Abstract
In title compound, C12H9IN2O2S, the nitro group is rotated slightly, by 8.91 (3)°, out of the plane of the aromatic ring to which it is bonded. Between the two aromatic rings the CSN plane is at a dihedral angle of 84.0 (7)° to the HNC plane. Molecules are linked by C—H⋯O interactions into a double helical supramolecular architecture. There are no iodo–nitro, π–π or C—H⋯π(arene) interactions.
Related literature
For related literature, see: Bernstein et al. (1995 ▶); Brito et al. (2004 ▶, 2005 ▶, 2006 ▶); Glidewell et al. (2003 ▶); Kuhle (1973 ▶); Pauling (1960 ▶).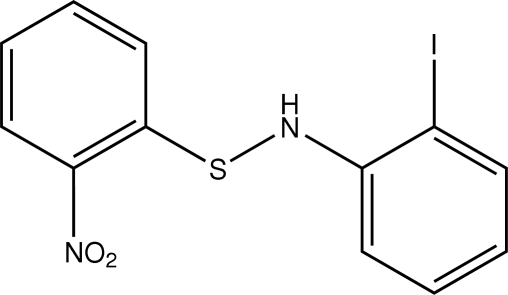
Experimental
Crystal data
C12H9IN2O2S
M r = 372.17
Trigonal,

a = 28.6221 (12) Å
c = 8.4062 (17) Å
V = 5963.9 (13) Å3
Z = 18
Mo Kα radiation
μ = 2.57 mm−1
T = 298 (2) K
0.47 × 0.32 × 0.20 mm
Data collection
Nonius KappaCCD area-detector diffractometer
Absorption correction: multi-scan (SORTAV; Blessing, 1995 ▶) T min = 0.380, T max = 0.600
11358 measured reflections
3251 independent reflections
2801 reflections with I > 2σ(I)
R int = 0.059
Refinement
R[F 2 > 2σ(F 2)] = 0.072
wR(F 2) = 0.205
S = 1.21
3251 reflections
166 parameters
H-atom parameters constrained
Δρmax = 1.04 e Å−3
Δρmin = −1.05 e Å−3
Data collection: COLLECT (Nonius, 2000 ▶); cell refinement: DENZO-SMN (Otwinowski & Minor, 1997 ▶); data reduction: DENZO-SMN; program(s) used to solve structure: SIR97 (Altomare et al., 1999 ▶); program(s) used to refine structure: SHELXL97 (Sheldrick, 2008 ▶); molecular graphics: ORTEP-3 for Windows (Farrugia, 1997 ▶) and PLATON (Spek, 2003 ▶); software used to prepare material for publication: WinGX (Farrugia, 1999 ▶).
Supplementary Material
Crystal structure: contains datablocks global, I. DOI: 10.1107/S1600536808019491/fl2204sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536808019491/fl2204Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Table 1. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| C10—H10⋯O1i | 0.93 | 2.55 | 3.445 (10) | 161 |
Symmetry code: (i)  .
.
Acknowledgments
This work was supported by a grant from the Universidad de Antofagasta (DI-1324–06). We thank the Spanish Research Council (CSIC) for providing us with a free-of-charge licence for the CSD system. AM and AR thank the Universidad de Antofagasta for PhD fellowships.
supplementary crystallographic information
Comment
Sulfenamides are important compounds with versatile industrial applications (Kuhle, 1973). Bond polarization in sulfenamide derivatives, resulting from the difference in electronegativity between sulfur and nitrogen, activates the S—N bond for attack by both nucleophiles and electrophiles, and appears to be the factor primarily responsible for the chemistry of these compounds. The title compound, (I), is a positional isomer of 4-Iodo -N-(2-nitrophenylsulfanyl)-aniline (Glidewell et al.,2003) and shows excellent agreement with its bonding geometries. The title compound is the result of the condensation reaction of 2-nitrophenylsulfenyl choride and 1-iodoaniline. Its structure is described here as part of our work involving the study of the synthesis and structural characterization of divalent-sulfur compounds (Brito et al., 2004, 2005, 2006). A view of the molecular structure of (I) is given in Fig.1. In (I) the 1-Iodo-benzene fragment is connected by an –NH—S– linker unit to the 2-nitrophenyl fragment. It contains an N atom as a chiral center, though the material is a racemic mixture. The nitro group is rotated by 8.91 (3)°. The S—N distance of 1.696 (6)Å is shorter than a normal S—N single-bond length (1.74 Å, Pauling, 1960), but is normal for this type of structure, many of which have S—N single bonds in the range 1.63–1.68 Å as a result of the π character of the S—N bond. The C7/S1/N1 plane makes a dihedral angle of 84.0 (7) ° with the H1/N1/C2 plane, in good agreement with the values of ~90.0 ° for the torsional ground state of this type species. The molecules are linked into a double helical supramolecular architecture with only hydrogen bonding contributing to the double helical arrangement (Bernstein et al., 1995). Atom C10 and nitro atom O1 in the molecule at (x, y, z) act as hydrogen-bond donor and acceptor respectively, Fig.2, Table 1. There are no iodo-nitro, π-π and C—H··· π (arene) interactions.
