Abstract
Human rhinovirus (HRV) infections are usually self-limited but may be associated with serious consequences, particularly in those with asthma and chronic respiratory disease. Effective antiviral agents are needed for preventing and treating HRV illnesses. Ruprintrivir (Agouron Pharmaceuticals, Inc., San Diego, Calif.) selectively inhibits HRV 3C protease and shows potent, broad-spectrum anti-HRV activity in vitro. We conducted three double-blind, placebo-controlled clinical trials in 202 healthy volunteers to assess the activity of ruprintrivir in experimental HRV infection. Subjects were randomized to receive intranasal ruprintrivir (8 mg) or placebo sprays as prophylaxis (two or five times daily [2×/day or 5×/day] for 5 days) starting 6 h before infection or as treatment (5×/day for 4 days) starting 24 h after infection. Ruprintrivir prophylaxis reduced the proportion of subjects with positive viral cultures (for 5×/day dosing groups, 44% for ruprintrivir treatment group versus 70% for placebo treatment group [P = 0.03]; for 2×/day dosing groups, 60% for ruprintrivir group versus 92% for placebo group [P = 0.004]) and viral titers but did not decrease the frequency of colds. Ruprintrivir treatment reduced the mean total daily symptom score (2.2 for ruprintrivir treatment group and 3.3 for the placebo treatment group [P = 0.014]) by 33%. Secondary endpoints, including viral titers, individual symptom scores, and nasal discharge weights, were also reduced by ruprintrivir treatment. Overall, ruprintrivir was well tolerated; blood-tinged mucus and nasal passage irritation were the most common adverse effects reported. Pharmacokinetic analysis of plasma and nasal ruprintrivir concentrations revealed intranasal drug residence with minimal systemic absorption. Results from these studies in experimental rhinoviral infection support continued investigation of intranasal ruprintrivir in the setting of natural HRV infection.
Human rhinoviruses (HRV) account for 40 to 50% of common colds on an annual basis and up to 80% of the colds during the autumn months in the Northern Hemisphere (2, 16). In healthy individuals, these infections are generally self-limiting and mild, although acute respiratory infections may be associated with substantial morbidity, loss of productivity, excess antibiotic use, and frequent self-medication with nonprescription remedies. HRV infection may also be complicated by acute sinusitis and otitis media and may cause exacerbations of asthma, chronic bronchitis, and cystic fibrosis, requiring acute care and hospital admission (7, 15, 20, 21, 24). For both otherwise healthy and high-risk individuals, antiviral treatment or prophylaxis would be desirable.
At this time, no antiviral agents are approved for the prevention or treatment of HRV infection. Several antiviral compounds with in vitro activity against HRV have been evaluated for the management of colds, including intranasal tremacamra, a soluble intercellular adhesion molecule 1 (ICAM-1); alpha interferon 2b; and the capsid binders, pirodavir and pleconaril (1, 8, 9-13, 22). While each of these investigational antiviral agents has important shortcomings, these studies have proven that prevention and early treatment of HRV colds are possible with antiviral compounds.
The HRV 3C protease is an enzyme responsible for the posttranslational cleavage of viral precursor polyproteins into their mature forms (19). Evaluation of the crystal structure of the HRV 3C protease has allowed the development of selective inhibitors targeting the enzyme's active site (17). Ruprintrivir (Agouron Pharmaceuticals, Inc., San Diego, Calif.) (formerly designated AG7088) is a potent, irreversible inhibitor of HRV 3C protease, developed through protein structure-based design methodologies. In vitro testing in cell protection assays has shown that ruprintrivir has a broad antipicornaviral spectrum, inhibiting the replication of all 48 HRV serotypes tested with a mean 50% effective concentration (EC50) of 0.023 μM (range, 0.003 to 0.081 μM), as well as replication of other picornaviruses (18). Ruprintrivir also has been shown to inhibit HRV replication in transformed human bronchial epithelial cells (BEAS-2B), an effect associated with decreased production of proinflammatory cytokines interleukin-6 and interleukin-8, which may have a role in the pathogenesis of rhinovirus symptoms (23, 25).
Ruprintrivir is a peptidomimetic compound (molecular weight, 598.7) with poor aqueous solubility and low oral bioavailability in animals (4). In healthy, uninfected volunteers, intranasal ruprintrivir spray was safe and well tolerated in doses of 4 mg or 8 mg 6×/day for 7 days (14). The present studies were designed to evaluate whether intranasal ruprintrivir could provide prophylactic and/or therapeutic benefit in experimentally infected volunteers.
MATERIALS AND METHODS
Subjects.
Subjects were healthy volunteers, 18 to 60 years of age, who were selected on the basis of a serum neutralizing antibody titer of ≤1:2 to at least one of the two rhinovirus challenge strains used. In addition, subjects were free of symptoms of an upper respiratory tract infection during the 2 weeks prior to screening. Female subjects who were nonpregnant, nonlactating, and either of nonchildbearing potential or using acceptable methods of contraception were included. Urine pregnancy tests were performed at the beginning of the study. Subjects were excluded from the study for the following: recent history of asthma or history of chronic respiratory disease; history of significant medical or psychiatric illness; dysfunctional taste or olfaction; alcohol or substance abuse; use of topical nasal decongestants within 48 h prior to randomization or any other intranasal medication during the 2 weeks prior to study entry; use of an investigational drug within 30 days prior to randomization; and unwillingness to abstain from tobacco use throughout the study period. Subjects also were excluded if a screening examination demonstrated abnormal nasal mucosa or clinically significant deviated nasal septum. All subjects provided written, informed consent as approved by the institutional review board at each study site. This research was performed in compliance with all relevant federal guidelines and institutional policies.
Challenge viruses.
The challenge viruses used for this study were obtained from safety-tested inoculum pools of rhinovirus (Hanks or HRV 39 strain) supplied by J. M. Gwaltney, Jr. (University of Virginia, Charlottesville). In a cytopathic inhibition assay using Hi-HeLa cells (18), the ruprintrivir EC50s against HRV 39 and Hanks strains were determined to be 0.032 and <0.003 μM, respectively. On day 0, all subjects were inoculated with a targeted total of 100 to 300 50% tissue culture infectious doses (TCID50) as nasal drops in a total volume of approximately 200 or 500 μl/nostril (site 3) of one of the two viruses. Subjects received the HRV challenge while in the supine position and were instructed to remain supine for 1 min after inoculation. The inoculation was repeated once at an interval of approximately 15 min. Based on back titration assays of the fresh inoculum pools performed at each site, the estimated delivered inocula were 30 TCID50 of HRV 39 and 300 TCID50 of HRV Hanks at study sites 1 and 2 and 10,000 TCID50 of HRV 39 and 158 TCID50 of HRV Hanks at study site 3.
Study medication.
Ruprintrivir nasal spray (a 2% suspension) and placebo (vehicle) were supplied by Agouron Pharmaceuticals, Inc., in a USP type I amber glass vial fitted with an intranasal delivery device (Valois VP-7 nasal spray pump) intended to administer 100 μl of the formulation per spray actuation. Subjects received two sprays per nostril, alternating between nostrils, for a total estimated delivered dose of 8 mg per administration.
