Abstract
The title compound, C14H18N2O3S, crystallizes in the thioamide form with an intramolecular N—H⋯O hydrogen bond associated with the thiourea unit. With the benzoic acid and the butyrylthioureido units, the molecule consists of two planar building blocks connected by the common NH function adjacent to the aromatic ring. The interplanar angle is 33.38 (3)°. Molecules are connected in chains parallel to [110] by classical hydrogen bonds of the N—H⋯O type from the other NH group to the benzoate C=O of a neighboring molecule.
Related literature
For related literature, see: del Campo et al. (2002 ▶); D’hooghe et al. (2005 ▶); Dušek (1985 ▶); Huebner et al. (1953 ▶); Rodriguez-Fernandez et al. (2005 ▶); Xu et al. (2004 ▶); Zeng et al. (2003 ▶).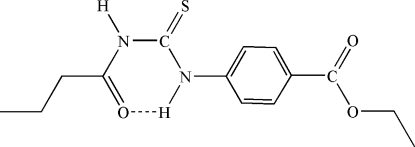
Experimental
Crystal data
C14H18N2O3S
M r = 294.36
Triclinic,

a = 7.9817 (4) Å
b = 9.8843 (6) Å
c = 11.0759 (6) Å
α = 114.472 (6)°
β = 101.156 (4)°
γ = 102.277 (5)°
V = 737.15 (7) Å3
Z = 2
Mo Kα radiation
μ = 0.23 mm−1
T = 100 (2) K
0.28 × 0.18 × 0.12 mm
Data collection
Oxford Diffraction Xcalibur S diffractometer
Absorption correction: multi-scan (CrysAlis RED; Oxford Diffraction, 2008 ▶) T min = 0.944, T max = 1.000 (expected range = 0.918–0.973)
15025 measured reflections
4104 independent reflections
3045 reflections with I > 2σ(I)
R int = 0.036
Refinement
R[F 2 > 2σ(F 2)] = 0.037
wR(F 2) = 0.096
S = 0.96
4104 reflections
191 parameters
1 restraint
H atoms treated by a mixture of independent and constrained refinement
Δρmax = 0.45 e Å−3
Δρmin = −0.24 e Å−3
Data collection: CrysAlis CCD (Oxford Diffraction, 2008 ▶); cell refinement: CrysAlis CCD; data reduction: CrysAlis RED (Oxford Diffraction, 2008 ▶); program(s) used to solve structure: SHELXS97 (Sheldrick, 2008 ▶); program(s) used to refine structure: SHELXL97 (Sheldrick, 2008 ▶); molecular graphics: XP (Siemens, 1994 ▶); software used to prepare material for publication: SHELXL97.
Supplementary Material
Crystal structure: contains datablocks I, global. DOI: 10.1107/S1600536808017868/im2072sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536808017868/im2072Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Table 1. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| N2—H02⋯O1 | 0.82 (2) | 1.92 (2) | 2.653 (1) | 148 (2) |
| N1—H01⋯O2i | 0.79 (1) | 2.20 (1) | 2.957 (1) | 160 (2) |
| C13—H13A⋯O1ii | 0.99 | 2.58 | 3.363 (2) | 136 |
| C1—H1B⋯Siii | 0.98 | 3.00 | 3.854 (2) | 147 |
| C13—H13B⋯Siv | 0.99 | 2.96 | 3.577 (1) | 122 |
| C14—H14C⋯Sv | 0.98 | 2.98 | 3.821 (2) | 144 |
Symmetry codes: (i)  ; (ii)
; (ii)  ; (iii)
; (iii)  ; (iv)
; (iv)  ; (v)
; (v)  .
