HpaA homologue in response to the presence of 4-HPA, enabling P. aeruginosa to utilize 4-HPA as a sole carbon source. Activation of the hpa regulon in response to its substrate also leads to increased aph(3′)-IIb expression, resulting in elevated resistance to aminoglycoside antibiotics. These results may help partially explain the intrinsic as well as adaptive aminoglycoside resistance in P. aeruginosa.
MATERIALS AND METHODS
Materials, strains and media.
All strains and plasmids used in this study are listed in Table 1. E. coli and P. aeruginosa strains were grown at 37°C in Luria-Bertani (LB) broth or M63 minimal medium (21). The M63 medium salt was supplemented with 1 μg of vitamin B12/ml and 0.2% of glycerol unless specified otherwise. The 4-hydroxylphenylacetate (4-HPA) and 3-hydroxylphenylacetate (3-HPA) were purchased from Sigma (St. Louis, Mo.). They were dissolved in water and adjusted to pH 7.0 with KOH before being added to the culture medium. Antibiotics and concentrations were as follows: for E. coli, ampicillin (100 μg/ml), kanamycin (50 μg/ml), tetracycline (20 μg/ml), gentamicin (10 μg/ml), spectinomycin (50 μg/ml), and streptomycin (25 μg/ml); and for P. aeruginosa, tetracycline (100 μg/ml), gentamicin (200 μg/ml), carbenicillin (150 μg/ml), spectinomycin (200 μg/ml), and streptomycin (200 μg/ml).
TABLE 1.
Strains, plasmids, and primers
| Strain, plasmid, or primer | Genotype or description | Source or reference |
|---|---|---|
| Strains | ||
| E. coli | ||
| DH5α | F− φ80 δlacZ ΔM15 endA1 recA1 hsdR17 supE44 thi-1 relA1 Δ(lacZYA-argF) gyrA96 deoR | GIBCO-BRL |
| P. aeruginosa | ||
| PAK | Wild-type clinical isolate | David Bradley |
| PAK1-3 | A spontaneous mutant derivative of PAK, Neor | 31 |
| PAK(aph) | PAK with aph mutated by gentamicin cassette insertion | This study |
| PAK(hpaA) | PAK with hpaA mutated by Ω insertion | This study |
| Plasmids | ||
| pUCP19 | Broad-host-range plasmid, Cbr | 32 |
| pWC001 | 2-kb DNA containing aph insert cloned into pUCP19 | This study |
| pEx18Tc | Sucrose selection suicide delivery plasmid, Tcr | 10 |
| pExaph | pEx18Tc containing 1.7-kb BamHI fragment from pWC001 | This study |
| pExaphG | pExaph with gentamicin cassette inserted at NsiI site disrupting aph gene | This study |
| pDN19lacΩ | A lacZ fusion plasmid vector, IncP, Spr/Smr/Tcr | 30 |
| pCR2.1-TOPO | TA-cloning vector, Apr Kmr | Invitrogen |
| pWC003 | 1.5-kb PCR product (HpaA5-Aph3) of hpaA cloned into pCR2.1-TOPO | This study |
| pWC011 | pDN19lacΩ containing 1.6-kb fragment from pWC003, aph::lacZ fusion | This study |
| pWC012 | pDN19lacΩ containing 1.1-kb fragment from pWC003, hpaA::lacZ fusion | This study |
| pWC013 | pDN19lacΩ containing 1.6-kb fragment from pWC003, PA4121::lacZ fusion with intact hpaA | This study |
| pWW001 | 1.0-kb PCR product (Aph5-Aph3) of aph cloned into pCR2.1-TOPO | This study |
| pWC014 | pDN19lac14 containing 1.1-kb fragment from pWW001, aph::lacZ fusion | This study |
| pWC018 | PA4121::lacZ fusion, without hpaA | This study |
| pEx18Ap | Sucrose selection suicide delivery plasmid, Apr | 10 |
| pExhpaA | pEx18Ap containing XbaI/SacI fragment from pWC003 | This study |
| pWC021 | pExhpaA with its BglII fragment replaced by Ω insertion | This study |
| Primers | ||
| HpaA5 | 5′ CGTTGACGATCACGTAGCCGGCGACAT 3′ | |
| Aph5 | 5′ GAGCGCCAGCCGATCCCCAACATCAAC 3′ | |
| Aph3 | 5′ CTCGCCAGCGGTAGCCGGCAAGGTAGT 3′ |
Mutations and plasmid construction.
Mutant strains of hpaA and aph in a strain PAK background were constructed using a sucrose selection suicide delivery system as described previously (10). For hpaA mutation, specific primers HpaA5 and Aph3 (Table 1) were used to amplify a 1.5-kb hpaA-containing region. The PCR product was ligated into TA cloning vector pCR2.1-TOPO (Invitrogen) to give rise to pWC003, from which the hpaA-containing fragment was subcloned into an XbaI/SacI site of sacB-containing vector pEx18Ap, resulting in pExhpaA. Then an Ω cassette was used to replace the BglII fragment of the hpaA gene in pExhpaA, and the resulting plasmid (pWC021) was used to transform wild-type PAK. Spectinomycin-streptomycin-carbenicillin-resistant single-crossover colonies were selected on plates followed by plating on LB agar containing spectinomycin-streptomycin and 5% sucrose. The resulting double-cross mutants were confirmed by PCR using primers Aph5 and Aph3.
