Abstract
The effects of moxifloxacin, a new methoxyfluoroquinolone, on the production of proinflammatory cytokines from human peripheral blood mononuclear cells (PBMCs) were evaluated. Moxifloxacin inhibited the production of tumor necrosis factor alpha (TNF-α) and/or interleukin-6 (IL-6) by PBMCs stimulated with lipopolysaccharide (LPS), lipoteichoic acid (LTA), and heat-killed bacteria in a concentration-dependent manner without cytotoxic effects. The addition of moxifloxacin reduced the population of cells positive for CD-14 and TNF-α and for CD-14 and IL-6 among the LPS- or LTA-stimulated PBMCs. By Western blot analysis, moxifloxacin pretreatment reduced the degradation of IκBα in LPS-stimulated PBMCs. In conclusion, moxifloxacin could interfere with NF-κB activation by inhibiting the degradation of IκBα and reduce the levels of production of proinflammatory cytokines.
Antibiotics are widely used as bacteriostatic or bactericidal drugs for therapy for bacterial infections. Besides the respective interactions between antibiotics and bacteria and between the immune system and bacteria, antibiotics also directly interact with the immune system. The immunomodulatory effects of antibiotics include alteration of phagocytosis, chemotaxis, endotoxin release, cytokine production, hematopoietic recovery after immunosuppression, and tumoricidal effects on certain cancer cells. Moreover some antibiotic agents can affect the life spans of cells through the induction or inhibition of apoptosis (6, 8-10, 22).
Among numerous antibiotics, quinolones exert various immunomodulatory effects and are widely used in clinical practice, and newer quinolones with enhanced potencies against microorganisms are continuously being developed (7, 14-17).
In this study, we wanted to evaluate the effects of moxifloxacin, a synthetic methoxyfluoroquinolone with a broad antibacterial spectrum used for numerous clinical indications, on the production of some proinflammatory cytokines from peripheral blood mononuclear cells (PBMCs). The immunomodulatory effects of moxifloxacin were compared to those of another fluoroquinolone, levofloxacin, and a β-lactam antibiotic, ceftriaxone. In addition, the pathway responsible for these changes was evaluated.
MATERIALS AND METHODS
Reagents and bacteria.
Moxifloxacin was obtained in powder form from Bayer (Leverkusen, Germany). Levofloxacin was obtained from Jae-Il, Pharmacia (Seoul, Korea). Ceftriaxone was purchased from Sigma (St. Louis, Mo.). Lipopolysaccharide (LPS; from Escherichia coli O111:B4) and lipoteichoic acid (LTA; from Enterococcus faecalis) were purchased from Sigma. Stock solutions of all agents were made in sterile distilled water.
Streptococcus pneumoniae and E. coli were also used as stimulants. They were isolated from the blood of patients in St. Mary's Hospital. E. coli was cultured in Mueller-Hinton broth (Becton Dickinson, Sparks, Md.) and S. pneumoniae was cultured in Mueller-Hinton broth with 5% lysed horse blood (Becton Dickinson, Cockeysville, Md.) at 37°C. The colonies were then harvested by centrifugation, washed twice in phosphate-buffered saline (PBS), killed by incubation at 65°C for 20 min, and stored at −70°C until use.
Isolation and stimulation of PBMCs.
Blood was obtained by venipuncture from healthy volunteers. PBMCs were separated on Ficoll-Paque (Sigma) density gradients, washed twice with calcium- and magnesium-free PBS, and resuspended in RPMI 1640 with l-glutamine containing 10% fetal bovine serum and 25 mM HEPES at a density of 106 cells/ml. The cells were exposed to 100 ng of LPS per ml, 1 μg of LTA per ml, or 107 CFU of heat-killed bacteria per ml with or without antibiotic treatment. Adequate concentrations of LPS, LTA, and bacteria were finally made with RPMI 1640, and an equal volume of each stimulus was added to same volume of the PBMC preparation.
ELISA for cytokine release.
Cells were seeded into 24-well plates at a density of 106 cells/well and were incubated for 6 h in the presence of LPS, LTA, and heat-killed S. pneumoniae or E. coli with or without antibiotics. The cell-free supernatant was collected by centrifugation and stored at −70°C. The concentrations of tumor necrosis factor alpha (TNF-α), and interleukin-6 (IL-6) were assayed with enzyme-linked immunosorbent assay (ELISA) kits (BD Pharmingen, San Diego, Calif.) according to the protocol of the manufacturer.