.
supplementary crystallographic information
Comment
Epoxy resins have the combination of good thermal and dimensional stability, excellent chemical and corrosion resistance, high tensile strength and modulus, and ease of handling and processability, ensuring their wide application in the aerospace and electronic industries in the form of structural adhesives, advanced composite matrices, and packaging materials (Dušek, 1985). The properties of cured epoxy polymers largely depend on the nature of chemical structure of the starting resins and curing agents. The title compound (I) is a precursor in an attempt to synthesize imidazole derivatives and transition metal complexes as epoxy resin curing agents and accelerators. Substituted thioureas are an important class of compounds, precursors or intermediates towards the synthesis of a variety of heterocyclic systems such as imidazole-2-thiones (Zeng et al., 2003), 2-imino-1,3-thiazolines (D'hooghe et al., 2005), pyrimidine-2-thiones and (benzothiazolyl)-4-quinazolinones. Thioureas are also known to exhibit a wide range of biological activities including antiviral, antibacterial, antifungal, antitubercular, antithyroidal, herbicidal and insecticidal activities (Huebner et al., 1953) and as agrochemicals (Xu et al., 2004). Among thiourea derivatives, acylthioureas, with O and S as potential donor sites, have been found to display a remarkably rich coordination chemistry. Such coordination compounds of thiourea have been studied for various biological systems (Rodriguez-Fernandez et al., 2005). In recent years some attention has also been paid to the potential use of acylthioureas as highly selective reagents for the enrichment and separation of metal cations (del Campo et al.,2002).
The title compound crystallizes in the thioamide form with an intramolecular hydrogen bond N2—H02···O1. Bond lengths and angles (cf. Supplementary Material) may be regarded as normal. The molecule consists of two planar building blocks: the butyrylthioureido group (S, C1–5, O1, N1, N2) and the benzoic acid moiety (C6–14, N2, O2, O3). Mean deviations from planarity for these moieties are 0.12 and 0.13 Å, respectively, and the interplanar angle is 33.38 (3)°. Molecules are connected to give infinite chains parallel to [110] by classical hydrogen bonds N1—H01···O2. These are in turn connected to antiparallel chains by the weak hydrogen bonds C13—H13A···O1. Additionally, there are three C—H···S contacts that may be borderline weak H bonds (Table 1, Fig. 2).
Experimental
A mixture of ammonium thiocyanate (26 mmol) and butanoyl chloride (26 mmol) in anhydrous acetone (70 ml) was stirred for 35 min. Then p-aminobenzoic acid ethyl ester (26 mmol) was added dropwise and the reaction mixture was refluxed for 2 h. After cooling, the reaction mixture was poured in acidified cold water. The resulting light green solid was filtered and washed with cold acetone.The product was recrystallized from ethanol as light greenish crystals (3.62 g, 91%), m.p. 412 K.
Refinement
The NH H atoms were refined freely but with distance restraints (command SADI). Methyl H atoms were included on the basis of idealized rigid groups (C—H 0.98 Å, H—C—H 109.5°) allowed to rotate but not tip. Other hydrogen atoms were included using a riding model with C—H 0.95 (aromatic) or 0.99 (methylene) Å. U(H) values were fixed at 1.5Uiso(C) of the parent C atom for methyl H, 1.2Uiso(C) for other H.
Figures
Fig. 1.
The molecular structure of the title compound in the crystal. Ellipsoids represent 50% probability levels.
Fig. 2.
Packing diagram of I showing classical and "weak" H bonds as thick or thin dashed bonds respectively. The double chain pattern is apparent.
