Abstract
A DNA fragment responsible for resistance to antimicrobial agents was cloned from the chromosomal DNA of Enterococcus faecalis ATCC 29212 by using drug-hypersensitive mutant Escherichia coli KAM32 as a host cell. Cells of E. coli KAM32 harboring a recombinant plasmid (pAEF82) carrying the DNA fragment became resistant to many structurally unrelated antimicrobial agents, such as norfloxacin, ciprofloxacin, doxycycline, acriflavine, 4′,6-diamidino-2-phenylindole, tetraphenylphosphonium chloride, daunorubicin, and doxorubicin. Since the sequence of the whole genome of E. faecalis is known, we sequenced several portions of the DNA insert in plasmid pAEF82 and identified two open reading frames within the insert. We designated the genes efrA and efrB. A search of the deduced amino acid sequences of EfrA and EfrB revealed that they are similar to each other and that they belong to the ATP-binding cassette (ABC) family of multidrug efflux transporters. Transformed E. coli KAM32 cells harboring efrAB showed energy-dependent efflux of acriflavine. The efflux activity was inhibited by reserpine, verapamil, and sodium-o-vanadate, known inhibitors of ABC efflux pumps.
The emergence of drug resistance in microorganisms and its association with serious infectious diseases have increased at alarming rates during the past several decades (12). Microorganisms have developed various mechanisms to resist the toxic effects of antibiotics and other chemicals (6, 18). These mechanisms include the inactivation of drugs by hydrolysis or modification, alteration of the targets, the creation of alternative pathways, inhibition of drug entry into cells, and active efflux of drugs (23). Among these, drug efflux, especially multidrug efflux, is emerging as a major problem in conferring multidrug resistance to bacterial cells because multidrug efflux pumps mediate extrusion of a wide variety of structurally unrelated antimicrobial agents (16, 25). These multidrug transporters are divided into two major classes on the basis of bioenergetics and structural criteria. One class consists of secondary multidrug transporters, which utilize the transmembrane electrochemical potential of a proton or sodium ion, and the other consists of the ATP-binding cassette (ABC) multidrug transporters, which utilize the free energy of ATP hydrolysis to drive the extrusion of drugs from cells (23).
Enterococci are gram-positive commensal bacteria that normally inhabit the gastrointestinal tracts of almost all animals and are the most abundant gram-positive cocci in humans (10, 22). Once regarded as a bacterial genus of little consequence in infectious diseases, the enterococci are increasingly recognized as the leading nosocomial pathogens because they cause serious diseases such as endocarditis, urinary tract infections, surgical wound infections, and bacteremia. Additionally, the enterococci are resistant to many antimicrobial agents used in hospitals (17, 28). As a result, few therapeutic options are available to treat infections caused by multidrug-resistant enterococci (28). In fact, the National Nosocomial Infections Surveillance system (24) ranks enterococci as the leading cause of surgical site infections and the third most common cause of both bloodstream and urinary tract infections. In addition, the risk of death associated with antibiotic-resistant enterococcal bacteremia is severalfold higher than that associated with antibiotic-susceptible enterococcal bacteremia (4).
Antibiotic resistance, together with the factors cytolysin, aggregation substance, gelatinase, and extracellular surface protein, is a known virulence factor suspected to be related to an enhanced ability to cause enterococcal diseases (9). Recently, Davis et al. (3) reported on the presence of 34 potential multidrug resistance-encoding genes in Enterococcus faecalis using bioinformatics approaches. Interestingly, among these are a large number (23 transporters) that are ABC multidrug transporters, suggesting the importance of this type of transporter in multidrug-resistant E. faecalis strains. The aim of this study was to analyze multidrug efflux pumps, especially ABC multidrug efflux pumps, from E. faecalis. Here we report on the cloning of the functional genes and the characterization of a novel ABC multidrug efflux pump, EfrAB, from E. faecalis.
MATERIALS AND METHODS
Bacteria and growth.
E. faecalis ATCC 29212 and Escherichia coli KAM32 (ΔacrB ΔydhE hsd negative)(2) were used in this study. E. faecalis cells were grown in brain heart infusion medium (Difco) and E. coli cells were grown in Luria (L) medium (15) at 37°C. Cell growth was monitored by measuring the optical density at 650 nm.
Cloning and sequencing.