Experimental
A sample of compound (I) was prepared by reaction of equimolar quantities of 2-nitrophenylsulfenyl chloride (0.01 mol,1.895 g) and 4-iodoaniline (0.01 mol, 2.190 g) in dichloromethane solution, in the presence of an excess of triethylamine. Purification was by thin-layer chromatography and crystals of (I) suitable for single-crystal X-ray diffraction were grown by slow evaporation of a solution in ethanol [m.p. 472 K]. FT—IR (KBr pellet, cm-1): ν (w, N—H amine) 3091, ν (w, S—N) 1039, ν (w, C—S) 731, ν (s, C—H disubstitution) 855, ν (versus, NO2) 1567.
Refinement
All H atoms were initially located in a difference Fourier map and were subsequently refined using a riding model, with C—H distances of 0.93 Å and Uiso(H)= 1.2 Ueq(C) for benzene H atoms and N—H = 0.86 Å for amino H atom and Uiso(H) = 1.2Ueq(N).
Figures
Fig. 1.
The molecule of compound (I), showing the atom-labelling scheme. Displacement ellipsoids are drawn at the 30% probability level and H atoms are shown as small spheres of arbitrary radii.
Fig. 2.
A portio nof the packing diagram for (I), showing the double helical arrangement of molecules along [001]. For the sake of clarity, H atoms not involved in the motif shown have been omitted. [Symmetry code: (i) 2/3 - y, -2/3 + x-y, -2/3 + z]
Crystal data
| C12H9IN2O2S | Z = 18 |
| Mr = 372.17 | F000 = 3240 |
| Trigonal, R3 | Dx = 1.865 Mg m−3 |
| Hall symbol: -R 3 | Melting point: 472 K |
| a = 28.6221 (12) Å | Mo Kα radiation λ = 0.71073 Å |
| b = 28.6221 (12) Å | Cell parameters from 909 reflections |
| c = 8.4062 (17) Å | θ = 1.4–28.5º |
| α = 90º | µ = 2.57 mm−1 |
| β = 90º | T = 298 (2) K |
| γ = 120º | Prism, yellow |
| V = 5963.9 (13) Å3 | 0.47 × 0.32 × 0.20 mm |
Data collection
| Nonius KappaCCD area-detector diffractometer | 2801 reflections with I > 2σ(I) |
| Radiation source: fine-focus sealed tube | Rint = 0.059 |
| Monochromator: graphite | θmax = 28.5º |
| φ scans, and ω scans with κ offsets | θmin = 1.4º |
| Absorption correction: multi-scan(SORTAV; Blessing, 1995) | h = −31→37 |
| Tmin = 0.380, Tmax = 0.600 | k = −36→35 |
| 11358 measured reflections | l = −7→11 |
| 3251 independent reflections |
Refinement
| Refinement on F2 | H-atom parameters constrained |
| Least-squares matrix: full | w = 1/[σ2(Fo2) + (0.087P)2 + 39.1076P] where P = (Fo2 + 2Fc2)/3 |
| R[F2 > 2σ(F2)] = 0.072 | (Δ/σ)max = 0.001 |
| wR(F2) = 0.205 | Δρmax = 1.04 e Å−3 |
| S = 1.21 | Δρmin = −1.05 e Å−3 |
| 3251 reflections | Extinction correction: SHELXL97 (Sheldrick, 2008), Fc*=kFc[1+0.001xFc2λ3/sin(2θ)]-1/4 |
| 166 parameters | Extinction coefficient: 0.0064 (7) |
Special details