The aph mutant was generated in a similar fashion. pWC001, a clone containing a partial hpaA-aph region, was digested with BamHI, and the resulting fragment was inserted into the same site of sucrose selection plasmid pEx18Tc to generate pExaph. pExaph was then digested with NsiI, and a gentamicin cassette was inserted. The resulting plasmid, pExaphG, was used to generate an aph knockout mutation by sucrose selection as described above. The aph mutation was confirmed by Southern blot analysis.
A 1.6-kb EcoRI fragment (containing an intact hpaA gene in the middle as well as the N terminus of aph and the open reading frame [ORF] PA4121 in the opposing direction at the 5′ and 3′ ends, respectively) was isolated from pWC003 and inserted into lacZ fusion vector pDN19lacΩ. The resulting plasmids pWC011 and pWC013 encode APH-LacZ and PA4121-LacZ fusions, respectively. To construct the aph-lacZ fusion without an hpaA gene, a 1.0-kb fragment was amplified using primer set Aph5-Aph3 and cloned into pCR2.1-TOPO to generate pWW001. A 1.1-kb EcoRI fragment was isolated from pWW001 and inserted into pDN19lacΩ, generating an aph::lacZ fusion construct named pWC014. To construct the PA4121-LacZ fusion without an hpaA gene, a BamHI-BglII fragment from pWC003 was inserted in front of the promoterless lacZ gene in pDN19lacΩ, generating pWC018. Similarly, a 1.1-kb EcoRI-BglII fragment from pWC003 was used to construct hpaA::lacZ fusion plasmid pWC012.
Neomycin resistance tests.
Two methods were used to determine inducible resistance to neomycin. First, a double-disk diffusion test was used for qualitative assays. Specified amounts of antibiotics and HPA (3-HPA or 4-HPA) solutions were dropped onto round sterile filter paper disks (7 mm in diameter) and air dried. Fresh bacterial cultures were spread onto M63 agar plates with sterile cotton swabs, and HPA disks were placed on the plates. Empty disks were used as controls. After 5 h of incubation at 37°C, the antibiotic disks were placed ca. 1.25 cm away from the HPA disks. Plates were further incubated at 37°C, and the inhibition zones were observed after 15 to 20 h. For a quantitative assay, bacteria were grown in LB broth overnight and the cell density was determined by measuring the optical density at 600 nm. After serial dilutions, bacterial cells were inoculated into the antibiotic gradients at a final density of 5 × 105 cells/ml. MICs were determined after 36 h of incubation at 37°C without agitation. All MIC tests in this report were done by using M63 medium containing 0.2% glycerol supplemented with various concentrations of 4-HPA.
Miscellaneous assays.
P. aeruginosa strains harboring various lacZ fusion constructs were grown overnight in M63 medium with or without 5 mM HPA unless specified otherwise, and their β-galactosidase activities were measured as described previously (16). Growth curves of the PAK strain and various mutant derivatives were generated by measuring the optical density at 600 nm of the cultures in M63 medium supplemented with 10 mM 4-HPA as a sole carbon source.
RESULTS
Isolation of aph and its upstream putative regulatory gene hpaA.
Wang et al. have previously described PAK1-3, a multidrug-resistant P. aeruginosa strain derived from strain PAK (31). As part of an effort to identify the mutant gene(s) responsible for the antibiotic resistance phenotype, we constructed a PAK1-3 genomic clone bank and introduced it into the PAK strain to select for clones conferring neomycin resistance (at 200 μg/ml). A total of seven positive colonies were obtained, from which the plasmids were isolated and analyzed by restriction enzyme digestion and sequencing. A single gene, aph(3′)-IIb (referred to in shortened form as aph hereafter), was found to be responsible for the resistance in all the clones. The aph gene encodes a 268-amino-acid-long aminoglycoside-phosphotransferase with 51.7% identity to APH(3′)-IIa from Tn5 (9). In all seven positive clones that we had isolated, the aph gene was preceded by a putative regulatory gene which is a homologue of hpaA from E. coli W (and which we call hpaA as well in this report).
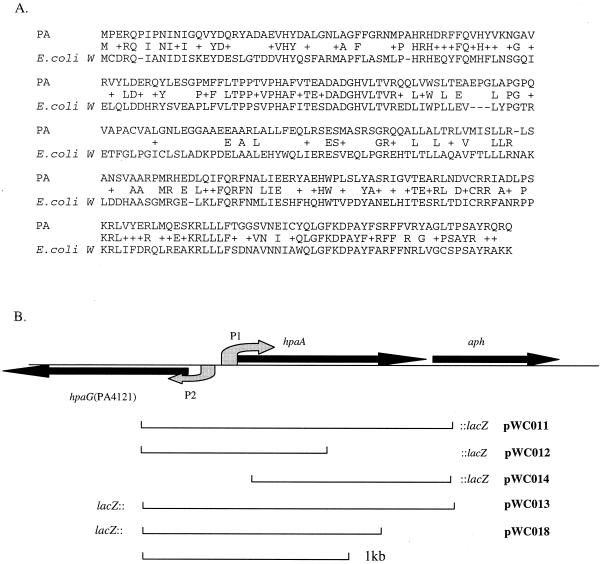
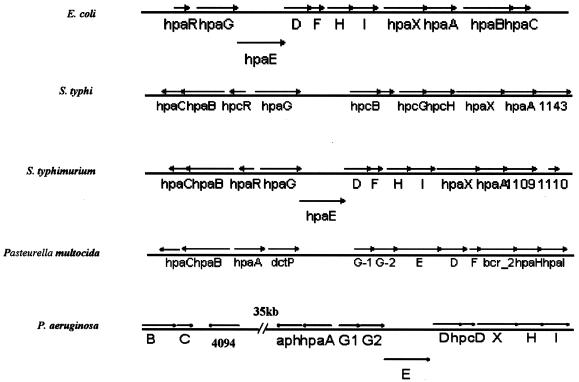
HpaA belongs to an AraC regulatory protein family and is required to activate the metabolic pathway genes of alternative carbon source 4-HPA in certain E. coli strains (20). As shown in Fig. 1A, HpaA proteins from E. coli and P. aeruginosa have a significant amount (63%) of sequence similarity. A series of HPA utilization genes were found around the hpaA locus (homologous to their counterparts in E. coli W) (19). Also in similarity to findings for E. coli, these genes form two separate operon-like structures, namely, the hydroxylase and meta operons. E. coli W harbors a positive regulator (HpaA) and a repressor (HpaR), controlling the hydroxylase operon and the meta operon, respectively. In P. aeruginosa, however, there is no HpaR homologue; instead, two AraC-type regulator genes were found in the hpa operons, namely, hpaA and PA4094 (Fig. 2). On the basis of the facts that PA4094 is adjacent to the hydroxylase operon (hpaBC), that hpaA is upstream of the meta operon, and that these two operons are far (ca. 35 kb) apart (Fig. 2), it is reasonable to speculate that in P. aeruginosa, PA4094 and hpaA regulate the two respective operons. The aph gene, seemingly so irrelevant to this pathway, is located immediately downstream of hpaA. As shown in Fig. 1B, the close proximity of hpaA and aph (51 bp apart), and the absence of any promoter-like element immediate upstream of the aph gene, introduces the possibility that these two genes might form an operon structure.
