Abstract
The spread of orf513-bearing class 1 integrons is associated with blaCTX-M-2 in gram-negative clinical isolates in Argentina, with In35 being the most frequently found integron (74%). Among 65 isolates without blaCTX-M-2, only one harbored a novel orf513-bearing class 1 integron with the dfrA3b gene. The finding of orf513 not associated with class 1 integrons in two gram-positive strains indicates the widespread occurrence of this putative site-specific recombinase.
Multiple-antibiotic resistance is common in clinical isolates from Argentina. Although integrons belonging to all classes have been found in clinical and environmental strains of multiresistant bacteria (21), class 1 integrons predominate among gram-negative microorganisms (10) and have been recently described in gram-positive bacteria (4, 17). Class 1 integrons are composed of three DNA segments, two that are conserved and one that includes the antibiotic resistance gene cassettes of various lengths and sequences. The 5′ conserved segment (5′-CS) includes the intI1 gene, and downstream of the last gene cassette, most of the studied class 1 integrons contain at least part of the 3′ conserved segment (3′-CS) formed by the qacEΔ1 gene, sul1, and orf5, of unknown function (14). Most class 1 integrons are found on defective transposons related to Tn402 that keep the tniA and tniBΔ1 genes. Several contain one or two insertion sequences between the conserved segments and the transposition genes.
A novel group of orf513-bearing class 1 integrons, also called unusual class 1 integrons, of which pDGO100 is the prototype was first described in 1990 (6). These integrons begin with typical class 1 integron structures with one or more gene cassettes located between the 5′-CSs and the 3′-CSs. These 3′-CSs, called the first 3′-CSs, include only the first 1,355 bases of a typical 3′-CS (6). In all studied orf513-bearing class 1 integrons, the first 3′-CSs end at the same point, 24 nucleotides (nt) after the stop codon of the sul1 gene, as described for In6 and In7 (16). Following the first 3′-CSs, there is a common region which includes orf513 (GenBank accession number L06418) and a region unique to each orf513-bearing class 1 integron. The unique regions differ in length and sequence, containing the antibiotic resistance gene dfrA10 (In7), catII (In6), blaDHA-1 (pSAL-1), blaCTX-M-9 (In60), or blaCTX-M-2 (In35 and InS21) (1, 5, 11, 16, 20). Adjacent to the unique regions are the second 3′-CSs with different deletions in the 5′ ends (1).
In Argentina, CTX-M-2 is by far the most frequent extended-spectrum β-lactamase, comprising 69% of all extended-spectrum β-lactamases found among clinical isolates in Argentinean hospitals (M. Galas, M. Rapoport, F. Pasteran, R. Melano, A. Petroni, P. Ceriana, A. Rossi, and the WHONET Group, Abstr. 39th Intersci. Conf. Antimicrob. Agents Chemother., abstr. 1474, 1999). A previous study with a small number of isolates reported that blaCTX-M-2 is always located at the same sequence position in orf513-bearing class 1 integrons with different arrays of cassettes in the variable regions (1). Nevertheless, genes encoding some different enzymes of the CTX-M family have been identified located near other genetic elements such as ISEcp1, IS26, and IS903C (2, 3, 7, 12) in isolates from Europe and Asia. The goals of the present study were (i) to examine the orf513-related structures and look for their association with resistance genes and (ii) to identify the different arrangements of cassettes in the variable regions of orf513-bearing class 1 integrons.
We studied 130 nonredundant multiresistant clinical isolates collected during nosocomial outbreaks at different hospitals in Buenos Aires, Argentina, between 1993 and 2000. Of these, 100 were gram-negative bacterial isolates resistant to β-lactams and aminoglycosides and were divided into blaCTX-M-2-positive (n = 35) and blaCTX-M-2-negative (n = 65) isolates (Table 1). Thirty were gram-positive bacterial isolates with diverse mechanisms of resistance: Enterococcus faecium, resistant to vancomycin (n = 8); beta-hemolytic Streptococcus, resistant to tetracycline and erythromycin (n = 6); Staphylococcus aureus, resistant to methicillin (n = 8); and coagulase-negative Staphylococcus, resistant to methicillin (n = 8). Isolates were identified by using the API systems (Biomerieux SA, Marcy-l'Etoile, France) and conventional biochemical tests. Susceptibility to antimicrobial agents in all the isolates was determined by the E-Test method (AB Biodisk, Solna, Sweden) according to the guidelines proposed by the manufacturer (Table 1). Bacterial DNA was extracted using standard techniques (13). Isolates were subjected to PCR analysis with internal primers for detection of the blaCTX-M-2 gene, orf513, class 1 integrons, and orf513-bearing class 1 integrons. The characterization of the different arrays of cassettes in the variable regions was performed by PCR mapping (Table 2) (8), and several PCR products obtained were sequenced to confirm the data.