Viability assay.
The viabilities of PBMCs treated with moxifloxacin were evaluated by the 3-(4,5-dimethylthiazole-2-y1)-2,5-diphenyltetrazolium bromide (MTT) assay (R&D Systems, Inc., Minneapolis, Minn.). In brief, aliquots of 104 cells/well were distributed in 96-well tissue culture plates (Nunc, Rosklide, Denmark) in 0.1 ml of 10% fetal calf serum-RPMI 1640 medium and incubated at 37°C with or without moxifloxain for 24 and 48 h. After incubation, MTT solution was added to the culture medium to achieve a final concentration of 1 mg/ml. After 2 h of incubation at 37°C, detergent solution was added to solubilize the colored formazan crystal produced from MTT. The absorbance was read at 550 nm with a spectrophotometer, and the percentage of viable cells was calculated.
Flow cytometry for detection of cytokine-producing PBMCs.
PBMCs were incubated in the absence or presence of moxifloxacin (10 μg/ml) and stimulant (1 μg of LTA per ml or 100 ng of LPS per ml) for 6 h at 37°C in 24-well plates. After incubation, 105 cells were harvested, washed twice with PBS, and distributed into polystyrene round-bottom tubes for immunolabeling. Two-color staining for the detection of cell surface markers (CD-14) and intracellular TNF-α or IL-6 was performed by the protocol of the manufacturer (BD Pharmingen). Flow cytometric analysis was performed on a FACScan instrument (Becton Dickinson, San Jose, Calif.). The percentage of double-stained cells (cells positive for CD-14 and TNF-α and for CD-14 and IL-6) among the stimulated PBMCs was calculated and compared by stimulus.
Western blot analysis of IκB.
PBMCs were exposed to 100 ng of LPS per ml with or without pretreatment with 10 μg of moxifloxacin per ml for 1 h. Nuclear extracts were harvested from the stimulated cells by the method described by Ichiyama et al. (5). The protein concentrations were determined with Bio-Rad (Hercules, Calif.) protein concentration reagents. Samples containing 30 μg of protein were separated by electrophoresis on a 10% polyacrylamide gel and were then transferred to a polyvinylidene difluoride membrane. After the membranes were washed three times in TBST (10 mM Tris-Cl [pH 8.0], 0.05% 150 mM NaCl, Tween 20), they were incubated overnight in a 1:1,000 dilution of rabbit polyclonal anti-IκBα antibodies (Cell Signaling Technology Inc., Beverly, Mass.) in TBST containing 5% skim milk at 4°C. After the membranes were washed in TBST, they were incubated in a 1:2,000 dilution of horseradish peroxidase-conjugated anti-rabbit immunoglobulin G (Santa Cruz Biotechnology, Santa Cruz, Calif.) for 2 h at room temperature. Chemiluminescent detection was performed with enhanced chemiluminescence (ECL) reagents (Amersham Pharmacia Biotech, Piscataway, N.J.) after the membranes were washed in TBST. Autoradiography of the membranes was performed with Hyperfilm-ECL (Amersham Pharmacia Biotech).
Statistical analysis.
All experiments were repeated at least three times. Statistical analysis was performed by analysis of variance by Tukey's method or the Scheffe test. A P value of <0.05 was judged to be statistically significant.
RESULTS
Moxifloxacin suppresses TNF-α and IL-6 release from PBMCs in a concentration-dependent manner without cytotoxicity.
To determine the effects of antibiotics on cytokine production, LPS, LTA, or bacteria were added to PBMCs with or without antibiotics. None of the antibiotics used in the experiments led to the production of cytokines by the PBMCs without stimulation. LPS, LTA, and bacteria induced TNF-α and IL-6 production by the PBMCs.
Addition of moxifloxacin, levofloxacin, or ceftriaxone to LPS-stimulated PBMCs decreased the level of production of TNF-α, and among these agents, moxifloxacin showed the greatest inhibitory effect. Moxifloxacin also decreased the levels of production of TNF-α by LTA- and heat-killed E. coli-stimulated PBMCs, but levofloxacin and ceftriaxone did not affect the levels of production of TNF-α by these cells. The level of TNF-α production by heat-killed S. pneumoniae-stimulated PBMCs did not show any significant change with the addition of antibiotics (Table 1).
TABLE 1.