Crystal data
| C14H18N2O3S | Z = 2 |
| Mr = 294.36 | F000 = 312 |
| Triclinic, P1 | Dx = 1.326 Mg m−3 |
| Hall symbol: -P 1 | Melting point: 412 K |
| a = 7.9817 (4) Å | Mo Kα radiation λ = 0.71073 Å |
| b = 9.8843 (6) Å | Cell parameters from 7295 reflections |
| c = 11.0759 (6) Å | θ = 2.8–30.7º |
| α = 114.472 (6)º | µ = 0.23 mm−1 |
| β = 101.156 (4)º | T = 100 (2) K |
| γ = 102.277 (5)º | Pyramid, colourless |
| V = 737.15 (7) Å3 | 0.28 × 0.18 × 0.12 mm |
Data collection
| Oxford Diffraction Xcalibur S diffractometer | 4104 independent reflections |
| Radiation source: Enhance (Mo) X-ray Source | 3045 reflections with I > 2σ(I) |
| Monochromator: graphite | Rint = 0.036 |
| Detector resolution: 16.1057 pixels mm-1 | θmax = 30.8º |
| T = 100(2) K | θmin = 2.8º |
| ω scans | h = −10→11 |
| Absorption correction: multi-scan(CrysAlis RED; Oxford Diffraction, 2008) | k = −14→13 |
| Tmin = 0.944, Tmax = 1.000 | l = −15→15 |
| 15025 measured reflections |
Refinement
| Refinement on F2 | Secondary atom site location: difference Fourier map |
| Least-squares matrix: full | Hydrogen site location: inferred from neighbouring sites |
| R[F2 > 2σ(F2)] = 0.037 | H atoms treated by a mixture of independent and constrained refinement |
| wR(F2) = 0.096 | w = 1/[σ2(Fo2) + (0.059P)2] where P = (Fo2 + 2Fc2)/3 |
| S = 0.96 | (Δ/σ)max = 0.001 |
| 4104 reflections | Δρmax = 0.45 e Å−3 |
| 191 parameters | Δρmin = −0.24 e Å−3 |
| 1 restraint | Extinction correction: none |
| Primary atom site location: structure-invariant direct methods |
Special details
| Geometry. All e.s.d.'s (except the e.s.d. in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell e.s.d.'s are taken into account individually in the estimation of e.s.d.'s in distances, angles and torsion angles; correlations between e.s.d.'s in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell e.s.d.'s is used for estimating e.s.d.'s involving l.s. planes.Least-squares planes (x,y,z in crystal coordinates) and deviations from them (* indicates atom used to define plane)- 7.3219 (0.0010) x + 3.9334 (0.0038) y + 4.1423 (0.0037) z = 2.1058 (0.0010)* -0.1410 (0.0006) S * -0.1556 (0.0012) C1 * -0.0747 (0.0014) C2 * 0.1521 (0.0012) C3 * 0.1191 (0.0012) C4 * -0.0053 (0.0010) C5 * 0.0111 (0.0008) O1 * 0.2033 (0.0010) N1 * -0.1089 (0.0009) N2Rms deviation of fitted atoms = 0.1249- 6.2227 (0.0013) x + 7.6804 (0.0015) y - 1.8402 (0.0041) z = 2.0195 (0.0017)Angle to previous plane (with approximate e.s.d.) = 33.38 (0.03)* -0.0510 (0.0011) C6 * -0.1492 (0.0011) C7 * -0.0777 (0.0011) C8 * 0.0751 (0.0012) C9 * 0.1972 (0.0012) C10 * 0.1468 (0.0011) C11 * 0.0732 (0.0011) C12 * 0.0367 (0.0013) C13 * -0.2621 (0.0011) C14 * 0.0788 (0.0009) O2 * 0.0421 (0.0010) O3 * -0.1099 (0.0009) N2Rms deviation of fitted atoms = 0.1266 |
| Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F2, conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > σ(F2) is used only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2 are statistically about twice as large as those based on F, and R- factors based on ALL data will be even larger. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| S | 0.47787 (5) | 0.62325 (4) | 0.72720 (3) | 0.02040 (10) | |
| O1 | 0.11708 (12) | 0.45529 (11) | 0.28568 (9) | 0.0208 (2) | |
| O2 | 1.03683 (13) | 1.25923 (10) | 0.60929 (9) | 0.0217 (2) | |
| O3 | 1.04639 (12) | 1.31498 (10) | 0.82958 (9) | 0.0200 (2) | |
| N1 | 0.20683 (14) | 0.45249 (13) | 0.49337 (11) | 0.0164 (2) | |
| H01 | 0.186 (2) | 0.3993 (17) | 0.5294 (16) | 0.020 (4)* | |
| N2 | 0.38248 (15) | 0.68105 (13) | 0.51142 (11) | 0.0168 (2) | |
| H02 | 0.318 (2) | 0.6327 (19) | 0.4292 (16) | 0.034 (5)* | |
| C1 | −0.2318 (2) | −0.03015 (17) | 0.08970 (15) | 0.0334 (4) | |
| H1A | −0.1512 | −0.0873 | 0.1061 | 0.050* | |
| H1B | −0.2954 | −0.0787 | −0.0107 | 0.050* | |
| H1C | −0.3200 | −0.0343 | 0.1396 | 0.050* | |
| C2 | −0.1213 (2) | 0.14034 (17) | 0.14262 (14) | 0.0313 (3) | |
| H2A | −0.0333 | 0.1442 | 0.0912 | 0.038* | |
| H2B | −0.2027 | 0.1971 | 0.1238 | 0.038* | |
| C3 | −0.02144 (18) | 0.22120 (15) | 0.29715 (13) | 0.0195 (3) | |
| H3A | −0.1110 | 0.2245 | 0.3480 | 0.023* | |
| H3B | 0.0497 | 0.1574 | 0.3162 | 0.023* | |
| C4 | 0.10397 (16) | 0.38601 (15) | 0.35434 (13) | 0.0161 (3) | |
| C5 | 0.35361 (16) | 0.58945 (14) | 0.57240 (12) | 0.0152 (2) | |
| C6 | 0.52972 (17) | 0.81999 (14) | 0.56141 (13) | 0.0158 (2) | |
| C7 | 0.60248 (17) | 0.84283 (15) | 0.46402 (13) | 0.0177 (3) | |
| H7 | 0.5545 | 0.7659 | 0.3681 | 0.021* | |
| C8 | 0.74473 (18) | 0.97773 (15) | 0.50722 (13) | 0.0174 (3) | |
| H8 | 0.7932 | 0.9937 | 0.4405 | 0.021* | |
| C9 | 0.81749 (17) | 1.09026 (14) | 0.64780 (13) | 0.0162 (3) | |
| C10 | 0.74195 (17) | 1.06811 (14) | 0.74442 (13) | 0.0176 (3) | |
| H10 | 0.7898 | 1.1453 | 0.8402 | 0.021* | |
| C11 | 0.59726 (18) | 0.93406 (15) | 0.70161 (13) | 0.0183 (3) | |