TABLE 1.
MIC90sa of antimicrobial agents for the studied multiresistant isolates
| Type of isolate (n = 100) | No. of blaCTX-M-2- negative isolates | No. of blaCTX-M-2- positive isolates | MIC90 (μg/ml)
|
|||||
|---|---|---|---|---|---|---|---|---|
| AMPb | CTX | CAZ | AMK | GEN | IPM | |||
| Acinetobacter spp. | 20 | 1 | >1,024 (512->1,024) | 256 (16-512) | 32 (8-512) | 16 (0.5-64) | 512 (2->512) | 1 (0.5-2) |
| Citrobacter freundii | 4 | 0 | >1,024 | 64 | (512->1,024) | (4-32) | (2-128) | (0.25-2) |
| Enterobacter cloacae | 4 | 1 | >1,024 | 256 (32-512) | 512 (1-512) | 4 (0.5-4) | 512 (8->512) | 0.5 (0.12-2) |
| Escherichia coli | 2 | 2 | >1,024 | 16 | (8-16) | 16 | 64 | 0.06 |
| Klebsiella pneumoniae | 10 | 10 | >1,024 | >512 (128->1024) | 512 (4-512) | 16 (4-16) | 64 (32-512) | 0.12 (<0.12-0.5) |
| Proteus mirabilis | 2 | 12 | >1,024 | >128 (8->512) | 1 (0.5-16) | 32 (8-64) | 128 (64-512) | 0.5 (0.5-2) |
| Pseudomonas aeruginosa | 10 | 4 | ND | ND | 32 (4-128) | 4 (4-64) | 128 (2->512) | 1 (0.5-2) |
| Salmonella spp. | 2 | 2 | >1,024 | (16-128) | (2-64) | (64-128) | (32-128) | (0.06-0.5) |
| Serratia marcescens | 7 | 3 | >1,024 | 16 (4-128) | 2 (0.5-32) | 32 (0.5-128) | 64 (16-256) | 0.5 (0.25-0.5) |
| Stenotrophomonas maltophilia | 4 | 0 | ND | ND | (1-128) | (64-256) | (32-64) | (64-128) |
MIC90 is the MIC at which 90% of the isolates tested are inhibited. Minimum and maximum MICs are shown in parentheses.
AMP, ampicillin; CTX, cefotaxime; CAZ, ceftazidime; AMK, amikacin; GEN, gentamicin; IPM, imipenem; ND, not determined.
TABLE 2.
Oligonucleotides used for PCR mapping
| Primer | Primer sequence (5′ to 3′) | Position | Accession no. or source |
|---|---|---|---|
| 1 | TCA CTT TAT CGG GAC CAC | blaCTX-M-2 | X92507 |
| 2 | ATG ACT CAG AGC ATT CGC | blaCTX-M-2 | X92507 |
| 3 | CAT TCT GCG GTC GGC TT | orfD | X72585 |
| 4 | CGC AAG TAA TCG CAA CAT CC | 3′-CS | U49101 |
| 5 | AGC CCC ATA CCT ACA AAG CC | 3′-CS | U49101 |
| 6 | ATG GTT TCA TGC GGG TT | orf513 | L06418 |
| 7 | CTG AGG GTG TGA GCG AG | orf513 | L06418 |
| 8 | GCG AAC ACT GCG GCG GTC AC | orf513 | L06418 |
| 9 | GAC GGT GTT CGG CAT TCT | 3′-CS | U49101 |
| 10 | TTT GAA GGT TCG ACA GC | 3′-CS | U49101 |
| 11 | AAA CAC GCC AGG CAT TC | aacA4 | AF231133 |
| 12 | CGC AGA TCA GTT GGA AG | aadA | AF326210 |
| 13 | CCG CAG CTA GAA TTT TG | aadB | X04555 |
| 14 | GCC TGA CGA TGC GTG GA | 5′-CS | M73819 |
| 15 | GAC TTG ACC TGA ATG TTT GG | 3′-CS | M73819 |
| 16 | CAT CGG TTT TGT AAG GTT | blaoxa-4 | This study |
All the isolates carrying blaCTX-M-2 harbored orf513-bearing class 1 integrons and, as described previously (1), this gene was always found located at the same position in these structures, with different arrays of cassettes in the variable regions. The cassette array aacA4-blaOXA-2-orfD was identified in 26 isolates (74%) harboring blaCTX-M-2. In the remaining nine isolates, the following cassettes were characterized: aadA1 in four isolates (11%), aadB-aadA1 in two isolates (6%), and aacA4, aacA4-aadA1, and orfD in one isolate each (Fig. 1). The presence of different types of cassettes was not correlated with the bacterial species. The arrangement of cassettes most frequently found, aacA4-blaOXA-2-orfD, has been recently described (1, 5). The sequence