Effect of moxifloxacin on production of TNF-α by PBMCsa
| Treatment | TNF-α concn (pg/ml) with the following stimulus:
|
|||
|---|---|---|---|---|
| LPS | E. coli | LTA | S. pneumoniae | |
| Stimulus only | 3,066 ± 249 | 1,372 ± 175 | 341 ± 30 | 1,159 ± 63 |
| Stimulus and: | ||||
| moxifloxacin (10 μg/ml) | 1,072 ± 23b | 720 ± 75b | 164 ± 22b | 912 ± 97 |
| levofloxacin (10 μg/ml) | 1,472 ± 45b | 908 ± 322 | 209 ± 82 | 920 ± 132 |
| ceftriaxone (250 μg/ml) | 1,208 ± 237b | 1,076 ± 16 | 264 ± 54 | 917 ± 433 |
The concentrations were measured by ELISA. The values are means ± standard errors of the means for quadruplicate measurements.
P < 0.05 compared to the result for each stimulus only.
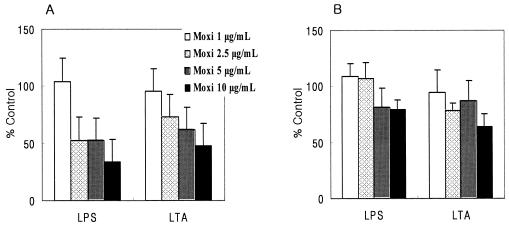
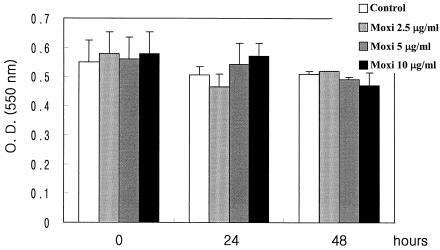
Addition of moxifloxacin decreased the level of production of IL-6 regardless of the stimulation used, especially when moxifloxacin was added to heat-killed bacterium-stimulated PBMCs. Although levofloxacin and ceftriaxone did not affect the production of IL-6 by LPS-, LTA-, and heat-killed E. coli-stimulated PBMCs, they significantly decreased the level of production of IL-6 by heat-killed S. pneumoniae-stimulated PBMCs (Table 2). Moxifloxacin inhibited the production of TNF-α and IL-6 in a dose-dependent fashion (Fig. 1). Moxifloxacin was not toxic for the cultured PBMCs at any concentration used, as determined by the MTT assay (Fig. 2). Levofloxacin and ceftriaxone also did not show any significant toxicity to the PBMCs (data not shown).
TABLE 2.
Effect of moxifloxacin on production of IL-6 by PBMCsa
| Treatment | IL-6 concn (pg/ml) with the following stimulus:
|
|||
|---|---|---|---|---|
| LPS | E. coli | LTA | S. pneumoniae | |
| Stimulus only | 24,405 ± 5,148 | 17,451 ± 6,484 | 5,931 ± 813 | 14,677 ± 1,289 |
| Stimulus and: | ||||
| moxifloxacin (10 μg/ml) | 19,200 ± 4,120c | 2,918 ± 1,123b,c,d | 3,755 ± 148 | 1,067 ± 74b,c |
| levofloxacin (10 μg/ml) | 23,381 ± 1,747 | 12,715 ± 2,085 | 5,717 ± 591 | 1,493 ± 196b,c |
| ceftriaxone (250 μg/ml) | 29,952 ± 7,043 | 18,517 ± 3,885 | 7,253 ± 296 | 4,352 ± 1,138b |
The concentrations were measured by ELISA. The values are means ± standard errors of the means for quadruplicate measurements.
P < 0.05 compared to the result for each stimulus only.
P < 0.05 compared to the result for ceftriaxone treatment.
P < 0.05 compared to the result for levofloxacin treatment.
FIG. 1.
Concentration-dependent effect of moxifloxacin (Moxi) on the production of TNF-α (A) and IL-6 (B). Data are expressed as the level of production of TNF-α or IL-6 in the presence of moxifloxacin as a percentage of the level of production of TNF-α or IL-6 by PBMCs stimulated with only LPS or LTA (100%).
FIG. 2.
Cytotoxic effect of moxifloxacin (Moxi) assessed by MTT assay. Data are expressed as the mean ± standard error of the mean absorbance for quadruplicate measurements. O.D. (550 nm), optical density at 550 nm.
Moxifloxacin inhibits cytokine production by CD-14-positive cells.