Study design.
Ruprintrivir or placebo was administered starting either 6 h prior to viral challenge (prophylaxis) or 24 h after viral challenge (treatment). The ruprintrivir dosing regimen used was dependent upon the study site where the subjects were enrolled. Ruprintrivir prophylaxis was administered every 4 h while awake (five times daily [5×/day]) at site 3 (R. B. Turner, Medical University of South Carolina) or every 12 h (2×/day) at site 2 (F. G. Hayden, University of Virginia). Ruprintrivir treatment was administered 5×/day at sites 1 (J. M. Gwaltney, Jr., University of Virginia), 2, and 3. Subjects were housed at the study site, where they remained for the duration of the study period. For each trial, subjects were randomized in a 1:1 ratio to receive ruprintrivir or placebo for 4 days (treatment) or 5 days (prophylaxis). Randomization for the prophylaxis studies was stratified by viral strain. Randomization for the treatment study was stratified by study site and viral strain. Study personnel directly supervised study drug administration.
Assessments.
Screening of all subjects was performed within 14 days of randomization and included a medical history and physical examination, nasal examination, rhinovirus serology (serum neutralizing antibody to specific challenge virus strains), clinical chemistry laboratory evaluations, complete blood count and hematology panel, and urinalysis. Laboratory assessments were also performed on days 0 and 5 (discharge) and at an exit evaluation 3 to 4 weeks after rhinovirus challenge. Safety and tolerability were assessed by observation and by volunteered reports of adverse effects, changes in physical examination, vital signs, nasal examinations, and routine hematology, chemistry, and urinalysis profiles.
Measurement of infection.
Nasal washings for virologic analyses, including qualitative and quantitative viral cultures and HRV RNA quantification, were collected from subjects each morning on days 0 through 5. The initial isolate from each subject was subjected to neutralization testing with type-specific antisera to confirm its serotype. By using a standard method, nasal washings were cultured for rhinovirus on human embryonic lung fibroblast cells (WI-38 strain) purchased from commercial sources (3). Following adsorption, the inoculum was washed two times with phosphate-buffered saline and replenished with medium. Once-frozen (−70°C) aliquots of nasal wash samples that were positive in qualitative viral culture were thawed and subjected to quantitative viral titer determination by culture of serial 10-fold dilutions in duplicate monolayers. The titer of the virus in the original nasal lavage was calculated as the dilution in which viral growth was last seen in the quantitative assay. If one of the undiluted monolayer cultures was positive and the other was negative, the titer assigned was 0.7 log10 TCID50/ml; if both were positive, the titer assigned was 1.2 log10 TCID50/ml. Based on the volume of the inoculum, the limit of detection was calculated to be ≤0.4 log10 TCID50/ml (for sites 1 and 2). If results on quantitative culture were negative, the results from the initial qualitative culture were reported. HRV RNA was quantified using the HRV-A TaqMan reverse transcription-PCR assay, which amplifies a conserved region of the 5′ untranslated region (G. Smith, S. Binford, P. Weady, and A. Patrick, unpublished results). Briefly, 560 μl of nasal lavage sample was extracted using a QIAamp viral RNA kit (Qiagen, Inc., Valencia, Calif.). The purified RNA was reverse transcribed using random hexamers followed by TaqMan PCR using a Prism 7700 instrument (Applied Biosystems, Foster City, Calif.).
Serum neutralizing antibody titers to the challenge virus were done at each site by standard methods (5). Serum specimens for antibody testing were collected during screening, immediately prior to virus challenge ´, and again 3 to 4 weeks later at the study exit visit (convalescent). Subjects with at least a fourfold increase in antibody titer to the challenge virus when the convalescent-phase serum sample was compared with the acute-phase serum sample were considered infected.
Measurement of illness.
The presence and severity of eight symptoms (sneezing, malaise, rhinorrhea, sore throat, headache, chilliness, nasal obstruction or congestion, and cough) were assessed by subjects on day 0 prior to viral challenge and then twice daily, once in the morning prior to nasal washing and again in the evening prior to the 7:00 p.m. dose of study drug. The severity of each symptom was rated on a five-point scale, ranging from 0 (none) to 4 (very severe). Subjects were instructed to score each symptom on the basis of the maximal severity experienced since the previous report. The sum of daily scores for all eight symptoms comprised the total daily symptom score; the sum of scores for sneezing, rhinorrhea, sore throat, nasal obstruction or congestion, and cough comprised the total daily respiratory symptom score. Nasal discharge weights were determined daily on days 0 to 4 by having subjects collect all tissues used for nose blowing.
Pharmacokinetics.
Samples were collected to determine drug residence in the nasal cavity and the extent of systemic exposure. Collection of nasal washes was performed on day 2 within 15 min prior to the fourth ruprintrivir dose for 5×/day dosing groups or 15 min prior to and 6 h after the first dose for the 2×/day dosing group. On day 3, blood samples were collected 15 min prior to and 1, 2, and 4 h following the third dose of ruprintrivir in 5×/day dosing groups and 15 min prior to and 1, 4, 8, and 12 h after the dose in the 2×/day dosing group.
Concentrations of ruprintrivir and its primary metabolite AG7185 in plasma and nasal washings were determined using TurbulonSpray liquid chromatography with tandem mass spectrometric detection (Alta Analytical Laboratory, El Dorado Hills, Calif.). The lower limits of detection of this assay were 0.2 ng/ml for plasma samples and 1.0 ng/ml for nasal wash samples. The following pharmacokinetic parameters were determined from the concentrations of ruprintrivir and AG7185 in plasma: maximum concentration of drug in plasma (Cmax), time to maximum concentration of drug in plasma (Tmax), and area under the concentration-versus-time curve (AUC) during the dosing interval. Cmax and Tmax estimates were obtained from the plasma drug concentration-versus-time curve. AUC was calculated using the log linear trapezoidal rule. Concentrations of ruprintrivir and AG7185 were measured in nasal washes from each nostril and were summed to obtain the amount of ruprintrivir and AG7185 recovered from the nose.
Study endpoints.
The primary efficacy measure for prophylaxis was defined prospectively as a reduction in the proportion of subjects with positive viral culture due to the rhinovirus challenge strain. The primary efficacy measure for treatment was defined by a reduction in the total mean symptom score for days 1 through 4 for subjects who became infected with the challenge virus. Infection was defined by a positive culture, fourfold or greater increase in virus-specific serum neutralizing antibody titers, or both. Secondary efficacy endpoints included the incidence of clinical colds, change in viral titer over time, mean total and respiratory symptom scores (for prophylaxis), change in individual symptom scores over time, and nasal discharge weights. The presence of a clinical cold was determined using the previously described modified Jackson criteria (6). For each subject, the area under the log10 viral titer-versus-time curve was computed. In a separate analysis, the change in viral RNA level over time was also determined for infected subjects.
Data analysis.