| H11 | 0.5448 | 0.9203 | 0.7675 | 0.022* | |
| C12 | 0.97646 (17) | 1.22913 (14) | 0.69088 (13) | 0.0168 (3) | |
| C13 | 1.20350 (18) | 1.45409 (16) | 0.88185 (14) | 0.0237 (3) | |
| H13A | 1.1674 | 1.5352 | 0.8632 | 0.028* | |
| H13B | 1.2942 | 1.4265 | 0.8350 | 0.028* | |
| C14 | 1.28094 (19) | 1.51477 (17) | 1.03564 (14) | 0.0267 (3) | |
| H14A | 1.1882 | 1.5369 | 1.0802 | 0.040* | |
| H14B | 1.3836 | 1.6116 | 1.0751 | 0.040* | |
| H14C | 1.3218 | 1.4356 | 1.0525 | 0.040* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| S | 0.02247 (18) | 0.01856 (17) | 0.01304 (16) | −0.00118 (13) | −0.00170 (12) | 0.00807 (12) |
| O1 | 0.0212 (5) | 0.0233 (5) | 0.0159 (5) | 0.0035 (4) | 0.0012 (4) | 0.0114 (4) |
| O2 | 0.0249 (5) | 0.0201 (5) | 0.0190 (5) | 0.0014 (4) | 0.0087 (4) | 0.0103 (4) |
| O3 | 0.0195 (5) | 0.0196 (5) | 0.0147 (4) | −0.0025 (4) | 0.0005 (4) | 0.0091 (4) |
| N1 | 0.0176 (5) | 0.0163 (5) | 0.0118 (5) | 0.0002 (4) | 0.0020 (4) | 0.0072 (4) |
| N2 | 0.0179 (5) | 0.0166 (5) | 0.0114 (5) | 0.0007 (4) | 0.0007 (4) | 0.0069 (4) |
| C1 | 0.0376 (9) | 0.0212 (7) | 0.0205 (7) | 0.0011 (6) | −0.0044 (6) | 0.0010 (6) |
| C2 | 0.0431 (9) | 0.0214 (7) | 0.0159 (7) | 0.0027 (6) | −0.0031 (6) | 0.0059 (6) |
| C3 | 0.0197 (6) | 0.0180 (6) | 0.0138 (6) | 0.0018 (5) | 0.0023 (5) | 0.0046 (5) |
| C4 | 0.0147 (6) | 0.0193 (6) | 0.0126 (6) | 0.0058 (5) | 0.0036 (5) | 0.0062 (5) |
| C5 | 0.0153 (6) | 0.0146 (6) | 0.0138 (6) | 0.0040 (5) | 0.0046 (5) | 0.0055 (5) |
| C6 | 0.0153 (6) | 0.0149 (6) | 0.0166 (6) | 0.0032 (5) | 0.0030 (5) | 0.0085 (5) |
| C7 | 0.0218 (7) | 0.0169 (6) | 0.0128 (6) | 0.0053 (5) | 0.0041 (5) | 0.0066 (5) |
| C8 | 0.0220 (6) | 0.0178 (6) | 0.0155 (6) | 0.0063 (5) | 0.0071 (5) | 0.0102 (5) |
| C9 | 0.0174 (6) | 0.0152 (6) | 0.0169 (6) | 0.0043 (5) | 0.0045 (5) | 0.0093 (5) |
| C10 | 0.0215 (6) | 0.0159 (6) | 0.0132 (6) | 0.0043 (5) | 0.0040 (5) | 0.0065 (5) |
| C11 | 0.0215 (6) | 0.0188 (6) | 0.0157 (6) | 0.0050 (5) | 0.0073 (5) | 0.0093 (5) |
| C12 | 0.0186 (6) | 0.0165 (6) | 0.0170 (6) | 0.0063 (5) | 0.0048 (5) | 0.0096 (5) |
| C13 | 0.0192 (7) | 0.0222 (7) | 0.0232 (7) | −0.0037 (5) | −0.0011 (5) | 0.0135 (6) |
| C14 | 0.0227 (7) | 0.0281 (8) | 0.0198 (7) | 0.0003 (6) | 0.0025 (5) | 0.0086 (6) |
Geometric parameters (Å, °)
| S—C5 | 1.6617 (13) | C13—C14 | 1.4939 (18) |
| O1—C4 | 1.2207 (15) | N1—H01 | 0.791 (13) |
| O2—C12 | 1.2114 (15) | N2—H02 | 0.823 (15) |
| O3—C12 | 1.3336 (15) | C1—H1A | 0.9800 |