Moxifloxacin treatment reduced the proportion of cells positive for both CD-14 and TNF-α from 49.72% ± 2.19% to 37.85% ± 0.96% (P < 0.05) among the LPS-stimulated PBMCs and from 34.50% ± 3.84% to 22.24% ± 1.04% among the LTA-stimulated PBMCs (P < 0.05). Moxifloxacin treatment also reduced the proportion of cells positive for both CD-14 and IL-6 from 55.66% ± 1.64% to 42.38% ± 2.09% (P < 0.05) among the LPS-stimulated PBMCs and from 38.22% ± 1.04% to 26.11% ± 0.68% among the LTA-stimulated PBMCs (P < 0.05) in quadruplicate experiments.
Moxifloxacin inhibits IκBα degradation.
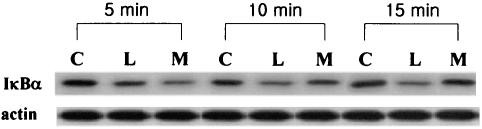
We hypothesized that moxifloxacin's modulatory effect might be associated with the nuclear factor κB (NF-κB) signaling pathway and performed a Western blot analysis to assess the level of IκBα degradation. Although moxifloxacin pretreatment initially (5 min) enhanced the level of IκBα degradation by LPS challenge, IκBα degradation started to be inhibited by moxifloxacin pretreatment 10 min after LPS challenge. After 15 min, the density of IκBα expression was nearly similar to that of control cells (Fig. 3). These findings suggest that moxifloxacin could interrupt NF-κB activation by inhibiting IκBα degradation.
FIG. 3.
Representative Western blot analysis of IκBα expression. PBMCs were pretreated with 10 μg of moxifloxacin per ml and then challenged with LPS (10 ng/ml). Lanes: C, controls; L, LPS-stimulated PBMCs; M, moxifloxacin-pretreated, LPS-stimulated PBMCs.
DISCUSSION
Quinolones, which exert their bactericidal effect by inhibiting DNA gyrase, are known to interfere with certain immune functions. A high concentration of ciprofloxacin can inhibit peripheral blood lymphocyte growth (3) or can enhance the level of production of cytokines, especially IL-2, gamma interferon, and IL-4 (18-20). The explanation for these phenomena is exposure of cells to DNA-damaging agents, which induces the functions of numerous genes that facilitate the repair of such lesions; but we must remember that most of these results were obtained with concentrations above 20 μg/ml, which cannot be reached in human blood. On the contrary, the newer quinolones, such as grepafloxacin and trovafloxacin, have immunosuppressive effects in vitro within a range of twofold of the peak level achieved in serum (3, 18, 19). The exact mechanisms of the difference between the immunostimulatory and the immunosuppressive effects due to the quinolone concentration are still uncertain. We wanted to evaluate the immunomodulatory effects of moxifloxacin and the pathways involved. In this study, moxifloxacin decreased the levels of production of TNF-α and IL-6 by LPS- and heat-killed bacterium-stimulated PBMCs in a concentration-dependent manner. The inhibitory effect of moxifloxacin on the production of IL-6 was greater than that of ceftriaxone. These results were similar to those reported by Purswani et al. (16), who assessed the inhibitory effect of trovafloxacin on the production of cytokines and who compared the effects of trovafloxacin to those of ceftriaxone and ciprofloxacin. Purswani et al. (16) showed that trovafloxacin and ciprofloxacin clearly had different effects on TNF-α and IL-6 production, but we could not find any consistent differences between the effects of moxifloxacin and levofloxacin. Moxifloxacin showed more inhibitory activity than levofloxacin only when it was added to heat-killed E. coli-stimulated PBMCs. The differences in the immunomodulating effects among the quinolones and the meaning of these differences are still hard to understand.
Although we confirmed these inhibitory effects with lower concentrations of moxifloxacin, we used the highest concentrations of antibiotics to maximize the differences in some experiments, and the moxifloxacin concentrations in tissues and cells commonly exceeded the peak levels achievable in serum (2.5 to 5.0 μg/ml) (11). Moxifloxacin inhibited cytokine production by CD-14-positive cells such as monocytes, a well-known source of the cytokine response to stimuli, such as bacteria (1, 21, 23). This inhibitory effect was not associated with cellular toxicity. The MTT assay did not show any significant difference in the responses of control and moxifloxacin-treated PBMCs at any concentration tested (Fig. 2). This means that moxifloxacin could directly interfere with the production of cytokines from the stimulated PBMCs, as Araujo et al. (1) have reported. They explained that the moxifloxacin-induced inhibition was a result of the direct interaction with LPS, its receptor, and/or its stimulatory pathway. We wanted to explain this by focusing on the NF-κB signaling pathway.
