Abstract
The title compound, 4C9H6O6·C6H8N6·2H2O, crystallizes in a layer structure where each sheet is composed of anellated hydrogen-bonded rings of six distinct sizes: R 2 2(16), R 3 3(18), R 4 4(12), R 4 4(18), R 4 4(22) and R 4 4(25). The two largest rings, viz. R 4 4(22) and R 4 4(25), are associated with O—H⋯N bonds from the carboxyl groups to the triazole rings. The typical head-to-tail carboxyl–carboxyl R 2 2(8) motif is not observed.
Related literature
For related literature, see: Althoff et al. (2006 ▶); Dale & Elsegood (2004 ▶); Dale et al. (2004 ▶); Dorn et al. (2005 ▶, 2006 ▶); Du et al. (2005 ▶); Etter et al. (1990 ▶); Fan et al. (2005 ▶); Goldberg & Bernstein (2007 ▶); Janiak (2000 ▶); Shattock et al. (2005 ▶); Turner et al. (2008 ▶); Wang & Wang (2005 ▶); Wisser & Janiak (2007a
▶,b
▶).
Experimental
Crystal data
4C9H6O6·C6H8N6·2H2O
M r = 1040.76
Triclinic,
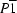
a = 9.7989 (1) Å
b = 10.7511 (2) Å
c = 12.6578 (2) Å
α = 108.801 (1)°
β = 98.737 (1)°
γ = 113.340 (1)°
V = 1097.44 (3) Å3
Z = 1
Mo Kα radiation
μ = 0.13 mm−1
T = 203 (2) K
0.37 × 0.05 × 0.02 mm
Data collection
Bruker APEXII CCD area-detector diffractometer
Absorption correction: multi-scan (SADABS; Sheldrick, 1996 ▶) T min = 0.952, T max = 0.997
20984 measured reflections
4824 independent reflections
3452 reflections with I > 2σ(I)
R int = 0.036
Refinement
R[F 2 > 2σ(F 2)] = 0.041
wR(F 2) = 0.105
S = 1.02
4824 reflections
359 parameters
H atoms treated by a mixture of independent and constrained refinement
Δρmax = 0.25 e Å−3
Δρmin = −0.24 e Å−3
Data collection: APEX2 (Bruker, 2006 ▶); cell refinement: SAINT (Bruker, 2006 ▶); data reduction: SAINT; program(s) used to solve structure: SHELXS97 (Sheldrick, 2008 ▶); program(s) used to refine structure: SHELXL97 (Sheldrick, 2008 ▶); molecular graphics: DIAMOND (Crystal Impact, 2006 ▶); software used to prepare material for publication: publCIF (Westrip, 2008 ▶).
Supplementary Material
Crystal structure: contains datablocks I, global. DOI: 10.1107/S1600536808015808/kj2089sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536808015808/kj2089Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Table 1. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| O2—H2⋯O11i | 0.92 (2) | 1.67 (2) | 2.594 (2) | 175 (2) |
| O4—H4⋯O8 | 0.88 (2) | 1.75 (2) | 2.626 (2) | 174 (2) |
| O6—H6⋯O1ii | 0.91 (2) | 1.84 (2) | 2.699 (2) | 157 (2) |
| O7—H7⋯N1 | 0.96 (2) | 1.75 (2) | 2.703 (2) | 172 (2) |
| O9—H9⋯O13 | 0.91 (3) | 1.63 (3) | 2.531 (2) | 170 (2) |
| O12—H12⋯N2iii | 0.90 (2) | 1.76 (2) | 2.644 (2) | 167 (2) |
| O13—H13A⋯O10iv | 0.91 (2) | 1.81 (3) | 2.711 (2) | 171 (2) |
| O13—H13B⋯O5 | 0.85 (3) | 1.92 (3) | 2.751 (2) | 167 (2) |
Symmetry codes: (i) 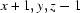 ; (ii)
; (ii)  ; (iii)
; (iii) 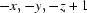 ; (iv)
; (iv) 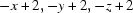 .
.