Chromosomal DNA was prepared from E. faecalis cells by the method of Berns and Thomas (1). The DNA was partially digested with Sau3AI, and the fragments from 4 to 10 kbp were separated by sucrose density gradient centrifugation. Plasmid pBR322 was digested with BamHI, dephosphorylated with bacterial alkaline phosphatase, and then ligated to the chromosomal DNA fragments with a ligation kit (version 2; TaKaRa Co.). Competent E. coli KAM32 cells were transformed with the recombinant plasmids and were spread on 1.5% agar plates containing L broth, 60 μg of ampicillin per ml, and various antimicrobial agents, which were used for selection: chloramphenicol (3 μg/ml), erythromycin (10 μg/ml), norfloxacin (0.07 μg/ml), acriflavine (5 μg/ml), ethidium bromide (12 μg/ml), and Hoechst 33342 (3 μg/ml). The plates were incubated at 37°C for 24 h. Candidate colonies that appeared on the plates were picked up and purified on a new plate containing the same drug, and plasmids were isolated from the candidate colonies. Each candidate plasmid was retransformed into KAM32 cells, and the cells were spread onto the same plate. Plasmids were isolated from each of the transformants that were retransformed and used for restriction mapping and sequencing.
Vectors pSTV29 and pBR322 are compatible in the same cell. pSTV29 carries the chloramphenicol resistance gene. Thus, chloramphenicol was used for selection of pSTV29-derived hybrid plasmid pAEF86 (which carries efrA). pBR322 carries the ampicillin resistance gene. Thus, ampicillin was used for selection of pBR322-derived hybrid plasmid pAEF87 (which carries efrB).
The nucleotide sequence of the gene was partially determined by the dideoxy chain termination method (27) with an automated DNA sequencer (ALF Express; Pharmacia Biotech).
Drug susceptibility tests.
The MICs of various antimicrobial agents were determined in Mueller-Hinton broth (Difco) containing different drugs at various concentrations (14). The cells were incubated in the test medium at 37°C for 24 h, and the growth was examined by visual inspection. The MIC was defined as the lowest concentration of a drug that inhibited visible growth.
Fluorometric assay of drug efflux.
Fluorometric assays of EfrAB-mediated efflux of acriflavine were carried out essentially as described previously (2). Acriflavine binds to DNA, resulting in fluorescence quenching (2). E. coli KAM32 cells harboring recombinant plasmids were grown in L broth at 37°C at the exponential phase of growth. The cells were harvested and washed with modified Tanaka buffer (pH 7.0) (32) containing 2 mM MgSO4. The cells were loaded with 4.2 μM acriflavine by incubation for 2 h at 37°C in the presence of 40 μM carbonylcyanide m-chlorophenylhydrazone. The cells were washed three times and resuspended in 0.1 Mmorpholinepropanesulfonic acid-tetramethylammonium hydroxide (pH 7.0) containing 2 mM MgSO4 and 4.2 μM acriflavine. Energy-dependent efflux was measured following the addition of lactate to a final concentration of 20 mM. Fluorometric measurements were performed at 25°C with a Hitachi 2000 fluorescence spectrophotometer. Excitation and emission wavelengths of 468 and 499 nm, respectively, were used.
To study the effects of the inhibitors on the efflux of acriflavine, the cell suspensions were preincubated in the presence of inhibitors for 5 min prior to the addition of lactate. The efflux rate was calculated from the increase (linear portion) in the fluorescence for 30 s.
RESULTS
Functional cloning and sequencing of genes for drug resistance
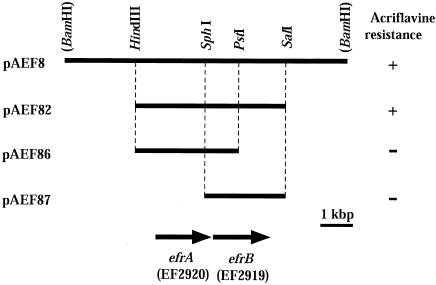
We obtained nine recombinant plasmids from a selection plate containing acriflavine. Restriction patterns, drug specificity, and partial sequencing of both ends of the insert DNAs revealed that these nine candidates showed three patterns. We determined the partial sequence of one of them, pAEF8, which conferred multidrug resistance to KAM32, and found that the plasmid carries putative ABC transporter genes (loci EF2920 and EF2919) with some other open reading frames (ORFs), according to the genome sequence information for E. faecalis V583 from The Institute for Genomic Research (TIGR; http://www.tigr.org). This plasmid was used for further analysis (Fig. 1). The insert in pAEF8 was about 9 kbp. A HindIII-SalI fragment (4.8 kbp) of pAEF8, which contains the EF2920 and EF2919 loci, was subcloned into a pUC19 vector. The resulting hybrid plasmid, pAEF82, was introduced into E. coli KAM32 cells. The transformants harboring plasmid pAEF82 showed resistance to acriflavine (Fig. 1). Neither plasmid pAEF86 carrying EF2920 nor plasmid pAEF87 carrying EF2919 conferred drug resistance. Thus, we concluded that both the EF2920 locus and the EF2919 locus are necessary and responsible for acriflavine resistance. We renamed these genes efrA and efrB (for E. faecalis multidrug resistance), respectively.
