Abstract
Prevention of sexually transmitted infections is a priority in developed and developing countries. One approach to prevention is the use of topical microbicides, and one promising approach is the use of dendrimers, highly branched macromolecules synthesized from a polyfunctional core. Three new dendrimer products developed to provide stable and cost-efficient microbicides were initially evaluated in vitro for anti-herpes simplex virus activity and then in vivo by using a mouse model of genital herpes. From these experiments one product, SPL7013, was chosen for further evaluation to define the dose and duration of protection. Unformulated SPL7013 provided significant protection from genital herpes disease and infection at concentrations as low as 1 mg/ml and for at least 1 h following topical (intravaginal) administration of 10 mg/ml. This compound was then formulated into three vehicles and further evaluated in mouse and guinea pig models of genital herpes infection. In the murine evaluations each of the formulations provided significant protection at concentrations of 10 and 50 mg/ml. Formulated compounds provided protection for at least 1 h at a concentration of 10 mg/ml. From these experiments formulation 2V was chosen for dose ranging experiments using the guinea pig model of genital herpes. The guinea pig evaluations suggested that doses of 30 to 50 mg/ml were required for optimal protection. From these studies a lead compound and formulation (2V of SPL7013) was chosen for ongoing evaluations in primate models of simian immunodeficiency virus and Chlamydia trachomatis infection.
The spread of sexually transmitted infections (STIs) continues to grow at an alarming rate. In the United States more than 12 million people are infected with STIs every year, accounting for 5 of the 10 most commonly reported infectious diseases (4). Globally the incidence of human immunodeficiency virus (HIV) infection continues to grow, with the most recent data from the United Nations showing that 40 million people worldwide are HIV positive. Similarly, infections with herpes simplex virus type 2 (HSV-2) continue to increase around the world at an alarming rate despite the availability of effective antivirals (11). Seroprevalence data suggest that >45 million patients are infected in the United States at this time, with projections for even further increases (5, 6). The high percentage of women infected with both HSV-2 and HIV is of particular concern. Because genital herpes can lead to an increased risk of HIV infections, prevention of genital herpesvirus infections may also impact the spread of HIV (5). Vaccines for STIs remain an important goal for reduction of the their spread; however, HIV vaccines remain an elusive goal, while the prospects for vaccines for other STIs, including genital herpesvirus and human papillomavirus infection, are more encouraging (8, 12).
Microbicides, defined as a chemical entity that can prevent or reduce transmission of STIs when applied to the vagina or rectum, represent an intriguing approach to the prevention of STIs. Most microbicide candidates act by disrupting the cell membrane or envelope of the pathogen (for example, detergents such as nonoxynol-9), by blocking receptor-ligand interactions (for example, sulfated compounds, such as PRO 2000), or by modifying the vaginal environment (for example, pH buffering agents such as Buffer-gel) (reviewed in references 10, 14, and 15).
Dendrimers are a relatively new class of macromolecules characterized by highly branched three-dimensional architectures that offer an alternative to polyanionic polymers. They are assembled in a precise stepwise manner, and this controlled synthesis allows the assembly of highly defined “nano-objects,” in contrast to the heterogenous nature of traditional polymer-based materials. Therefore, we applied this technology to prepare defined macromolecular polyanions that would retain good levels of activity against the early stages of viral infection and have optimum physical properties (i.e., low systemic absorption, water solubility, ease of formulation, etc.) for microbicide development. In vitro and in vivo studies on a selection of these compounds have been reported previously and showed that they are potent inhibitors of a range of sexually transmitted diseases. Several compounds inhibited the replication of HIV type 1 with a 50% effective concentration (EC50) of <1 μg/ml (19), while members of this same class of dendrimer were also effective in vitro against HSV-1 and HSV-2 (3). These compounds appeared to inhibit the early stages of virus replication although there was some evidence of effects on the late stages of viral replication (17, 19). In addition, the compounds were nontoxic to the cells up to the highest concentration tested, 100 μg/ml (3).