Acknowledgments
Support through DFG grant No. Ja466/14-1 is acknowledged.
supplementary crystallographic information
Comment
Hydrogen bonding within crystalline systems is of timely interest for the rational design of organized solids (Althoff et al., 2006; Dorn et al., 2005; Dorn et al. 2006; Wisser & Janiak, 2007a,b). Co-crystallization of benzene-di, -tri- and -tetra-carboxylic acids, like trimesic acid or hemimellitic acid with solvent molecules or nitrogen bases is the focus of permanent and recent research activities (Dale & Elsegood, 2004; Dale et al., 2004; Du et al., 2005; Fan et al., 2005; Goldberg & Bernstein, 2007; Shattock et al., 2005; Turner et al., 2008; Wang & Wang, 2005). Co-crystal structures of trimesic acid (benzene-1,3,5-tricarboxylic acid) have been reported with 2,5-bis(3- and 4-pyridyl)-1,3,4-oxadiazole (two-dimensional sheet, Du et al., 2005), 3,6-bis(3'-pyridyl)-1,2,4,5-tetrazine (one-dimensional ribbon, Wang & Wang, 2005), 1,2-bis(4-pyridyl)ethane (two-dimensional 6,3- and 10,3-network with interpenetration, Shattock et al., 2005), mono- and bis(methanol) (one-dimensional tape, Dale et al., 2004), acetic acid (Goldberg & Bernstein, 2007) and dihydrate (three-dimensional network, Fan et al., 2005).
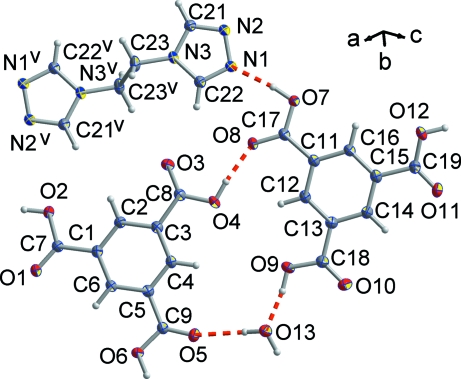
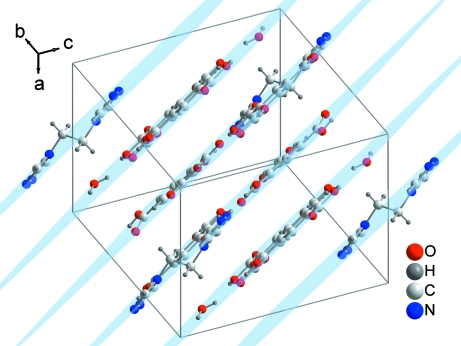
The hydrogen-bonded sheet in the title compound contains several different motifs that engage all of the strong hydrogen bond donors and acceptors available (Fig. 1 and 2). The hydrogen bond distances in the sheet are spread over a narrow range, with D···A distances from 2.53 to 2.75 Å. The sheet is constructed of six distinct hydrogen-bonded rings of sizes R22(16), R33(18), R44(12), R44(18), R44(22) and R44(25), using Etter's graph set analysis (Etter et al., 1990). The two largest rings R44(22) and R44(25) are associated with the O—H···N bonds from the carboxylic acid groups to the triazole rings. All N1 and N2 nitrogen atoms of the 1,2-bis(1,2,4-triazol-4-yl)ethane molecule act as hydrogen-bond acceptors. The smallest ring R44(12) incorporates two water molecules and two carboxylic acid groups. The 18-membered R33(18) and R44(18) rings are constructed from one water molecule in combination with three and four carboxylic acid groups, respectively. These water and carboxylic acid containing motifs are different from those seen in the structure of the trimesic acid dihydrate (Fan et al., 2005). Also, formation of the common R22(8) head-to-tail carboxylic acid–acid graph-set motif is apparently prevented in the structure of the title compound by the water and the bis-triazole molecule. No relevant π–π or C—H···π interactions are found between molecules in adjacent sheets (Fig. 3) (Janiak, 2000).
Experimental
A mixture of trimesic acid, H3btc (210 mg, 1.00 mmol), 1,2-bis(1,2,4-triazol-4-yl)ethane, btre (164 mg, 1.00 mmol) and water (15 ml) was stirred for 30 min at room temperature, transferred to a Teflon-lined stainless-steel autoclave and heated at 453 K for 3 d. Then the autoclave was cooled to room temperature at a rate of 2.8 K h-1. A colorless crystalline product was filtered off, washed with distilled water and dried in air (yield 135 mg, 52% based on H3btc). Elemental analysis C21H18N3O13 (520.38) calcd. C 48.47, H 3.49, N 8.07; found: C 47.84, H 3.49, N 8.02%.
Refinement
Hydrogen atoms for aromatic CH and aliphatic CH2 were positioned geometrically (C—H = 0.94 Å for aromatic CH, C—H = 0.98 Å for CH2) and refined using a riding model. Protic hydrogen atoms of the carboxyl groups and of the water of crystallization were found and refined with Uiso(H) = 1.5Ueq(O).