FIG. 1.
Restriction map of plasmid pAEF8 and its derivatives. Horizontal bars indicate DNA regions derived from E. faecalis chromosomal DNA. The two arrows indicate the positions and directions of the efrA and efrB genes. The plus signs on the right indicate that E. coli KAM32 cells harboring each plasmid grew on an L medium plate containing 5 μg of acriflavine per ml. Minus signs indicate that cells did not grow on the same plate.
According to the genome sequence of E. faecalis V583 from TIGR (http://www.tigr.org), the efrA and efrB genes specified putative 572- and 589-amino-acid proteins, respectively. Both efrA and efrB genes have promoter-like sequences in their upstream regions and ribosome-binding sequences (Shine-Dalgarno sequences) (29), each of which is followed by a start codon (ATG). Both genes are followed by a transcription terminator-like (inverted repeat) sequence. The two genes overlap by 1 nucleotide.
Hydropathy analysis by the method of Kyte and Doolittle (13) suggested that both EfrA and EfrB possess six putative transmembrane segments followed by hydrophilic segments (data not shown). The hydrophilic segments of both EfrA and EfrB contained putative ATP-binding domains, Walker A and Walker B motifs, and ATP signature sequences (8, 34).
Drug susceptibility studies with E. coli.
To investigate the contribution of EfrAB to drug resistance, efrAB was expressed in E. coli KAM32, which is hypersensitive to many drugs due to deficiencies in the major multidrug efflux pumps AcrAB and YdhE (2). The drug susceptibilities of E. coli KAM32 cells harboring pAEF82 (which carries efrAB) and pUC18 (the control) are shown in Table 1. In KAM32 cells, plasmid pAEF82 conferred resistance to several structurally unrelated drugs: norfloxacin, ciprofloxacin, doxycycline, acriflavine, 4′,6-diamidino-2-phenylindole (DAPI), and tetraphenylphosphonium chloride. We also observed some elevated (twofold) resistance to arbekacin, novobiocin, daunorubicin, doxorubicin, ethidium bromide, and safranin O. This slightly elevated resistance was reproducible. Therefore, we conclude that the EfrA and EfrB proteins confer multidrug resistance, perhaps a multidrug efflux pump. No significant changes in the MICs of the other drugs tested were observed (Table 1).
TABLE 1.
MICs of various drugs for E. coli KAM32/pUC18 and KAM32/pAEF82
| Drug | MIC (μg/ml)
|
||
|---|---|---|---|
| KAM32/pUC18 | KAM32/pAEF82 | Relative fold resistance | |
| Norfloxacin | 0.015 | 0.06 | 4 |
| Ciprofloxacin | 0.002 | 0.008 | 4 |
| Doxycycline | 0.25 | 1 | 4 |
| Arbekacin | 0.5 | 1 | 2 |
| Novobiocin | 4 | 8 | 2 |
| Chloramphenicol | 1 | 1 | 1 |
| Erythromycin | 4 | 4 | 1 |
| Gentamicin | 0.5 | 0.5 | 1 |
| Tetracycline | 0.5 | 0.5 | 1 |
| Triclosan | 0.12 | 0.12 | 1 |
| Daunorubicin | 2 | 4 | 2 |
| Doxorubicin | 2 | 4 | 2 |
| Acriflavine | 2 | 16 | 8 |
| DAPI | 0.12 | 1.0 | 8 |
| Tetraphenylphosphonium chloride | 8 | 32 | 4 |
| Ethidium bromide | 4 | 8 | 2 |
| Safranin O | 4 | 8 | 2 |
| Hoechst 33342 | 1 | 1 | 1 |
| Pyronin Y | 1 | 1 | 1 |
| Rhodamine-6G | 8 | 8 | 1 |
Both EfrA and EfrB are necessary for function.
