Abstract
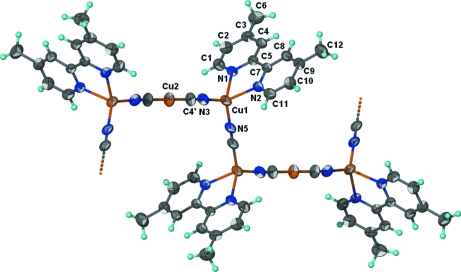
In the title compound, [Cu3(CN)3(C12H12N2)2], two 2,2′-bipyridine N,N′-chelated CuI atoms are linked by a cyanide bridge that lies about a center of inversion; the CuI atom exists in a tetrahedral coordination geometry. This dinuclear entity is linked to another CuI atom that lies on a twofold rotation axis by another cyanide bridge, these bridges giving rise to the formation of a linear chain motif.
Related literature
Some copper(I) cyanide adducts with 2,2′-bipyridine-like ligands that adopt chain structures in which the cyanide group functions as a bridge are triscyano-bis(2,2′-biquinoline)tricopper (Chesnut et al., 2001 ▶; Dessy et al., 1985 ▶), tetrakiscyano(2,2′-biquinoline)tetracopper (Chesnut & Zubieta, 1998 ▶) and biscyano-(4,4′-diphenyl-2,2′-bipyridine)dicopper (Chesnut et al., 2001 ▶).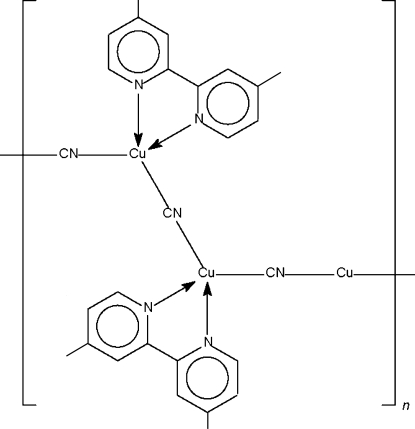
Experimental
Crystal data
[Cu3(CN)3(C12H12N2)2]
M r = 637.15
Monoclinic,

a = 10.7196 (7) Å
b = 12.3700 (9) Å
c = 20.9182 (14) Å
β = 100.146 (1)°
V = 2730.4 (3) Å3
Z = 4
Mo Kα radiation
μ = 2.34 mm−1
T = 295 (2) K
0.30 × 0.20 × 0.16 mm
Data collection
Bruker SMART APEX diffractometer
Absorption correction: multi-scan (SADABS; Sheldrick, 1996 ▶) T min = 0.540, T max = 0.705
8685 measured reflections
3125 independent reflections
2491 reflections with I > 2σ(I)
R int = 0.024
Refinement
R[F 2 > 2σ(F 2)] = 0.038
wR(F 2) = 0.110
S = 1.03
3125 reflections
170 parameters
H-atom parameters constrained
Δρmax = 0.53 e Å−3
Δρmin = −0.21 e Å−3
Data collection: SMART (Bruker, 2002 ▶); cell refinement: SAINT (Bruker, 2002 ▶); data reduction: SAINT; program(s) used to solve structure: SHELXS97 (Sheldrick, 2008 ▶); program(s) used to refine structure: SHELXL97 (Sheldrick, 2008 ▶); molecular graphics: X-SEED (Barbour, 2001 ▶); software used to prepare material for publication: publCIF (Westrip, 2008 ▶).
Supplementary Material
Crystal structure: contains datablocks global, I. DOI: 10.1107/S1600536808022964/rk2103sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536808022964/rk2103Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Acknowledgments
The authors thank Shantou University and the University of Malaya for supporting this study.
supplementary crystallographic information
Comment
The cyanide group functions as a bridging group in a number of copper(I) adducts of 2,2'-bipyridine type of N-heterocycles. Those who crystal structure have been described include the triscyano-bis(2,2'-biquinoline)tricopper (Chesnut et al., 2001; Dessy et al., 1985), tetrakiscyano(2,2'-biquinoline)tetracopper (Chesnut & Zubieta, 1998) and biscyano-(4,4'-diphenyl-2,2'-bipyridine)dicopper (Chesnut et al., 2001).
The copper(I) cyanide adduct with 4,4'-dimethyl-2,2'-biyridine (Scheme 1, Fig. 1) adopts a similar chain motif. Two N-heterocycle-chelated copper(I) atoms are linked by a cyanide bridge that lies about a center-of-inversion; the copper(I) atom exists in a tetrahedral coordination geometry. This dinuclear entity is linked to copper(I) atom that lies on a twofold rotation axis by another cyanide bridge.