FIG. 1.
(A) Alignment of HpaA from P. aeruginosa and E. coli. Letters between two lines of a sequence designate identical amino acids; a plus sign indicates two similar amino acids. (B) A schematic representation of the hpaA-aph locus in P. aeruginosa and the structures of lacZ fusion plasmids pWC011, pWC012, pWC013, pWC014, and pWC018. Filled straight arrows represent ORFs and transcription direction; curved arrows shaded gray show the putative promoters.
FIG. 2.
Genomic structures of hpa regulons found in various microorganisms. All the ORFs were drawn according to their actual sizes within each regulon. Letters B, C, D, E, F, H, I, and X stand for ORFs hpaB, -C, -D, -E, -F, -H, -I, and -X, respectively.
Regulation of the aph gene by the autoregulator HpaA.
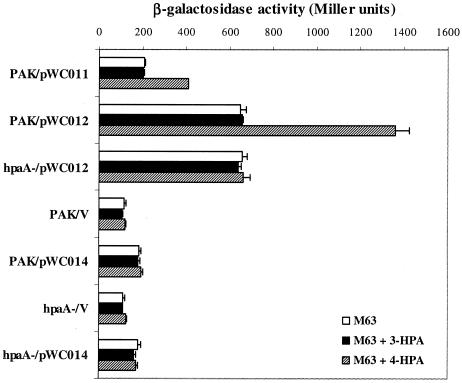
We first tested the expression of hpaA by using an hpaA::lacZ fusion plasmid (pWC012). Strain PAK and the hpaA mutant containing pWC012 were grown in M63 minimal medium supplemented with 5 mM 4-HPA or left untreated, and β-galactosidase assays were performed. As shown in Fig. 3, no differences in the β-galactosidase activities were observed in the PAK and the hpaA backgrounds when cells were grown without 4-HPA. In the presence of 5 mM 4-HPA, however, a significant induction of hpaA expression was observed in wild-type PAK and no induction was detected in the hpaA background, suggesting that HpaA activates its own expression in response to the presence of the 4-HPA substrate.
FIG. 3.
Induction of hpaA and aph in P. aeruginosa with 4-HPA requires the presence of intact hpaA genes. Cells were grown overnight in M63 medium with or without supplementation of 5 mM 3-HPA or 4-HPA. Data represent the averages of the results of six independent β-galactosidase activity experiments. pWC011, pWC012, and pWC014 are lacZ fusion plasmids for hpaA-aph, hpaA, and aph, respectively (Fig. 1B); PAK/V and hpaA-/V are vector controls containing pDN19lacΩ.
Next, we tested the effect of hpaA on aph expression. As shown in Fig. 3, when induced by 5 mM 4-HPA the expression of aph::lacZ in the presence of hpaA (pWC011) (as measured by β-galactosidase activity levels) increased more than twofold. In the absence of hpaA (i.e., pWC014 in an hpaA mutant background), however, the β-galactosidase activity did not respond to the induction by 4-HPA and remained at a level very close to that of pWC011 without 4-HPA induction, which was significantly higher than that of the vector control. These results suggested that there are two layers of control over aph expression: one provided by the hpaA promoter, which responds to the presence of 4-HPA, and another one probably residing in the hpaA-aph intergenic region and acting constitutively. In agreement with this hypothesis, the expression of aph::lacZ (pWC014) in an hpaA mutant background did not decrease in the presence or absence of 4-HPA, suggesting that HpaA exerts no control on the second aph promoter.
4-HPA induces neomycin resistance in P. aeruginosa.
