Abstract
Resiquimod is a Toll-like receptor 7 (TLR7) and TLR8 agonist that is a potent inducer of alpha interferon (IFN-α) and other cytokines. The effects of multiple applications of resiquimod gel were assessed in a randomized, single-blind, dose-ranging, placebo-controlled study with 41 healthy subjects. Over a 3-week period, 1-g doses of resiquimod or vehicle gel (3:1 randomization) were applied to a 50-cm2 area of the upper arm according to the following regimens: 0.25% applied for 8 h two times per week, 0.05% applied for 8 h two times per week, 0.05% applied for 8 h three times per week, and 0.01% applied for 24 h three times per week. Skin biopsy specimens were obtained prior to the application of the first dose and after the completion of application of the last dose. Dosing with 0.01 and 0.05% resiquimod was well tolerated, but that with 0.25% was not; a dose-response relationship for local adverse effects was observed. The level of systemic exposure during multiple topical dosings was <1% of the applied dose. A significant increase in responders after completion of application of the last dose was observed for serum IFN and the interleukin-1 (IL-1) receptor antagonist (P < 0.01, Fisher's exact test). Increased levels of mRNA for IL-6, IL-8, IFN-α, and Mx (an IFN-α-inducible protein) were seen in posttreatment biopsy specimens from the group receiving 0.25% resiquimod compared to the levels seen in specimens from the group receiving vehicle only (P < 0.01, Wilcoxon rank sum test). A dose-response-related increase in CD3-positive cells consistent with T-lymphocyte infiltration and a decrease in CD1a-positive cells, consistent with emigration of Langerhans' cells, were observed in treated skin. This study suggests that resiquimod is a potent topically active immune response modifier that significantly enhances the cutaneous immune response.
Resiquimod (R-848; S-28463; 4-amino-2-ethoxymethyl-α,α-dimethyl-1H-imidazo[4,5-c]quinoline-1-ethanol) induces endogenous production of alpha interferon (IFN-α), interleukin 12 (IL-12), tumor necrosis factor alpha, and other cytokines from peripheral blood mononuclear cells, monocytes, and dendritic cells (DCs) (16, 19). In comparison to the related imidazoquinoline imiquimod (R-837), resiquimod is approximately 100 times more effective on a weight basis in inducing cytokines in vitro and in vivo (15, 16). The relative profile of induced cytokines is different: in peripheral blood mononuclear cell cultures, resiquimod induces larger amounts of IL-12 directly and larger amounts of IFN-γ indirectly compared with the levels induced by imiquimod (19). Resiquimod is also more effective in enhancing antigen presentation by DCs (3). Induction of these cytokines by both of these small molecules appears to involve the Toll-like receptor (TLR) signaling pathway, as induction is absent in TLR7- and MyD88-deficient mice (8) and present in HEK293 cells only when these cells have been transfected with human TLR8 (11).
A 5% imiquimod cream formulation (Aldara) is used for the treatment of external anogenital warts (2, 4). After topical application, cytokine-specific mRNA is induced; local inflammation may also be observed during treatment of anogenital warts, consistent with the pharmacologic effect of the induced cytokines, some of which have proinflammatory properties (4, 18). Although resiquimod has been less well studied, in animals it has been shown to induce local cytokines in the skin after topical administration (9), as well as to induce systemic cytokines and biomarkers after oral administration (16). In rats, approximately 8.5% of a topical application of a 14C-labeled resiquimod gel formulation was absorbed (A. M. Draper, G. L. Carlson, M. Berge, C. Powers, A. Ginkel, and V. L. Horton, Abstr. Am. Int. Soc. Study Xenobiot., abstr. 339, 1996). In a study of a single dose of resiquimod gel applied to healthy human skin, minimal effects were observed with respect to local tolerance and local cytokine mRNA induction at resiquimod concentrations of up to 0.25% (D. Sauder, M. Tomai, D. McDermott, M. Smith, T. Senta, and T. C. Meng, Abstr. 38th Intersci. Conf. Antimicrob. Agents Chemother., abstr. A090, 1998).
To assess the effects of topical application of resiquimod in humans, a randomized, single-blind, placebo-controlled study of the application of resiquimod gel to healthy human skin was conducted with healthy adults. In addition to safety assessments, levels of resiquimod and the metabolite S-28371 in serum and urine were measured to determine resiquimod pharmacokinetics after topical administration. Serum cytokine and cytokine-inducible biomarker levels were measured to evaluate systemic pharmacodynamic effects, while assays for cytokine mRNA levels and immunohistologic analysis of dermal biopsy specimens were performed to investigate local pharmacodynamic effects.
MATERIALS AND METHODS
Study population.
The study protocol was approved by the Research Consultants' Review Committee, Austin, Tex. The study was conducted under Good Clinical Practice guidelines. All study subjects gave written informed consent prior to the initiation of study procedures. The study was conducted at the Phase I unit of PPD Pharmaco, Austin, Tex.