Experimental
4,4'-Dimethyl-2,2'-bipyridine (0.055 g, 0.3 mmol), cuprous cyanide (0.009 g, 0.1 mmol) and acetonitrile (8 ml) were placed in a 15-ml, teflon-lined autoclave. It was heated at 453 K for 72 hours, then was cooled to 333 K at a rate of 5 K per hour and then kept at this temperature for a further 10 hours before being cooled to room temperature. Red prisms were in 50% yield based on the N-heterocycle.
Refinement
The component atoms of the cyanide groups were each refined as a 50%:50% mixture of carbon and nitrogen. The pair of C/N atoms were restrained to the same site and also to have the same temperature factors. Hydrogen atoms were placed at calculated positions in the riding model approximation with C—H = 0.93–0.98 Å and Uiso(H) = 1.2–1.5Ueq(C).
Figures
Fig. 1.
Thermal ellipsoid plot (Barbour, 2001) of a portion of the linear chain motif; probability levels are set at 50%.
Crystal data
| [Cu3(CN)3(C12H12N2)2] | F000 = 1288 |
| Mr = 637.15 | Dx = 1.550 Mg m−3 |
| Monoclinic, C2/c | Mo Kα radiation λ = 0.71073 Å |
| Hall symbol: -C 2yc | Cell parameters from 2750 reflections |
| a = 10.7196 (7) Å | θ = 2.5–27.0º |
| b = 12.3700 (9) Å | µ = 2.34 mm−1 |
| c = 20.9182 (14) Å | T = 295 (2) K |
| β = 100.146 (1)º | Block, red |
| V = 2730.4 (3) Å3 | 0.30 × 0.20 × 0.16 mm |
| Z = 4 |
Data collection
| Bruker SMART APEX diffractometer | 3125 independent reflections |
| Radiation source: fine-focus sealed tube | 2491 reflections with I > 2σ(I) |
| Monochromator: graphite | Rint = 0.024 |
| T = 295(2) K | θmax = 27.5º |
| φ and ω scans | θmin = 2.5º |
| Absorption correction: multi-scan(SADABS; Sheldrick, 1996) | h = −13→13 |
| Tmin = 0.540, Tmax = 0.705 | k = −16→15 |
| 8685 measured reflections | l = −16→27 |
Refinement
| Refinement on F2 | Secondary atom site location: difference Fourier map |
| Least-squares matrix: full | Hydrogen site location: inferred from neighbouring sites |
| R[F2 > 2σ(F2)] = 0.038 | H-atom parameters constrained |
| wR(F2) = 0.110 | w = 1/[σ2(Fo2) + (0.065P)2 + 0.8323P] where P = (Fo2 + 2Fc2)/3 |
| S = 1.03 | (Δ/σ)max = 0.001 |
| 3125 reflections | Δρmax = 0.53 e Å−3 |
| 170 parameters | Δρmin = −0.21 e Å−3 |
| Primary atom site location: structure-invariant direct methods | Extinction correction: none |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | Occ. (<1) | |
| Cu1 | 0.74268 (3) | 0.41078 (2) | 0.072850 (16) | 0.04716 (14) | |
| Cu2 | 0.5000 | 0.41827 (4) | 0.2500 | 0.05469 (16) | |
| N1 | 0.75592 (19) | 0.54384 (16) | 0.01065 (10) | 0.0420 (5) | |
| N2 | 0.92273 (18) | 0.47469 (15) | 0.11124 (9) | 0.0406 (4) | |
| N3 | 0.6442 (3) | 0.41773 (18) | 0.14085 (14) | 0.0519 (6) | 0.50 |