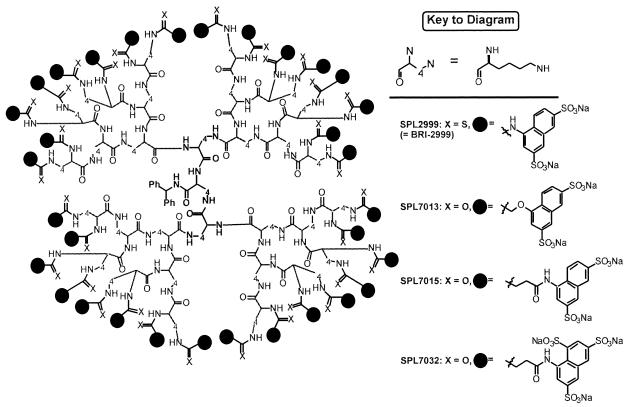
Recently, dendrimers dissolved in saline (i.e., unformulated) were used in in vivo evaluations of activity in a mouse model of genital herpes (3). These early dendrimer-based microbicide candidates were prepared by assembling aromatic-sulfonic acid or aromatic-carboxylic acid units to the outer surface of lysine- or polyamidoamine-based dendrimers via a thiourea linkage (for example, see compound SPL2999 in Fig. 1, which has previously been referred to as BRI-2999) (3, 7). Since that time we have further refined the dendrimer architecture in order to produce compounds in a Good Laboratory Practice/Current Good Manufacturing Practice environment with the required stability and cost-of-production profile necessary for a microbicide candidate. This work resulted in the identification of three new microbicide candidates, SPL7013, SPL7015, and SPL7032 (Fig. 1), which are all prepared from the same lysine dendrimer and where the earlier thiourea linkage has been replaced by a more stable amide bond.
FIG. 1.
Molecular structures of SPL2999, SPL7013, SPL7015, and SPL7032. The lysine dendrimer generations are built out from a central core (boldface), through a series of layers or generations. The outer sulfonic acid surface is attached via thiourea (SPL2992) or amide (SPL7013, SPL7015, and SPL7032) linkages.
This paper reports on the in vivo evaluation of these stable, expanded-spectrum dendrimers when used in mouse and guinea pig models of HSV-2-induced genital herpesvirus infection. In addition we report the in vivo activity of three prototype formulations of the selected development candidate, SPL7013. This formulated preparation is currently undergoing in vivo evaluations in macaques for efficacy against simian/human immunodeficiency virus and Chlamydia trachomatis.
MATERIALS AND METHODS
Viruses. HSV-2 strain G was used for in vitro assays (7), while HSV-2 strain 186 was used for mouse inoculations (3) and strain MS was used for guinea pig inoculations (2). All viruses were prepared as described previously (2, 3, 7).
Dendrimers.
A powder form of the dendrimers was supplied by Starpharma (Prahran, Australia). Solutions (1 to 100 mg/ml) were prepared in phosphate-buffered saline (PBS) and stored at room temperature.
Formulations.
Formulation development work on SPL7013 was performed at the Center for Pharmaceutical Science and Technology at the University of Kentucky. Methylparaben (NF), propylparaben (NF), edetate disodium dihydrate (EDTA; USP), propylene glycol (USP), glycerin (USP), and sodium hydroxide (NF) were obtained from Spectrum Quality Products, Inc. (New Brunswick, N.J.). Carbopol 971P (NF) was purchased from BF Goodrich Specialty Chemicals (Cleveland, Ohio).
A range of excipients were initially investigated, but ultimately research focused on carbopol-based aqueous gels due to their mucoadhesive properties and their use in vaginal products and other microbicide formulations. As shown in Table 1, three different gel prototype SPL7013 formulations (1V, 2V, and 3V) were developed containing 5, 1, 0.1, and 0% (wt/wt) SPL7013. The prototypes differed only in the final percentage (weight per weight) of propylene glycol and glycerin. All of the gel formulations contained a final carbopol 971P (NF) concentration of 4.75 to 5% (wt/wt) depending on the final concentration of SPL7013 in the gel. Carbopol 971P (NF) is a cross-linked acrylic acid listed in the USP Monograph as Carbomer 941. After initial evaluations, 1 to 5% SPL7013-containing formulation 2V was also prepared.