For the primary and secondary efficacy endpoints, both intent-to-treat and efficacy-evaluable analyses were performed. However, since the results were nearly identical, only efficacy-evaluable results are presented here. For the prophylaxis studies, evaluable subjects had a negative nasal washing culture prior to viral inoculation and completed all study medication and procedures through day 5. For the treatment study, evaluable subjects included those who had a negative nasal culture prior to viral challenge, completed all study medication and procedures through day 5, and had evidence of infection with the challenge virus strain. The safety analysis included all subjects who received study drug. The null hypothesis in the prophylaxis study that the percentage of subjects experiencing positive culture in the ruprintrivir group was equal to that in the placebo group was tested using a chi-square test (2×/day; no stratification) or Cochran-Mantel-Haenszel test (5×/day; stratified by virus strain), as appropriate. In the treatment study, mean total symptom scores for days 1 through 4 were compared using analysis of variance (ANOVA) or analysis of covariance (ANCOVA) to test the null hypothesis that the mean total symptom score in the ruprintrivir treatment group was equal to that of the placebo group. The ANCOVA model for this dosing group included effects for treatment, study site, and challenge virus strain and was adjusted for baseline values. The baseline value was the last value reported after the virus challenge and before the first treatment dose. The frequencies of antibody response, infection, and clinical cold were summarized by treatment and analyzed by the chi-square test or Cochran-Mantel-Haenszel test, as appropriate. Mean respiratory symptom scores for days 1 through 4, daily total symptom scores, daily and cumulative nasal discharge weights, area under the log10 viral titer-versus-time curve, and log10 viral titer and viral RNA by day were summarized by treatment and compared using ANOVA or ANCOVA, as appropriate.
In this proof-of-principle study, all P values reported are one sided, and a one-sided P value of <0.05 was considered to be statistically significant. All statistical analyses were performed using SAS software version 6.12 (SAS Institute, Inc., Cary, N.C.).
RESULTS
Subjects.
A total of 202 subjects (101 subjects each in the ruprintrivir and placebo groups) were enrolled in these studies. All randomized subjects completed study drug administration, and safety was evaluated in all subjects. Of the 202 subjects enrolled, 194 could be evaluated for efficacy (see below).
The demographic characteristics of the subjects, including age, sex, and race, are summarized by treatment group in Table 1. The age of subjects at sites 1 and 2 (Charlottesville, Va.) were, on average, 10 years lower than at site 3 (Medical University of South Carolina), reflecting an increased use of student volunteers at the Charlottesville sites. A history of allergy was reported in 7 (28%) subjects in the 5×/day prophylaxis study, 5 (19%) subjects in 2×/day prophylaxis study, and 14 (28%) subjects in the 5×/day treatment study receiving ruprintrivir and in 24 (24%) of all subjects receiving placebo.
TABLE 1.
Demographic and baseline characteristics of the subjects by treatment group
| Characteristic | Value for treatment group
|
|||||
|---|---|---|---|---|---|---|
| 5×/day prophylaxis
|
2×/day prophylaxis
|
5×/day treatment
|
||||
| Placebo (n = 27) | Ruprintrivir (n = 25) | Placebo (n = 25) | Ruprintrivir (n = 26) | Placebo (n = 49) | Ruprintrivir (n = 50) | |
| Age (yr) | ||||||
| Mean (SD) | 32.7 (10.4) | 31.5 (9.9) | 21.2 (4.1) | 21.2 (3.1) | 22.3 (5.7) | 23.3 (6.7) |
| Range | 18-58 | 20-54 | 18-38 | 18-31 | 18-44 | 18-43 |
| Sex (n [%]) | ||||||
| Women | 15 (56) | 17 (68) | 17 (68) | 18 (69) | 29 (59) | 23 (46) |
| Men | 12 (44) | 8 (32) | 8 (32) | 8 (31) | 20 (41) | 27 (54) |
| Race (n [%]) | ||||||
| White | 20 (74) | 18 (72) | 19 (76) | 19 (73) | 39 (80) | 45 (90) |
| Black | 5 (19) | 6 (24) | 2 (8) | 3 (12) | 6 (12) | 1 (2) |
| Asian | 2 (7) | 1 (4) | 0 | 2 (8) | 2 (4) | 3 (6) |
| Hispanic | 0 | 0 | 2 (8) | 1 (4) | 1 (2) | 0 |
| Other | 0 | 0 | 2 (8) | 1 (4) | 1 (2) | 1 (2) |
| Virus strain | ||||||
| Hanks | 22 | 21 | 25 | 26 | 17 | 18 |
| HRV 39 | 5 | 4 | 0 | 0 | 32 | 32 |
| History of allergy (n [%]) | 3 (11) | 7 (28) | 10 (40) | 5 (19) | 11 (22) | 14 (28) |
Prophylactic efficacy.
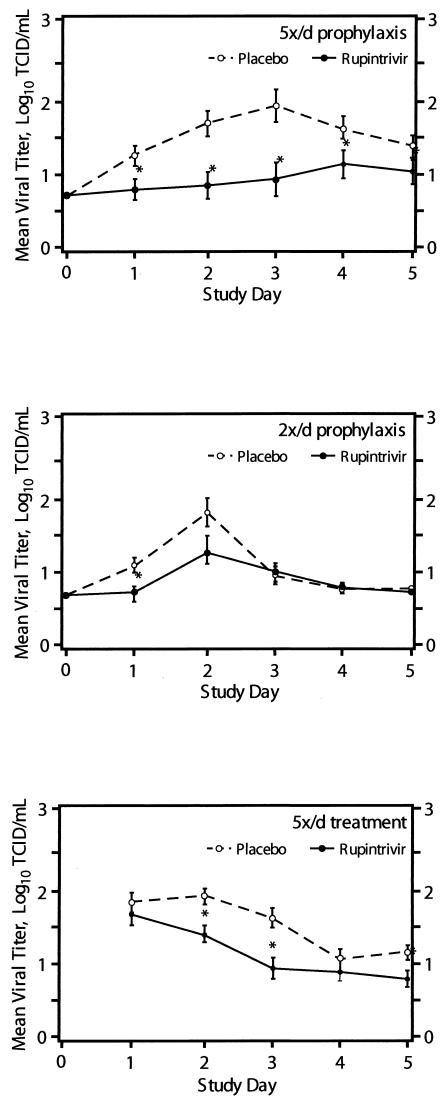
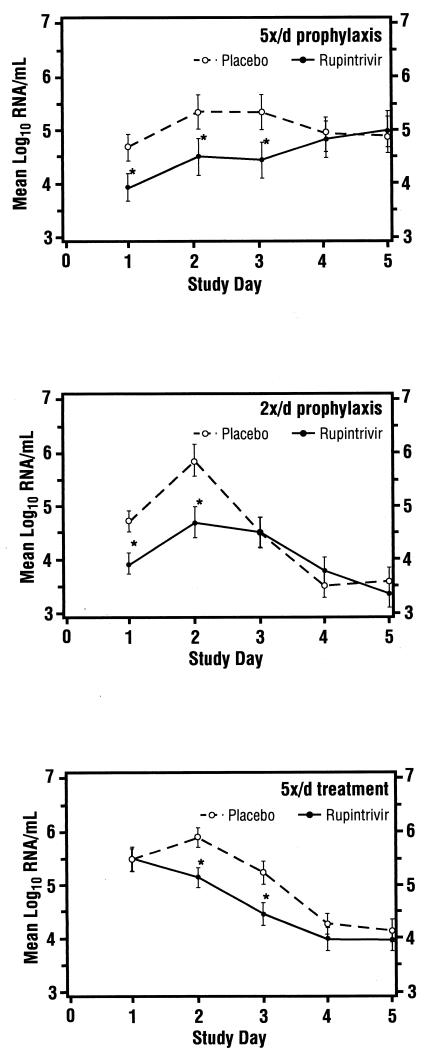
All subjects in the 5×/day study were evaluated for efficacy. One subject in the 2×/day study (ruprintrivir arm) was excluded from efficacy analyses due to a positive culture prior to virus inoculation. Table 2 summarizes primary and secondary measures of efficacy for evaluable subjects in the two prophylaxis studies. In both groups, the proportion of subjects with one or more positive viral cultures was significantly reduced by treatment with ruprintrivir compared with treatment with placebo. In the 5×/day study, this frequency was reduced by 37%, and in the 2×/day study, it was reduced by 35%. In both prophylaxis studies, subjects receiving ruprintrivir demonstrated significantly lower viral titers and RNA levels than subjects receiving placebo (Fig. 1 and 2). Since the quantification of RNA is not sensitive to the presence of ruprintrivir, the correlation between these two independent measurements suggests that drug carryover did not confound the infectious viral titer results obtained. Analyses of area under the log10 viral titer-versus-time curve revealed a significantly lower AUC in subjects receiving ruprintrivir versus placebo (Table 2). Of note, much higher copy numbers of RNA (approximately 5 to 6 log10 units at peak) were detected than by infectious virus titers (approximately 2 log10 units at peak), but the patterns were similar over time.