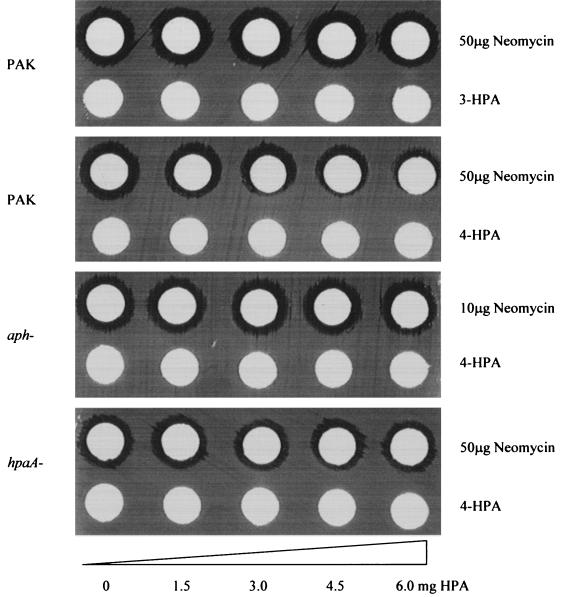
Since aph transcription can be activated by adding 5 mM 4-HPA in the culture medium, we further tested whether the presence of 4-HPA in culture medium changes the level of bacterial resistance to neomycin. This was first investigated with a double-disk diffusion assay. As shown in Fig. 4, the presence of 4-HPA decreased the size of the inhibition zones formed by neomycin on the PAK bacterial lawn in a concentration-dependent fashion. However, 4-HPA failed to interfere with the formation of a neomycin inhibition zone on either hpaA or aph mutant strains. Apparently 4-HPA is able to induce P. aeruginosa aminoglycoside resistance in an HpaA-dependent manner.
FIG. 4.
The results of a double-disk diffusion assay show that neomycin resistance in P. aeruginosa can be induced by the presence of 4-HPA in a concentration-dependent manner. This result was not observed for the isogenic aph or hpaA mutant strains or when 3-HPA is used as inducer. The amounts of neomycin and 3- or 4-HPA used in these tests are indicated (see Materials and Methods for details).
The ability of 4-HPA to induce aph expression (and, hence, bacterial resistance to neomycin) was further characterized by a MIC test. A neomycin gradient was generated in M63 minimal medium supplemented with 4-HPA at concentrations ranging from 0 to 10 mM. Overnight cultures of PAK, hpaA, and aph strains were inoculated into neomycin gradients at final densities of 5 × 105 cells/ml, and bacterial growth was scored after 24 and 36 h of incubation. As shown in Table 2 for the wild-type strain PAK, increasing the concentration of 4-HPA from 0 to 10 mM led to increases (from 30 to 250 μg/ml) in the level of bacterial resistance to neomycin. Under the same conditions, no induction of resistance was observed in either an hpaA or aph mutant background. The neomycin resistance level for the aph mutant strain was (as expected) dramatically decreased, dropping from 30 μg of neomycin/ml for the wild-type strain to 5 μg/ml for the aph mutant strain, suggesting that the aph gene does play an important role in the intrinsic resistance of P. aeruginosa to aminoglycosides. For the hpaA mutant strain, an apparent defect in bacterial growth in 4-HPA-containing minimal medium was observed. After an extended period (36 h) of incubation, which enabled us to score the growth, no induction of neomycin resistance by 4-HPA was observed. However, this resistance level (30 μg of neomycin/ml) was still significantly higher than that for the aph mutant strain (5 μg of neomycin/ml), supporting the previous conclusion that low-level constitutive expression of aph does exist.
TABLE 2.
MICs of neomycin for PAK and mutants
| Strain | MIC (μg/ml) of neomycin supplemented with:
|
||
|---|---|---|---|
| No 4-HPA | 1 mM 4-HPA | 10 mM 4-HPA | |
| PAK | 30 | 130 | 250 |
| PAK(aph) | 5 | 5 | 5 |
| PAK(hpaA) | 30 | 30 | 30 |
HpaA controls metabolism of 4-HPA, while APH is not required.
We were intrigued by the fact that aph is a total stranger to the hpa regulon, since none of the hpa regulon-harboring bacteria contained the aph homologue. To determine whether HpaA regulates the meta operon, we analyzed the requirement of this special gene cluster in the metabolism of 4-HPA as a sole carbon source.
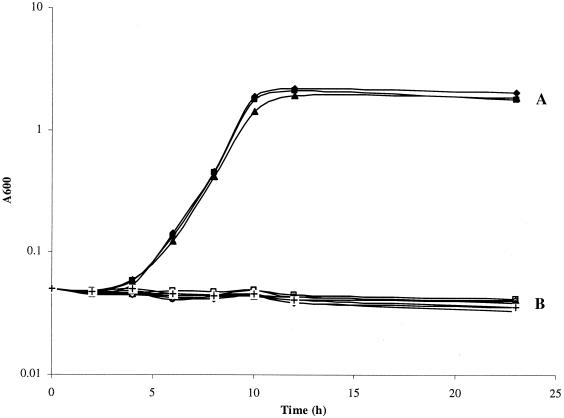
Strains of PAK, aph, and hpaA individually or the hpaA mutant harboring pWC011 or pWC014 were inoculated into M63 minimal medium with or without 10 mM 4-HPA as the sole carbon source. The results shown in Fig. 5 encouraged several conclusions. (i) The P. aeruginosa PAK strain was capable of utilizing 4-HPA as a sole carbon source for growth. (ii) The PAK strain with an hpaA mutation lost the ability to utilize 4-HPA, and this defect was complemented by pWC011 carrying an intact copy of hpaA but not by pWC014 lacking the hpaA gene. (iii) An aph mutation had no effect on the bacterial ability to utilize 4-HPA as the sole carbon source. While comparing the growth levels of the PAK strain in M63 medium with glucose, glycerol, or 4-HPA as a sole carbon source at the same molar concentrations, we found that 4-HPA served as the best carbon source, giving the PAK strain the fastest growth rate and highest stationary phase cell density (data not shown).
FIG. 5.