NF-κB is a ubiquitous and important transcription factor for genes that encode proinflammatory cytokines, such as IL-1, IL-6, IL-8, and TNF-α. The prototype of NF-κB is a heterodimer consisting of p50 and p65 bound by members of the IκB family, including IκBα, in the cytoplasm. NF-κB activation requires degradation of the IκB protein. Phosphorylation of IκBα by drugs, cytokines, bacterial products, and viruses rapidly leads to IκB degradation and the translocation of NF-κB to the nucleus. Activation of NF-κB results in the binding of specific promoter elements and the expression of mRNAs for proinflammatory cytokine genes (2, 12, 13).
As shown in Fig. 3, at 5 min after LPS challenge, PBMCs pretreated with 10 μg of moxifloxacin per ml showed more degradation of IκBα than PBMCs not treated with moxifloxacin. However, the levels of expression of IκBα by moxifloxacin-pretreated LPS-stimulated PBMCs started to increase and were similar to those of control cells after 15 min. These findings could be the explanation for the decreased levels of TNF-α and IL-6 production by stimulated PBMCs achieved with moxifloxacin treatment. Ichiyama et al. (5) reported that clarithromycin inhibited NF-κB activation in pulmonary epithelial cells, but this inhibition was not linked to the preservation of IκBα degradation. Macrolides and quinolones might inhibit NF-κB activation by different pathways, but we could not hypothesize what this pathway is because the result could be affected by certain characteristics of the stimuli (such as characteristics related to the bacterium itself or to some bacterial component) and the target cells. Recently, Hoffmann et al. (4) have made an interesting suggestion concerning a negative-feedback loop within the NF-κB and IκBα signaling pathway.
Although an assay to evaluate the alteration of the dynamics of IκBα and a direct assay for NF-κB were not performed in this study, we can assume from the results of Western blot analysis that moxifloxacin inhibits the degradation of IκBα and exerts inhibitory effects on the production of proinflammatory cytokines. Further studies on the alteration of NF-κB and IκBα by repeated exposure to quinolones and the effect on the production of anti-inflammatory cytokines and chemokines will be helpful to extend our knowledge about immunomodulating antibiotics.
In conclusion, moxifloxacin has an inhibitory effect on the production of TNF-α and IL-6 from human PBMCs stimulated with bacteria and bacterial components by inhibiting the degradation of IκBα.
REFERENCES
- 1.Araujo, F. G., T. L. Slifer, and J. S. Remington. 2002. Effect of moxifloxacin on secretion of cytokines by human monocytes stimulated with lipopolysaccharide. Clin. Microbiol. Infect. 8:26-30. [DOI] [PubMed] [Google Scholar]
- 2.Brummer, E., A. Maqbool, and D. A. Stevens. 2001. Protection of bronchoalveolar macrophages by granulocyte-macrophage colony-stimulating factor against dexamethasone suppression of fungicidal activity for Aspergillus fumigatus conida. Med. Mycol. 39:509-515. [DOI] [PubMed] [Google Scholar]
- 3.Forsgren, A., S. F. Scholossman, and T. F. Tedder. 1987. 4-Quinolone drugs affect cell cycle progression and function of human lymphocytes in vitro. Antimicrob. Agents Chemother. 31:768-773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hoffmann, A., A. Levchenko, M. L. Scott, and D. Baltimore. 2002. The decoding IκB-NF-κB signaling module: temporal control and selective gene activation. Science 298:1241-1245. [DOI] [PubMed] [Google Scholar]
- 5.Ichiyama, T., M. Nishikawa, T. Yoshitomi, S. Hasegawa, T. Matsubara, T. Hayashi, and S. Furukawa. 2001. Clarithromycin inhibits NF-κB activation in human peripheral blood mononuclear cells and pulmonary epithelial cells. Antimicrob. Agents Chemother. 45:44-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jun, Y. T., H. J. Kim, M. J. Song, J. H. Lim, D. G. Lee, K. J. Han, S. M. Choi, J. H. Yoo, W. S. Shin, and J. H. Choi. 2003. In vitro effects of ciprofloxacin and roxithromycin on apoptosis of Jurkat T lymphocytes. Antimicrob. Agents Chemother. 47:1161-1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khan, A. A., T. R. Slifer, and J. S. Remington. 1998. Effect of trovafloxacin on production of cytokines by human monocytes. Antimicrob. Agents Chemother. 42:1713-1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Labro, M. T. 1997. The prohost effect of antimicrobial agents as a predictor of clinical outcome. J. Chemother. 9(Suppl. 1):100-108. [PubMed] [Google Scholar]
- 9.Labro, M. T. 1998. Antibacterial agents-phagocytes: new concepts for old in immunomodulation. Int. J. Antimicrob. Agents 10:11-21. [DOI] [PubMed] [Google Scholar]
- 10.Labro, M. T. 2000. Interference of antibacterial agents with phagocyte functions: immunomodulation or “immuno-fairy tales”? Clin. Microbiol. Rev. 13:615-650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.MacGowan, A. P. 1999. Pharmacodynamics of moxifloxacin, p. 5-15. In D. Adams and R. Finch (ed.), Moxifloxain in practice. Maxim Medical, Oxford, United Kingdom.