| O3—C13 | 1.4576 (15) | C1—H1B | 0.9800 |
| N1—C5 | 1.3850 (16) | C1—H1C | 0.9800 |
| N1—C4 | 1.3856 (16) | C2—H2A | 0.9900 |
| N2—C5 | 1.3443 (16) | C2—H2B | 0.9900 |
| N2—C6 | 1.4161 (16) | C3—H3A | 0.9900 |
| C1—C2 | 1.520 (2) | C3—H3B | 0.9900 |
| C2—C3 | 1.5071 (18) | C7—H7 | 0.9500 |
| C3—C4 | 1.5044 (17) | C8—H8 | 0.9500 |
| C6—C11 | 1.3929 (17) | C10—H10 | 0.9500 |
| C6—C7 | 1.3930 (17) | C11—H11 | 0.9500 |
| C7—C8 | 1.3833 (17) | C13—H13A | 0.9900 |
| C8—C9 | 1.3926 (17) | C13—H13B | 0.9900 |
| C9—C10 | 1.3944 (17) | C14—H14A | 0.9800 |
| C9—C12 | 1.4846 (17) | C14—H14B | 0.9800 |
| C10—C11 | 1.3896 (17) | C14—H14C | 0.9800 |
| C12—O3—C13 | 116.02 (10) | C2—C1—H1C | 109.5 |
| C5—N1—C4 | 129.07 (11) | H1A—C1—H1C | 109.5 |
| C5—N2—C6 | 127.24 (11) | H1B—C1—H1C | 109.5 |
| C3—C2—C1 | 111.68 (12) | C3—C2—H2A | 109.3 |
| C4—C3—C2 | 114.61 (11) | C1—C2—H2A | 109.3 |
| O1—C4—N1 | 122.53 (12) | C3—C2—H2B | 109.3 |
| O1—C4—C3 | 123.91 (11) | C1—C2—H2B | 109.3 |
| N1—C4—C3 | 113.56 (11) | H2A—C2—H2B | 107.9 |
| N2—C5—N1 | 114.66 (11) | C4—C3—H3A | 108.6 |
| N2—C5—S | 126.56 (9) | C2—C3—H3A | 108.6 |
| N1—C5—S | 118.75 (9) | C4—C3—H3B | 108.6 |
| C11—C6—C7 | 120.20 (11) | C2—C3—H3B | 108.6 |
| C11—C6—N2 | 122.15 (11) | H3A—C3—H3B | 107.6 |
| C7—C6—N2 | 117.61 (11) | C8—C7—H7 | 120.1 |
| C8—C7—C6 | 119.86 (11) | C6—C7—H7 | 120.1 |
| C7—C8—C9 | 120.51 (11) | C7—C8—H8 | 119.7 |
| C8—C9—C10 | 119.35 (12) | C9—C8—H8 | 119.7 |
| C8—C9—C12 | 118.79 (11) | C11—C10—H10 | 119.7 |
| C10—C9—C12 | 121.84 (11) | C9—C10—H10 | 119.7 |
| C11—C10—C9 | 120.51 (12) | C10—C11—H11 | 120.2 |
| C10—C11—C6 | 119.52 (11) | C6—C11—H11 | 120.2 |
| O2—C12—O3 | 124.13 (12) | O3—C13—H13A | 110.2 |
| O2—C12—C9 | 123.78 (11) | C14—C13—H13A | 110.2 |
| O3—C12—C9 | 112.08 (10) | O3—C13—H13B | 110.2 |
| O3—C13—C14 | 107.33 (10) | C14—C13—H13B | 110.2 |
| C5—N1—H01 | 115.6 (11) | H13A—C13—H13B | 108.5 |
| C4—N1—H01 | 114.8 (11) | C13—C14—H14A | 109.5 |
| C5—N2—H02 | 109.4 (11) | C13—C14—H14B | 109.5 |
| C6—N2—H02 | 121.4 (11) | H14A—C14—H14B | 109.5 |
| C2—C1—H1A | 109.5 | C13—C14—H14C | 109.5 |
| C2—C1—H1B | 109.5 | H14A—C14—H14C | 109.5 |
| H1A—C1—H1B | 109.5 | H14B—C14—H14C | 109.5 |
| C1—C2—C3—C4 | 174.75 (13) | C7—C8—C9—C10 | −1.97 (19) |
| C5—N1—C4—O1 | −10.3 (2) | C7—C8—C9—C12 | 176.71 (11) |
| C5—N1—C4—C3 | 168.79 (12) | C8—C9—C10—C11 | 0.99 (19) |
| C2—C3—C4—O1 | 4.85 (19) | C12—C9—C10—C11 | −177.65 (12) |