Growth curves of strains PAK, PAK(aph), and PAK(hpaA) as well as those of strain PAK(hpaA) containing pWC011 (hpaA clone) or pWC014 (control) in M63 medium supplemented with 10 mM 4-HPA as the sole carbon source or left untreated. Group A includes strains PAK, PAK(aph), and PAK(hpaA)/pWC011 in M63 medium with 4-HPA. Group B includes strains PAK, PAK(aph), and PAK(hpaA)/pWC011 in M63 medium without 4-HPA and also strains PAK(hpaA) and PAK(hpaA)/WC014 in M63 medium with and without 4-HPA.
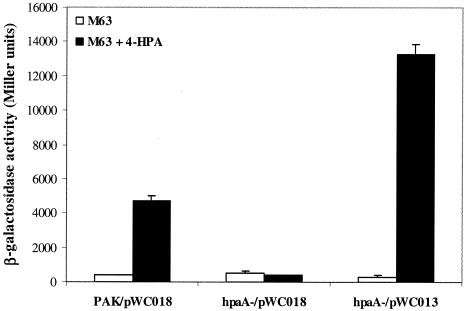
To test the role of HpaA in meta operon regulation, we constructed two PA4121::lacZ fusion plasmids, pWC013 and pWC018, for which PA4121 is the first gene of the meta operon in P. aeruginosa and shares homology with E. coli HpaG. pWC013 contains an intact copy of hpaA, while pWC018 does not have the hpaA gene (Fig. 1B). PAK and hpaA mutant strains containing these two plasmids were grown in M63 medium supplemented with 5 mM 4-HPA or left untreated, and β-galactosidase activities were measured. In similarity to the results seen with hpaA and aph, expression of PA4121 is activated by the HpaA in a 4-HPA-dependent manner (Fig. 6). A mutation in the hpaA gene abolished this induction, and this defect was complemented by the hpaA gene in pWC013. These results indicate that the meta operon of P. aeruginosa is positively regulated by HpaA (unlike the results seen with E. coli W, in which it was repressed by HpaR).
FIG. 6.
Expression of PA4121 is under the positive regulation of HpaA in response to the presence of 4-HPA. Cells were grown in M63 medium with (filled bars) or without (empty bars) 5 mM 4-HPA, and β-galactosidase activities were measured. pWC013 and pWC018 are both lacZ fusion plasmids for gene PA4121, and pWC013 contains an intact copy of hpaA (Fig. 1B).
An unmarked deletion mutant of hpaA in the strain PAK background behaved exactly the same as the insertional mutant with respect to its basal and inducible (by HPA analogues) levels of resistance to neomycin as well as with respect to the expression of hpaA downstream genes (namely, aph and PA4121) (data not shown). Therefore, genes carried on the Ω fragment did not contribute to the neomycin resistance.
The 4-HPA analogue 3-HPA is incapable of inducing the hpaA-aph operon.
The results of studies with Pseudomonas putida and Acinetobacter spp. have suggested that fluorescent pseudomonads utilize 3-HPA and 4-HPA by two different pathways and that these two analogous compounds share the same pathway in E. coli (4, 28). We first tested the growth of PAK and another standard P. aeruginosa strain (PAO1) on M63 medium with 3-HPA as the sole carbon source. Surprisingly, after 36 h of incubation at 37°C, no apparent growth was detected, suggesting that P. aeruginosa is not able to utilize 3-HPA as the sole carbon source.
When 3-HPA was used as an inducer, no expression of the hpaA::lacZ and aph::lacZ fusions was observed (Fig. 3). Furthermore, when 3-HPA was used in double-disk diffusion assays (as shown in Fig. 4), no noticeable effect was observed compared to that seen with 4-HPA, suggesting that the induction effect of 4-HPA is highly specific (possibly through a specific interaction between 4-HPA molecules and HpaA protein) (see Discussion).
The presence of an hpaA-aph locus and induction of neomycin resistance in various P. aeruginosa isolates.
We wanted to test whether the aph gene is commonly located behind the hpaA gene on the chromosome of various P. aeruginosa isolates. A PCR approach was employed to survey clinical and environmental isolates. Specific primers for a 1-kb DNA fragment encompassing the whole hpaA coding region and part of the aph coding region were targeted for PCR amplifications to determine the presence of the hpaA-aph gene cluster. Strain PAK was included as a positive control, while E. coli O157 and water were used as negative controls. From all 58 isolates, including 19 CF isolates, 20 non-CF isolates, and 19 environmental isolates, 1-kb fragments were amplified (data not shown). Furthermore, 56 of these strains were capable of utilizing 4-HPA, as determined by growth on M63 medium supplemented with 4-HPA as a sole carbon source. These results suggest that the coexistence of hpaA and aph is a common feature in P. aeruginosa.
Out of the 58 P. aeruginosa isolates, 48 exhibited higher levels of resistance to neomycin than the PAK strain (for which the neomycin MIC is ca. 10 μg/ml in standard MHB medium) and the other 10 showed a level of resistance similar to or even lower than that of strain PAK. Utilizing the double-disk diffusion assay, we further assessed the ability of 4-HPA to induce aph expression in these 58 P. aeruginosa isolates. A total of 45 strains with various levels of resistance to neomycin were tested; the others (mostly CF isolates) were excluded due to their apparent growth defect on M63 plates or to extremely high resistance to neomycin. Out of the 27 clinical isolates, 19 (including 5 CF and 14 non-CF isolates) showed induction of resistance in the presence of 4-HPA. However, only 3 out of 18 environmental isolates responded positively to the presence of 4-HPA. Thus, a significantly (P = 6.5864 × 10−5) higher portion of the clinical isolates than environmental isolates showed 4-HPA-induced resistance, implying that 4-HPA-mediated induction of aph gene plays a role in the life of clinical P. aeruginosa isolates, possibly by providing a selection advantage in the challenge of repetitive antibiotic chemotherapy.