TABLE 2.
Prophylactic antiviral efficacy of ruprintrivir in experimental rhinovirus infection
| Characteristic | Value for treatment group
|
|||||
|---|---|---|---|---|---|---|
| 5×/day
|
2×/day
|
|||||
| Ruprintrivir (n = 25) | Placebo (n = 27) | P valuef | Ruprintrivir (n = 25) | Placebo (n = 25) | P valuef | |
| No. (%) with positive viral culture [95% CI]a | 11 (44) [27, 63] | 19 (70) [52, 84] | 0.03 | 15 (60) [41, 77] | 23 (92) [75, 98] | 0.004 |
| No. (%) with antibody responseb [95% CI] | 9 (36) [20, 55] | 9 (33) [19, 52] | 0.60 | 18 (72) [52, 86] | 20 (80) [61, 91] | 0.25 |
| No. (%) infectedc [95% CI] | 14 (56) [37, 73] | 21 (78) [59, 89] | 0.05 | 20 (80) [61, 91] | 23 (92) [75, 98] | 0.11 |
| No. (%) with coldd [95% CI] | 6 (43) [21, 67] | 11 (52) [32, 72] | 0.24 | 10 (50) [30, 70] | 13 (57) [37, 74] | 0.33 |
| Mean AUC normalized by day (log10 TCID · day/ml) (SD) | 0.74 (0.1) | 1.4 (0.8) | <0.001 | 0.91 (0.3) | 1.07 (0.3) | 0.03 |
| Mean daily total symptom score/day (SD) | 2.4 (2.6) | 3.5 (3.9) | 0.12 | 1.9 (2.0) | 2.4 (2.2) | 0.18 |
| Mean daily respiratory symptom score/daye (SD) | 1.9 (2.4) | 2.8 (3.1) | 0.12 | 1.5 (1.5) | 1.9 (1.7) | 0.18 |
| Mean cumulative nasal discharge weight (g) (days 0-4) (SD) | 11.3 (14.3) | 24.9 (57.4) | 0.12 | 16.4 (24.7) | 28.0 (23.7) | 0.05 |
95% confidence intervals (95% CI) are shown as percentages in brackets.
Defined as at least a fourfold rise in antibody titer to the challenge virus.
Subjects with positive viral culture and/or antibody response.
Number of colds in those infected, defined by modified Jackson criteria.
Includes sneezing, rhinorrhea, sore throat, nasal obstruction or congestion, and cough.
P values comparing values for ruprintrivir-treated subjects to placebo-treated subjects. All P values are one sided; a one-sided P value of <0.05 was considered to be statistically significant.
FIG. 1.
Mean log10 viral titer in nasal lavage fluid over time for subjects for whom efficacy could be evaluated (5×/day [5x/d] prophylaxis, 2×/day prophylaxis, and 5×/day treatment groups). Values are given as means ± standard errors (error bars) by the least-square method. Values for the ruprintrivir-treated subjects that were significantly different (one-sided P values of <0.05) from the values for the placebo-treated subjects by ANOVA with effects for treatment (all groups), site (5x/d treatment group), and challenge virus strain (5x/d prophylaxis and treatment groups) and by ANCOVA with effects for treatment, study site, and challenge virus strain adjusted for baseline values (days 2 to 5 of 5x/d treatment group) are indicated by asterisks.
FIG. 2.
Mean log10 viral RNA/ml in nasal lavage fluid over time for subjects for whom efficacy could be evaluated (5×/day [5x/d] prophylaxis, 2×/day prophylaxis, and 5×/day treatment groups). Values are given as means ± standard errors (error bars) by the least-square method. Values for the ruprintrivir-treated subjects that were significantly different (one-sided P values of <0.05) from the values for the placebo-treated subjects by ANOVA with effects for treatment (all groups), site (5x/d treatment group), and challenge virus strain (5x/d prophylaxis and treatment groups) and by ANCOVA with effects for treatment, study site, and challenge virus strain adjusted for baseline values (days 2 to 5 of 5x/d treatment group) are indicated by asterisks.
No important differences were observed between ruprintrivir and placebo treatment groups with regard to rates of seroconversion or clinical colds, although the overall infection rate was reduced by 28% in the 5×/day ruprintrivir treatment group compared to the rate for the placebo treatment group.