Plasmid pAEF82 contains two ORFs, efrA and efrB, according to the genome sequence information for E. faecalis from TIGR (http://www.tigr.org). These two ORFs overlap by 1 nucleotide. It seemed possible that one long ORF encompasses both efrA and efrB. On the other hand, if two ORFs actually exist, then only one of them may be enough to confer drug resistance. Thus, we tested these possibilities. We constructed plasmids carrying either efrA or efrB. Each gene was located under the control of the lac promoter in the pSTV29 vector or under the control of the tet promoter in the pBR322 vector, resulting in plasmids pAEF86 (which carries efrA) and pAEF87 (which carries efrB), respectively (Fig. 1). E. coli KAM32 cells transformed with either plasmid pAEF86 or pAEF87 did not show elevated levels of acriflavine or DAPI resistance (Table 2). E. coli KAM32 cells cotransformed with these two plasmids showed elevated levels of resistance to acriflavine and DAPI. Thus, it is very likely that efrA and efrB are two ORFs. It is clear that both EfrA and EfrB are necessary for the observed drug resistance, perhaps for the function of a multidrug efflux pump.
TABLE 2.
Retrieval of drug resistance by cotransformation
| Drug | MIC (μg/ml)
|
|||
|---|---|---|---|---|
| KAM32/ pSTV29 | KAM32/ pAEF86 (efrA)a | KAM32/ pAEF87 (efrB) | KAM32/pAEF86/pAEF87 (efrA + efrB) | |
| Acriflavine | 2 | 2 | 2 | 8 |
| DAPI | 0.12 | 0.12 | 0.12 | 0.5 |
The gene(s) carried by the plasmid(s) is indicated in parentheses.
EfrAB mediates efflux of acriflavine.
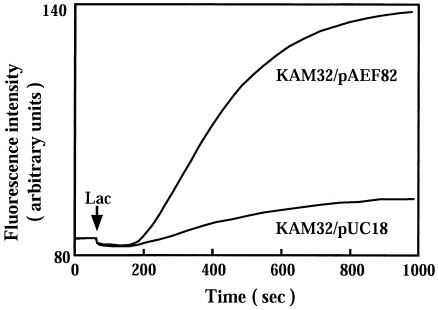
We measured acriflavine efflux with E. coli KAM32/pAEF82 cells. The fluorescence of acriflavine decreases when it binds to DNA (2). Lactate was added as an energy source to the assay mixture, which contained energy-starved and acriflavine-loaded cells. As shown in Fig. 2, addition of lactate elicited strong efflux of acriflavine in KAM32/pAEF82 cells but not in control KAM32/pUC18 cells. This result indicates that EfrAB is an energy-dependent efflux pump.
FIG. 2.
Fluorometric assay of acriflavine efflux. Acriflavine efflux from E. coli KAM32/pUC18 cells or KAM32/pAEF82 (which carries efrAB) cells was measured. Energy-starved and acriflavine-loaded cells were prepared. Sodium lactate (Lac; final concentration, 20 mM) was added at the time indicated by the arrow to energize the cells. Acriflavine efflux is represented by a rapid increase in fluorescence.
Inhibition of EfrAB-mediated drug efflux.
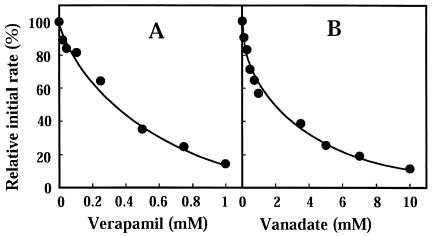
A calcium blocker, verapamil, is known to be a potent inhibitor of a wide range of multidrug efflux pumps, including ATP-dependent transporters as well as other primary active transporters (5, 19, 20). The effect of verapamil on acriflavine efflux via EfrAB was investigated in E. coli KAM32/pAEF82 cells. Verapamil inhibited acriflavine efflux, and the concentration causing 50% inhibition was 0.3 mM (Fig. 3A). A plant alkaloid, reserpine, also inhibited the efflux activity (data not shown).
FIG. 3.