DISCUSSION
Aminoglycoside-phosphotransferase APH(3′)-IIb of P. aeruginosa was shown by Hächler et al. to specifically confer resistance to a group of aminoglycoside antibiotics, including kanamycin A and B, neomycin B and C, butirosin, and seldomycin F5 (9). However, Hächler et al. were not able to use its putative promoter to express the aph gene in E. coli and no P. aeruginosa expression data were presented. Using a genetic approach, we showed that the aph(3′)-IIb gene of P. aeruginosa strain PAK is positively controlled by its upstream AraC-type regulator HpaA. The hpaA promoter drives transcription of aph, and this transcription is auto-regulated by HpaA in response to the presence of 4-HPA. Also, aminoglycoside resistance in strain PAK is clearly inducible by 4-HPA in an HpaA-dependent manner. More significantly, the coexistence of hpaA and aph in P. aeruginosa is not limited to strains PAK and PAO1; instead, it is a common feature in both clinical and environmental isolates of P. aeruginosa. By showing the growth of strain PAK in 4-HPA-based medium, we demonstrated the ability of P. aeruginosa to utilize 4-HPA as a sole carbon source in an HpaA-dependent manner. 4-HPA is actually a better carbon source than glucose or glycerol (data not shown). Therefore, hpaA-aph could be an important operon for P. aeruginosa, not only conferring P. aeruginosa resistance to some of the important aminoglycoside antibiotics (e.g., neomycin and kanamycin) but also enabling it to adjust this resistance according to certain environmental signals, especially in vivo (when P. aeruginosa is infecting human or animal hosts), for P. aeruginosa has been shown to utilize aromatic compounds found in mammalian nervous system through an hpa pathway (6, 21).
Data obtained from 3-HPA in this study were also quite interesting. As a 4-HPA analogue, 3-HPA was suggested to be degraded by the same metabolic pathway as 4-HPA in E. coli but by different pathways in fluorescent pseudomonads and Acinetobacter spp. (4, 28). However, P. aeruginosa strain PAK cannot utilize 3-HPA and it has no significant inducing effect on hpaA-aph expression either. Further study is needed to clarify this issue.
A discrepancy has been noticed among the results of the hpaA-aph PCR survey (positive results for all 58 isolates), the 4-HPA utilization test (56 positive results out of 58), and the double-disk diffusion assay (22 isolates responsive out of 45). This can be tentatively explained by the following possible factors: (i) mutations in the hpaA-aph region, which make this operon either nonfunctional or constitutive; (ii) bacterial defect in the uptake of the 4-HPA; and (iii) other mechanisms of aminoglycoside resistance that mask the aph-mediated resistance. For example, alterations in the antibiotic targets likely mask the effect of aph induction by 4-HPA.
It has been suggested that E. coli W might have acquired its hpa catabolic cassette horizontally from other organisms, as evidenced by the fact that homologues flanking the hpa regulon are found in E. coli K-12, which does not have the hpa regulon (19). We have looked into all other organisms with available genomic sequence data and compared the P. aeruginosa hpa regulon with its counterparts in these organisms (Fig. 2). The fact that the two P. aeruginosa hpa operons are farther separated than their counterparts in E. coli W (and that they possess two activators instead of a repressor and an activator) distinguishes P. aeruginosa from most other hpa-containing bacteria, including E. coli, Salmonella enterica serovar Typhi, and S. enterica serovar Typhimurium, whose hpa regulons have both hpaA and hpaR homologues.
Although β-lactamase genes are almost ubiquitously present on the chromosomes of enterobacteria, few are in salmonellae, most of which are plasmid borne (2, 13). On the chromosome of S. enterica serovar Typhimurium LT2, however, an ORF (no. 1109) encoding a probable β-lactamase is found localized downstream of the hpaA homologue, with a 14-bp intergenic region (15), resembling the hpaA-aph structure in P. aeruginosa. The same structure is also found in the S. enterica serovar Typhi CT18 chromosome, in which an almost identical ORF 1143, sharing 99% amino acid identity with ORF 1109 in S. enterica serovar Typhimurium, was located immediately downstream to the hpaA homologue (17) (Fig. 2). Therefore, it is possible that in salmonellae, the chromosomal β-lactamase (in similarity to that of P. aeruginosa) is inducible only under certain environmental conditions. The presence of the probable β-lactamase ORF behind the hpaA homologous gene may prove an interesting lead for the study of the possible correlation of hpa pathways and inducible antibiotic resistance in these bacteria.
Antibiotic-modifying enzymatic genes have been suggested to have evolved through two different pathways, either being acquired from antibiotic-producing microorganisms that need to defend against their own metabolic by-products or originating from normal metabolic genes and having undergone series of mutations (26). The aac(6′)-Ic gene of Serratia marcescens is found in all S. marcescens strains, while its expression is silent in the aminoglycoside-susceptible ones (27). This gene was suggested to have evolved from a normal metabolic gene, although no physiological evidence is yet available (26). Meanwhile, E. coli W has been found to contain (near the hpa regulon) a pac gene encoding a penicillin G acylase, which is believed to hydrolyze esters of 4-HPA and phenylacetic acids, therefore expanding the substrate spectrum of the hpa pathway (22, 23). Although our data indicate that aph is not required for the utilization of 4-HPA in P. aeruginosa, we certainly have not tested all the possible substrates suitable for the hpa pathway; also, our approach would probably have been incapable of detecting any effect of aph mutation if the APH were only to play a compensatory role in the hpa pathway.