| N4 | 0.5915 (2) | 0.4184 (2) | 0.18400 (12) | 0.0502 (6) | 0.50 |
| N5 | 0.7489 (2) | 0.28632 (17) | 0.01721 (11) | 0.0487 (6) | 0.50 |
| C3' | 0.6442 (3) | 0.41773 (18) | 0.14085 (14) | 0.0519 (6) | 0.50 |
| C4' | 0.5915 (2) | 0.4184 (2) | 0.18400 (12) | 0.0502 (6) | 0.50 |
| C5' | 0.7489 (2) | 0.28632 (17) | 0.01721 (11) | 0.0487 (6) | 0.50 |
| C1 | 0.6717 (3) | 0.5725 (2) | −0.04135 (14) | 0.0531 (6) | |
| H1 | 0.5932 | 0.5384 | −0.0485 | 0.064* | |
| C2 | 0.6951 (3) | 0.6501 (2) | −0.08494 (13) | 0.0562 (7) | |
| H2 | 0.6325 | 0.6680 | −0.1200 | 0.067* | |
| C3 | 0.8105 (3) | 0.7011 (2) | −0.07670 (12) | 0.0493 (6) | |
| C4 | 0.8992 (2) | 0.67121 (18) | −0.02273 (12) | 0.0450 (6) | |
| H4 | 0.9791 | 0.7030 | −0.0154 | 0.054* | |
| C5 | 0.8687 (2) | 0.59363 (16) | 0.02044 (12) | 0.0385 (5) | |
| C6 | 0.8409 (4) | 0.7858 (2) | −0.12307 (15) | 0.0699 (9) | |
| H6A | 0.8117 | 0.7623 | −0.1669 | 0.105* | |
| H6B | 0.9309 | 0.7970 | −0.1164 | 0.105* | |
| H6C | 0.7997 | 0.8523 | −0.1156 | 0.105* | |
| C7 | 0.9594 (2) | 0.56084 (18) | 0.07973 (11) | 0.0391 (5) | |
| C8 | 1.0699 (2) | 0.61388 (19) | 0.10154 (13) | 0.0461 (6) | |
| H8 | 1.0910 | 0.6739 | 0.0789 | 0.055* | |
| C9 | 1.1516 (3) | 0.5801 (2) | 0.15691 (14) | 0.0523 (6) | |
| C10 | 1.1131 (3) | 0.4918 (2) | 0.18924 (14) | 0.0559 (7) | |
| H10 | 1.1634 | 0.4661 | 0.2270 | 0.067* | |
| C11 | 1.0006 (3) | 0.4430 (2) | 0.16498 (13) | 0.0516 (6) | |
| H11 | 0.9766 | 0.3837 | 0.1873 | 0.062* | |
| C12 | 1.2752 (3) | 0.6366 (3) | 0.18039 (18) | 0.0781 (10) | |
| H12A | 1.3038 | 0.6203 | 0.2255 | 0.117* | |
| H12B | 1.2635 | 0.7133 | 0.1751 | 0.117* | |
| H12C | 1.3372 | 0.6125 | 0.1556 | 0.117* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| Cu1 | 0.0535 (2) | 0.0446 (2) | 0.0483 (2) | −0.01261 (12) | 0.02281 (16) | −0.00397 (12) |
| Cu2 | 0.0494 (3) | 0.0793 (4) | 0.0406 (3) | 0.000 | 0.0223 (2) | 0.000 |
| N1 | 0.0483 (11) | 0.0401 (10) | 0.0402 (12) | −0.0070 (8) | 0.0149 (9) | −0.0055 (8) |
| N2 | 0.0469 (10) | 0.0416 (10) | 0.0376 (11) | −0.0031 (8) | 0.0193 (9) | 0.0007 (8) |
| N3 | 0.0530 (13) | 0.0527 (14) | 0.0522 (15) | −0.0098 (10) | 0.0152 (12) | −0.0068 (10) |
| N4 | 0.0469 (13) | 0.0700 (16) | 0.0387 (13) | −0.0049 (10) | 0.0214 (11) | −0.0049 (10) |
| N5 | 0.0560 (13) | 0.0453 (12) | 0.0514 (15) | −0.0154 (10) | 0.0281 (11) | −0.0066 (9) |
| C3' | 0.0530 (13) | 0.0527 (14) | 0.0522 (15) | −0.0098 (10) | 0.0152 (12) | −0.0068 (10) |
| C4' | 0.0469 (13) | 0.0700 (16) | 0.0387 (13) | −0.0049 (10) | 0.0214 (11) | −0.0049 (10) |
| C5' | 0.0560 (13) | 0.0453 (12) | 0.0514 (15) | −0.0154 (10) | 0.0281 (11) | −0.0066 (9) |
| C1 | 0.0539 (15) | 0.0559 (15) | 0.0491 (16) | −0.0090 (12) | 0.0082 (13) | −0.0071 (12) |