Inhibition of acriflavine efflux. Acriflavine efflux was measured as described in the legend to Fig. 2. Various concentrations of verapamil or sodium o-vanadate were added to the assay mixture and preincubated with the cells for 5 min. Sodium lactate (final concentration, 20 mM) was added to initiate the assay. The relative initial velocity of acriflavine efflux was measured. The initial velocity observed in the absence of inhibitor was 100%.
Since EfrAB is a member of the ABC family of transporters, it is likely that ATP is the energy source for transport. Sodium-o-vanadate is an inhibitor of ATPase and inhibits the activities of MDR1, HorA, and LmrA (7, 26, 33). We tested the effect of sodium-o-vanadate on acriflavine efflux. Sodium-o-vanadate strongly inhibited acriflavine efflux, and the concentration causing 50% inhibition was 1.9 mM (Fig. 3B).
Sequence similarity.
EfrA and EfrB showed 32% sequence identity and 42% similarity. The SwissProt database was searched for sequences similar to the amino acid sequence of EfrAB. Several multidrug efflux proteins of the ABC type and putative proteins from species ranging from bacteria to humans showed sequence similarity (similarity, 40 to 70%) (Table 3). The putative pump ABC(1) and the ABC(2) pump of Clostridium perfringens showed the highest levels of identity and similarity with EfrA and EfrB, respectively. This suggests that a similar two-component multidrug efflux pump is present in C. perfringens. LmrA of Lactococcus lactis showed 28% identity and 51% similarity with EfrA and 27% identity and 48% similarity with EfrB (Table 3).
TABLE 3.
Homologs of EfrAB
| Transporter | Length (amino acids) | Organism | EfrA
|
EfrB
|
SwissProt database accession no. | ||
|---|---|---|---|---|---|---|---|
| % Identity | % Similarity | % Identity | % Similarity | ||||
| EfrA | 572 | Enterococcus faecalis | 100 | 100 | 32 | 42 | This study |
| EfrB | 589 | Enterococcus faecalis | 32 | 42 | 100 | 100 | This study |
| ABC(1) | 577 | Clostridum perfringens | 57 | 77 | 30 | 52 | BAB80085 |
| ABC(2) | 593 | Clostridum perfringens | 29 | 50 | 51 | 68 | BAB80086 |
| VcaM | 619 | Vibrio cholerae non-O1 | 37 | 58 | 28 | 48 | AB073220 |
| YvcC | 589 | Bacillus subtilis | 28 | 50 | 32 | 53 | D70031 |
| MsbA | 582 | Escherichia coli | 28 | 50 | 31 | 54 | P27299 |
| HorA | 583 | Lactobacillus brevis | 28 | 50 | 30 | 50 | AB005752 |
| LmrA | 590 | Lactococcus lactis | 28 | 51 | 27 | 48 | U63741 |
| AbcA | 575 | Staphylococcus aureus | 26 | 48 | 28 | 53 | U29478 |
| MDR1(N)a | 659 | Homo sapiens | 29 | 50 | 28 | 48 | P08183 |
| MDR1(C)a | 621 | Homo sapiens | 30 | 48 | 28 | 47 | P08183 |
N, N-terminal half; C, C-terminal half.
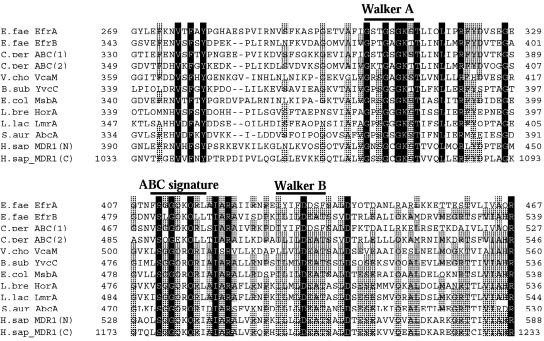
The Walker A and B motifs (34) and the ABC signature sequence characteristics (8) of the ABC transporters were conserved in the hydrophilic domains of EfrA and EfrB, similar to various ABC efflux pumps and putative pumps (Fig. 4).
FIG. 4.
Multiple-sequence alignments of EfrA, EfrB, and similar or putative proteins. The amino acid sequence alignments of the Walker A motif, the Walker B motif, and the ABC signature sequences are shown. The sequence of human MDR1 was divided into an N-terminal half (N) and a C-terminal half (C). Identical and similar residues are indicated with black and gray backgrounds, respectively. Gaps in the alignment are indicated by hyphens. The numbers on the right and left of the sequences indicate the beginning and the end of each sequence, respectively. The sequences were aligned by using the EMBL CLUSTAL W program (available at the website http://www.ch.embnet.org/software/ClustalW.html) and the GENETIX-MAC program (version 10.1). The names of the organisms from which the proteins are derived are spelled out in Table 3.