Figures
Fig. 1.
Fully labelled displacement ellipsoid diagram (at 50% probability) of the asymmetric unit. Symmetry code (v) 1-x, -y, -z.
Fig. 2.
The hydrogen-bonded sheet in the structure of 2(C6H3-1,3,5-(COOH)3).0.5(C6H8N6).H2O with graph set pattern of hydrogen-bonded rings in violet. Hydrogen bond data is given in Table 1. Additional symmetry code (v) 1-x, -y, -z.
Fig. 3.
Packing of the hydrogen-bonded sheets parallel to the (-2,3,-2)-plane with a d-spacing (distance of neighboring sheets) of 3.155 Å.
Crystal data
| 4C9H6O6·C6H8N6·2H2O | Z = 1 |
| Mr = 1040.76 | F000 = 538 |
| Triclinic, P1 | Dx = 1.575 Mg m−3 |
| Hall symbol: -P 1 | Mo Kα radiation λ = 0.71073 Å |
| a = 9.7989 (1) Å | Cell parameters from 5028 reflections |
| b = 10.7511 (2) Å | θ = 2.2–31.5º |
| c = 12.6578 (2) Å | µ = 0.13 mm−1 |
| α = 108.801 (1)º | T = 203 (2) K |
| β = 98.737 (1)º | Needle, colourless |
| γ = 113.340 (1)º | 0.37 × 0.05 × 0.02 mm |
| V = 1097.44 (3) Å3 |
Data collection
| Bruker APEXII CCD area-detector diffractometer | 4824 independent reflections |
| Radiation source: fine-focus sealed tube | 3452 reflections with I > 2σ(I) |
| Monochromator: graphite | Rint = 0.036 |
| T = 203(2) K | θmax = 27.1º |
| ω scans | θmin = 1.8º |
| Absorption correction: multi-scan(SADABS; Sheldrick, 1996) | h = −12→12 |
| Tmin = 0.952, Tmax = 0.997 | k = −13→13 |
| 20984 measured reflections | l = −16→16 |
Refinement
| Refinement on F2 | Secondary atom site location: difference Fourier map |
| Least-squares matrix: full | Hydrogen site location: inferred from neighbouring sites |
| R[F2 > 2σ(F2)] = 0.041 | H atoms treated by a mixture of independent and constrained refinement |
| wR(F2) = 0.105 | w = 1/[σ2(Fo2) + (0.0562P)2 + 0.0985P] where P = (Fo2 + 2Fc2)/3 |
| S = 1.02 | (Δ/σ)max < 0.001 |
| 4824 reflections | Δρmax = 0.25 e Å−3 |
| 359 parameters | Δρmin = −0.24 e Å−3 |
| Primary atom site location: structure-invariant direct methods | Extinction correction: none |
Special details
| Experimental. IR (KBr) 3512m (νCOO-H), 3427m (νCOO-H), 3122m, 1886m, 1704 s, (νasymCO2), 1539m (νasymCO2),1452 s (νsymCO2), 1356w (νsymCO2), 1320w (δOH···O),1285m, 1225m, 1190m, 1071m, 1020m, 986m, 936m (γOH···O), 905m, 870w, 844w, 814w, 745 s, 683 s, 666 s, 936m, 605w, 570w, 510m, 448m cm-1.Thermogravimetric analysis (simultaneous thermoanalysis apparatus STA 409 C from Netzsch under nitrogen with a heating rate of 10 K min-1 in the range of 323 to 920 K): A sample of the compound shows the first weight loss in the temperature range 450–490 K which corresponds to the removal of the water molecule (obs. 3.67, calcd. 3.45%). From 550 to 610 K a less well resolved weight loss of about 17% occurs which is assigned to the half btre molecule (calcd. 15.8%). A third weight loss in the range 610–650 K of around 40% is assigned to the removal of one H~3~btc molecule (calcd. 40.3%). A weight loss continues to 920 K where 18.6% of the original mass is retained. |