| C2 | 0.0655 (16) | 0.0599 (16) | 0.0415 (15) | 0.0020 (13) | 0.0047 (13) | 0.0003 (12) |
| C3 | 0.0678 (16) | 0.0432 (12) | 0.0409 (14) | 0.0022 (12) | 0.0206 (13) | 0.0002 (10) |
| C4 | 0.0525 (14) | 0.0423 (13) | 0.0451 (14) | −0.0055 (10) | 0.0221 (11) | −0.0005 (10) |
| C5 | 0.0457 (12) | 0.0370 (11) | 0.0365 (13) | −0.0020 (9) | 0.0175 (10) | −0.0035 (9) |
| C6 | 0.098 (2) | 0.0630 (18) | 0.0550 (19) | 0.0058 (16) | 0.0312 (17) | 0.0142 (14) |
| C7 | 0.0456 (13) | 0.0367 (11) | 0.0400 (13) | −0.0031 (9) | 0.0216 (11) | −0.0021 (9) |
| C8 | 0.0501 (14) | 0.0424 (12) | 0.0482 (15) | −0.0047 (10) | 0.0153 (12) | 0.0046 (10) |
| C9 | 0.0506 (14) | 0.0537 (15) | 0.0530 (17) | −0.0049 (11) | 0.0103 (12) | −0.0013 (11) |
| C10 | 0.0554 (15) | 0.0640 (17) | 0.0473 (16) | 0.0013 (13) | 0.0066 (12) | 0.0101 (13) |
| C11 | 0.0628 (16) | 0.0484 (13) | 0.0474 (15) | −0.0050 (12) | 0.0201 (13) | 0.0069 (12) |
| C12 | 0.0605 (18) | 0.080 (2) | 0.086 (2) | −0.0172 (16) | −0.0074 (17) | 0.0093 (19) |
Geometric parameters (Å, °)
| Cu1—N1 | 2.118 (2) | C4—C5 | 1.395 (3) |
| Cu1—N2 | 2.109 (2) | C4—H4 | 0.9300 |
| Cu1—N3 | 1.917 (3) | C5—C7 | 1.491 (3) |
| Cu1—N5 | 1.938 (2) | C6—H6A | 0.9600 |
| Cu2—N4 | 1.829 (2) | C6—H6B | 0.9600 |
| Cu2—N4i | 1.829 (2) | C6—H6C | 0.9600 |
| N1—C1 | 1.333 (3) | C7—C8 | 1.361 (4) |
| N1—C5 | 1.340 (3) | C8—C9 | 1.388 (4) |
| N2—C11 | 1.336 (3) | C8—H8 | 0.9300 |
| N2—C7 | 1.347 (3) | C9—C10 | 1.385 (4) |
| N3—N4 | 1.146 (4) | C9—C12 | 1.502 (4) |
| N5—C5'ii | 1.154 (4) | C10—C11 | 1.364 (4) |
| C1—C2 | 1.377 (4) | C10—H10 | 0.9300 |
| C1—H1 | 0.9300 | C11—H11 | 0.9300 |
| C2—C3 | 1.372 (4) | C12—H12A | 0.9600 |
| C2—H2 | 0.9300 | C12—H12B | 0.9600 |
| C3—C4 | 1.392 (4) | C12—H12C | 0.9600 |
| C3—C6 | 1.503 (4) | ||
| N3—Cu1—N5 | 124.25 (9) | N1—C5—C4 | 121.7 (2) |
| N3—Cu1—N2 | 106.70 (9) | N1—C5—C7 | 116.1 (2) |
| N5—Cu1—N2 | 113.61 (9) | C4—C5—C7 | 122.2 (2) |
| N3—Cu1—N1 | 121.75 (9) | C3—C6—H6A | 109.5 |
| N5—Cu1—N1 | 103.61 (9) | C3—C6—H6B | 109.5 |
| N2—Cu1—N1 | 77.69 (7) | H6A—C6—H6B | 109.5 |
| N4—Cu2—C4'i | 179.88 (15) | C3—C6—H6C | 109.5 |
| C4'i—Cu2—N4i | 0.00 (12) | H6A—C6—H6C | 109.5 |
| C1—N1—C5 | 117.7 (2) | H6B—C6—H6C | 109.5 |
| C1—N1—Cu1 | 126.79 (17) | N2—C7—C8 | 121.9 (2) |
| C5—N1—Cu1 | 114.80 (16) | N2—C7—C5 | 114.8 (2) |
| C11—N2—C7 | 116.8 (2) | C8—C7—C5 | 123.3 (2) |
| C11—N2—Cu1 | 127.21 (16) | C7—C8—C9 | 121.3 (2) |
| C7—N2—Cu1 | 115.86 (16) | C7—C8—H8 | 119.4 |
| N4—N3—Cu1 | 175.6 (3) | C9—C8—H8 | 119.4 |
| N3—N4—Cu2 | 177.1 (3) | C10—C9—C8 | 116.5 (2) |
| C5'ii—N5—Cu1 | 178.3 (3) | C10—C9—C12 | 121.9 (3) |
| N5ii—N5—Cu1 | 178.3 (3) | C8—C9—C12 | 121.6 (3) |