| Geometry. All e.s.d.'s (except the e.s.d. in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell e.s.d.'s are taken into account individually in the estimation of e.s.d.'s in distances, angles and torsion angles; correlations between e.s.d.'s in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell e.s.d.'s is used for estimating e.s.d.'s involving l.s. planes. |
| Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F2, conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > σ(F2) is used only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2 are statistically about twice as large as those based on F, and R- factors based on ALL data will be even larger. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| I1 | 1.003222 (18) | 0.07997 (2) | 0.27240 (7) | 0.0680 (3) | |
| S1 | 0.81458 (6) | −0.00495 (6) | 0.12352 (17) | 0.0458 (4) | |
| N1 | 0.8810 (2) | 0.0367 (2) | 0.1625 (7) | 0.0565 (13) | |
| H1 | 0.9042 | 0.0289 | 0.1244 | 0.068* | |
| N2 | 0.70588 (19) | −0.0418 (2) | −0.0484 (7) | 0.0529 (12) | |
| O1 | 0.7146 (2) | −0.0679 (2) | 0.0488 (7) | 0.0679 (13) | |
| O2 | 0.6610 (2) | −0.0547 (3) | −0.0985 (9) | 0.0865 (19) | |
| C1 | 0.9522 (2) | 0.1110 (2) | 0.3204 (7) | 0.0450 (12) | |
| C2 | 0.8998 (2) | 0.0847 (2) | 0.2574 (6) | 0.0427 (11) | |
| C3 | 0.8677 (3) | 0.1073 (3) | 0.2903 (7) | 0.0498 (13) | |
| H3 | 0.8328 | 0.0909 | 0.25 | 0.06* | |
| C4 | 0.8862 (3) | 0.1532 (3) | 0.3809 (8) | 0.0559 (15) | |
| H4 | 0.8642 | 0.1679 | 0.3997 | 0.067* | |
| C5 | 0.9383 (3) | 0.1777 (3) | 0.4448 (8) | 0.0596 (17) | |
| H5 | 0.9507 | 0.2083 | 0.5081 | 0.072* | |
| C6 | 0.9706 (3) | 0.1569 (2) | 0.4143 (7) | 0.0518 (14) | |
| H6 | 1.0053 | 0.1733 | 0.4562 | 0.062* | |
| C7 | 0.80300 (19) | 0.02629 (18) | −0.0433 (6) | 0.0340 (9) | |
| C8 | 0.75198 (19) | 0.00701 (19) | −0.1113 (6) | 0.0365 (10) | |
| C9 | 0.7438 (2) | 0.0326 (3) | −0.2401 (7) | 0.0482 (13) | |
| H9 | 0.7093 | 0.0191 | −0.2816 | 0.058* | |
| C10 | 0.7861 (3) | 0.0775 (3) | −0.3060 (7) | 0.0549 (15) | |
| H10 | 0.7809 | 0.0938 | −0.3946 | 0.066* | |
| C11 | 0.8368 (3) | 0.0982 (2) | −0.2383 (7) | 0.0495 (13) | |
| H11 | 0.8656 | 0.1294 | −0.2798 | 0.059* | |
| C12 | 0.8450 (2) | 0.0729 (2) | −0.1094 (6) | 0.0391 (10) | |
| H12 | 0.8795 | 0.0875 | −0.0663 | 0.047* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| I1 | 0.0502 (3) | 0.0627 (4) | 0.0973 (5) | 0.0329 (2) | −0.0026 (2) | 0.0077 (2) |
| S1 | 0.0515 (8) | 0.0395 (7) | 0.0445 (7) | 0.0213 (6) | −0.0044 (6) | 0.0046 (5) |
| N1 | 0.060 (3) | 0.049 (3) | 0.057 (3) | 0.025 (2) | −0.028 (3) | −0.012 (2) |
| N2 | 0.033 (2) | 0.045 (3) | 0.067 (3) | 0.009 (2) | 0.004 (2) | −0.008 (2) |
| O1 | 0.055 (3) | 0.052 (3) | 0.076 (3) | 0.011 (2) | 0.014 (2) | 0.021 (2) |
