Abstract
We have evaluated the efficacy of voriconazole (VRC) in a murine model of systemic infection by Scedosporium apiospermum. The survival of mice treated with VRC at 5, 20, or 40 mg/kg/day was greater than that of the control group (P ≤ 0.0009). VRC reduced the tissue burden in the spleen and brain (P < 0.001 in both organs) in comparison with that of the control group.
Scedosporium apiospermum is an opportunistic filamentous fungus that causes severe infections, not only in immunodepressed patients but also in the immunocompetent. These infections are generally treated with amphotericin B (AMB) alone or in combination with other antifungal drugs, but outcomes are often unsuccessful. In vitro studies have revealed that this fungus is resistant to the available antifungal drugs, and voriconazole (VRC) is one of the few drugs that have shown some in vitro activity against this fungus (3, 4, 11). Further studies in appropriate animal models are needed to confirm its in vivo activity. However, concentrations of VRC in the serum of mice are very low, at times undetectable because of the rapid clearance of the drug. Some authors have demonstrated the inhibitory effect of grapefruit juice on the cytochrome P450 enzymes that are involved in the metabolism of VRC (5), which can be useful for increasing the concentrations of such drugs in murine serum (15). In the present study, we have evaluated the efficacy of VRC in a systemic S. apiospermum infection of immunodepressed mice drinking grapefruit juice instead of water.
The clinical strain S. apiospermum FMR 6694 was used in this study. The fungus was stored in slant cultures covered with sterile paraffin oil and subcultured on potato dextrose agar plates at 35°C for 7 days. In a previous in vitro study, the MIC of VRC against this strain was 1 μg/ml (data not shown). Preparation of the inoculum was done as previously described (14). Male OF-1 mice weighing 30 g (Charles River, Barcelona, Spain) were used in this study. Groups of 10 animals were housed under standard conditions, with drink and feed supplied ad libitum. Conditions were approved by the Animal Welfare Committee of the Rovira i Virgili University. Animals were immunodepressed by intraperitoneal (i.p.) administration of a single dose of 200 mg of cyclophosphamide (Genoxal, Laboratorios Funk, Barcelona, Spain) per kg plus intravenous (i.v.) administration of 150 mg of 5-fluorouracil (Productos Roche, Madrid, Spain) per kg 1 day prior to infection. VRC was provided by Pfizer (Madrid, Spain). Stock solutions of this drug were prepared in polyethylene glycol 200 (PEG 200). AMB was purchased from Squibb Industria Farmacéutica and reconstituted in accordance with the manufacturer's instructions.
Groups of 10 animals were infected with an inoculum of 104 CFU given i.v. via the lateral tail vein. Mice treated with VRC received the drug at 5, 20, or 40 mg/kg/day perorally (p.o.) by gavage; these doses were tested twice. AMB deoxycholate was administered at 0.8 mg/kg of body weight per day i.v. or at 1.5 mg/kg of body weight per day i.p. The control group received PEG 200 p.o. All treatments were begun 1 day after challenge and administered daily for 10 days. Animals were checked daily for 14 days. Three days prior to infection, the mice that received VRC or PEG 200 were given grapefruit juice (Hero España, Murcia, Spain) in place of water.
In the fungal-burden study, groups of 10 animals were infected with an inoculum of 3.4 × 103 CFU i.v. The control group received PEG 200, and the treatment groups received AMB i.v. at 0.8 mg/kg/day and VRC p.o. at 5, 20, and 40 mg/kg/day, respectively. All treatments were administered daily for 10 days, and approximately 24 h after administration of the last dose, the surviving animals were sacrificed by inhalation of halothane. The brain, kidneys, and spleen were removed aseptically, weighed, and homogenized in 2 ml of 0.9% saline. Serial 10-fold dilutions of these homogenates were placed on potato dextrose agar plates and incubated at 35°C. After 72 to 96 h, the number of CFU was determined. Survival rates were evaluated by the Kaplan-Meyer test, and organ burdens were compared by the Mann-Whitney U test with GraphPad Prism software.
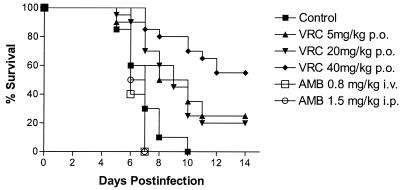
Figure 1 shows the survival curves of the different groups of mice included in the survival study. Untreated animals began to die on day 5 postinfection, and on day 10, no animals were alive. The mean survival time (MST) of the control group was 6.90 days. Similar mortality rates were found in animals treated with AMB i.p. at 1.5 mg/kg (MST = 6.50 days) and with AMB i.v. at 0.8 mg/kg (MST = 6.40 days).
FIG. 1.
Survival curves of male immunodepressed OF-1 mice infected i.v. with S. apiospermum and left untreated, given PEG 200, or treated with AMB at a dose of 0.8 mg/kg i.v. or 1.5 mg/kg i.p. or with VRC at 5, 20, or 40 mg/kg p.o. Treatments were started on day 1 postinfection and continued for 10 days.