| Geometry. All e.s.d.'s (except the e.s.d. in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell e.s.d.'s are taken into account individually in the estimation of e.s.d.'s in distances, angles and torsion angles; correlations between e.s.d.'s in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell e.s.d.'s is used for estimating e.s.d.'s involving l.s. planes. |
| Refinement. Refinement of F^2^ against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F^2^, conventional R-factors R are based on F, with F set to zero for negative F^2^. The threshold expression of F^2^ > σ(F^2^) is used only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F^2^ are statistically about twice as large as those based on F, and R- factors based on ALL data will be even larger. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| O1 | 1.35077 (13) | 0.71622 (12) | 0.20868 (9) | 0.0281 (3) | |
| O2 | 1.12433 (15) | 0.50380 (13) | 0.11195 (10) | 0.0342 (3) | |
| H2 | 1.169 (2) | 0.490 (2) | 0.0526 (19) | 0.051* | |
| O3 | 0.72628 (14) | 0.32810 (13) | 0.28747 (10) | 0.0357 (3) | |
| O4 | 0.75183 (14) | 0.47603 (13) | 0.46864 (10) | 0.0295 (3) | |
| H4 | 0.658 (3) | 0.402 (2) | 0.4509 (17) | 0.044* | |
| O5 | 1.23563 (17) | 0.97506 (14) | 0.69889 (11) | 0.0479 (4) | |
| O6 | 1.41605 (14) | 1.03213 (13) | 0.61107 (11) | 0.0335 (3) | |
| H6 | 1.473 (3) | 1.117 (2) | 0.6791 (19) | 0.050* | |
| C1 | 1.15857 (19) | 0.65177 (17) | 0.30595 (13) | 0.0226 (3) | |
| C2 | 1.01588 (19) | 0.54260 (17) | 0.29865 (13) | 0.0241 (4) | |
| H2A | 0.9603 | 0.4520 | 0.2306 | 0.031 (5)* | |
| C3 | 0.95434 (19) | 0.56593 (17) | 0.39110 (13) | 0.0224 (3) | |
| C4 | 1.03774 (18) | 0.69911 (17) | 0.49313 (13) | 0.0230 (3) | |
| H4A | 0.9963 | 0.7159 | 0.5555 | 0.028* | |
| C5 | 1.18299 (19) | 0.80705 (17) | 0.50200 (13) | 0.0228 (3) | |
| C6 | 1.24244 (19) | 0.78375 (17) | 0.40836 (13) | 0.0230 (3) | |
| H6A | 1.3397 | 0.8576 | 0.4144 | 0.028* | |
| C7 | 1.22269 (19) | 0.62928 (17) | 0.20573 (13) | 0.0235 (3) | |
| C8 | 0.79976 (19) | 0.44429 (17) | 0.37606 (14) | 0.0237 (3) | |
| C9 | 1.2777 (2) | 0.94488 (18) | 0.61364 (14) | 0.0260 (4) | |
| O7 | 0.23309 (14) | 0.11931 (13) | 0.42638 (10) | 0.0327 (3) | |
| H7 | 0.230 (2) | 0.064 (2) | 0.349 (2) | 0.049* | |
| O8 | 0.47138 (14) | 0.26410 (13) | 0.43007 (10) | 0.0369 (3) | |
| O9 | 0.82100 (16) | 0.72343 (14) | 0.77430 (11) | 0.0405 (3) | |
| H9 | 0.916 (3) | 0.808 (3) | 0.814 (2) | 0.061* | |
| O10 | 0.75891 (16) | 0.79239 (14) | 0.93837 (11) | 0.0487 (4) | |