DISCUSSION
We investigated the efflux activity of acriflavine, an optimal substrate for EfrAB, and clearly demonstrated the energy-dependent efflux of this antimicrobial agent out of cells. So far, we have not succeeded in observing the ATP-driven efflux of acriflavine or ATPase activity with EfrAB. However, the efflux activity was strongly inhibited by known inhibitors of ABC multidrug efflux pumps, such as verapamil, reserpine, and an ATPase inhibitor, sodium-o-vanadate, suggesting that drug efflux through EfrAB is driven by ATP.
LmrA, an experimentally well-characterized bacterial ABC multidrug transporter from L. lactis, is composed of a transmembrane domain and an ATP-binding domain and functions as a homodimer (33). Neither efrA nor efrB was able to confer drug resistance when either one of these genes was introduced into host cells, while drug resistance was retrieved when these two genes were both introduced into the cells (Table 2). This suggests that EfrA and EfrB form a heterodimer which functions as a multidrug efflux pump.
Recently, the presence of 34 putative multidrug transporters in E. faecalis was suggested from genome sequencing information (3). Among these, ABC transporters constitute the majority (23 transporters; about 68% of the multidrug efflux pumps). This is clearly distinct from the information on the proportion of ABC transporters from other bacterial genome sequences: 19% in E. coli, 17% in Mycobacterium tuberculosis, 11% in Bacillus subtilis, and 3% in Pseudomonas aeruginosa (21, 31). This probably suggests the physiological importance of ABC efflux transporters in E. faecalis. E. faecalis shows fairly high levels of resistance to many antimicrobial agents, presumably due to the presence of many multidrug efflux pumps. However, only two multidrug efflux pumps have been reported in enterococci. One is EmeA, a NorA homolog and a member of the MFS family, which was identified as the first example of a multidrug transporter in E. faecalis (11, 14). The other is Lsa, which confers resistance to clindamycin and quinupristin-dalfopristin (30). Lsa is an ABC protein and contains two ATP-binding cassettes and no obvious transmembrane domain. Thus, it seems that Lsa is not an integral membrane protein. It is not yet clear whether Lsa is really an efflux pump. Thus, the EfrAB protein reported in this study is the first ABC multidrug efflux pump from E. faecalis for which efflux activity has been demonstrated experimentally.
In this study, E. coli was used as the host cell for the cloning and expression of E. faecalis genes; we thus questioned whether the promoter of efrAB is functional in E. coli cells. This issue was investigated further. In plasmid pAEF82, efrAB is under the control of the lac promoter of the pUC19 vector, but the original promoter region was also preserved. In order to show that the original promoter of efrAB is functional, another hybrid plasmid with the same insert region as pAEF82, but in the opposite direction of the insert DNA, was prepared in the pUC18 vector. E. coli KAM32 cells transformed with this plasmid showed almost the same profiles of resistance to acriflavine and the other drugs tested (data not shown). In addition, the addition of isopropyl-β-d-thiogalactopyranoside, an inducer of the lac promoter, had no effect on either plasmid. These results indicate that EfrAB is expressed through an original promoter of its own, not the lac promoter, and that the promoter of efrAB is functional in E. coli.
Although it is sometimes difficult, we nonetheless succeeded in cloning the genes responsible for a multidrug efflux pump from the gram-positive organism E. faecalis by using a gram-negative organism, E. coli, as the host. It has been suggested that more than 30 multidrug efflux genes are present in E. faecalis (3), but so far we have succeeded in cloning only 3 of them. Drug-hypersensitive strain E. coli KAM32 is a very suitable host for the cloning of genes that encode drug resistance. Construction of a drug-hypersensitive gram-positive bacterial host would be valuable for analyses of drug resistance determinants from gram-positive bacteria. Such attempts are now under way.
Acknowledgments
We thank M. Varela of Eastern New Mexico University for critical reading of the manuscript.
This research was supported by a grant from the Ministry of Education, Science, Sport and Culture of Japan.