Study subjects had to be healthy, between the ages of 18 and 50 years, and nonsmokers during the previous 12 months; to have a weight ≥70 kg (males) or ≥55 kg (females); and to be within 10% of ideal body weight relative to height and frame size and free of significant abnormalities and tattoos at the application site. Subjects were excluded if they had a positive serum hepatitis B surface antigen test or a positive urine screen for drugs or alcohol; had received IFN-α or an IFN-α inducer within the previous 8 weeks; had received any investigational, immunomodulator, steroid, antiviral, or cytotoxic drug or any drug known to have major organ toxicity within the previous 4 weeks; had received any medication (except acetaminophen) within the previous 1 week; had used acetaminophen or ingested any caffeine-containing food or beverage within 72 h of administration of dose 1; had donated one of more pints of whole blood within the previous 30 days; or had experienced a bacterial or viral infection within the previous 4 weeks. In addition, subjects were excluded if they had a history of recent allergy or asthma, seizure disorders, keloid formation, or chemical exposure or dependency (including alcohol). Women were required to be either surgically sterilized or at least 2 years beyond menopause in order to participate in this study.
Study designs.
Resiquimod gel and matching vehicle were provided by 3M Pharmaceuticals, Saint Paul, Minn.
The four treatment regimens studied were 0.25% resiquimod for 8 h two times per week for 3 weeks (0.25Resi2x), 0.05% resiquimod for 8 h two times per week for 3 weeks (0.05Resi2x), 0.05% resiquimod for 8 h three times per week for 3 weeks (0.05Resi3x), and 0.01% resiquimod for 24 h three times per week for 3 weeks (0.01Resi3x). Subjects were enrolled into sequential dosing cohorts, and each consisted of four male and four female subjects. Within each regimen six of the subjects were randomly assigned to active treatment with resiquimod gel and two (one male and one female) were randomly assigned to vehicle. The study subjects and the clinic personnel administering the study drug were blinded to treatment assignment.
Each dose consisted of 1 g of gel. Dose applications were separated from each other by at least 36 h. Prior to application of study drug, dermal biopsy specimens were obtained from the area adjacent to the planned application site. Clinic staff then applied the study drug to a 50-cm2 area of the skin on the upper arm of the subject and lightly rubbed the study drug for even coverage, and the drug was allowed to air dry for at least 30 min. The application site was covered with loose mesh gauze to protect the site. Eight or 24 h after application of study drug (depending on the dosing regimen), the study drug was removed by washing with soap and water followed by washing with isopropyl alcohol. Each dose of study drug was applied to the same site. Dermal biopsy specimens were obtained from the site of application 24 h after application of the last dose of study drug. Subjects were domiciled for the first dose and the last dose (through 48 h after dose application). All study drug dosing occurred in the clinic and was applied by clinic staff.
Safety parameters were assessed by performance of a physical examination, complete blood count and differential, chemistry panel, urinalysis, and electrocardiogram and by determination of vital signs, adverse events, and concomitant medication usage. Specific defined local adverse events, including erythema, edema, induration, vesicles, erosions, ulcerations, excoriation, and scabbing at the application site, were collected separately from other adverse events. The presence of these defined local skin reactions (LSRs) were assessed prior to the application of each dose, after removal of the first and final doses, and approximately 24 h after application of the study drug for all doses. Each of these LSRs was graded by both the subject and the investigator by using the following scale: none, mild (visible LSR without discomfort or with minimal discomfort which does not disrupt normal activities), moderate (visible LSR with considerable discomfort which interferes with or restricts but does not disrupt normal activities), and severe (visible LSR with considerable discomfort which disrupts normal activities).
To assess the pharmacokinetics of systemic exposure after topical administration of resiquimod gel, the levels of resiquimod and the metabolite S-28371 in serum were assessed at 0 h (predosing), 12 and 24 h after application of the first dose, and after application of the final dose. Urine was collected over 48 h after application of the first dose and after application of the final dose. After solid-phase extraction of the samples, resiquimod and S-28371 were quantified by separation by reversed-phase liquid chromatography and fluorescence detection. The bioanalytical method was validated with respect to linearity, specificity, intra- and interday precision and accuracy, recovery, and stability. The lower limits of quantitation were 20 pg/ml in serum and 10 pg/ml in urine.
Systemic pharmacodynamics were assessed by measurement of serum cytokine and biomarker levels. Serum IFN activity was measured predosing and at 2, 6, 12, 24, and 48 h after application of the first and final doses by bioassay by using a reduction in cytopathic effect (5). Serum 2′,5′-oligoadenylate synthetase (2′,5′-AS) activity was measured predosing and at 24 and 48 h after application of the first and final doses by radioimmunassay (20). Serum IL-1 receptor antagonist (IL-1RA) levels were measured predosing and at 4, 8, 12, and 24 h after application of the first and final doses by enzyme-linked immunosorbent assay (R&D Systems Inc., Minneapolis, Minn.). Serum neopterin levels were measured predosing and at 8, 12, 24, and 48 h after application of the first and final doses by radioimmunoassay (Henning, Berlin, Germany).