| O11 | 0.23548 (16) | 0.46243 (14) | 0.93776 (11) | 0.0443 (4) | |
| O12 | 0.10133 (15) | 0.22723 (13) | 0.79990 (11) | 0.0360 (3) | |
| H12 | 0.029 (3) | 0.214 (2) | 0.8372 (19) | 0.054* | |
| C11 | 0.40237 (18) | 0.33372 (17) | 0.60392 (13) | 0.0226 (3) | |
| C12 | 0.54215 (19) | 0.46733 (17) | 0.66014 (14) | 0.0248 (4) | |
| H12A | 0.6137 | 0.4932 | 0.6193 | 0.030* | |
| C13 | 0.57695 (19) | 0.56305 (17) | 0.77641 (14) | 0.0252 (4) | |
| C14 | 0.47026 (19) | 0.52582 (17) | 0.83609 (14) | 0.0259 (4) | |
| H14A | 0.4929 | 0.5908 | 0.9144 | 0.031* | |
| C15 | 0.33011 (19) | 0.39277 (17) | 0.78027 (14) | 0.0242 (4) | |
| C16 | 0.29563 (19) | 0.29562 (17) | 0.66414 (13) | 0.0226 (3) | |
| H16A | 0.2012 | 0.2052 | 0.6267 | 0.027* | |
| C17 | 0.37241 (19) | 0.23634 (17) | 0.47946 (14) | 0.0255 (4) | |
| C18 | 0.7287 (2) | 0.70561 (18) | 0.83851 (15) | 0.0295 (4) | |
| C19 | 0.2168 (2) | 0.36255 (19) | 0.84716 (14) | 0.0276 (4) | |
| O13 | 1.09496 (16) | 0.94370 (15) | 0.86765 (12) | 0.0442 (4) | |
| H13A | 1.134 (3) | 1.031 (3) | 0.932 (2) | 0.066* | |
| H13B | 1.129 (3) | 0.959 (3) | 0.813 (2) | 0.066* | |
| C21 | 0.2033 (2) | −0.17823 (19) | 0.03864 (14) | 0.0307 (4) | |
| H21A | 0.1519 | −0.2613 | −0.0349 | 0.037* | |
| C22 | 0.3793 (2) | 0.02501 (18) | 0.18472 (14) | 0.0277 (4) | |
| H22A | 0.4747 | 0.1115 | 0.2332 | 0.033* | |
| C23 | 0.4714 (2) | −0.0680 (2) | 0.01332 (16) | 0.0338 (4) | |
| H23A | 0.4241 | −0.1585 | −0.0605 | 0.041* | |
| H23B | 0.5608 | −0.0657 | 0.0623 | 0.041* | |
| N1 | 0.25099 (16) | −0.01876 (15) | 0.21283 (12) | 0.0281 (3) | |
| N2 | 0.13810 (16) | −0.14927 (15) | 0.11899 (12) | 0.0293 (3) | |
| N3 | 0.35506 (16) | −0.07258 (15) | 0.07521 (11) | 0.0272 (3) |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| O1 | 0.0239 (6) | 0.0268 (6) | 0.0238 (6) | 0.0049 (5) | 0.0126 (5) | 0.0063 (5) |
| O2 | 0.0292 (7) | 0.0311 (7) | 0.0215 (6) | 0.0013 (6) | 0.0140 (5) | 0.0010 (5) |
| O3 | 0.0264 (7) | 0.0300 (7) | 0.0276 (7) | −0.0011 (6) | 0.0100 (5) | 0.0032 (6) |
| O4 | 0.0195 (6) | 0.0265 (6) | 0.0300 (6) | 0.0014 (5) | 0.0130 (5) | 0.0073 (5) |
| O5 | 0.0467 (9) | 0.0343 (7) | 0.0338 (7) | 0.0005 (6) | 0.0268 (7) | −0.0017 (6) |
| O6 | 0.0236 (7) | 0.0270 (6) | 0.0248 (6) | −0.0018 (5) | 0.0099 (5) | −0.0015 (5) |
| C1 | 0.0207 (8) | 0.0239 (8) | 0.0197 (8) | 0.0080 (7) | 0.0084 (7) | 0.0075 (7) |
| C2 | 0.0208 (9) | 0.0237 (8) | 0.0187 (8) | 0.0054 (7) | 0.0065 (7) | 0.0049 (7) |
| C3 | 0.0187 (8) | 0.0223 (8) | 0.0225 (8) | 0.0070 (7) | 0.0074 (7) | 0.0081 (7) |
| C4 | 0.0209 (8) | 0.0235 (8) | 0.0221 (8) | 0.0079 (7) | 0.0111 (7) | 0.0082 (7) |
| C5 | 0.0207 (9) | 0.0219 (8) | 0.0228 (8) | 0.0079 (7) | 0.0091 (7) | 0.0078 (7) |
| C6 | 0.0178 (8) | 0.0226 (8) | 0.0229 (8) | 0.0050 (7) | 0.0093 (7) | 0.0076 (7) |