Aminoglycoside-modifying enzyme genes have not been found to be generally regulated. So far, only two of them, the aac(6′)-Ic gene of Serratia marcescens and the aac(2′)-Ia gene of Providencia stuartii, are known to be under regulation, and the exact regulatory factor(s) is yet to be discovered (26, 27). Our study of hpaA-aph provides a novel regulation model for aminoglycoside-modifying genes: a surrogate type of activator is recruited from another regulatory pathway. This also raised a possible mechanism for the bacteria to acquire increased antibiotic resistance through mutation: mutations within the hpaA coding region or its promoter region which cause constitutive activation of hpaA by affecting either HpaA-(4-HPA) interaction or HpaA-DNA interaction. It has been mentioned by Hächler et al. that since they found that all 10 tested strains had aph genes present, aph is likely a ubiquitous gene in P. aeruginosa (9). Our study suggests that not just the presence of aph alone but also the coexistence of hpaA and aph seems to be a common feature in P. aeruginosa; more importantly, a higher proportion of clinical strains than environmental isolates showed increased neomycin resistance in response to the presence of 4-HPA. We postulate that at least some of the clinical strains might have acquired elevated aminoglycoside resistance through an hpaA-aph induction pathway.
Acknowledgments
We thank Reuben Ramphal for the gift of the clinical isolates and Weihui Wu for technique assistance.
This work is supported by the American Cancer Society and the Cystic Fibrosis Foundation.
REFERENCES
- 1.Bodey, G. P., R. Bolivar, V. Fainstein, and L. Jadeja. 1983. Infections caused by Pseudomonas aeruginosa. Rev. Infect. Dis. 5:279-313. [DOI] [PubMed] [Google Scholar]
- 2.Bradford, P. A. 2001. Extended-spectrum β-lactamases in the 21st century: characterization, epidemiology, and detection of this important resistance threat. Clin. Microbiol. Rev. 14:933-951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burlingame, R., and P. J. Chapman. 1983. Catabolism of phenylpropionic acid and its 3-hydroxy derivative by Escherichia coli. J. Bacteriol. 155:113-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cooper, R. A., and M. A. Skinner. 1980. Catabolism of 3- and 4-hydroxyphenylacetate by the 3,4-dihydroxyphenylacetate pathway in Escherichia coli. J. Bacteriol. 143:302-306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cuskey, S. M., and R. H. Olsen. 1988. Catabolism of aromatic biogenic amines by Pseudomonas aeruginosa PAO1 via meta cleavage of homoprotocatechuic acid. J. Bacteriol. 170:393-399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cuskey, S. M., V. Peccoraro, and R. H. Olsen. 1987. Initial catabolism of aromatic biogenic amines by Pseudomonas aeruginosa PAO: pathway description, mapping of mutations, and cloning of essential genes. J. Bacteriol. 169:2398-2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Galimand, M., T. Lambert, G. Gerbaud, and P. Courvalin. 1993. Characterization of the aac(6′)-Ib gene encoding an aminoglycoside 6′-N-acetyltransferase in Pseudomonas aeruginosa BM2656. Antimicrob. Agents Chemother. 37:1456-1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Govan, J. R., and V. Deretic. 1996. Microbial pathogenesis in cystic fibrosis: mucoid Pseudomonas aeruginosa and Burkholderia cepacia. Microbiol. Rev. 60:539-574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hächler, H., P. Santanam, and F. H. Kayser. 1996. Sequence and characterization of a novel chromosomal aminoglycoside phosphotransferase gene, aph(3′)-IIb, in Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 40:1254-1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hoang, T. T., R. R. Karkhoff-Schweizer, A. J. Kutchma, and H. P. Schweizer. 1998. A broad-host-range Flp-FRT recombination system for site-specific excision of chromosomally-located DNA sequences: application for isolation of unmarked Pseudomonas aeruginosa mutants. Gene 212:77-86. [DOI] [PubMed] [Google Scholar]
- 11.Kominos, S. D., C. E. Copeland, B. Grosiak, and B. Postic. 1972. Introduction of Pseudomonas aeruginosa into a hospital via vegetables. Appl. Microbiol. 24:567-570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kucera, M., and O. Lysenko. 1968. The mechanism of pathogenicity of Pseudomonas aeruginosa. V. Isolation and properties of the proteinases toxic for larvae of the greater wax moth Galleria mellonella L. Folia Microbiol. 13:288-294. [DOI] [PubMed] [Google Scholar]
- 13.Livermore, D. M. 1995. β-Lactamases in laboratory and clinical resistance. Clin. Microbiol. Rev. 8:557-584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mahajan-Miklos, S., M. W. Tan, L. G. Rahme, and F. M. Ausubel. 1999. Molecular mechanisms of bacterial virulence elucidated using a Pseudomonas aeruginosa-Caenorhabditis elegans pathogenesis model. Cell 96:47-56. [DOI] [PubMed] [Google Scholar]