To assess the local biological response to resiquimod application, dermal biopsy specimens were taken before application of the first dose and 24 h after application of the last dose. After the skin was cleaned and anesthetized (2% lidocaine), two 4-mm dermal punch biopsy specimens were obtained. One of the biopsy samples was quick-frozen in liquid nitrogen and stored at −70°C until RNA was extracted. RNA was extracted by using RNA STAT-60 (Tel-Test, Inc., Friendswood, Tex.), with minor modifications (14). Total mRNA was reverse transcribed (12). Semiquantitative PCR was performed with oligonucleotide primer sets either selected and purchased from Clontech Laboratories (Palo Alto, Calif.) or synthesized by Dalton Chemical Laboratories (Toronto, Ontario, Canada). Where applicable, the primer sets were designed to cross intron-exon boundaries to allow differentiation of PCR products from genomic DNA contaminants. The sequences of the primer sets are as follows: for glyceraldehyde-3-phosphate dehydrogenase (G3PDH), 5′-TGAAGGTCGGAGTCAACGGATTTGGT and 3′-CATGTGGGCCATGAGGTCCACCAC; for IFN-α, 5′-TGATGGCAACCAGTTCCAGAAGGCTCAAG and 3′ACAACCTCCCAGGCACAAGGGCTGTATTT; for IL-6, 5′-ATGAACTCCTTCTCCACAAGCGC and 3′-GAAGAGCCCTCAGGCTGGACTG; for IL-8, 5′-ATGACTTCCAAGCTGGCCGTGGCT and 3′-TCTCAGCCCTCTTCAAAAACTTCTC; and for Mx (an IFN-α-inducible protein), 5′-GTGTGGAGCAGGACCTGGCCCTGCCAG and 3′-CTGCCTCTGGATGTACTTCTTGATGAG. cDNA was amplified as described previously(13) by using the following cycle: denaturation for 1 min at 94°C, annealing for 1 min at either 60°C (G3PDH, IL-6, IL-8) or 66°C (IFN-α, Mx), and primer extension for 1 min at 72°C. The optimum numbers of cycles were determined in pilot experiments and were 25 cycles for IFN-α, 28 cycles for IL-6 and IL-8, 24 to 28 cycles for G3PDH, and 34 cycles for Mx. The samples were then separated by electrophoresis, and the PCR products were quantified by densitometry after silver staining (7). The relative PCR product yields among the different samples were determined by normalizing the cytokine densitometric values to those for G3PDH.
The second 4-mm dermal punch biopsy specimen was obtained for immunohistology. One half was placed in 10% buffered formalin and stored at room temperature for no more than 24 h, while the other half was placed in optimum-cutting tissue compound (Miles, Inc., Elkhart, Ind.), snap-frozen in liquid nitrogen, and stored at −70°C. Formalin-fixed sections were embedded in paraffin before they were cut; frozen sections were cut on a cryostat, thaw-mounted onto gelatin-coated slides, and stored at −20°C. Primary antibodies were either murine or rabbit anti-human monoclonal antibodies. Murine anti-CD4, murine anti-CD8, murine anti-HLA-DR, murine anti-leukocyte common antigen/CD45, and rabbit anti-human CD3 were obtained from DAKO (Mississauga, Ontario, Canada). Murine anti-CD1a was obtained from Immunotech (Marseille, France). Secondary antibodies were either biotinylated goat anti-mouse immunoglobulin G (IgG; Zymed Laboratories, Inc., San Francisco, Calif.) or biotinylated goat anti-rabbit IgG (Biogenex, San Ramon, Calif.). After deparaffinization, the sections were quenched in 3% hydrogen peroxide in methanol for 15 min, blocked in 0.5% casein and 0.05% thimerosal in water for 15 min, labeled with primary antibody (dilution, 1:50 to 1:200) for 2 h, washed, and labeled with secondary antibody (1:100 dilution for CD3, 1:200 dilution for the others) for 1 h. All incubations were performed at room temperature in a humidified chamber; washes were performed with 50 mM Tris-HCl (pH 7.6). Immunoperoxidase labeling was performed by the biotin-streptavidin peroxidase technique with diaminobenzidine according to the manufacturer’s instructions(Research Genetics, Inc., Huntsville, Ala.), and the sections were counterstained with Harris hematoxylin. Coded immunoperoxidase-stained sections were analyzed at ×200 magnification, and stained cells from at least five high-power fields per slide were enumerated to quantify cell phenotypes.
All personnel conducting laboratory analyses were blinded to subject treatment assignments.
Statistical methods.
No sample size calculations were performed before the study was conducted, as the studies were expected to provide only general trends. For each serum cytokine marker, a response was determined for each subject and a Fisher's exact test was used to test for differences across treatment groups. For dermal cytokines and dermal immunohistologic markers, changes from prestudy values were calculated and pairwise comparisons of each resiquimod group to the vehicle group were done by using Wilcoxon rank sum tests. The presence of adverse events considered to be related to systemic cytokine effects was correlated with the change from the prestudy value for each serum cytokine (maximum change at dose 1 or the last dose), dermal cytokine mRNA, and dermal immunohistologic marker by using a Spearman rank correlation. The incidence of adverse events was summarized by treatment group. For each LSR, the incidence at each visit was summarized by treatment group. A cumulative LSR score was calculated by summing all LSR scores at day 3 (after first dose), day 11 (after the application of three doses for those receiving the study drug two times per week and after the application of five doses for those receiving the study drug three times per week regimens), and day 20 (after application of the last dose) for each subject.