| O2 | 0.036 (2) | 0.075 (4) | 0.124 (5) | 0.009 (2) | −0.009 (3) | −0.004 (3) |
| C1 | 0.052 (3) | 0.046 (3) | 0.038 (3) | 0.025 (2) | 0.004 (2) | 0.011 (2) |
| C2 | 0.057 (3) | 0.046 (3) | 0.031 (2) | 0.030 (2) | −0.004 (2) | −0.001 (2) |
| C3 | 0.059 (3) | 0.054 (3) | 0.044 (3) | 0.034 (3) | −0.001 (2) | −0.002 (2) |
| C4 | 0.066 (4) | 0.060 (4) | 0.050 (3) | 0.037 (3) | 0.014 (3) | −0.003 (3) |
| C5 | 0.073 (4) | 0.048 (3) | 0.044 (3) | 0.020 (3) | 0.015 (3) | −0.003 (2) |
| C6 | 0.052 (3) | 0.045 (3) | 0.044 (3) | 0.013 (2) | 0.006 (2) | 0.006 (2) |
| C7 | 0.036 (2) | 0.030 (2) | 0.034 (2) | 0.0157 (18) | 0.0034 (18) | −0.0011 (17) |
| C8 | 0.032 (2) | 0.033 (2) | 0.041 (2) | 0.0138 (18) | 0.0014 (18) | −0.0049 (18) |
| C9 | 0.048 (3) | 0.054 (3) | 0.050 (3) | 0.032 (3) | −0.008 (2) | −0.007 (2) |
| C10 | 0.076 (4) | 0.056 (3) | 0.044 (3) | 0.041 (3) | −0.005 (3) | 0.001 (3) |
| C11 | 0.060 (3) | 0.042 (3) | 0.040 (3) | 0.020 (3) | 0.009 (2) | 0.009 (2) |
| C12 | 0.039 (2) | 0.036 (2) | 0.037 (2) | 0.014 (2) | 0.0032 (19) | −0.0011 (18) |
Geometric parameters (Å, °)
| I1—C1 | 2.092 (6) | C4—H4 | 0.93 |
| S1—N1 | 1.696 (6) | C5—C6 | 1.352 (10) |
| S1—C7 | 1.781 (5) | C5—H5 | 0.93 |
| N1—C2 | 1.441 (7) | C6—H6 | 0.93 |
| N1—H1 | 0.86 | C7—C12 | 1.390 (7) |
| N2—O1 | 1.216 (8) | C7—C8 | 1.399 (7) |
| N2—O2 | 1.219 (7) | C8—C9 | 1.392 (8) |
| N2—C8 | 1.458 (7) | C9—C10 | 1.365 (10) |
| C1—C6 | 1.391 (9) | C9—H9 | 0.93 |
| C1—C2 | 1.402 (8) | C10—C11 | 1.386 (10) |
| C2—C3 | 1.392 (8) | C10—H10 | 0.93 |
| C3—C4 | 1.375 (9) | C11—C12 | 1.387 (8) |
| C3—H3 | 0.93 | C11—H11 | 0.93 |
| C4—C5 | 1.400 (11) | C12—H12 | 0.93 |
| N1—S1—C7 | 102.9 (3) | C5—C6—C1 | 120.3 (6) |
| C2—N1—S1 | 122.0 (5) | C5—C6—H6 | 119.8 |
| C2—N1—H1 | 119 | C1—C6—H6 | 119.8 |
| S1—N1—H1 | 119 | C12—C7—C8 | 116.5 (5) |
| O1—N2—O2 | 123.7 (6) | C12—C7—S1 | 120.5 (4) |
| O1—N2—C8 | 117.8 (5) | C8—C7—S1 | 122.9 (4) |
| O2—N2—C8 | 118.5 (6) | C9—C8—C7 | 121.8 (5) |
| C6—C1—C2 | 121.3 (6) | C9—C8—N2 | 118.4 (5) |
| C6—C1—I1 | 119.6 (5) | C7—C8—N2 | 119.7 (5) |
| C2—C1—I1 | 119.1 (4) | C10—C9—C8 | 120.5 (5) |
| C3—C2—C1 | 117.1 (5) | C10—C9—H9 | 119.8 |
| C3—C2—N1 | 122.3 (5) | C8—C9—H9 | 119.8 |
| C1—C2—N1 | 120.6 (5) | C9—C10—C11 | 118.8 (6) |
| C4—C3—C2 | 121.6 (6) | C9—C10—H10 | 120.6 |
| C4—C3—H3 | 119.2 | C11—C10—H10 | 120.6 |
| C2—C3—H3 | 119.2 | C10—C11—C12 | 120.8 (5) |
| C3—C4—C5 | 119.9 (6) | C10—C11—H11 | 119.6 |
| C3—C4—H4 | 120.1 | C12—C11—H11 | 119.6 |
| C5—C4—H4 | 120.1 | C11—C12—C7 | 121.5 (5) |
| C6—C5—C4 | 119.8 (6) | C11—C12—H12 | 119.3 |
| C6—C5—H5 | 120.1 | C7—C12—H12 | 119.3 |
| C4—C5—H5 | 120.1 | ||
| C7—S1—N1—C2 | 83.9 (5) | C12—C7—C8—C9 | 1.0 (7) |
| C6—C1—C2—C3 | 1.3 (8) | S1—C7—C8—C9 | 178.9 (4) |