reported for the blaOXA-2 gene in InS21 of a Salmonella enterica serovar Infantis isolate from the province of Santa Fe, Argentina, is that of a pseudogene (GenBank file AJ311891) (5). In contrast, the blaOXA-2 gene sequenced in In35 of pMAR-12 (1), identical to that reported previously (GenBank file M95287)(15), was active as demonstrated by the presence of a band of pI 7.7 showing β-lactamase activity in isoelectric focusing experiments. Also, other blaOXA-2 sequences in Klebsiella pneumoniae and Salmonella enterica serovar Typhimurium isolates were identical to that of the complete gene reported previously (M95287) (15). The orf513-bearing class 1 integron In35 carrying the blaCTX-M-2 gene was found located in different conjugative plasmids, as shown by different restriction patterns obtained with HindIII (data not shown).
FIG. 1.
(A) Structure of In35 containing the blaCTX-M-2 gene. Orf3::QacEΔ1 is a fusion protein (GenPept AAM03346). (B) Percentages of the different arrangements of cassettes characterized in the variable regions (solid bars) of the unusual class 1 integrons harboring blaCTX-M-2.
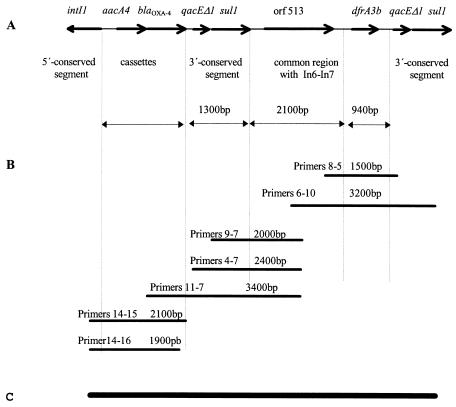
All the strains without blaCTX-M-2 carried at least one class 1 integron (data not shown). In addition, 23% (15 of 65) of the isolates carried class 2 integrons. Only one of the 65 isolates studied (1.5%) harbored a novel orf513-bearing class 1 integron that was characterized in a Citrobacter freundii isolate and was termed In38, with the aacA4-blaOXA-4 cassette array within the variable region. This novel genetic structure (Fig. 2) has, like others already described, a common region that includes orf513. This region starts 24 nt after the sul1 gene stop codon in the first 3′-CS and ends with the same segment of 28 nt described for In6 and In35 (1, 19). The unique region located between the common region that includes orf513 and the second 3′-CS is an open reading frame of 714 nt that starts 123 nt downstream of the end of the common region and shows no similarity to any reported sequence. The first 47 amino acids of the product of this uncharacterized open reading frame have 96% identity with the N-terminal protein sequence of dihydrofolate reductase type IIIb from an isolate of Shigella sonnei (PIR accession number A37174) (18), and the corresponding gene has been named the dfrA3b gene. The last 96 nt of the unique region and the following partial second 3′-CS of this orf513-bearing class 1 integron have 100% identity with the sequence of In6 reported by Valentine et al. (GenBank accession number U04278) (19). No duplications of the common region were observed at the beginning of the unique region, as described for In6. Deletions in the second 3′-CSs have been described to be different in length, but in the case of this new structure the second 3′-CS starts at the same point as that described for In6 (Fig. 3) (19).
FIG. 2.
Characterization of the genetic structure of In38 from an isolate of Citrobacter freundii. (A) Region characterized by PCR mapping. (B) Lengths of the PCR products obtained with different combinations of primers listed in Table 2.(C) Representation of sequence reported in this study (GenBank accession number AY162283) relative to structures depicted in panels A and B.