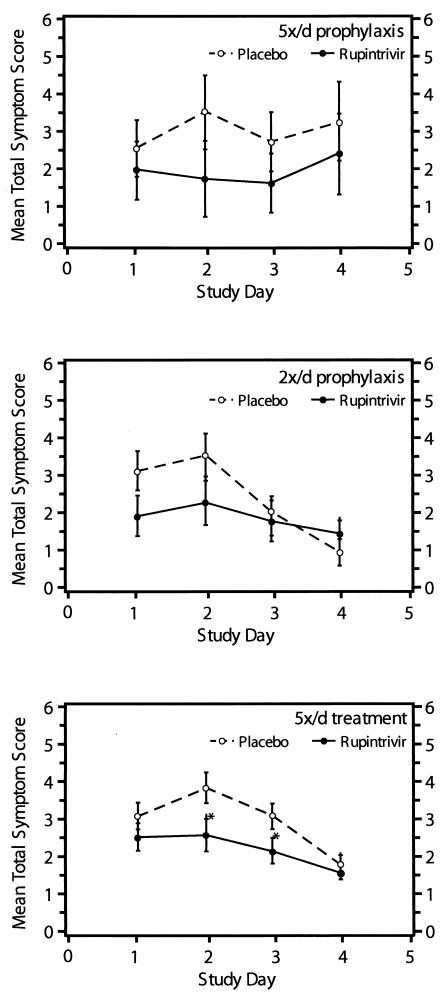
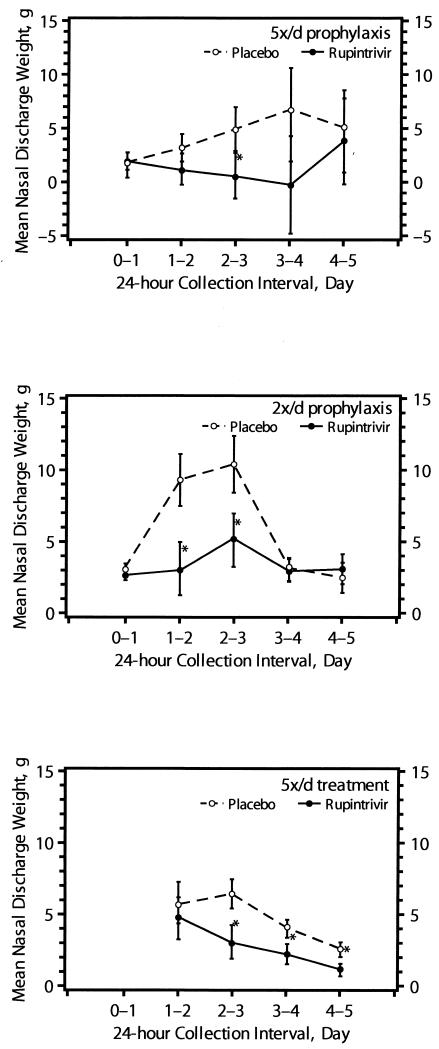
Total symptom scores by day (Fig. 3) tended to be lower in ruprintrivir-treated subjects in both prophylactic trials. In addition, total respiratory symptom scores (which included sneezing, rhinorrhea, sore throat, nasal obstruction or congestion, and cough) tended to be lower in ruprintrivir-treated subjects than in placebo-treated subjects (Table 2). Overall, nasal discharge weights per day (Fig. 4) were lower for ruprintrivir-treated subjects than for placebo-treated subjects and significantly reduced at day 2 to 3 in the 5×/day group and at day 1 to 2 and day 2 to 3 in the 2×/day group. Cumulative (i.e., total across time) nasal discharge weights were reduced by 55% in 5×/day and 41% in 2×/day ruprintrivir prophylaxis groups, but the difference between groups was significant only for subjects in the 2×/day prophylactic study (Table 2).
FIG. 3.
Mean total symptom score over time for subjects for whom efficacy could be evaluated (5×/day [5x/d] prophylaxis, 2×/day prophylaxis, and 5×/day treatment groups). For both treatment and prophylaxis groups, baseline values were virtually 0. Values are given as means ± standard errors (error bars) by the least-square method. Values for the ruprintrivir-treated subjects that were significantly different (one-sided P values of <0.05) from the values for the placebo-treated subjects by ANOVA for treatment (5×/day prophylaxis and 2×/day prophylaxis groups) and challenge virus strain (5×/day prophylaxis group) and by ANCOVA with effects for treatment, study site, challenge virus strain adjusted for baseline values are indicated by asterisks.
FIG. 4.
Mean nasal discharge weight over time for subjects in whom efficacy could be evaluated (5×/day [5x/d] prophylaxis, 2×/day prophylaxis, and 5×/day treatment groups). Values are given as means ± standard errors (error bars) by the least-square method. Values for the ruprintrivir-treated subjects that were significantly different (one-sided P values of <0.05) from the values for the placebo-treated subjects by ANOVA with effects for treatment (all treatment groups), study site (5x/d treatment group), and challenge virus strain (5x/d prophylaxis and treatment groups) are indicated by asterisks.
Treatment efficacy.
In the treatment study, six ruprintrivir-treated subjects and one placebo-treated subject were excluded from the efficacy evaluation due to no evidence of infection or a positive culture prior to virus challenge, respectively. In this study, 39 of 44 (89%) infected subjects receiving ruprintrivir and 45 of 48 (94%) infected subjects receiving placebo shed challenge virus (Table 3). For these subjects, all 44 (100%) subjects receiving ruprintrivir and all 48 (100%) subjects receiving placebo tested positive for viral RNA by the HRV-A TaqMan RT-PCR assay.
TABLE 3.
Effects of intranasal ruprintrivir on virologic and illness measures in subjects receiving early treatment for experimental rhinovirus infection
| Characteristic | Value for treatment group
|
||
|---|---|---|---|
| Ruprintrivir (n = 44) | Placebo (n = 48) | P valuee | |
| No. (%) with positive viral culture [95% CI]a | 39 (89) [76, 95] | 45 (94) [83, 98] | 0.19 |
| No. (%) with antibody responseb [95% CI] | 21 (48) [34, 62] | 26 (54) [40, 67] | 0.29 |
| No. (%) infectedc | 44 (100) | 48 (100) | NA |
| No. (%) with coldd [95% CI] | 23 (52) [38, 66] | 30 (63) [48, 75] | 0.14 |
| Mean AUC normalized by day (log10 TCID · day/ml) (SD) | 1.1 (0.5) | 1.6 (0.8) | <0.001 |
| Mean daily total symptom score/day (SD) | 2.2 (2.4) | 3.3 (2.5) | 0.01 |
| Mean daily respiratory symptom score/daye (SD) | 1.9 (1.7) | 2.8 (2.0) | 0.007 |
| Mean cumulative nasal discharge weight (g) (days 0-4) (SD) | 11.1 (14.6) | 18.5 (18.1) | 0.01 |
95% confidence intervals (95% CI) are shown as percentages in brackets.
Defined as at least a fourfold rise in antibody titer to the challenge virus.
Subjects with positive viral culture and/or antibody response.
Number of colds in those infected, defined by modified Jackson criteria.
Includes sneezing, rhinorrhea, sore throat, nasal obstruction or congestion, and cough. P values comparing values for ruprintrivir-treated subjects to placebo-treated subjects. All P values are one sided; a one-sided P value of <0.05 was considered to be statistically significant. NA, not available.