TABLE 1.
Excipients for three prototype vaginal microbicide placebo gels
| Excipient | Prototype gel | Amt (wt/wt) (%) | Function |
|---|---|---|---|
| Water for injection, USP | All | 100a | Vehicle |
| Methylparaben, NF | All | 0.18 | Antimicrobial preservative |
| Propylparaben, NF | All | 0.02 | Antimicrobial preservative |
| EDTA, USP | All | 0.1 | Antioxidant |
| Carbopol 971, NF | All | 5.0 | Gelling agent |
| Propylene glycol, USP | 1V | 5.0 | Emollient |
| 2V | 1.0 | ||
| 3V | 0 | ||
| Glycerin, USP | 1V | 5.0 | Emollient |
| 2V | 1.0 | ||
| 3V | 0 | ||
| 2 N NaOH to pH 4.5 | All | 9.0 | pH-adjusting agent |
Make up to 100%.
The gels were made with a Caframo (Wiarton, Ontario, Canada) stirrer, model BDC-1850, by adding the required amount of purified water, EDTA, and then propylene glycol and glycerin. Next, carbopol 971P (NF) was slowly added to avoid clumping, and the formed gel was mixed until the polymer was fully hydrated (∼1 h). The pH was adjusted to 4.5 with 2 N NaOH, and then methylparaben and propylparaben were added and mixed until dissolved. The gel was then made to weight with purified water, and the final pH was adjusted to 4.5 with either 2 N NaOH or 1 N HCl. The viscosity of all gels was measured with a cone and plate rheometer, model RVDV III+ (Brookfield Engineering; Middleboro, Mass.), at 25°C for 5 min at 1.7 rpm by using spindle CPE-52. For all gels, the viscosity of the formulations was in the range of 30,000 to 43,000 cP under the conditions described above.
Each formulation was assessed for toxicity in a 5-day rabbit vaginal-irritation study prior to evaluation. Each of the placebo prototype gels and those gels containing 1 and 5% (wt/wt) SPL7013 elicited the same level of minimal irritation in a 5-day repeat dose rabbit vaginal model (data not shown).
In vitro evaluations.
Confluent Vero cell monolayers in six-well plates were incubated in duplicate with different concentrations of dendrimers ranging from 0.01 to 30 μg/ml at 37°C for 1 h. One hundred PFU of HSV-2 strain G were then added to the cells, and the samples were incubated at 37°C for 1 h. After the inoculum was removed, the cells were washed with PBS and overlaid with 0.5% methylcellulose for a plaque assay. After 2 days the monolayers were fixed with 10% formalin and stained with 0.5% crystal violet as previously described (7). EC50 values were calculated with the Statview computer program.
The cytotoxicity of the compounds was also evaluated by using Vero cells following incubation with various concentrations of the test compounds for 2 days and examination using the neutral-red uptake assay as previously described (7).
Animal models.
All animal protocols were approved by the Cincinnati Children's Hospital Animal Use and Care Committee. All procedures complied with the relevant federal and institutional policies.
Mouse model of genital HSV-2 infection.