The treatments with VRC at 5, 20, and 40 mg/kg/day were tested twice. Data from the two experiments were not statistically significantly different and are grouped in a single curve (Fig. 1). Survival of animals treated with VRC increased significantly, with MSTs of 9.2, 9.45, and 11.9 days with doses of 5, 20, and 40 mg/kg/day, respectively (VRC at 5 mg/kg/day versus the control, P < 0.0001; VRC at 5 mg/kg/day versus both AMB treatments, P ≤ 0.0187; VRC at 20 mg/kg/day versus the control and AMB treatments, P ≤ 0.0009; VRC at 40 mg/kg/day compared to the control and to both AMB treatments, P < 0.0001). VRC at 40 mg/kg was more effective at prolonging survival than both VRC at 5 mg/kg/day (P = 0.018) and VRC at 20 mg/kg/day (P = 0.009). No significant differences were found between the group that received VRC at 5 mg/kg and that which received VRC at 20 mg/kg (P = 0.95). The most-affected organs in untreated animals were the brain and kidneys. In the spleen, CFU counts were a bit lower. AMB did not reduce the tissue burden in any of the organs studied (Table 1). VRC at 5, 20, or 40 mg/kg/day significantly reduced tissue burden in the brain in comparison to both the control mice (P < 0.02 in all cases) and the AMB-treated mice (P < 0.009 in all cases). The tissue burden in the kidneys was reduced somewhat in animals treated with VRC at any dose, but the differences from the control group were only significant in the animals receiving VRC at 20 mg/kg. In the spleens of animals treated with VRC at any dose, CFU counts were significantly lower than in those untreated or treated with AMB (P < 0.05).
TABLE 1.
Semiquantitative results of organ cultures of mice treated with antifungal therapy begun 24 h after challenge and sacrificed 24 h after completion of therapy
| Treatment (no. of animals) | Mean log10 no. of CFU/g (CI)a
|
||
|---|---|---|---|
| Kidneys | Spleen | Brain | |
| Control (9) | 4.49 (4.24-4.73) | 4.64 (4.30-5.04) | 5.55 (5.29-5.80) |
| AMB, 0.8 mg/kg (5) | 5.14 (4.53-5.73) | 4.82 (4.49-5.13) | 5.68 (5.40-5.97) |
| VRC, 5 mg/kg (4) | 4.43 (3.71-5.14) | 4.27b,c (3.67-4.87) | 4.91b,c (3.54-6.27) |
| VRC, 20 mg/kg (5) | 3.92b,c (3.65-4.18) | 4.25b,c (3.88-4.61) | 4.65b,c (4.09-5.20) |
| VRC, 40 mg/kg (9) | 3.39 (0.87-5.91) | 3.83b,c (3.12-4.54) | 4.25b,c (3.77-4.74) |
CI, confidence interval.
P < 0.05 versus AMB-treated group.
P < 0.05 versus control group.
In this study, we have used a murine model of systemic S. apiospermum infection previously developed by us but with a different lethal dose (2, 14) to test the efficacy of VRC. González et al. (7) recently described a similar model, although they used a less virulent strain because, even though the inoculum was the same, only 25% of the mice died after 25 days. The differences in mortality could be due to the different immunosuppression regimens used in the two studies. We used cyclophosphamide plus 5-fluorouracil, and González et al. (7) used cyclophosphamide alone. In previous studies, we demonstrated some variability in the virulence of different S. apiospermum strains (14), which could be another reason for the mentioned differences between the models. The ability of grapefruit juice to improve the pharmacological availability of different drugs in mice has been extensively documented (5, 15). Some authors (10) have reported that the effect of grapefruit juice is merely due to an increase in the intestinal absorption of VRC. Although the effect of grapefruit juice remains controversial, this pharmacokinetic interaction proved to be useful in our case and previously in resolving experimental blastomycosis in mice (16). In a previous study, we demonstrated that grapefruit juice did not exert any in vitro or in vivo inhibitory effect against this fungus (data not shown). The increase in survival was markedly evident in animals that received VRC at 40 mg/kg, half of which survived after 14 days. VRC administered at lower doses had a moderate but significant effect in increasing survival. In experimental fusariosis in mice receiving grapefruit juice by gavage, other authors (8) have previously observed a partial response due to VRC at doses lower than 40 mg/kg/day. In immunocompromised patients, S. apiospermum usually presents tropism for the central nervous system (CNS), causing primary brain abscesses that are usually fatal (9, 13). In approximately half of the reported cases, no site of S. apiospermum infection other than the CNS was found (1). Although correlations between results obtained with animal models and the course of infections in humans is not always good, this study and others (2, 7, 14) confirm this CNS tropism. In the present model, only animals receiving VRC were able to reduce the fungal load in the brain. Although few data still exist, clinical findings on the role of VRC in human scedosporiosis are very positive and seem to confirm experimental studies. In recent years, several human cases of disseminated infection by this fungus have been treated with VRC with very favorable outcomes (6, 12, 13, 17).