RESULTS
Subject disposition.
The initial design of this study called for a total of 32 subjects, 8 for each dosing regimen, enrolled in cohorts in a dose-escalating study beginning with 0.25Resi2x. However, because of the limited tolerance of that regimen, the dosing regimens for subsequent cohorts were altered. In addition, posttreatment biopsy specimens for all eight subjects in the original 0.05Resi2x cohort were damaged; therefore, a second cohort that received 0.05Resi2x was enrolled. Nonbiopsy results for the subjects who received resiquimod in both 0.05Resi2x cohorts were combined for the 0.05Resi2x group. The results for subjects who received the vehicle in each of the cohorts were combined within the vehicle group. In addition, one subject who was discontinued for a non-adverse-event-related reason was replaced. Therefore, a total of 41 subjects were enrolled in five cohorts representing five treatment groups, including the vehicle (Table 1). The first subject was enrolled on 20 November 1996, and the last subject completed the study on 5 June 1997. During the study, one subject was discontinued for each of the following reasons: LSRs (0.25Resi2x cohort), positive urine test for cannabis (0.05Resi2x cohort), urinary tract infection (0.05Resi3x cohort), and asymptomatic ventricular trigeminy (0.05Resi3x cohort). The investigators attributed only the discontinuation for LSRs to study drug. No deaths or serious adverse events occurred during the study. Thirty-seven of 41 subjects completed all study procedures.
TABLE 1.
Subject characteristics
| Characteristic | 0.25Resi2x (n = 6) | 0.05Resi3x (n = 6) | 0.05Resi2x (n = 12)a | 0.01Resi3x (n = 7) | Vehicle (n = 10)b | Total (n = 41) |
|---|---|---|---|---|---|---|
| Sex (no. [%] of subjects) | ||||||
| Male | 4 (67) | 3 (50) | 5 (42) | 3 (43) | 5 (50) | 20 (49) |
| Female | 2 (33) | 3 (50) | 7 (58) | 4 (57) | 5 (50) | 21 (51) |
| Ethnicity (no. [%] of subjects) | ||||||
| African American | 0 | 0 | 1 (8) | 1 (14) | 1 (10) | 3 (7) |
| Caucasian | 5 (83) | 5 (83) | 9 (75) | 6 (86) | 7 (70) | 32 (78) |
| Hispanic | 1 (17) | 1 (17) | 2 (17) | 0 | 2 (20) | 6 (15) |
| Mean ± SD age (yr [range]) | 36.7 ± 10.3 (26-50) | 48.3 ± 11.3 (27-59) | 42.3 ± 13.4 (22-59) | 51.1 ± 10.8 (27-58) | 43.5 ± 11.4 (24-60) | 44.2 ± 12.1 (22-60) |
Includes resiquimod-treated subjects from both cohorts assigned to 0.05Resi2x.
Data for vehicle-treated subjects from each cohort are pooled.
Concentrations of resiquimod and S-28731 in serum.
None of the subjects in any of the groups had quantifiable concentrations of resiquimod or S-28371 in serum following administration of the first and last doses, with the exception of the samples for a subject in the 0.25Resi2x group after administration of the last dose. This subject had resiquimod concentrations of 134, 230, and 152 pg/ml before administration of the last dose and 8 and 12 h after administration of the last dose, respectively.
Concentrations of resiquimod in urine.
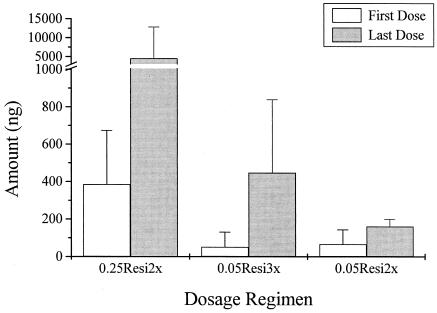
High intersubject variabilities in the urinary excretion of resiquimod were observed. The mean 24-h levels (0 to 48 h) of resiquimod and S-28371 in urine after administration of the first and last doses are depicted in Fig. 1. The 0.25Resi2x and 0.05Resi3x groups had approximately 10-fold increases in the levels in urine after administration of the last dose compared with the level after administration of the first dose. The 0.05Resi2x group also had increases, although they were not as great. Mean levels could not be determined for the 0.01Resi3x group because most values were below the limits of detection. As assessed by the amounts of resiquimod and S-28371 recovered in urine, systemic exposure during multiple topical dosing was minimal, accounting for <1% of the applied dose.
FIG. 1.
Mean ± standard deviation total amounts of resiquimod and S-28371 combined, after sample hydrolysis, excreted in urine (0 to 48 h) during multiple topical dosing of resiquimod. Mean levels could not be determined for those receiving the 0.01Resi3x regimen because most values were below the detection limits.
Systemic cytokines and inducible markers.