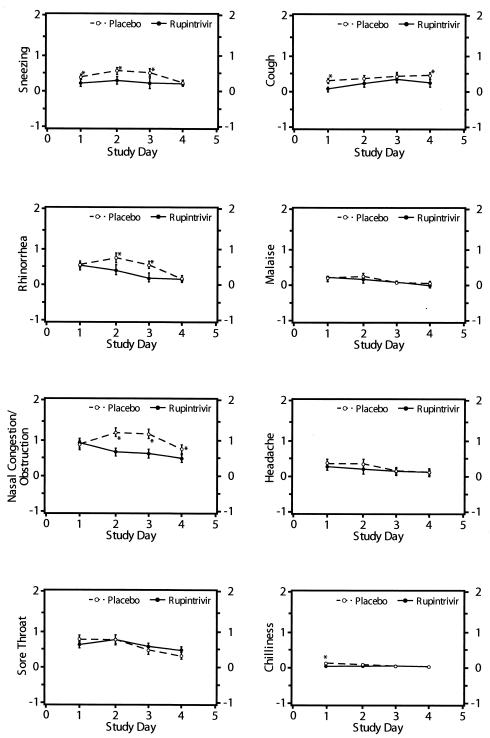
In these subjects, the frequency of illness was not reduced by ruprintrivir. However, the severity of illness was significantly lower in ruprintrivir recipients than in those receiving placebo. The mean daily total symptom score over the 4 treatment days was 33% lower in ruprintrivir-treated subjects than that of placebo-treated subjects (2.2 versus 3.3, respectively [P = 0.01]). Similarly, total symptom scores by day of treatment were lower in ruprintrivir-treated subjects on days 2 and 3 (P < 0.05) (Fig. 3). Significantly lower scores for the individual symptoms of sneezing, rhinorrhea, and nasal obstruction or congestion were observed in ruprintrivir-treated subjects compared with placebo-treated subjects on days 1 to 3, 2 or 3, and 2 to 4, respectively (Fig. 5). In addition, ruprintrivir treatment significantly lowered nasal discharge weights during days 2 or 3, 3 or 4, and 4 or 5 (Fig. 4), and cumulative nasal discharge weights measured over the 4 days of treatment were 40% lower (Table 3). Subjects receiving ruprintrivir also demonstrated significantly lower viral titers and RNA levels than placebo-treated subjects on days 2, 3, and 5 and on days 2 and 3, respectively (Fig. 1 and 2).
FIG. 5.
Mean scores for individual symptoms (sneezing, cough, rhinorrhea, malaise, nasal congestion or obstruction, headache, sore throat, and chilliness) over time for subjects in whom efficacy could be evaluated (5×/day treatment group). Symptom scores before challenge were 0 for all subjects, except for one subject who had a baseline rhinorrhea score of 1. Values are given as means ± standard errors (error bars) by the least-square method. Values for the ruprintrivir-treated subjects that were significantly different (one-sided P values of <0.05) from the values for the placebo-treated subjects by ANCOVA with effects for treatment, study site, and challenge virus strain adjusted for baseline values are indicated by asterisks.
Tolerability.
There were no serious adverse effects reported, and there were not any withdrawals due to the development of adverse effects. Overall, 58 of 202 evaluable subjects experienced treatment-emergent adverse effects. Of these 58 subjects, 19 reported local effects, including blood-tinged mucus (n = 11), nasal passage irritation (n = 3), sinus pain (n = 3), postnasal drip (n = 2), and nasal dryness (n = 1). (Note that a subject could report more than one adverse effect.) Local nasal effects were more common in subjects receiving 5×/day dosing (prophylaxis, 19%; treatment, 8%) than in subjects receiving 2×/day prophylaxis (2%). Local nasal effects were slightly more common in ruprintrivir-treated subjects than placebo-treated subjects (11% versus 8%).
Adverse events reported by ≥5% of subjects are summarized by study (prophylaxis or treatment study) in Table 4. In the 5×/day prophylaxis study, adverse events included blood-tinged mucus (11% for placebo-treated subjects versus 16% for ruprintrivir-treated subjects) and nasal passage irritation (7% for placebo-treated subjects versus 4% for ruprintrivir-treated subjects). In the 2×/day prophylaxis study, no individual adverse event was reported by >5% of subjects receiving either ruprintrivir or placebo. In the 5×/day treatment study, adverse events occurring in >5% of subjects included ear disorder (4% for placebo-treated subjects versus 8% for ruprintrivir-treated subjects), blood-tinged mucus (2% for placebo-treated subjects versus 6% for ruprintrivir-treated subjects), and nausea (2% for placebo-treated subjects versus 6% for ruprintrivir-treated subjects). Ear disorder was defined as stuffy, clogged, or popping ears and was considered related to HRV infection.
TABLE 4.
Adverse effects reported in ≥5% of subjects receiving ruprintrivir or placebo for experimental rhinovirus infection
| Adverse effect | No. (%) of subjects in treatment group
|
|||||
|---|---|---|---|---|---|---|
| 5×/day prophylaxis
|
2×/day prophylaxis
|
5×/day treatment
|
||||
| Placebo (n = 27) | Ruprintrivir (n = 25) | Placebo (n = 25) | Ruprintrivir (n = 26) | Placebo (n = 49) | Ruprintrivir (n = 50) | |
| Subjects with adverse eventsa | 10 (37) | 10 (40) | 5 (20) | 4 (15) | 12 (24) | 17 (34) |
| Blood-tinged mucus | 3 (11) | 4 (16) | 0 | 0 | 1 (2) | 3 (6) |
| Nasal passage irritation | 2 (7) | 1 (4) | 0 | 0 | 0 | 0 |
| Ear disorderb | 0 | 0 | 0 | 0 | 2 (4) | 4 (8) |
| Nausea | 1 (4) | 0 | 0 | 0 | 1 (2) | 3 (6) |
Subjects who experienced more than one adverse event were counted once.
Stuffy, clogged, or popping ears.
The only clinically relevant changes in physical examination were tonsillar exudate and cervical adenopathy in one placebo-treated subject on day 4 of the study. No clinically significant differences were observed between ruprintrivir- and placebo-treated subjects regarding vital signs, day 5 nasal examination, hematology, and clinical chemistry laboratory evaluations, and urinalysis findings (data not shown). Urinalysis detected 16 subjects (6 ruprintrivir-treated and 10 placebo-treated subjects) with trace proteinuria or with a score of 1+ for proteinuria on day 5; this resolved in all four cases for which a repeat urinalysis was performed.
Pharmacokinetics.
The concentrations of ruprintrivir and its metabolite AG7185 in plasma were determined on day 3 of dosing. Nonmeasurable plasma ruprintrivir concentrations were observed in 63% of ruprintrivir-treated subjects; in the remainder, ruprintrivir concentrations were low (≤0.92 ng/ml). Plasma ruprintrivir AUC values ranged from 0.12 to 3.04, 0 to 1.41, and 0.11 to 1.66 ng · h/ml for 5×/day prophylaxis, 2×/day prophylaxis, and 5×/day treatment groups, respectively, confirming the minimal systemic exposure associated with multiple doses of ruprintrivir. Measurable concentrations of AG7185 were observed in all ruprintrivir-treated subjects; all AG7185 concentrations were ≤12.18 ng/ml.