| C7 | 0.0211 (9) | 0.0219 (8) | 0.0209 (8) | 0.0058 (7) | 0.0084 (7) | 0.0065 (7) |
| C8 | 0.0191 (8) | 0.0245 (8) | 0.0240 (8) | 0.0069 (7) | 0.0083 (7) | 0.0100 (7) |
| C9 | 0.0248 (9) | 0.0218 (8) | 0.0250 (8) | 0.0062 (7) | 0.0131 (7) | 0.0065 (7) |
| O7 | 0.0237 (7) | 0.0300 (6) | 0.0214 (6) | −0.0013 (5) | 0.0110 (5) | 0.0004 (5) |
| O8 | 0.0273 (7) | 0.0332 (7) | 0.0261 (6) | −0.0017 (6) | 0.0167 (6) | 0.0013 (5) |
| O9 | 0.0282 (7) | 0.0291 (7) | 0.0359 (7) | −0.0047 (6) | 0.0161 (6) | 0.0014 (6) |
| O10 | 0.0416 (8) | 0.0347 (7) | 0.0305 (7) | −0.0044 (6) | 0.0152 (6) | −0.0049 (6) |
| O11 | 0.0399 (8) | 0.0402 (8) | 0.0307 (7) | 0.0046 (6) | 0.0237 (6) | 0.0018 (6) |
| O12 | 0.0269 (7) | 0.0324 (7) | 0.0349 (7) | 0.0026 (6) | 0.0202 (6) | 0.0079 (6) |
| C11 | 0.0199 (9) | 0.0220 (8) | 0.0225 (8) | 0.0073 (7) | 0.0090 (7) | 0.0081 (7) |
| C12 | 0.0223 (9) | 0.0240 (8) | 0.0255 (8) | 0.0077 (7) | 0.0123 (7) | 0.0095 (7) |
| C13 | 0.0234 (9) | 0.0218 (8) | 0.0242 (8) | 0.0072 (7) | 0.0094 (7) | 0.0063 (7) |
| C14 | 0.0243 (9) | 0.0239 (8) | 0.0220 (8) | 0.0075 (7) | 0.0101 (7) | 0.0051 (7) |
| C15 | 0.0223 (9) | 0.0251 (8) | 0.0245 (8) | 0.0096 (7) | 0.0109 (7) | 0.0101 (7) |
| C16 | 0.0197 (8) | 0.0208 (8) | 0.0223 (8) | 0.0060 (7) | 0.0083 (7) | 0.0072 (7) |
| C17 | 0.0219 (9) | 0.0237 (8) | 0.0240 (8) | 0.0051 (7) | 0.0104 (7) | 0.0082 (7) |
| C18 | 0.0273 (10) | 0.0236 (9) | 0.0274 (9) | 0.0054 (8) | 0.0115 (8) | 0.0060 (7) |
| C19 | 0.0242 (9) | 0.0311 (9) | 0.0227 (8) | 0.0092 (8) | 0.0109 (7) | 0.0094 (7) |
| O13 | 0.0374 (8) | 0.0341 (7) | 0.0289 (7) | −0.0054 (6) | 0.0157 (6) | 0.0019 (6) |
| C21 | 0.0262 (9) | 0.0272 (9) | 0.0209 (8) | 0.0011 (7) | 0.0097 (7) | 0.0032 (7) |
| C22 | 0.0236 (9) | 0.0258 (8) | 0.0229 (8) | 0.0040 (7) | 0.0103 (7) | 0.0062 (7) |
| C23 | 0.0336 (10) | 0.0365 (10) | 0.0329 (9) | 0.0138 (8) | 0.0233 (8) | 0.0144 (8) |
| N1 | 0.0233 (8) | 0.0249 (7) | 0.0228 (7) | 0.0023 (6) | 0.0103 (6) | 0.0049 (6) |
| N2 | 0.0223 (8) | 0.0280 (7) | 0.0234 (7) | 0.0022 (6) | 0.0105 (6) | 0.0053 (6) |
| N3 | 0.0239 (8) | 0.0268 (7) | 0.0235 (7) | 0.0055 (6) | 0.0132 (6) | 0.0079 (6) |
Geometric parameters (Å, °)
| O1—C7 | 1.2182 (19) | O12—C19 | 1.298 (2) |
| O2—C7 | 1.3221 (19) | O12—H12 | 0.90 (2) |
| O2—H2 | 0.92 (2) | C11—C12 | 1.390 (2) |
| O3—C8 | 1.2147 (19) | C11—C16 | 1.395 (2) |
| O4—C8 | 1.3217 (19) | C11—C17 | 1.489 (2) |
| O4—H4 | 0.88 (2) | C12—C13 | 1.391 (2) |
| O5—C9 | 1.2065 (19) | C12—H12A | 0.9400 |
| O6—C9 | 1.3204 (19) | C13—C14 | 1.388 (2) |
| O6—H6 | 0.91 (2) | C13—C18 | 1.497 (2) |
| C1—C2 | 1.390 (2) | C14—C15 | 1.389 (2) |
| C1—C6 | 1.391 (2) | C14—H14A | 0.9400 |
| C1—C7 | 1.492 (2) | C15—C16 | 1.395 (2) |