The serum cytokine responses are depicted in Table 2. For this analysis, any increase in IFN activity from the baseline level was considered a response, while a doubling of the response over the baseline level was considered a response for neopterin, 2′,5′-AS, and IL-1RA. No differences in the percentages of responders after the administration a single dose were seen among the treatment groups. A significant increase in the number of responders was observed after administration of the last dose for both IFN and IL-1RA (P < 0.01, Fisher's exact test), but no significant increase was seen for neopterin or 2′,5′-AS. The presence of an adverse event considered attributable to systemic cytokine effects as a result of study drug administration (e.g., fever, myalgias, arthralgias, and neutropenia) was significantly correlated with a maximum change in the levels of IFN, 2′,5′-AS, and IL-1RA in serum after administration of the last dose (P < 0.05 for each marker, Spearman rank correlation).
TABLE 2.
Responders for serum immune markers after application of the first dose and the final dose, by treatment group
| Dose and marker | No. (%) of subjects who were respondersa
|
|||||
|---|---|---|---|---|---|---|
| 0.25Resi2x (n = 6) | 0.05Resi3x (n = 6) | 0.05Resi2x (n = 12)c | 0.01Resi3x (n = 7) | Vehicle (n = 10)d | P valueb | |
| First dose | ||||||
| IFN | 0 | 0 | 0 | 0 | 0 | 1.000 |
| Neopterin | 0 | 0 | 1 (8) | 0 | 0 | 1.000 |
| 2′,5′-AS | 3 (50) | 1 (17) | 2 (17) | 2 (29) | 3 (30) | 0.649 |
| IL-1RA | 0 | 0 | 0 | 0 | 0 | 1.000 |
| Final dose | ||||||
| IFN | 4 (67) | 1 (17) | 0 | 0 | 1 (10) | 0.006 |
| Neopterin | 1 (17) | 1 (17) | 1 (9) | 0 | 1 (10) | 1.000 |
| 2′,5′-AS | 4 (67) | 2 (33) | 3 (27) | 2 (40) | 4 (40) | 0.650 |
| IL-1RA | 5 (83) | 2 (33) | 0 | 0 | 1 (10) | <0.001 |
A responder is defined as a subject with any increase in the IFN level over the baseline level and a doubling of the neopterin, 2′,5′-AS, or IL-1RA levels over the baseline levels.
Fisher's exact test.
Includes resiquimod-treated subjects from both cohorts assigned to 0.05Resi2x.
Data for vehicle-treated subjects from each cohort are pooled.
Local biological response. (i) Expression of mRNA for cytokines and inducible markers.
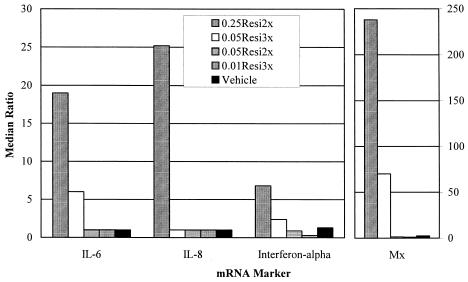
Median changes in relative cytokine mRNA levels in dermal biopsy specimens are depicted in Fig. 2. There were significant changes in the levels of mRNA for IL-6, IL-8, IFN-α, and Mx compared with the prestudy levels (P < 0.01, Wilcoxon rank sum test) when the 0.25Resi2x group was compared to the vehicle group. There were also increases in IL-6, IFN-α, and Mx mRNA levels for the 0.05Resi3x group, although only the level of Mx mRNA was significantly (P < 0.05) different from that for the vehicle group. No other significant changes were observed. The presence of an adverse event considered attributable to systemic cytokine effects as a result of study drug administration was significantly correlated with the change in the ratio of the levels of mRNA for IL-6, IL-8, and Mx in dermal biopsy specimens compared with the levels prestudy (P < 0.05 for each marker, Spearman rank correlation).
FIG. 2.
Median change in relative cytokine mRNA levels in dermal skin biopsy specimens expressed as the ratio of the mRNA levels after administration of the last dose divided by the levels before administration of the first dose (y axis shows median ratio compared to baseline). Individual cytokine levels, as determined by semiquantitative reverse transcriptase PCR, were first normalized to the G3PDH mRNA levels from the respective samples.
(ii) Immunohistology.
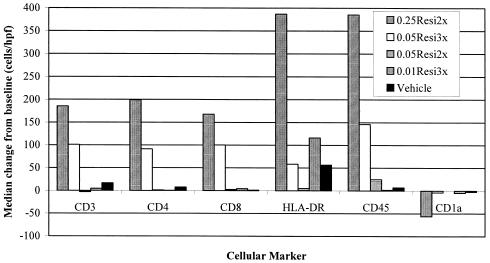
Dermal immunohistology revealed a marked increase in the number of cells per high-power field that were positive for CD3, CD4, CD8, CD45, and HLA-DR as well as a decrease in the number of cells positive for CD1a (Fig. 3). There was a statistically significant difference in the levels of each of these markers when the absolute changes from the prestudy levels between the 0.25Resi2x group and the vehicle group were compared (P < 0.05, Wilcoxon rank sum test). There were no other statistically significant changes for the other groups, although some changes were seen for the 0.05Resi3x group.
FIG. 3.
Median change in the number of immunopositive cells per high-power field in dermal biopsy specimens from before the administration of the first dose to after the administration of the last dose. The numbers of cells in a minimum of five fields were enumerated for each biopsy sample and for each marker.
LSRs.