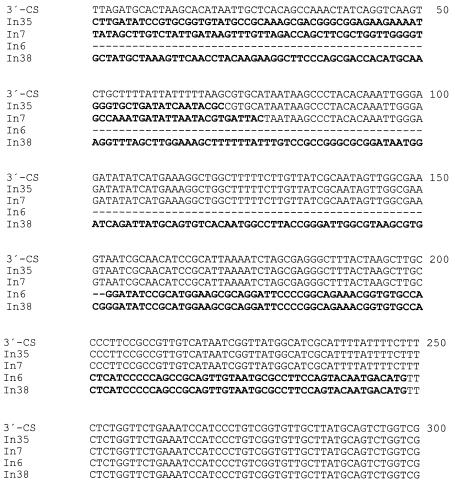
FIG. 3.
Deletionsat the 5′ ends of the second 3′-CSs in In35 (blaCTX-M-2), In7 (dfrA10), In6 (catII), and In38 (dfrA3b). Base 1 corresponds to the first base of a typical 3′-CS. Nucleotides that differ from those in a typical 3′-CS are shown in bold.
The entire gene of orf513 was detected in one Enterococcus faecium isolate and in one group G Streptococcus isolate. The analysis of the sequence revealed that it was identical to that of the orf513 gene described in pDGO100 (GenBank accession number L06418). These isolates did not harbor either int1 or the sulI gene, and the putative association of orf513 with resistance genes was not determined. This finding indicates the widespread occurrence of this putative site-specific recombinase in the bacterial population and demonstrates that it is not associated solely with class 1 integrons. Further analysis to determine the environment of orf513 in these gram-positive isolates is in progress in our laboratory.
It is noteworthy that only 5 of 39 multiresistant nonfermenting isolates, one Acinetobacter and four Pseudomonas aeruginosa isolates, harbored orf513-bearing class 1 integrons. In this regard, one possible explanation is that chromosomal resistance mechanisms such as efflux pumps are more common than plasmid-mediated resistance factors in these genera in this bacterial population.
In conclusion, almost all orf513-bearing class 1 integrons are associated with blaCTX-M-2 in the gram-negative bacterial population under study and the sequences adjacent to the blaCTX-M-2 gene are conserved in all the studied isolates. As has been described for class 1 integrons (9), it seems that once located in these orf513-bearing class 1 integrons, the whole genetic structures are transferred among different plasmids, thus enabling them to be disseminated. Therefore, the capture of the blaKLUA-1 gene from the chromosome of Kluyvera ascorbata by an as yet unknown mechanism that possibly involves orf513 has taken place once, and since that event, the genehas spread through different plasmids under selection due to antimicrobial pressure. These findings may explain the unusual distribution of β-lactamases among the bacterial population in Argentina.
Nucleotide sequence accession number.
The sequence of In38 has been submitted to GenBank under accession number AY162283.
Acknowledgments
We are grateful to P. Jeric for providing some of the gram-positive isolates.
This work was supported by a grant from ANPCyT, PICT99 07064, Buenos Aires, Argentina, to M.C. and D.C.
REFERENCES
- 1.Arduino, S. M., P. H. Roy, G. A. Jacoby, B. E. Orman, S. A. Piñeiro, and D. Centrón. 2002. blaCTX-M-2 is located in an unusual class 1 integron (In35) which includes Orf513. Antimicrob. Agents Chemother. 46:2303-2306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cao, V., T. Lambert, and P. Courvalin. 2002. ColE1-like plasmid pIP843 of Klebsiella pneumoniae encoding extended-spectrum β-lactamase CTX-M-17. Antimicrob. Agents Chemother. 46:1212-1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chanawong, A., F. H. M'Zali, J. Heritage, J.-H. Xiong, and P. M. Hawkey. 2002. Three cefotaximases, CTX-M-9, CTX-M-13, and CTX-M-14, among Enterobacteriaceae in the People's Republic of China. Antimicrob. Agents Chemother. 46:630-637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clark, N. C., O. Olsvik, J. M. Swenson, C. A. Spiegel, and F. Tenover. 1999. Detection of a streptomycin/spectinomycin adenylyltransferase gene (aadA) in Enterococcus faecalis. Antimicrob. Agents Chemother. 43:157-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Di Conza, J., J. Ayala, P. Power, M. Mollerach, and G. Gutkind. 2002. Novel class 1 integron (InS21) carrying blaCTX-M-2 in Salmonella enterica serovar Infantis. Antimicrob. Agents Chemother. 46:2257-2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hall, R. M., and H. W. Stokes. 1990. The structure of a partial duplication in the integron of plasmid pDGO100. Plasmid 23:76-79. [DOI] [PubMed] [Google Scholar]