- 12.Maniatis, T. 1997. Catalysis by a multiprotein IκB kinase complex. Science 278:818-819. [DOI] [PubMed] [Google Scholar]
- 13.May, M. J., and S. Ghosh. 1998. Signal transduction through NF-κB. Immunol. Today 19:80-88. [DOI] [PubMed] [Google Scholar]
- 14.Morikawa, K., H. Watabe, M. Araake, and S. Morikawa. 1996. Modulatory effect of antibiotics on cytokine production by human monocytes in vitro. Antimicrob. Agents Chemother. 40:1366-1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ono, Y., Y. Ohmoto, K. Ono, Y. Sakada, and K. Murata. 2000. Effect of grepafloxacin on cytokine production in vivo. J. Antimicrob. Chemother. 46:91-94. [DOI] [PubMed] [Google Scholar]
- 16.Purswani, M., S. Eckert, H. Arora, R. Johann-Liang, and G. J. Noel. 2000. The effect of three broad-spectrum antimicrobials on mononuclear cell responses to encapsulated bacteria: evidence for down-regulation of cytokine mRNA transcription by trovafloxacin. J. Antimicrob. Chemother. 46:921-929. [DOI] [PubMed] [Google Scholar]
- 17.Riesbeck, K., and A. Forsgren. 1994. Limited effects of temifloxacin compared with ciprofloxacin on T-lymphocyte function. Antimicrob. Agents Chemother. 38:879-882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Riesbeck, K., and A. Forsgren. 1994. Increased interleukin 2 transcription in murine lymphocytes by ciprofloxacin. Immunopharmacology 27:155-164. [DOI] [PubMed] [Google Scholar]
- 19.Riesbeck, K., M. Sigvardsson, T. Leanderson, and A. Forsgren. 1994. Superinduction of cytokine gene transcription by ciprofloxacin. J. Immunol. 153:343-352. [PubMed] [Google Scholar]
- 20.Riesbeck, K., A. Forsgren, A. Henriksson, and A. Bredberg. 1998. Ciprofloxacin induces an immunomodulatory stress response in human T lymphocytes. Antimicrob. Agents Chemother. 42:1923-1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roberts, F. A., G. J. Richardson, and S. M. Michalek. 1997. Effects of Porphyromonas gingivalis and Escherichia coli lipopolysaccharides on mononuclear phagocytes. Infect. Immun. 65:3248-3254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shalit, I., Y. Kletter, D. Halperin, D. Waldman, E. Vasserman, A. Nagler, and I. Fabian. 2001. Immunomodulatroy effects of moxifloxacin in comparison to ciprofloxacin and G-CSF in a murine model of cyclophosphamide-induced leukopenia. Eur. J. Haematol. 66:287-296. [DOI] [PubMed] [Google Scholar]
- 23.Tohru, K., H. Koichi, H. Yoshihiro, G. Kazunori, K. Takao, T. Hiroshi, T. Yutaka, W. Akira, and N. Toshihiro. 2002. Clarithromycin suppresses lipopolysaccharide-induced interleukin-8 production by human monocytes through AP-1 and NF-κB transcription factors. J. Antimicrob. Chemother. 49:745-755. [DOI] [PubMed] [Google Scholar]