| N1—C1—C2 | 123.4 (2) | C11—C10—C9 | 119.2 (3) |
| N1—C1—H1 | 118.3 | C11—C10—H10 | 120.4 |
| C2—C1—H1 | 118.3 | C9—C10—H10 | 120.4 |
| C3—C2—C1 | 120.2 (3) | N2—C11—C10 | 124.3 (2) |
| C3—C2—H2 | 119.9 | N2—C11—H11 | 117.8 |
| C1—C2—H2 | 119.9 | C10—C11—H11 | 117.8 |
| C2—C3—C4 | 116.8 (2) | C9—C12—H12A | 109.5 |
| C2—C3—C6 | 122.2 (3) | C9—C12—H12B | 109.5 |
| C4—C3—C6 | 120.9 (2) | H12A—C12—H12B | 109.5 |
| C3—C4—C5 | 120.2 (2) | C9—C12—H12C | 109.5 |
| C3—C4—H4 | 119.9 | H12A—C12—H12C | 109.5 |
| C5—C4—H4 | 119.9 | H12B—C12—H12C | 109.5 |
| N3—Cu1—N1—C1 | −81.2 (2) | C1—N1—C5—C7 | 178.9 (2) |
| N5—Cu1—N1—C1 | 64.9 (2) | Cu1—N1—C5—C7 | −9.9 (2) |
| N2—Cu1—N1—C1 | 176.6 (2) | C3—C4—C5—N1 | 1.8 (3) |
| N3—Cu1—N1—C5 | 108.54 (18) | C3—C4—C5—C7 | −178.5 (2) |
| N5—Cu1—N1—C5 | −105.30 (17) | C11—N2—C7—C8 | −0.4 (3) |
| N2—Cu1—N1—C5 | 6.36 (15) | Cu1—N2—C7—C8 | 176.04 (18) |
| N3—Cu1—N2—C11 | 54.7 (2) | C11—N2—C7—C5 | −179.5 (2) |
| N5—Cu1—N2—C11 | −85.9 (2) | Cu1—N2—C7—C5 | −3.0 (2) |
| N1—Cu1—N2—C11 | 174.5 (2) | N1—C5—C7—N2 | 8.7 (3) |
| N3—Cu1—N2—C7 | −121.34 (17) | C4—C5—C7—N2 | −171.0 (2) |
| N5—Cu1—N2—C7 | 98.09 (17) | N1—C5—C7—C8 | −170.4 (2) |
| N1—Cu1—N2—C7 | −1.55 (15) | C4—C5—C7—C8 | 10.0 (4) |
| C5—N1—C1—C2 | 0.0 (4) | N2—C7—C8—C9 | 1.3 (4) |
| Cu1—N1—C1—C2 | −170.0 (2) | C5—C7—C8—C9 | −179.7 (2) |
| N1—C1—C2—C3 | 1.1 (4) | C7—C8—C9—C10 | −1.5 (4) |
| C1—C2—C3—C4 | −0.7 (4) | C7—C8—C9—C12 | 178.5 (3) |
| C1—C2—C3—C6 | 179.7 (3) | C8—C9—C10—C11 | 1.0 (4) |
| C2—C3—C4—C5 | −0.7 (4) | C12—C9—C10—C11 | −179.1 (3) |
| C6—C3—C4—C5 | 179.0 (2) | C7—N2—C11—C10 | −0.2 (4) |
| C1—N1—C5—C4 | −1.4 (3) | Cu1—N2—C11—C10 | −176.2 (2) |
| Cu1—N1—C5—C4 | 169.76 (17) | C9—C10—C11—N2 | −0.1 (5) |
Symmetry codes: (i) −x+1, y, −z+1/2; (ii) −x+3/2, −y+1/2, −z.
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: RK2103).
References
- Barbour, L. J. (2001). J. Supramol. Chem., 1, 189–191.
- Bruker (2002). SMART and SAINT Bruker AXS Inc., Madison, Winconsin, USA.
- Chesnut, D. J., Kusnetzow, A., Birge, R. & Zubieta, J. (2001). J. Chem. Soc. Dalton Trans. pp. 2581–2586.
- Chesnut, D. J. & Zubieta, J. (1998). J. Chem. Soc. Chem. Commun. pp. 1707–1708.
- Dessy, G., Fares, V., Imperatori, P. & Morpurgo, G. O. (1985). J. Chem. Soc. Dalton Trans. pp. 1285–1288.
- Sheldrick, G. M. (1996). SADABS University of Göttingen, Germany.
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Westrip, S. P. (2008). publCIF In preparation.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablocks global, I. DOI: 10.1107/S1600536808022964/rk2103sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536808022964/rk2103Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report