| C2—C3 | 1.393 (2) | C15—C19 | 1.494 (2) |
| C2—H2A | 0.9400 | C16—H16A | 0.9400 |
| C3—C4 | 1.395 (2) | O13—H13A | 0.91 (2) |
| C3—C8 | 1.491 (2) | O13—H13B | 0.85 (3) |
| C4—C5 | 1.394 (2) | C21—N2 | 1.300 (2) |
| C4—H4A | 0.9400 | C21—N3 | 1.353 (2) |
| C5—C6 | 1.393 (2) | C21—H21A | 0.9400 |
| C5—C9 | 1.489 (2) | C22—N1 | 1.306 (2) |
| C6—H6A | 0.9400 | C22—N3 | 1.358 (2) |
| O7—C17 | 1.3090 (19) | C22—H22A | 0.9400 |
| O7—H7 | 0.96 (2) | C23—N3 | 1.472 (2) |
| O8—C17 | 1.2230 (18) | C23—C23i | 1.513 (3) |
| O9—C18 | 1.304 (2) | C23—H23A | 0.9800 |
| O9—H9 | 0.91 (3) | C23—H23B | 0.9800 |
| O10—C18 | 1.210 (2) | N1—N2 | 1.3784 (18) |
| O11—C19 | 1.2201 (19) | ||
| C7—O2—H2 | 110.6 (13) | C14—C13—C12 | 119.73 (15) |
| C8—O4—H4 | 107.2 (13) | C14—C13—C18 | 119.44 (14) |
| C9—O6—H6 | 112.6 (13) | C12—C13—C18 | 120.83 (14) |
| C2—C1—C6 | 119.32 (14) | C13—C14—C15 | 120.10 (15) |
| C2—C1—C7 | 120.95 (14) | C13—C14—H14A | 120.0 |
| C6—C1—C7 | 119.73 (14) | C15—C14—H14A | 120.0 |
| C1—C2—C3 | 120.72 (14) | C14—C15—C16 | 120.34 (14) |
| C1—C2—H2A | 119.6 | C14—C15—C19 | 117.53 (14) |
| C3—C2—H2A | 119.6 | C16—C15—C19 | 122.06 (15) |
| C2—C3—C4 | 119.85 (14) | C15—C16—C11 | 119.51 (15) |
| C2—C3—C8 | 117.56 (14) | C15—C16—H16A | 120.2 |
| C4—C3—C8 | 122.59 (14) | C11—C16—H16A | 120.2 |
| C5—C4—C3 | 119.53 (14) | O8—C17—O7 | 122.24 (14) |
| C5—C4—H4A | 120.2 | O8—C17—C11 | 122.17 (15) |
| C3—C4—H4A | 120.2 | O7—C17—C11 | 115.59 (13) |
| C6—C5—C4 | 120.21 (14) | O10—C18—O9 | 125.16 (16) |
| C6—C5—C9 | 119.87 (14) | O10—C18—C13 | 121.74 (15) |
| C4—C5—C9 | 119.88 (13) | O9—C18—C13 | 113.10 (14) |
| C1—C6—C5 | 120.34 (14) | O11—C19—O12 | 125.08 (15) |
| C1—C6—H6A | 119.8 | O11—C19—C15 | 120.02 (15) |
| C5—C6—H6A | 119.8 | O12—C19—C15 | 114.90 (14) |
| O1—C7—O2 | 123.19 (14) | H13A—O13—H13B | 110 (2) |
| O1—C7—C1 | 124.36 (15) | N2—C21—N3 | 110.82 (14) |
| O2—C7—C1 | 112.45 (13) | N2—C21—H21A | 124.6 |
| O3—C8—O4 | 123.84 (15) | N3—C21—H21A | 124.6 |
| O3—C8—C3 | 123.02 (14) | N1—C22—N3 | 110.10 (15) |
| O4—C8—C3 | 113.14 (14) | N1—C22—H22A | 124.9 |
| O5—C9—O6 | 122.58 (16) | N3—C22—H22A | 124.9 |
| O5—C9—C5 | 124.55 (15) | N3—C23—C23i | 111.15 (18) |
| O6—C9—C5 | 112.86 (13) | N3—C23—H23A | 109.4 |
| C17—O7—H7 | 107.8 (13) | C23i—C23—H23A | 109.4 |
| C18—O9—H9 | 112.4 (14) | N3—C23—H23B | 109.4 |
| C19—O12—H12 | 113.6 (14) | C23i—C23—H23B | 109.4 |
| C12—C11—C16 | 119.86 (14) | H23A—C23—H23B | 108.0 |
| C12—C11—C17 | 117.73 (14) | C22—N1—N2 | 107.37 (13) |
| C16—C11—C17 | 122.41 (14) | C21—N2—N1 | 106.94 (13) |
| C11—C12—C13 | 120.45 (14) | C21—N3—C22 | 104.77 (13) |
| C11—C12—H12A | 119.8 | C21—N3—C23 | 127.89 (14) |
| C13—C12—H12A | 119.8 | C22—N3—C23 | 127.25 (14) |
Symmetry codes: (i) −x+1, −y, −z.