As previously described (2, 3) female Swiss Webster mice weighing 18 to 21 g (Harlan, Indianapolis, Ind.) were given 0.1 ml of a suspension containing 3 mg of medroxyprogesterone acetate (Upjohn Pharmacia, Kalamazoo, Mich.) by subcutaneous injection 7 days and 1 day prior to challenge to increase susceptibility to vaginal HSV infection. Animals were then anesthetized, and the vaginas were swabbed with a calcium alginate swab prior to intravaginal inoculation of the formulated or unformulated dendrimer or placebo in a volume of 15 μl. Following various defined intervals the animals were then challenged with 15 μl of a suspension containing 104 PFU of HSV-2 strain 186 applied intravaginally without removal of the preceding treatment material. Vaginal swabs were collected from all animals on day 2 after inoculation and stored frozen (−80°C) until assayed for the presence of virus on susceptible rabbit kidney cells. Animals were then monitored daily for 21 days for evidence of herpetic disease, including hair loss and erythema around the perineum, chronic urinary incontinence, hind-limb paralysis, and death. For the purpose of these studies animals that did not develop symptoms were defined as infected if virus was isolated from the vaginal swab specimens collected on day 2 after inoculation (2, 3).
Guinea pig model of genital herpes.
As previously described, Hartley guinea pigs weighing 275 to 300 g (Charles River Breeding Laboratory, Wilmington, Mass.) were treated intravaginally with 200 μl of formulated dendrimer or placebo, followed by intravaginal inoculation with 200 μl of a suspension containing 106 PFU of HSV-2 strain MS without removal of the preceding treatment material (2). Vaginal swabs were obtained on days 1 and 2 postinoculation and stored frozen (−80°) until assayed for the presence of virus on susceptible rabbit kidney cells. For the purpose of these studies animals that did not develop symptoms were defined as infected if virus was isolated from the vaginal swab specimens collected on day 1 or 2 after inoculation (2).
Statistics.
Incidence data were compared by Fisher's exact test. All comparisons were two-sided. No corrections were made for multiple comparisons.
RESULTS
In vitro.
All three compounds had similar in vitro activities against HSV-2, with no evidence of toxicity even at the highest concentration tested, 1,000 μg/ml (Table 2).
TABLE 2.
Antiviral activity of dendrimers against HSV-2 determined by plaque reduction assaya
Evaluations were repeated five times for SPL7013, twice for SPL7015, and once for SPL7032.
CC50, cytotoxic concentration.
The standard deviation was 0.03 μg/ml.
Animal models. (i) Unformulated dendrimers.
In the initial experiment 10% solutions of SPL7013, -7015, and -7032 (Fig. 1) were evaluated in mice. Significant protection by each compound against disease and infection was observed (Table 3) when the time from treatment to virus challenge was minimal (20 s). From this and similar comparisons and because of the ease of manufacturing, cost, and stability, SPL7013 was chosen for further development.
TABLE 3.
Evaluation of three expanded-spectrum dendrimer products against genital herpes in miceb
| Treatment | Concn (mg/ml) | Fraction (%) of animals protected against:
|
|
|---|---|---|---|
| Disease | Infection | ||
| SPL7013 | 100 | 11/11 (100)a | 11/11 (100)a |
| SPL7015 | 100 | 9/12 (75)a | 8/12 (67)a |
| SPL7032 | 100 | 11/12 (92)a | 11/12 (92)a |
| PBS | 0/12 (0) | 0/12(0) | |
P < 0.005 versus PBS.
Mice were treated 20 s prior to challenge.
In the subsequent experiments either the effect of drug concentration or the duration of protection was evaluated. As seen in Table 4 compound SPL7013 provided significant protection at concentrations as low as 1 mg/ml when the time from treatment to challenge was minimal. As seen in Table 5 this compound, at a concentration of 10 mg/ml, provided significant protection from disease for at least 1 h following administration.
TABLE 4.
Effect of concentration on protection from genital herpes by dendrimer SPL7013 in micec
| Treatment | Concn (mg/ml) | Fraction (%) of animals protected against:
|
|
|---|---|---|---|
| Disease | Infection | ||
| SPL7013 | 100 | 12/12 (100)a | 12/12 (100)a |
| SPL7013 | 10 | 11/12 (92)a | 10/12 (83)a |
| SPL7013 | 1 | 8/12 (67)b | 6/12 (50)b |
| PBS | 0/12 (0) | 0/12(0) | |
P < 0.001 versus PBS.