REFERENCES
- 1.Berns, K. I., and C. A. J. Thomas. 1965. Isolation of high molecular weight DNA from Haemophilus influenzae. J. Mol. Biol. 11:117-120. [DOI] [PubMed] [Google Scholar]
- 2.Chen, J., Y. Morita, M. N. Huda, T. Kuroda, T. Mizushima, and T. Tsuchiya. 2002. VmrA, a member of a novel class of Na+-coupled multidrug efflux pumps from Vibrio parahaemolyticus. J. Bacteriol. 184:572-576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Davis, D. R., J. B. McAlpine, C. J. Pazoles, M. K. Talbot, E. A. Alder, C. White, B. M. Jonas, B. E. Murray, G. M. Weinstock, and B. L. Rogers. 2001. Enterococcus faecalis multi-drug resistance transporters: application for antibiotic discovery. J. Mol. Microbiol. Biotechnol. 3:179-184. [PubMed] [Google Scholar]
- 4.Edmond, M. B., J. F. Ober, J. D. Dawson, D. L. Weinbaum, and R. P. Wenzel. 1996. Vancomycin-resistant enterococcal bacteremia: natural history and attributable mortality. Clin. Infect. Dis. 23:1234-1239. [DOI] [PubMed] [Google Scholar]
- 5.Endicott, J. A., and V. Ling. 1989. The biochemistry of P-glycoprotein-mediated multidrug resistance. Annu. Rev. Biochem. 58:137-171. [DOI] [PubMed] [Google Scholar]
- 6.Hayes, J. D., and C. R. Wolf. 1990. Molecular mechanisms of drug resistance. Biochem. J. 272:281-295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Horio, M., M. M. Gottesman, and I. Pastan. 1988. ATP-dependent transport of vinblastine in vesicles from human multidrug resistant cells. Proc. Natl. Acad. Sci. USA 85:3580-3584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hyde, S. C., P. Emsley, M. J. Hartshorn, M. M. Mimmack, U. Gileadi, S. R. Pearce, M. P. Gallagher, D. R. Gill, R. E. Hubbard, and C. F. Higgins. 1990. Structural model of the ATP-binding proteins associated with cystic fibrosis, multidrug resistance and bacterial transport. Nature 346:362-365. [DOI] [PubMed] [Google Scholar]
- 9.Ike, Y., D. Clewell, R. Segarra, and M. Gilmore. 1990. Genetic analysis of the pAD1 hemolysin/bacteriocin determinant in Enterococcus faecalis: Tn917 insertional mutagenesis and cloning. J. Bacteriol. 172:155-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jett, B. D., M. M. Huycke, and M. S. Gilmore. 1994. Virulence of enterococci. Clin. Microbiol. Rev. 7:462-478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jonas, B. M., B. E. Murray, and G. M. Weinstock. 2001. Characterization of emeA, a norA homolog and multidrug resistance efflux pump, in Enterococcus faecalis. Antimicrob. Agents Chemother. 45:3574-3579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jones, R. N. 2001. Resistance patterns among nosocomial pathogens: trends over the past few years. Chest 119:397S-404S. [DOI] [PubMed] [Google Scholar]
- 13.Kyte, J., and R. F. Doolittle. 1982. A simple method for displaying the hydropathic character of a protein. J. Mol. Biol. 157:105-132. [DOI] [PubMed] [Google Scholar]
- 14.Lee, E. W., J. Chen, M. N. Huda, T. Kuroda, T. Mizushima, and T. Tsuchiya. 2003. Functional cloning and expression of emeA, and characterization of EmeA, a multidrug efflux pump from Enterococcus faecalis. Biol. Pharm. Bull. 26:266-270. [DOI] [PubMed] [Google Scholar]
- 15.Lennox, E. S. 1955. Transduction of linked genetic characters of host by bacteriophage P1. Virology 1:190-206. [DOI] [PubMed] [Google Scholar]
- 16.Levy, S. B. 1992. Active efflux mechanisms for antimicrobial resistance. Antimicrob. Agents Chemother. 36:695-703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moellering, R. C., Jr. 1998. Vancomycin-resistant enterococci. Clin. Infect. Dis. 26:1196-1199. [DOI] [PubMed] [Google Scholar]
- 18.Neu, H. C. 1992. The crisis in antibiotic resistance. Science 257:1064-1073. [DOI] [PubMed] [Google Scholar]