Except for two subjects in the 0.25Resi2x group, all LSRs that occurred were graded by the investigator as mild in intensity; the two subjects who were exceptions had moderate erythema, erosions, vesicles, ulceration, and scabbing. Except for one of the subjects in the 0.25Resi2x group, who had moderate discomfort while lying on the application site, the subjects graded all LSRs that occurred as mild in intensity. Higher incidences of induration, erosion, excoriation or flaking, and scabbing were seen in the 0.25Resi2x and 0.05Resi3x groups (Table 3). A cumulative scoring of LSRs after days 3, 11, and 20 suggested a dose relationship.
TABLE 3.
Incidence of LSRs and adverse events during study period
| Reaction or event | No. (%) of subjects
|
||||
|---|---|---|---|---|---|
| 0.25Resi2x (n = 6) | 0.05Resi3x (n = 6) | 0.05Resi2x (n = 12)a | 0.01Resi3x (n = 7) | Vehicle (n = 10)b | |
| LSRs | |||||
| Erythema | 6 (100) | 6 (100) | 9 (75) | 4 (57) | 6 (60) |
| Edema | 0 | 2 (33) | 1 (8) | 0 | 0 |
| Induration | 6 (100) | 4 (67) | 4 (33) | 1 (14) | 0 |
| Vesiclesc | 5 (83) | 6 (100) | 7 (58) | 3 (43) | 7 (70) |
| Erosions | 2 (33) | 2 (33) | 0 | 0 | 0 |
| Ulcerations | 0 | 1 (17) | 0 | 0 | 0 |
| Excoriation | 5 (83) | 3 (50) | 0 | 0 | 1 (10) |
| Scabbing | 6 (100) | 4 (67) | 1 (8) | 1 (14) | 1 (10) |
| Adverse eventsd | |||||
| Pruritis at biopsy site | 0 | 1 (17) | 1 (8) | 0 | 2 (20) |
| Burning sensation at dose site | 2 (33) | 0 | 0 | 0 | 0 |
| Erythema due to tape irritation | 0 | 2 (33) | 3 (25) | 0 | 4 (40) |
| Pruritis at dose site | 5 (83) | 3 (50) | 4 (33) | 0 | 0 |
| Diaphoresis | 1 (17) | 0 | 0 | 1 (14) | 0 |
| Vasovagal episode | 0 | 1 (17) | 1 (8) | 0 | 0 |
| Chills | 1 (17) | 0 | 0 | 0 | 1 (10) |
| Fever | 6 (100) | 5 (83) | 9 (75) | 5 (71) | 8 (80) |
| Tiredness | 0 | 2 (33) | 2 (17) | 0 | 1 (10) |
| Headache | 2 (33) | 2 (33) | 2 (17) | 2 (29) | 0 |
| Arthralgia | 2 (33) | 1 (17) | 0 | 0 | 0 |
| Myalgia | 1 (17) | 2 (33) | 0 | 1 (14) | 0 |
| Pain at biopsy site | 0 | 0 | 2 (17) | 0 | 1 (10) |
| Drowsiness | 0 | 0 | 0 | 1 (14) | 1 (10) |
| Neutropenia | 3 (50) | 0 | 0 | 1 (14) | 0 |
Includes resiquimod-treated subjects from both cohorts assigned to 0.05Resi2x.
Data for vehicle-treated subjects from each cohort are pooled.
Papules were included in this category by the assessing investigative staff.
Only adverse events present in more than one subject are listed.
Adverse events.
Twenty-seven (87%) of 31 subjects receiving resiquimod and 8 (80%) of 10 subjects receiving vehicle reported at least one adverse event (without respect to causality). Adverse events that occurred in more than one study subject are shown in Table 2. The most common adverse event was fever (temperature greater than 37.1°C) (Table 2).
Two subjects in the 0.25Resi2x group experienced repeated, transient, dosing-related symptoms including fever and chills, as well as decreased circulating neutrophil counts (nadir values, 1,040 and 1,010 cells/μl, respectively). The effects on erythrocyte, lymphocyte, and platelet counts were not clinically meaningful in these subjects. One of these subjects was the only subject with detectable levels of resiquimod in serum. The other subject was the only subject with a moderate-grade LSR. This subject discontinued treatment prior to administration of the last dose, and the serum resiquimod concentration after the administration of multiple doses could not be assessed.