P < 0.05 versus PBS.
Mice were treated 20 s prior to challenge.
TABLE 5.
Duration of protection by dendrimer SPL7013 against genital herpes In mice
| Treatment | Concn (mg/ml) | Time (min) treated prior to challenge | Fraction (%) of animals protected against:
|
|
|---|---|---|---|---|
| Disease | Infection | |||
| SPL7013 | 10 | 5 | 14/16 (88)a | 14/16 (88)a |
| SPL7013 | 10 | 30 | 13/16 (81)a | 12/16 (75)a |
| PBS | 5 | 0/16 (0) | 0/16 (0) | |
| SPL7013 | 10 | 60 | 5/15 (33)b | 4/15 (27) |
| PBS | 5 | 0/15 (0) | 0/15 (0) | |
P < 0.001 versus PBS.
P < 0.05 versus PBS.
(ii) Formulated dendrimers.
Three different formulations of dendrimer SPL7013 were then prepared at the University of Kentucky at concentrations of 1 and 5% (Table 1). In the initial experiment each formulation of 1% SPL7013 was evaluated in the mouse model of genital HSV infection. As seen in Table 6 each formulation provided significant protection when administered 5 min prior to intravaginal challenge. Note also that the placebo formulation provided some protection against disease but not infection. This is most likely due to the buffering effect of the formulation in maintaining the acid pH of the vagina. In the subsequent experiment the duration of protection out to 30 min after treatment with the 5% concentration of each formulation was evaluated. Again, significant protection against infection and disease was provided by each formulation (Table 7). The 2V formulation was chosen for further evaluation and was shown to provide significant protection at a concentration of 1% for 30 min in two experiments and for at least 1 h after application in the one experiment where this was evaluated (Table 8).
TABLE 6.
Evaluation of three 1% formulations of dendrimer SPL7013 against genital herpes in micee
| Treatment | Concn (mg/ml) | Fraction (%) of animals protected against:
|
|
|---|---|---|---|
| Disease | Infection | ||
| SPL7013 formulation 1V | 10 | 11/16 (69)a,c | 11/16 (69)a,d |
| Placebo 1V | 3/16 (19) | 1/16 (6) | |
| SPL7013 formulation 2V | 10 | 12/16 (75)a | 12/16 (75)a,c |
| Placebo 2V | 7/16 (44)b | 4/16 (25) | |
| SPL7013 formulation 3V | 10 | 13/15 (87)a,c | 12/15 (80)a,c |
| Placebo 3V | 6/16 (38)b | 4/16 (25) | |
| PBS | 1/16 (6) | 1/16 (6) | |
P < 0.001 versus PBS.
P < 0.05 versus PBS.
P < 0.05 versus placebo.
P < 0.001 versus placebo.
Mice were treated 5 min prior to challenge.
TABLE 7.
Evaluation of three 5% formulations of dendrimer SPL7013 against genital herpes in mice
| Treatment | Concn (mg/ml) | Time (min) treated prior to challenge | Fraction (%) of animals protected against:
|
|
|---|---|---|---|---|
| Disease | Infection | |||
| SPL7013 formulation 1V | 50 | 30 | 10/16 (63)a | 10/16 (63)a |
| SPL7013 formulation 2V | 50 | 30 | 15/16 (94)a | 15/16 (94)a |
| SPL7013 formulation 3V | 50 | 30 | 16/16 (100)a | 16/16 (100)a |
| SPL7013 formulation 1V | 50 | 5 | 14/16 (88)a | 13/16 (81) |
| SPL7013 formulation 2V | 50 | 5 | 15/16 (94)a | 15/16 (94)a |
| SPL7013 formulation 3V | 50 | 5 | 15/16 (94)a | 15/16 (94)a |
| PBS | 5 | 0/16 (0) | 0/16 (0) | |
P < 0.001 versus PBS.
TABLE 8.