- 19.Neyfakh, A. A., C. M. Borsch, and G. W. Kaatz. 1993. Fluoroquinolone resistance protein NorA of Staphylococcus aureus is a multidrug efflux transporter. Antimicrob. Agents Chemother. 37:128-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Neyfakh, A. A., V. E. Bidnenko, and L. B. Chen. 1991. Efflux-mediated multidrug resistance in Bacillus subtilis: similarities and dissimilarities with the mammalian system. Proc. Natl. Acad. Sci. USA 88:4781-4785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nishino, K., and A. Yamaguchi. 2001. Analysis of a complete library of putative drug transporter genes in Escherichia coli. J. Bacteriol. 183:5803-5812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Noble, C. J. 2000. Cariage of group D streptococci in the human bowel. J. Clin. Pathol. 31:513-522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Putman, M., H. W. van Veen, and W. N. Konings. 2000. Molecular properties of bacterial multidrug transporters. Microbiol. Mol. Biol. Rev. 64:672-693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Richards, M. J., J. R. Edwards, D. H. Culver, and R. P. Gaynes. 2000. Nosocomial infections in combined medical-surgical intensive care units in the United States. Infect. Control Hosp. Epidemiol. 21:510-515. [DOI] [PubMed] [Google Scholar]
- 25.Saier, M. H., Jr., R. Tam, A. Reizer, and J. Reizer. 1994. Two novel families of bacterial membrane proteins concerned with nodulation, cell division and transport. Mol. Microbiol. 11:841-847. [DOI] [PubMed] [Google Scholar]
- 26.Sakamoto, K., A. Margolles, H. W. van Veen, and W. N. Konings. 2001. Hop resistance in the beer spoilage bacterium Lactobacillus brevis is mediated by the ATP-binding cassette multidrug transporter HorA. J. Bacteriol. 183:5371-5375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sanger, F., S. Nicklen, and A. R. Coulson. 1977. DNA sequencing with chain-terminating inhibitors. Proc. Natl. Acad. Sci. USA 74:5463-5467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shepard, B. D., and M. S. Gilmore. 2002. Antibiotic-resistant enterococci: the mechanisms and dynamics of drug introduction and resistance. Microbes Infect. 4:215-224. [DOI] [PubMed] [Google Scholar]
- 29.Shine, J., and L. Dalgarno. 1974. The 3′-terminal sequence of Escherichia coli 16S ribosomal RNA: complementarity to nonsense triplets and ribosome binding sites. Proc. Natl. Acad. Sci. USA 71:1342-1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Singh, K. V., G. M. Weinstock, and B. E. Murray. 2002. An Enterococcus faecalis ABC homologue (Lsa) is required for the resistance of this species to clindamycin and quinupristin-dalfopristin. Antimicrob. Agents Chemother. 46:1845-1850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stover, C. K., X. Q. Pham, A. L. Erwin, S. D. Mizoguchi, P. Warrener, M. J. Hickey, F. S. Brinkman, W. O. Hufnagle, D. J. Kowalik, M. Lagrou, R. L. Garber, L. Goltry, E. Tolentino, S. Westbrock-Wadman, Y. Yuan, L. L. Brody, S. N. Coulter, K. R. Folger, A. Kas, K. Larbig, R. Lim, K. Smith, D. Spencer, G. K. Wong, Z. Wu, I. T. Paulsen, J. Reizer, M. H. Saier, R. E. Hancock, S. Lory, and M. V. Olson. 2000. Complete genome sequence of Pseudomonas aeruginosa PAO1, an opportunistic pathogen. Nature 406:959-964. [DOI] [PubMed] [Google Scholar]
- 32.Tanaka, S., S. A. Lerner, and E. C. C. Lin. 1967. Replacement of a phosphoenolpyruvate-dependent phosphotransferase by a nicotinamide adenine dinucleotide-linked dehydrogenase for the utilization of mannitol. J. Bacteriol. 93:642-648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van Veen, H. W., A. Margolles, M. Muller, C. F. Higgins, and W. N. Konings. 2000. The homodimeric ATP-binding cassette transporter LmrA mediates multidrug transport by an alternating two-site (two-cylinder engine) mechanism. EMBO J. 19:2503-2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Walker, J. E., M. Saraste, M. J. Runswick, and N. J. Gay. 1982. Distantly related sequences in the alpha- and beta-subunits of ATP synthase, myosin, kinases and other ATP-requiring enzymes and a common nucleotide binding fold. EMBO J. 1:945-951. [DOI] [PMC free article] [PubMed] [Google Scholar]