Hydrogen-bond geometry (Å, °)
| D—H···A | D—H | H···A | D···A | D—H···A |
| O2—H2···O11ii | 0.92 (2) | 1.67 (2) | 2.594 (2) | 175 (2) |
| O4—H4···O8 | 0.88 (2) | 1.75 (2) | 2.626 (2) | 174 (2) |
| O6—H6···O1iii | 0.91 (2) | 1.84 (2) | 2.699 (2) | 157 (2) |
| O7—H7···N1 | 0.96 (2) | 1.75 (2) | 2.703 (2) | 172 (2) |
| O9—H9···O13 | 0.91 (3) | 1.63 (3) | 2.531 (2) | 170 (2) |
| O12—H12···N2iv | 0.90 (2) | 1.76 (2) | 2.644 (2) | 167 (2) |
| O13—H13A···O10v | 0.91 (2) | 1.81 (3) | 2.711 (2) | 171 (2) |
| O13—H13B···O5 | 0.85 (3) | 1.92 (3) | 2.751 (2) | 167 (2) |
Symmetry codes: (ii) x+1, y, z−1; (iii) −x+3, −y+2, −z+1; (iv) −x, −y, −z+1; (v) −x+2, −y+2, −z+2.
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: KJ2089).
References
- Althoff, G., Ruiz, J., Rodríguez, V., López, G., Pérez, J. & Janiak, C. (2006). CrystEngComm, 8, 662–665.
- Bruker (2006). APEX2 and SAINT Bruker AXS Inc., Madison, Wisconsin, USA.
- Crystal Impact (2006). DIAMOND Crystal Impact GbR, Bonn, Germany.
- Dale, S. H. & Elsegood, M. R. J. (2004). Acta Cryst. C60, o444–o448. [DOI] [PubMed]
- Dale, S. H., Elsegood, M. R. J. & Richards, S. J. (2004). Chem. Commun. pp. 1278–1279. [DOI] [PubMed]
- Dorn, T., Chamayou, A.-C. & Janiak, C. (2006). New J. Chem.30, 156–167.
- Dorn, T., Janiak, C. & Abu-Shandi, K. (2005). CrystEngComm, 7, 663–641.
- Du, M., Zhang, Z.-H. & Zhao, X.-J. (2005). Cryst. Growth Des.5, 1247–1254.
- Etter, M. C., MacDonald, J. C. & Bernstein, J. (1990). Acta Cryst. B46, 256–262. [DOI] [PubMed]
- Fan, Z.-Z., Li, X.-H. & Wang, G.-P. (2005). Acta Cryst. E61, o1607–o1608.
- Goldberg, I. & Bernstein, J. (2007). Chem. Commun. pp. 132–143. [DOI] [PubMed]
- Janiak, C. (2000). J. Chem. Soc. Dalton Trans. pp. 3885–3896.
- Shattock, T. R., Vishweshwar, P., Wang, Z. & Zaworotko, M. J. (2005). Cryst. Growth Des.5, 2046–2049.
- Sheldrick, G. M. (1996). SADABS University of Göttingen, Germany.
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Turner, D. R., Pek, S. N. & Batten, S. R. (2008). New J. Chem.32, 719–726.
- Wang, W.-J. & Wang, J.-S. (2005). Mol. Cryst. Liq. Cryst.440, 147–152.
- Westrip, S. P. (2008). publCIF In preparation.
- Wisser, B. & Janiak, C. (2007a). Acta Cryst. E63, m1732–m1733.
- Wisser, B. & Janiak, C. (2007b). Acta Cryst. E63, o2871–o2872.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablocks I, global. DOI: 10.1107/S1600536808015808/kj2089sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536808015808/kj2089Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report