Evaluation of duration of protection of 1% 2V formulation of SPL7013 against genital herpes in mice
| Treatment | Concn (mg/ml) | Time (min) treated prior to challenge | Fraction (%) of animals protected against:
|
|
|---|---|---|---|---|
| Disease | Infection | |||
| SPL7013 formulation 2V | 10 | 5 | 8/15 (53)a | 8/15 (53)a |
| SPL7013 formulation 2V | 10 | 30 | 9/15 (60)a | 8/15 (53)a |
| SPL7013 formulation 2V | 10 | 60 | 6/15 (40)b | 6/15 (40)b |
| PBS | 5 | 0/15 (0) | 0/15 (0) | |
| SPL7013 formulation 2V | 10 | 5 | 8/12 (67)a | 8/12 (67)a |
| SPL7013 formulation 2V | 10 | 30 | 8/12 (67)a | 8/12 (67)a |
| PBS | 5 | 0/12 (0) | 0/12 (0) | |
P < 0.01 versus PBS.
P < 0.05 versus PBS.
The 2V formulation of SPL7013 was further evaluated in the guinea pig model of genital herpes because this model, it is felt, better mimics human disease (13). In the initial experiments 1 to 5% concentrations of SPL7013 in formulation 2V were applied 5 min prior to virus challenge. As seen in Table 9, protection appeared to be dose dependant, with increased protection at 3 to 5% concentrations. The experiment was repeated to determine if the decreased activity of the 30-mg/ml dose would be confirmed. Repeat experiments showed that protection with this concentration was not diminished in comparison to that with lesser concentrations. Thus, the second experiment confirmed the high protection rates provided by the 3 and 5% concentrations and were consistent with dose-dependant activity. The activity seen in the placebo recipients in the first experiment is consistent with that observed in some of the mouse studies (Table 6) with formulation 2V.
TABLE 9.
Evaluation of protection of different concentrations of formulation 2V of SPL7013 against genital herpes in guinea pigsd
| Treatment | Concn (mg/ml) | Fraction (%) of animals protected against:
|
|
|---|---|---|---|
| Disease | Infection | ||
| SPL7013 formulation 2V | 10 | 7/15 (47) | 5/15 (33) |
| SPL7013 formulation 2V | 20 | 10/15 (67) | 9/15 (60)a |
| SPL7013 formulation 2V | 30 | 7/15 (47) | 7/15 (47)a |
| SPL7013 formulation 2V | 40 | 11/15 (73)a | 10/15 (67)a |
| SPL7013 formulation 2V | 50 | 12/15 (80)a | 11/15 (73)a |
| Placebo gel | 10/15 (67) | 8/15 (53)a | |
| PBS | 4/15 (27) | 1/15 (7) | |
| SPL7013 formulation 2V | 30 | 16/18 (89)b,c | 15/18 (83)b,c |
| SPL7013 formulation 2V | 50 | 17/18 (94)b,c | 16/18 (89)b,c |
| Placebo gel | 3/18 (17) | 2/18 (11) | |
| PBS | 4/18 (22) | 3/18 (17) | |
P < 0.05 versus PBS.
P < 0.001 versus PBS.
P < 0.001 versus placebo.
Animals were treated 5 min prior to challenge.
DISCUSSION
The continued HIV epidemic and ongoing increases in the prevalence of genital HSV-2 and other STIs underscore the need for a safe effective user-controlled strategy to prevent these infections. Microbicides offer one such strategy. Because of the lack of efficacy and possible deleterious effects of N-9, a nonionic surfactant that disrupts lipid membranes, such as viral envelopes (16, 18), compounds that inhibit binding, such as polyanions, rather than acting as detergents are receiving increased attention (reviewed in references 10, 14, and 15). One potential drawback of the polyanions in clinical development as topical microbicides is that they are mixtures of compounds. PRO 2000 (1, 9), for example, is a polymer mixture of between 4 and 6 kDa, and Carraguard contains various carbohydrates with various levels of sulfation. In contrast, SPL7013 has been characterized by mass spectrometry, capillary electrophoresis, and high-pressure liquid chromatography, and in-process controls have been developed to tightly control the synthesis. As a result SPL7013 has entered full preclinical development as a topical microbicide.