DISCUSSION
Topical resiquimod gel was well tolerated when it was applied to healthy human skin as multiple doses of 1 g of 0.01% resiquimod for 24 h three times per week for 3 weeks and as multiple doses of 0.05% resiquimod for 8 h two or three times per week for 3 weeks. A dose-response relationship for LSRs was observed when a cumulative scoring system was used. Although only one subject reported an LSR of moderate severity, overall assessment of the LSRs and the presence of systemic signs and symptoms consistent with systemic induction of cytokines in two subjects suggested that the 0.25% resiquimod gel might not be adequately tolerable for future studies. The local effects observed for 0.25% resiquimod in this multiple-dose study were markedly different from those observed in the single-dose study, in which 0.25% resiquimod was tolerated but which also resulted in minimal evidence of cytokine production (Sauder et al., 38th ICAAC). This may be a result of increased local concentrations of resiquimod, changes in local penetration, an influx of cells capable of responding to resiquimod, or the increased responsiveness of target cells after multiple doses. Systemic accumulation of resiquimod after topical dosing was not observed in this study. The systemic effects observed during multiple dosing of 0.25% resiquimod may be related to spillover into the systemic circulation of cytokines induced locally rather than to systemic induction. A resiquimod concentration of 5 to 6 ng/ml in serum is usually required to induce cytokines systemically (I. Soria, unpublished data). The transient decrease in circulating neutrophil levels observed in a few subjects after multiple applications of resiquimod may be related to cytokine induction in the skin. In contrast, an increase in circulating neutrophil levels with a decrease in lymphocyte counts has been observed after oral administration of resiquimod in humans (T. Meng, unpublished data). This suggests that the cells are adhering or migrating, depending on where the cytokines are being induced, rather than resiquimod having a direct effect on cell production or survival. Tumor necrosis factor induces endothelial cell-leukocyte adhesion molecule type 1 expression, which can result in neutrophil adherence, while IL-8 induces neutrophil transmigration across endothelial cells (1a, 17).
The changes in cytokine mRNA levels in treated skin are consistent with those observed with imiquimod in a placebo-controlled study of patients with anogenital warts, in which significant increases in the levels of mRNA for IFN-α, IFN-γ, and 2′,5′-AS and a decrease for CD1a were observed (1, 18). The dose-dependent increases in the levels of CD3-, CD4-, and CD8-positive cells are consistent with the influx of cells associated with cellular immunity into treated skin. The decrease in the levels of CD1a-positive cells is consistent with activation of Langerhans' cells, which are bone marrow-derived DCs, by resiquimod and their emigration from the skin. Emigration of Langerhans' cells from the skin to the regional lymph nodes has been observed in mice following imiquimod application (14). Langerhans' cells, along with other types of DCs, play a major role in both the innate and the acquired host immune responses. The DC lineage appears to affect the response to resiquimod. Resiquimod can activate NFκb via TLR7 and induce type I IFN production from plasmacytoid DCs, as well as induce maturation of these cells, as determined by measurement of the levels of cytokine production, costimulatory marker expression, and CCR7 expression and improved survival (6, 10). In contrast, IL-12 monocyte-derived DCs preferentially secrete IL-12 in response to resiquimod (10).
These studies suggest that resiquimod is a topically active immunomodulator that can activate local cytokine mRNA production and initiate a cutaneous immune response.
Acknowledgments
We acknowledge Thomas L. Hunt for contributions to data collection and Jennie G. Jacobson, Lester Harrison, Adele Hoglin, and Becky Hansen for help with manuscript preparation.
This study was sponsored by 3M Pharmaceuticals.
M. H. Smith, T. Senta-McMillian, I. Soria, and T. Meng are employed by 3M Pharmaceuticals.
REFERENCES
- 1.Arany, I., S. K. Tyring, M. A. Stanley, M. A. Tomai, R. L. Miller, M. H. Smith, D. J. McDermott, and H. B. Slade. 1999. Enhancement of the innate and cellular immune response in patients with genital warts treated with topical imiquimod cream 5%. Antivir. Res. 43:55-63. [DOI] [PubMed] [Google Scholar]
- 1a.Barker, J. N. W. N., M. L. Jones, R. S. Mitra, E. Crockett-Torabe, J. C. Fantone, S. L. Kunkel, J. S. Warren, V. M. Dixit, and B. J. Nickoloff. 1991. Modulation of keratinocyte-derived interleukin-8 which is chemotactic for neutrophils and T lymphocytes. Am. J. Pathol. 139:869-876. [PMC free article] [PubMed] [Google Scholar]