- 15.McClelland, M., K. E. Sanderson, J. Spieth, S. W. Clifton, P. Latreille, L. Courtney, S. Porwollik, J. Ali, M. Dante, F. Du, S. Hou, D. Layman, S. Leonard, C. Nguyen, K. Scott, A. Holmes, N. Grewal, E. Mulvaney, E. Ryan, H. Sun, L. Florea, W. Miller, T. Stoneking, M. Nhan, R. Waterston, and R. K. Wilson. 2001. Complete genome sequence of Salmonella enterica serovar Typhimurium LT2. Nature 413:852-856. [DOI] [PubMed] [Google Scholar]
- 16.Miller, J. H. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 17.Parkhill, J., G. Dougan, K. D. James, N. R. Thomson, D. Pickard, J. Wain, C. Churcher, K. L. Mungall, S. D. Bentley, M. T. Holden, M. Sebaihia, S. Baker, D. Basham, K. Brooks, T. Chillingworth, P. Connerton, A. Cronin, P. Davis, R. M. Davies, L. Dowd, N. White, J. Farrar, T. Feltwell, N. Hamlin, A. Haque, T. T. Hien, S. Holroyd, K. Jagels, A. Krogh, T. S. Larsen, S. Leather, S. Moule, P. O'Gaora, C. Parry, M. Quail, K. Rutherford, M. Simmonds, J. Skelton, K. Stevens, S. Whitehead, and B. G. Barrell. 2001. Complete genome sequence of a multiple drug resistant Salmonella enterica serovar Typhi CT18. Nature 413:848-852. [DOI] [PubMed] [Google Scholar]
- 18.Poirel, L., T. Lambert, S. Türkoglü, E. Ronco, J.-L. Gaillard, and P. Nordmann. 2001. Characterization of class 1 integrons from Pseudomonas aeruginosa that contain the blaVIM-2 carbapenem-hydrolyzing β-lactamase gene and of two novel aminoglycoside resistance gene cassettes. Antimicrob. Agents Chemother. 45:546-552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Prieto, M. A., E. Diaz, and J. L. Garcia. 1996. Molecular characterization of the 4-hydroxyphenylacetate catabolic pathway of Escherichia coli W: engineering a mobile aromatic degradative cluster. J. Bacteriol. 178:111-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Prieto, M. A., and J. L. Garcia. 1997. Identification of a novel positive regulator of the 4-hydroxyphenylacetate catabolic pathway of Escherichia coli. Biochem. Biophys. Res. Commun. 232:759-765. [DOI] [PubMed] [Google Scholar]
- 21.Prieto, M. A., and J. L. Garcia. 1997. Identification of the 4-hydroxyphenylacetate transport gene of Escherichia coli W: construction of a highly sensitive cellular biosensor. FEBS Lett. 414:293-297. [DOI] [PubMed] [Google Scholar]
- 22.Prieto, M. A., and J. L. Garcia. 1994. Molecular characterization of 4-hydroxyphenylacetate 3-hydroxylase of Escherichia coli. A two-protein component enzyme. J. Biol. Chem. 269:22823-22829. [PubMed] [Google Scholar]
- 23.Prieto, M. A., A. Perez-Aranda, and J. L. Garcia. 1993. Characterization of an Escherichia coli aromatic hydroxylase with a broad substrate range. J. Bacteriol. 175:2162-2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schwocho, L. R., C. P. Schaffner, G. H. Miller, R. S. Hare, and K. J. Shaw. 1995. Cloning and characterization of a 3-N-aminoglycoside acetyltransferase gene, aac(3)-Ib, from Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 39:1790-1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shaw, K. J., H. Munayyer, P. N. Rather, R. S. Hare, and G. H. Miller. 1993. Nucleotide sequence analysis and DNA hybridization studies of the ant(4′)-IIa gene from Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 37:708-714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shaw, K. J., P. N. Rather, R. S. Hare, and G. H. Miller. 1993. Molecular genetics of aminoglycoside resistance genes and familial relationships of the aminoglycoside-modifying enzymes. Microbiol. Rev. 57:138-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shaw, K. J., P. N. Rather, F. J. Sabatelli, P. Mann, H. Munayyer, R. Mierzwa, G. L. Petrikkos, R. S. Hare, G. H. Miller, P. Bennett, et al. 1992. Characterization of the chromosomal aac(6′)-Ic gene from Serratia marcescens. Antimicrob. Agents Chemother. 36:1447-1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sparnins, V. L., P. J. Chapman, and S. Dagley. 1974. Bacterial degradation of 4-hydroxyphenylacetic acid and homoprotocatechuic acid. J. Bacteriol. 120:159-167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stover, C. K., X. Q. Pham, A. L. Erwin, S. D. Mizoguchi, P. Warrener, M. J. Hickey, F. S. Brinkman, W. O. Hufnagle, D. J. Kowalik, M. Lagrou, R. L. Garber, L. Goltry, E. Tolentino, S. Westbrock-Wadman, Y. Yuan, L. L. Brody, S. N. Coulter, K. R. Folger, A. Kas, K. Larbig, R. Lim, K. Smith, D. Spencer, G. K. Wong, Z. Wu, I. T. Paulsen, J. Reizer, M. H. Saier, R. E. Hancock, S. Lory, and M. V. Olson. 2000. Complete genome sequence of Pseudomonas aeruginosa PAO1, an opportunistic pathogen. Nature 406:959-964. [DOI] [PubMed] [Google Scholar]
- 30.Totten, P. A., and S. Lory. 1990. Characterization of the type a flagellin gene from Pseudomonas aeruginosa PAK. J. Bacteriol. 172:7188-7199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang, Y., U. Ha, L. Zeng, and S. Jin. 2003. Regulation of membrane permeability by a two-component regulatory system in Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 47:95-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.West, S. E., H. P. Schweizer, C. Dall, A. K. Sample, and L. J. Runyen-Janecky. 1994. Construction of improved Escherichia-Pseudomonas shuttle vectors derived from pUC18/19 and sequence of the region required for their replication in Pseudomonas aeruginosa. Gene 148:81-86. [DOI] [PubMed] [Google Scholar]