In this paper we have shown that dendrimer SPL7013 provides protection from infection and disease in the mouse model of genital herpes even at concentrations as low as 1 mg/ml and for at least 1 h after administration. Similarly, after formulation this candidate microbicide remained active when used in the guinea pig model of genital herpes. Thus, despite the increased size, vaginal vault area, and higher dose of virus used in the guinea pig model, the high activity was maintained. Note also that, although good activity was maintained after formulation, there was no obvious advantage to the formulated product. Continuing evaluations are aimed at determining if the formulated products have advantages either in the duration of protection or dose effects in both the mouse and guinea pig models. Further, whether there might be advantages in larger animals, such as the primates that are currently being evaluated and humans, remains to be determined. The goal of the formulation should be to increase the spread of the material so it is more effective, increase the time it is present in the vaginal cavity through mucoadhesive or other properties to increase the duration of protection, or provide additional activity, for example, by maintaining the vaginal pH.
From both the mouse and guinea pig evaluations it appears that concentrations of 3% or higher of the formulated product may be necessary for optimal protection. Because of the encouraging results with this formulated dendrimer in the experiments presented here, evaluations in monkey models of simian/human immunodeficiency virus and chlamydia are ongoing. Dendrimer SPL7013 is one of the leading candidates to fulfill the difficult requirements of a microbicide to be safe yet active against a number of STIs.
Acknowledgments
This work was supported by NIAID contract AI 15439 and SBIR grant R43 AI 47548.
REFERENCES
- 1.Bourne, N., D. I. Bernstein, J. Ireland, A. J. Sonderfan, A. T. Profy, and L. R. Stanberry. 1999. The topical microbicide PRO 2000 protects against genital herpes infection in a mouse model. J. Infect. Dis. 180:203-205. [DOI] [PubMed] [Google Scholar]
- 2.Bourne, N., J. Ireland, L. R. Stanberry, and D. I. Bernstein. 1999. Effect of undecylenic acid as a topical microbicide against genital herpes infection in mice and guinea pigs. Antivir. Res. 40:139-144. [DOI] [PubMed] [Google Scholar]
- 3.Bourne, N., L. R. Stanberry, E. R. Kern, G. Holan, B. Matthews, and D. I. Bernstein. 2000. Dendrimers, a new class of candidate topical microbicides with activity against herpes simplex virus infection. Antimicrob. Agents Chemother. 44:2471-2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ebrahim, S. H., T. A. Peterman, A. A. Zaidi, and M. L. Kamb. 1997. Mortality related to sexually transmitted diseases in US women, 1973 through 1992. Am. J. Public Health 87:938-944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fisman, D. N., M. Lipsitch, E. W. Hook III, and S. J. Goldie. 2002. Projection of the future dimensions and costs of the genital herpes simplex type 2 epidemic in the United States. Sex. Transm. Dis. 29:608-622. [DOI] [PubMed] [Google Scholar]
- 6.Fleming, D. T., G. M. McQuillan, R. E. Johnson, A. J. Nahmias, S. O. Aral, F. K. Lee, and M. E. St. Louis. 1997. Herpes simplex virus type 2 in the United States, 1976 to 1994. N. Engl. J. Med. 337:1105-1111. [DOI] [PubMed] [Google Scholar]