To determine ruprintrivir residence in the nasal cavity following intranasal administration, nasal washes were collected from a subset of subjects on day 2 of dosing prior to the fourth dose (5×/day dosing) or prior to and 6 h following the first dose (2×/day dosing). Prior to the fourth dose, the amount of ruprintrivir recovered from the nasal washings varied substantially, ranging from 2.6 to 59.8 μg in the 5×/day prophylaxis group and from 0.005 to 62.7 μg in the 5×/day treatment group. In the 2×/day prophylaxis group, the amount of ruprintrivir recovered also varied, with 0 to 1.05 μg predose to 0 to 26.2 μg at 6 h after dosing. Drug concentrations in nasal washings were not corrected for dilution factors.
DISCUSSION
In this series of proof-of-principle studies, we have shown that intranasal administration of the novel rhinovirus 3C protease inhibitor ruprintrivir was well tolerated and provided significant antiviral effects. Specifically, ruprintrivir was effective in reducing the incidence of positive viral culture and measures of quantitative viral replication when used prophylactically or therapeutically and in attenuating symptom severity when used for early treatment. Although none of the studies showed significant reductions in the frequency of clinical colds, virologic and clinical secondary endpoints demonstrated trends favoring ruprintrivir over placebo administration. While the prophylaxis studies did not have the power to detect differences in secondary endpoints, data supporting the antiviral efficacy of ruprintrivir were observed, particularly reductions in viral titers over time, RNA levels, and daily nasal discharge weights.
The possibilities that reductions in viral titer observed for ruprintrivir-treated subjects might have been influenced by the inhibitory effect of residual ruprintrivir in the nasal wash (i.e., drug carryover) and that ruprintrivir may have interfered with viral replication, leading to false-negative viral cultures, cannot be excluded. Because this 3C protease inhibitor does not affect early events (e.g., attachment and penetration) but rather blocks a late step in replication, removal of drug from inoculated monolayers by washing after the adsorption period should be an effective means of avoiding carryover effects. Indeed, the correspondence between viral RNA levels (whose quantification is not sensitive to ruprintrivir) and viral titers indicate that drug carryover was not a confounding factor in the current studies.
As in previous studies, intranasal ruprintrivir was well tolerated. Study drug-related adverse events were generally mild in severity and local in nature (blood-tinged mucus and nasal irritation). Subjects who received the study drug 2×/day rather than 5×/day experienced fewer study drug-related adverse events, including blood-tinged mucus and nasal passage irritation. This observation is consistent with the conclusion that mucosal irritation related to repetitive nasal spray administration, rather than direct drug effect, was the likely cause for the adverse events noted.
Following intranasal administration of ruprintrivir, low concentrations of ruprintrivir and its metabolite AG7185 in plasma were observed, a finding that suggests that systemic exposure is minimal following multiple doses of drug and is consistent with the lack of systemic adverse events observed. Plasma AG7185 concentrations were higher than those observed in phase I studies; this result may be attributed to the differences in populations studied. In phase I studies, healthy volunteers had normal mucosa, whereas rhinovirus-infected volunteers in the present studies might have had some degree of loss of mucosal integrity due to infectious rhinitis. Significant amounts of ruprintrivir were recovered from the nose on day 2 of study in all dosing groups. While this is of particular interest in subjects given only two doses a day, the relationship between detectable drug levels in nasal washings and antiviral effect remains unclear. It is possible that alternative formulations that enhance delivery of ruprintrivir to the nasal epithelium or promote prolonged drug retention at higher levels may provide greater antiviral effects.
It is instructive to consider the results of these studies in the context of earlier trials conducted with intranasal antirhinovirus compounds having different mechanisms of antiviral action. The magnitude of the clinical effects observed in this study of early treatment (24 h postinoculation) is broadly comparable to that seen with intranasal administration of tremacamra, a soluble ICAM-1 receptor decoy, initiated at 4 h before or 12 h after infection and greater than those observed with administration of intranasal alpha interferon 2b or the capsid-binding agent pirodavir at 24 h after inoculation (9, 11, 22). Early intranasal tremacamra spray administration reduced cold frequency, symptom scores, and nasal mucus weights by 23, 45, and 56%, respectively, compared to treatment with placebo, and treatment with interferon drops reduced the values by 2, 20, and 52%, respectively; pirodavir did not affect these endpoints. Of note, neither alpha interferon 2b nor pirodavir alone was found to be therapeutically effective in treating natural rhinovirus colds, and it is likely that combinations of antiviral agents and antimediator drugs will be needed to provide maximal therapeutic benefit (8, 12, 13). With respect to prophylactic activity, intranasal administration of various interferons and pirodavir (6×/day, but not 3×/day) has been shown to reduce the frequency of experimental colds, and intranasal alpha interferon 2b reduces the risk of natural rhinovirus illness (1, 9). In contrast, intranasal ruprintrivir exerted significant antiviral effects but did not diminish the frequency of experimental colds. This finding is unexpected, and the explanation for it is unclear.
Ruprintrivir is formulated as a suspension intended for intranasal delivery directly to the site of viral replication and represents the first protease inhibitor to be evaluated clinically for in vivo activity against HRV infection. The broad antipicornavirus spectrum of ruprintrivir, combined with its antiviral activity and safety in these studies of experimental rhinovirus infection, indicate that it warrants further testing for the management of natural rhinovirus infections. To effectively inhibit the inflammatory cascade of the common cold, ruprintrivir treatment will need to be initiated rapidly after cold symptoms are recognized. This may be the greatest challenge for clinicians in the implementation of treatment with ruprintrivir or other antirhinoviral agents.
Acknowledgments
Funding for this research was provided by Agouron Pharmaceuticals, Inc., a Pfizer Company. F. G. Hayden serves as a paid consultant to Agouron/Pfizer.
We thank the volunteers who participated in these studies, the study staff, Mark Denzel of Agouron for study management support, and Annkatrin Petersen of Agouron for critical review of the manuscript.
REFERENCES
- 1.Arruda, E., and F. G. Hayden. 1995. Clinical studies of antiviral agents for picornaviral infections, p. 321-356. In D. J. Jeffries and E. DeClercq (ed.), Antiviral chemotherapy. John Wiley & Sons, Inc., New York, N.Y.
- 2.Arruda, E., A. Pitkaranta, T. J. Witek, Jr., C. A. Doyle, and F. G. Hayden. 1997. Frequency and natural history of rhinovirus infections in adults during autumn. J. Clin. Microbiol. 35:2864-2868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Couch, R. B., and A. L. Atmar. 1999. Rhinoviruses, p. 787-802. In E. H. Lennette (ed.), Laboratory diagnosis of viral infections. Marcel Dekker, New York, N.Y.