| I1—C1—C2—C3 | −179.0 (4) | C12—C7—C8—N2 | 180.0 (5) |
| C6—C1—C2—N1 | −179.0 (5) | S1—C7—C8—N2 | −2.1 (7) |
| I1—C1—C2—N1 | 0.8 (7) | O1—N2—C8—C9 | 170.0 (6) |
| S1—N1—C2—C3 | −16.1 (8) | O2—N2—C8—C9 | −8.4 (8) |
| S1—N1—C2—C1 | 164.2 (4) | O1—N2—C8—C7 | −9.0 (8) |
| C1—C2—C3—C4 | −0.2 (9) | O2—N2—C8—C7 | 172.6 (6) |
| N1—C2—C3—C4 | −179.9 (6) | C7—C8—C9—C10 | 1.0 (9) |
| C2—C3—C4—C5 | −1.2 (10) | N2—C8—C9—C10 | −178.0 (5) |
| C3—C4—C5—C6 | 1.4 (10) | C8—C9—C10—C11 | −2.5 (9) |
| C4—C5—C6—C1 | −0.3 (9) | C9—C10—C11—C12 | 2.2 (9) |
| C2—C1—C6—C5 | −1.0 (9) | C10—C11—C12—C7 | −0.3 (9) |
| I1—C1—C6—C5 | 179.2 (5) | C8—C7—C12—C11 | −1.3 (7) |
| N1—S1—C7—C12 | 1.6 (5) | S1—C7—C12—C11 | −179.3 (4) |
| N1—S1—C7—C8 | −176.3 (4) |
Hydrogen-bond geometry (Å, °)
| D—H···A | D—H | H···A | D···A | D—H···A |
| C10—H10···O1i | 0.93 | 2.55 | 3.445 (10) | 161 |
Symmetry codes: (i) −y+2/3, x−y−2/3, z−2/3.
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: FL2204).
References
- Altomare, A., Burla, M. C., Camalli, M., Cascarano, G. L., Giacovazzo, C., Guagliardi, A., Moliterni, A. G. G., Polidori, G. & Spagna, R. (1999). J. Appl. Cryst.32, 115–119.
- Bernstein, J., Davis, R. E., Shimoni, L. & Chang, N.-L. (1995). Angew. Chem. Int. Ed. Engl.34, 1555–1573.
- Blessing, R. H. (1995). Acta Cryst. A51, 33–38. [DOI] [PubMed]
- Brito, I., López-Rodríguez, M., Vargas, D. & León, Y. (2006). Acta Cryst. E62, o914–o916. [DOI] [PubMed]
- Brito, I., Vargas, D., León, Y., Cárdenas, A., López-Rodríguez, M. & Wittke, O. (2004). Acta Cryst. E60, o1668–o1670.
- Brito, I., Vargas, D., Reyes, A., Cárdenas, A. & López-Rodríguez, M. (2005). Acta Cryst. C61, o234–o236. [DOI] [PubMed]
- Farrugia, L. J. (1997). J. Appl. Cryst.30, 565.
- Farrugia, L. J. (1999). J. Appl. Cryst.32, 837–838.
- Glidewell, C., Low, J. N., Skakle, J. M. S. & Wardell, J. L. (2003). Acta Cryst. C59, o95–o97. [DOI] [PubMed]
- Kuhle, E. (1973). The Chemistry of the Sulfenic Acids, pp. 60–74. Stuttgart: G. Thieme.
- Nonius (2000). COLLECT Nonius BV, Delft, The Netherlands.
- Otwinowski, Z. & Minor, W. (1997). Methods in Enzymology, Vol. 276, Macromolecular Crystallography, Part A, edited by C. W. Carter Jr & R. M. Sweet, pp. 307–326. New York; Academic Press.
- Pauling, L. (1960). The Nature of the Chemical Bond, 3rd ed., pp. 257–264. Ithaca: Cornell University Press.
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Spek, A. L. (2003). J. Appl. Cryst.36, 7–13.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablocks global, I. DOI: 10.1107/S1600536808019491/fl2204sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536808019491/fl2204Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report