- 7.Gong, Y., B. Matthews, D. Cheung, T. Tam, I. Gadawski, D. Leung, G. Holan, J. Raff, and S. Sacks. 2002. Evidence of dual sites of action of dendrimers: SPL-2999 inhibits both virus entry and late stages of herpes simplex virus replication. Antivir. Res. 55:319-329. [DOI] [PubMed] [Google Scholar]
- 8.Koutsky, L. A., K. A. Ault, C. M. Wheeler, D. R. Brown, E. Barr, F. G. Alvarez, L. M. Chiacchierini, and K. U. Jansen. 2002. A controlled trial of a human papillomavirus type 16 vaccine. N. Engl. J. Med. 347:1645-1651. [DOI] [PubMed] [Google Scholar]
- 9.Mayer, K. H., S. A. Karim, C. Kelly, L. Maslankowski, H. Rees, A. T. Profy, J. Day, J. Welch, and Z. Rosenberg. 2003. Safety and tolerability of vaginal PRO 2000 gel in sexually active HIV-uninfected and abstinent HIV-infected women. AIDS 17:321-329. [DOI] [PubMed] [Google Scholar]
- 10.McCormack, S., R. Hayes, C. J. Lacey, and A. M. Johnson. 2001. Microbicides in HIV prevention. BMJ 322:410-413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smith, J. S., and N. J. Robinson. 2002. Age-specific prevalence of infection with herpes simplex virus types 2 and 1. J. Infect. Dis. 186(Suppl.):S3-S28. [DOI] [PubMed] [Google Scholar]
- 12.Stanberry, L. R., S. L. Spruance, A. L. Cunningham, D. I. Bernstein, A. Mindel, S. Sacks, S. Tyring, F. Y. Aoki, M. Slaoui, M. Denis, P. Vandepapeliere, and G. Dubin. 2002. Glycoprotein-D-adjuvant vaccine to prevent genital herpes. N. Engl. J. Med. 347:1652-1661. [DOI] [PubMed] [Google Scholar]
- 13.Stanberry, L. R. 1991. Evaluation of herpes simplex virus vaccines in animals: the guinea pig vaginal model. Rev. Infect. Dis. 11(Suppl.):S920-S923. [DOI] [PubMed] [Google Scholar]
- 14.Stone, A. 2002. Microbicides: a new approach to preventing HIV and other sexually transmitted infections. Nat. Rev. Drug Discov. 1:977-985. [DOI] [PubMed] [Google Scholar]
- 15.Turpin, J. A. 2002. Considerations and development of topical microbicides to inhibit the sexual transmission of HIV. Expert Opin. Investig. Drugs 11:1077-1097. [DOI] [PubMed] [Google Scholar]
- 16.Van Damme, L., G. Ramjee, M. Alary, B. Vuylsteke, V. Chandeying, P. Rees, L. Sirivongrangson, V. Mukenge-Tshibaka, C. Ettiegne-Traore, C. Uaheowitchai, S. S. Karim, B. Masse, J. Perriens, M. Laga, and the COL-1492 Study Group. 2002. Effectiveness of COL-1492, a nonoxynol-9 vaginal gel, on HIV-1 transmission in female sex workers: a randomized controlled trial. Lancet 360:971-977. [DOI] [PubMed] [Google Scholar]
- 17.Wald, A., and K. Link. 2002. Risk of human immunodeficiency virus in herpes simplex virus type 2-seropositive persons: a meta analysis. J. Infect. Dis. 185:45-52. [DOI] [PubMed] [Google Scholar]
- 18.Wilkinson, D., M. Tholandi, G. Ramjee, and G. W. Rutherford. 2002. Nonoxynol-9 spermicide for prevention of vaginally acquired HIV and other sexually transmitted infections: systematic review and meta-analysis of randomized controlled trials including more than 5000 women. Lancet Infect. Dis. 2:613-617. [DOI] [PubMed] [Google Scholar]
- 19.Witvrouw, M., V. Fikkert, W. Pluymers, B. Matthews, K. Mardel, D. Schols, J. Raff, Z. Debyser, E. De Clercq, G. Holan, and C. Pannecouque. 2000. Polyanionic (i.e., polysulfonate) dendrimers can inhibit the replication of human immunodeficiency virus by interfering with both virus adsorption and later steps (reverse transcriptase/integrase) in the virus replicative cycle. Mol. Pharmacol. 58:1100-1108. [PubMed] [Google Scholar]