- 7.Karim, A., L. Poirel, S. Nagarajan, and P. Nordmann. 2001. Plasmid-mediated extended-spectrum β-lactamase (CTX-M-3-like) from India and gene association with insertion sequence ISEcp1. FEMS Microbiol. Lett. 201:237-241. [DOI] [PubMed] [Google Scholar]
- 8.Lévesque, C., L. Piché, C. Larose, and P. H. Roy. 1995. PCR mapping of integrons reveals several novel combinations of resistance genes. Antimicrob. Agents Chemother. 39:185-191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Martinez Freijo, P., A. C. Fluit, F.-J. Schmitz, J. Verhoef, and M. E. Jones. 1999. Many class 1 integrons comprise distinct stable structures occurring in different species of Enterobacteriaceae isolated from widespread geographic regions in Europe. Antimicrob. Agents Chemother. 43:686-689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peters, E. D. J., M. A. Leverstein-van Hall, A. T. A. Box, J. Verhoef, and A. C. Fluit. 2001. Novel gene cassettes and integrons. Antimicrob. Agents Chemother. 45:2961-2964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sabaté, M., F. Navarro, E. Miró, S. Campoy, B. Mirelis, J. Barbé, and G. Prats. 2002. Novel complex sul1-type integron in Escherichia coli carrying blaCTX-M-9. Antimicrob. Agents Chemother. 46:2656-2661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saladin, M., V. T. B. Cao, T. Lambert, J.-L. Donay, J.-L. Herrmann, Z. Ould-Hocine, C. Verdet, F. Delisle, A. Philippon, and G. Arlet. 2002. Diversity of CTX-M β-lactamases and their promoter regions from Enterobacteriaceae isolated in three Parisian hospitals. FEMS Microbiol. Lett. 209:161-168. [DOI] [PubMed] [Google Scholar]
- 13.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 14.Stokes, H. W., and R. M. Hall. 1989. A novel family of potentially mobile DNA elements encoding site-specific gene-integration functions: integrons. Mol. Microbiol. 3:1669-1683. [DOI] [PubMed] [Google Scholar]
- 15.Stokes, H. W., and R. M. Hall. 1992. The integron In1 in plasmid R46 includes two copies of the OXA-2 gene cassette. Plasmid 28:225-234. [DOI] [PubMed] [Google Scholar]
- 16.Stokes, H. W., C. Tomaras, Y. Parsons, and R. M. Hall. 1993. The partial 3′-conserved segment duplications in the integrons In6 from pSa and In7 from pDGO100 have a common origin. Plasmid 30:39-50. [DOI] [PubMed] [Google Scholar]
- 17.Tauch, A., S. Gotker, A. Puhler, J. Kalinowski, and G. Thierbach. 2002. The 27.8-kb R-plasmid pTET3 from Corynebacterium glutamicum encodes the aminoglycoside adenyltransferase gene cassette aadA9 and the regulated tetracycline efflux system Tet 33 flanked by active copies of the widespread insertion sequence IS6100. Plasmid 48:117-129. [DOI] [PubMed] [Google Scholar]
- 18.Thomson, C. J., N. Barg, and S. G. B. Amyes. 1990. N-terminal amino acid sequence of the novel type IIIb trimethoprim-resistant plasmid-encoded dihydrofolate reductase from Shigella sonnei. J. Gen. Microbiol. 136:673-677. [DOI] [PubMed] [Google Scholar]
- 19.Valentine, C. R., M. J. Heinrich, S. L. Chissoe, and B. A. Roe. 1994. DNA sequence of direct repeats of the sulI gene of plasmid pSa. Plasmid 32:222-227. [DOI] [PubMed] [Google Scholar]
- 20.Verdet, C., G. Arlet, G. Barnaud, P. H. Lagrange, and A. Philippon. 2000. A novel integron in Salmonella enterica serovar Enteritidis, carrying the blaDHA-1 gene and its regulator gene ampR, originated from Morganella morganii. Antimicrob. Agents Chemother. 44:222-225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.White, P. A., C. J. McIver, and W. D. Rawlinson. 2001. Integrons and gene cassettes in the Enterobacteriaceae. Antimicrob. Agents Chemother. 45:2658-2661. [DOI] [PMC free article] [PubMed] [Google Scholar]