- 4.Dragovich, P. S., T. J. Prins, R. Zhou, S. E. Webber, J. T. Marakovits, S. A. Fuhrman, A. K. Patick, D. A. Matthews, C. A. Lee, C. E. Ford, B. J. Burke, P. A. Rejto, T. F. Hendrickson, T. Tuntland, E. L. Brown, J. W. Meador III, R. A. Ferre, J. E. Harr, M. B. Kosa, and S. T. Worland. 1999. Structure-based design, synthesis and biological evaluation of irreversible human rhinovirus 3C protease inhibitors. 4. Incorporation of P1 lactam moieties as L-glutamine replacements. J. Med. Chem. 42:1213-1224. [DOI] [PubMed] [Google Scholar]
- 5.Gwaltney, J. M., Jr., R. J. Colonno, V. V. Hamparian, and R. B. Turner. 1989. Rhinoviruses, p. 579-614. In N. J. Schmidt and R. W. Emmons (ed.), Diagnostic procedures for viral, rickettsial, and chlamydial infections, 6th ed. American Public Health Association, Washington, D.C.
- 6.Gwaltney, J. M., Jr., P. B. Moskalski, and J. O. Hendley. 1980. Interruption of experimental rhinovirus transmission. J. Infect. Dis. 142:811-815. [DOI] [PubMed] [Google Scholar]
- 7.Gwaltney, J. M., Jr., and R. R. Rueckert. 1997. Rhinovirus, p. 1025-1047. In D. D. Richman, R. J. Whitley, and F. G. Hayden (ed.), Clinical virology. Churchill Livingstone, New York, N.Y.
- 8.Gwaltney, J. M., Jr., B. Winther, J. T. Patrie, and J. O. Hendley. 2002. Combined antiviral-antimediator treatment for the common cold. J. Infect. Dis. 186:147-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hayden, F. G., K. Andries, and P. A. Janssen. 1992. Safety and efficacy of intranasal pirodavir (R77975) in experimental rhinovirus infection. Antimicrob. Agents Chemother. 36:727-732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hayden, F. G., T. Coats, K. Kim, H. A. Hassman, M. M. Blatter, B. Zhang, and S. Liu. 2002. Oral pleconaril treatment of picornavirus-associated viral respiratory illness in adults: efficacy and tolerability in phase II clinical trials. Antiviral Ther. 7:53-65. [PubMed] [Google Scholar]
- 10a.Hayden, F. G., D. T. Herrington, T. L. Coats, K. Kim, E. C. Cooper, S. A. Villano, S. Liu, S. Hudson, D. C. Pevear, M. Collett, M. McKinlay, and the Pleconaril Respiratory Infection Study Group. 2003. Efficacy and safety of oral pleconaril for treatment of colds due to picornaviruses in adults: results of 2 double-blind, randomized, placebo-controlled trials. Clin. Infect. Dis. 36:1523-1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hayden, F. G., and J. M. Gwaltney, Jr. 1984. Intranasal interferon-alpha 2 treatment of experimental rhinoviral colds. J. Infect. Dis. 150:174-180. [DOI] [PubMed] [Google Scholar]
- 12.Hayden, F. G., G. J. Hipskind, D. H. Woerner, G. F. Eisen, M. Janssens, P. A. Janssen, and K. Andries. 1995. Intranasal pirodavir (R77,975) treatment of rhinovirus colds. Antimicrob. Agents Chemother. 39:290-294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hayden, F. G., D. L. Kaiser, and J. K. Albrecht. 1988. Intranasal recombinant alfa-2b interferon treatment of naturally occurring common colds. Antimicrob. Agents Chemother. 32:224-230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hsyu, P. H., Y. K. Pathavala, M. Gersten, C. A. Penning, and B. M. Kerr. 2002. Pharmacokinetics and safety of an antiviral agent, ruprintrivir, in healthy volunteers. Antimicrob. Agents Chemother. 46:392-397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Johnston, S. L., P. K. Pattemore, G. Sanderson, S. Smith, F. Lampe, L. Josephs, P. Symington, S. O'Toole, S. H. Myint, D. A. J. Tyrrell, and S. T. Holgate. 1995. Community study of viral infections in exacerbations of asthma in 9-11 year old children. Br. Med. J. 310:1225-1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Makela, M. J., T. Puhakka, O. Ruuskanen, M. Leinonen, P. Saikku, M. Kimpimaki, S. Blomqvist, T. Hyypia, and P. Arstila. 1998. Viruses and bacteria in the etiology of the common cold. J. Clin. Microbiol. 36:539-542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matthews, D. A., W. W. Smith, R. A. Ferre, B. Condon, G. Budahazi, W. Sisson, J. E. Villafranca, C. A. Janson, H. E. McElroy, C. L. Gribskov, and S. Worland. 1994. Structure of human rhinovirus 3C protease reveals a trypsin-like polypeptide fold, RNA-binding site, and means for cleaving precursor polyprotein. Cell 77:761-771. [DOI] [PubMed] [Google Scholar]
- 18.Patick, A. K., S. L. Binford, M. A. Brothers, R. L. Jackson, C. E. Ford, M. D. Diem, F. Maldonado, P. S. Dragovich, R. Zhou, T. J. Prins, S. A. Fuhrman, J. W. Meador, L. S. Zalman, D. A. Matthews, and S. T. Worland. 1999. In vitro antiviral activity of AG7088, a potent inhibitor of human rhinovirus 3C protease. Antimicrob. Agents Chemother. 43:2444-2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Patick, A. K., and K. E. Potts. 1998. Protease inhibitors as antiviral agents. Clin. Microbiol. Rev. 11:614-627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pitkaranta, A., E. Arruda, H. Malmberg, and F. G. Hayden. 1997. Detection of rhinovirus in sinus brushings of patients with acute community-acquired sinusitis by reverse transcription-PCR. J. Clin. Microbiol. 35:1791-1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pitkaranta, A., A. Virolainen, J. Jero, E. Arruda, and F. G. Hayden. 1998. Detection of rhinovirus, respiratory syncytial virus, and coronavirus infections in acute otitis media by reverse transcriptase polymerase chain reaction. Pediatrics 102:291-295. [DOI] [PubMed] [Google Scholar]
- 22.Turner, R. B., M. T. Wecker, G. Pohl, T. J. Witek, E. McNally, R. St. George, B. Winther, and F. G. Hayden. 1999. Efficacy of tremacamra, a soluble intercellular adhesion molecule 1, for experimental rhinovirus infection: a randomized clinical trial. JAMA 281:1797-1804. [DOI] [PubMed] [Google Scholar]
- 23.Turner, R. B., K. W. Weingand, C. H. Yeh, and D. W. Leedy. 1998. Association between interleukin-8 concentration in nasal secretions and severity of symptoms of experimental rhinovirus colds. Clin. Infect. Dis. 26:840-846. [DOI] [PubMed] [Google Scholar]
- 24.Wedzicha, J. A. 2002. Exacerbations: etiology and pathophysiologic mechanisms. Chest 121:136S-141S. [DOI] [PMC free article] [PubMed]
- 25.Zalman, L. S., M. A. Brothers, P. S. Dragovich, R. Zhou, T. J. Prins, S. T. Worland, and A. K. Patick. 2000. Inhibition of human rhinovirus-induced cytokine production by AG7088, a human rhinovirus 3C protease inhibitor. Antimicrob. Agents Chemother. 44:1236-1241. [DOI] [PMC free article] [PubMed] [Google Scholar]