On the basis of our results and those obtained by other authors, VRC seems to be an option for the treatment of severe scedosporiosis.
Acknowledgments
This work was supported by a grant from the Fondo de Investigaciones Sanitarias from the Ministerio de Sanidad y Consumo of Spain (PI 020114).
REFERENCES
- 1.Berenguer, J., J. Diaz-Mediavilla, D. Urra, and P. Muñoz. 1989. Central nervous system infection caused by Pseudallescheria boydii. Rev. Infect. Dis. 11:890-896. [DOI] [PubMed] [Google Scholar]
- 2.Cano, J., J. Guarro, E. Mayayo, and J. Fernández-Ballart. 1992. Experimental infection with Scedosporium inflatum. J. Med. Vet. Mycol. 30:413-420. [PubMed] [Google Scholar]
- 3.Carrillo, A. J., and J. Guarro. 2001. In vitro activities of four novel triazoles against Scedosporium species. Antimicrob. Agents Chemother. 45:2151-2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Espinel-Ingroff, A., K. Boyle, and D. J. Sheehan. 2001. In vitro antifungal activities of voriconazole and reference agents as determined by NCCLS methods: review of the literature. Mycopathologia 150:101-115. [DOI] [PubMed] [Google Scholar]
- 5.Fuhr, U. 1998. Drug interactions with grapefruit juice: extent, probable mechanism and clinical relevance. Drug Safety 18:251-272. [DOI] [PubMed] [Google Scholar]
- 6.Girmenia, C., G. Luzi, M. Monaco, and P. Martino. 1998. Use of voriconazole in treatment of Scedosporium apiospermum infection: case report. J. Clin. Microbiol. 36:1436-1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.González, G. M., R. Tijerina, L. Najvar, M. Rinaldi, I. T. Yeh, and J. R. Graybill. 2002. Experimental murine model of disseminated Pseudallescheria infection. Med. Mycol. 40:243-248. [DOI] [PubMed] [Google Scholar]
- 8.Graybill, J. R., L. K. Najvar, G. M. Gonzalez, S. Hernandez, and R. Bocanegra. 2003. Improving the mouse model for studying the efficacy of voriconazole. J. Antimicrob. Chemother. 51:1373-1376. [DOI] [PubMed] [Google Scholar]
- 9.Horré, R., and G. S. de Hoog. 1998. Primary cerebral infections by melanized fungi: a review. Stud. Mycol. 43:176-193. [Google Scholar]
- 10.MacCallum, D. M., and F. C. Odds. 2002. Influence of grapefruit juice on itraconazole plasma levels in mice and guinea pigs. J. Antimicrob. Chemother. 50:219-224. [DOI] [PubMed] [Google Scholar]
- 11.Meletiadis, J., J. F. Meis, J. W. Mouton, J. L. Rodríguez-Tudela, J. P. Donnelly, and P. E. Verweij. 2002. In vitro activities of new and conventional antifungal agents against clinical Scedosporium isolates. Antimicrob. Agents Chemother. 42:62-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Muñoz, P., M. Marín, P. Tornero, P. Martín Rabadán, M. Rodríguez-Creixéms, and E. Bouza. 2000. Successful outcome of Scedosporium apiospermum disseminated infection treated with voriconazole in a patient receiving corticosteroid therapy. Clin. Infect. Dis. 31:1499-1501. [DOI] [PubMed] [Google Scholar]
- 13.Nesky, M. A., and E. C. McDougal. 2000. Pseudallescheria boydii brain abscess successfully treated with voriconazole and surgical drainage: case report and literature review of central nervous system pseudallescheriasis. Clin. Infect. Dis. 31:673-677. [DOI] [PubMed] [Google Scholar]
- 14.Ortoneda, M., F. J. Pastor, E. Mayayo, and J. Guarro. 2002. Comparison of the virulence of Scedosporium prolificans strains from different origins in a murine model. J. Med. Microbiol. 51:924-928. [DOI] [PubMed] [Google Scholar]
- 15.Sugar, A. M., and X.-P. Liu. 2000. Effect of grapefruit juice on serum voriconazole concentrations in the mouse. Med. Mycol. 38:209-212. [DOI] [PubMed] [Google Scholar]
- 16.Sugar, A. M., and X.-P. Liu. 2000. Efficacy of voriconazole in treatment of murine pulmonary blastomycosis. Antimicrob. Agents Chemother. 45:601-604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Walsh, T. J., I. Lutsar, T. Driscoll, B. Dupont, M. Roden, P. Ghahramani, M. Hodges, A. H. Groll, and J. R. Perfect. 2002. Voriconazole in the treatment of aspergillosis, scedosporiosis and other invasive fungal infections in children. Pediatr. Infect. Dis. 21:240-248. [DOI] [PubMed] [Google Scholar]