| C2—C3—C4—N1 | −174.24 (12) | C9—C10—C11—C6 | 1.1 (2) |
| C6—N2—C5—N1 | −174.22 (11) | C7—C6—C11—C10 | −2.31 (19) |
| C6—N2—C5—S | 4.13 (19) | N2—C6—C11—C10 | −179.92 (12) |
| C4—N1—C5—N2 | 10.80 (19) | C13—O3—C12—O2 | 1.00 (18) |
| C4—N1—C5—S | −167.69 (10) | C13—O3—C12—C9 | 179.95 (10) |
| C5—N2—C6—C11 | −42.44 (19) | C8—C9—C12—O2 | 7.60 (19) |
| C5—N2—C6—C7 | 139.90 (13) | C10—C9—C12—O2 | −173.76 (13) |
| C11—C6—C7—C8 | 1.35 (19) | C8—C9—C12—O3 | −171.36 (12) |
| N2—C6—C7—C8 | 179.06 (11) | C10—C9—C12—O3 | 7.28 (17) |
| C6—C7—C8—C9 | 0.81 (19) | C12—O3—C13—C14 | −169.36 (11) |
Hydrogen-bond geometry (Å, °)
| D—H···A | D—H | H···A | D···A | D—H···A |
| N2—H02···O1 | 0.82 (2) | 1.92 (2) | 2.653 (1) | 148 (2) |
| N1—H01···O2i | 0.79 (1) | 2.20 (1) | 2.957 (1) | 160 (2) |
| C13—H13A···O1ii | 0.99 | 2.58 | 3.363 (2) | 136 |
| C1—H1B···Siii | 0.98 | 3.00 | 3.854 (2) | 147 |
| C13—H13B···Siv | 0.99 | 2.96 | 3.577 (1) | 122 |
| C14—H14C···Sv | 0.98 | 2.98 | 3.821 (2) | 144 |
Symmetry codes: (i) x−1, y−1, z; (ii) −x+1, −y+2, −z+1; (iii) x−1, y−1, z−1; (iv) x+1, y+1, z; (v) −x+2, −y+2, −z+2.
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: IM2072).
References
- Campo, R. del, Criado, J. J., Hermosa, M. R., Jimenez-Sanchez, A., Manzano, J. L., Mante, E., Rodriguez-Fernandez, E. & Sanz, F. (2002). J. Inorg. Biochem.89, 74–82. [DOI] [PubMed]
- D’hooghe, M., Waterinckx, A. & De Kimpe, N. (2005). J. Org. Chem.70, 227–232. [DOI] [PubMed]
- Dušek, K. (1985). Adv. Polym. Sci.78, 115–118.
- Huebner, O. F., Marsh, J. L., Mizzoni, R. H., Mull, R. P., Schrooder, D. C., Troxell, H. A. & Scholz, C. R. (1953). J. Am. Chem. Soc.75, 2274–2275.
- Oxford Diffraction (2008). CrysAlis RED Oxford Diffraction Ltd, Abingdon, Oxfordshire, England.
- Rodriguez-Fernandez, E., Manzano, J. L., Benito, J. J., Hermosa, R., Monte, E. & Criado, J. J. (2005). J. Inorg. Biochem.99 , 1558–1572. [DOI] [PubMed]
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Siemens (1994). XP Siemens Analytical X-ray Instruments Inc., Madison, Wisconsin, USA.
- Xu, Y., Hua, W., Liu, X. & Zhu, D. (2004). Chin. J. Org. Chem.24, 1217–1222.
- Zeng, R. S., Zou, J. P., Zchen, S. J. & Shen, Q. (2003). Org. Lett.61, 1657–1659. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablocks I, global. DOI: 10.1107/S1600536808017868/im2072sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536808017868/im2072Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report