- 2.Beutner, K. R., S. K. Tyring, K. F. Trofatter, Jr., J. M. Douglas, S. Spruance, M. L. Owens, T. L. Fox, A. J. Hougham, and K. A. Schmitt. 1998. Imiquimod, a patient-applied immune-response modifier for treatment of external genital warts. Antimicrob. Agents Chemother. 42:789-794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burns, R. P., B. Ferbel, M. Tomai, R. Miller, and A. A. Gaspari. 2000. The imidazoquinolines, imiquimod and R-848, induce functional, but not phenotypic, maturation of human epidermal Langerhans' cells. Clin. Immunol. 94:13-23. [DOI] [PubMed] [Google Scholar]
- 4.Edwards, L., A. Ferenczy, L. Eron, D. Baker, M. L. Owens, T. L. Fox, A. J. Hougham, and K. A. Schmitt. 1998. Self administered topical 5% imiquimod cream for external anogenital warts. Arch. Dermatol. 134:25-30. [DOI] [PubMed] [Google Scholar]
- 5.Gibson, S. J., L. M. Imbertson, T. L. Wagner, T. L. Testerman, M. J. Reiter, R. M. Miller, and M. A. Tomai. 1995. Cellular requirements for cytokine production in response to the immunomodulators imiquimod and S-27609. J. Interferon Cytokine Res. 15:537-545. [DOI] [PubMed] [Google Scholar]
- 6.Gibson, S. J., J. M. Lindh, T. R. Riter, R. M. Gleason, L. M. Rogers, A. E. Fuller, J. L. Oesterich, K. B. Gorden, X. Qiu, S. W. McKane, R. J. Noelle, R. L. Miller, R. M. Kedl, P. Fitgerald-Bocarsly, M. A. Tomai, and J. P. Vasilakos. 2002. Plasmacytoid dendritic cells produce cytokines and mature in response to the TLR7 agonists, imiquimod and resiquimod. Cell. Immunol. 218:74-86. [DOI] [PubMed] [Google Scholar]
- 7.Gottlieb, M., and M. Charko. 1987. Silver staining of native and denatured eukaryotic DNA in agarose gels. Anal. Biochem. 165:33-37. [DOI] [PubMed] [Google Scholar]
- 8.Hemmi, H., T. Kaisho, O. Takeuchi, S. Sato, H. Sanjo, K. Hoshino, T. Horiuchi, H. Tomizawa, K. Takeda, and S. Akira. 2002. Small anti-viral compounds activate immune cells via the TLR7 MyD88-dependent signaling pathway. Nat. Immunol. 3:196-200. [DOI] [PubMed] [Google Scholar]
- 9.Imbertson, L. M., J. M. Beaurline, A. M. Couture, S. J. Gibson, R. M. A. Smith, R. L. Miller, M. J. Reiter, T. L. Wagner, and M. A. Tomai. 1998. Cytokine induction in hairless mouse and rat skin after topical application of the immune response modifiers imiquimod and S-28463. J. Investig. Dermatol. 110:734-739. [DOI] [PubMed] [Google Scholar]
- 10.Ito, T., R. Amakawa, T. Kaisho, H. Hemmi, K. Tajima, K. Uehira, Y. Ozaki, H. Tomizawa, S. Akira, and S. Fukuhara. 2002. Interferon-α and interleukin-12 are induced differentially by toll-like receptor 7 ligand in human blood dendritic cell subsets. J. Exp. Med. 11:1507-1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jurk, M., F. Heil, J. Vollmer, C. Schetter, A. M. Krieg, H. Wagner, G. Lipford, and S. Bauer. 2002. Human TLR7 or TLR8 independently confer responsiveness to the antiviral compound R-848. Nat. Immunol. 3:499. [DOI] [PubMed] [Google Scholar]
- 12.Kondo, S., T. Kono, D. N. Sauder, and R. C. Mckenzie. 1993. Il-8 gene expression and production in human keratinocytes and their modulation by UVB. J. Investig. Dermatol. 101:690-694. [DOI] [PubMed] [Google Scholar]
- 13.Saiki, R. K., D. H. Gelfand, S. Stoffel, S. J. Scharf, R. Higuchi, G. T Horn, K. B. Mullis, and H. A. Erlich. 1988. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science 239:487-491. [DOI] [PubMed] [Google Scholar]
- 14.Suzuki, H., B. Wang, G. M. Shivji, P. Toto, P. Amerio, M. A. Tomai, R. L. Miller, and D. N. Sauder. 2000. Imiquimod, a topical immune response modifier, induces migration of Langerhans cells. J. Investig. Dermatol. 114:135-141. [DOI] [PubMed] [Google Scholar]
- 15.Testerman, T. L., J. F. Gerster, L. M. Imbertson, M. J. Reiter, R. L. Miller, S. J. Gibson, T. L. Wagner, and M. A. Tomai. 1995. Cytokine induction by the immunomodulators imiquimod and S-27609. J. Leukoc. Biol. 58:365-372. [DOI] [PubMed] [Google Scholar]
- 16.Tomai, M. A., S. J. Gibson, L. M. Imbertson, R. L. Miller, P. E. Myhre, M. J. Reiter, J. M. Beaurline, J. F. Gerster, and V. L. Horton. 1995. Immunomodulating and antiviral activities of the imidazoquinolines S-28463. Antivir. Res. 28:253-264. [DOI] [PubMed] [Google Scholar]
- 17.Tonneson, M. G. 1989. Neutrophil-endothelial cell interactions: mechanisms of neutrophil adherence to vascular endothelium. J. Investig. Dermatol. 93(2 Suppl.):53S-58S. [DOI] [PubMed] [Google Scholar]
- 18.Tyring, S. K., I. Arany, M. A. Stanley, M. A. Tomai, R. L. Miller, M. H. Smith, D. J. McDermott, and H. B. Slade. 1998. A randomized, controlled, molecular study of condylomata acuminata clearance during treatment with imiquimod. J. Infect. Dis. 187:551-555. [DOI] [PubMed] [Google Scholar]
- 19.Wagner, T. L., V. L. Horton, G. L. Carlson, P. E. Myhre, S. J. Gibson, L. M. Imbertson, and M. A. Tomai. 1997. Induction of cytokines in cynomolgus monkeys by the immune response modifiers, imiquimod, S-27609 and S-28463. Cytokine 9:837-845. [DOI] [PubMed] [Google Scholar]
- 20.Witt, P. L., P. J. Ritch, D. Reding, T. L. McAuliffer, S. E. Grossberg, and E. C. Borden. 1993. Phase I trial of an oral immunomodulator and interferon inducer in cancer patients. Cancer Res. 53:5176-5180. [PubMed] [Google Scholar]