Abstract
Garenoxacin is a new des-F(6)-quinolone with broad-spectrum activity against both gram-positive cocci and gram-negative bacilli. We used the neutropenic murine thigh infection model to characterize the time course of antimicrobial activity of garenoxacin and determine which pharmacokinetic-pharmacodynamic (PK-PD) parameter best correlated with efficacy. Serum drug levels following three fourfold-escalating single-dose levels of garenoxacin were measured by microbiologic assay. In vivo postantibiotic effects (PAEs) were determined after doses of 16 and 64 mg/kg of body weight. Mice had 106.5 to 106.7 CFU of Streptococcus pneumoniae strain ATCC 10813 or Staphylococcus aureus strain ATCC 33591 per thigh when they were treated for 24 h with garenoxacin at a dose of 4 to 128 mg/kg/day fractionated for 3-, 6-, 12-, and 24-hour dosing regimens. Nonlinear regression analysis was used to determine which PK-PD parameter best correlated with the measurement of CFU/thigh at 24 h. Pharmacokinetic studies yielded peak/dose values of 0.2 to 0.3, area under the concentration-time curve (AUC)/dose values of 0.1 to 0.5, and half-lives of 0.7 to 1.6 h. Garenoxacin produced in vivo PAEs of 1.4 to 8.2 h with S. pneumoniae ATCC 10813, 7.6 to >12.4 h with S. aureus ATCC 25923, and 0 to 1.5 h with Klebsiella pneumoniae ATCC 43816. The 24-h AUC/MIC ratio was the PK-PD parameter that best correlated with efficacy (R2 = 71 to 90% for the two organisms compared with 43 to 56% for the peak/MIC ratio and 47 to 75% for percent time above the MIC [% T>MIC]).In subsequent studies we used the neutropenic murine thigh infection model to determine if the magnitude of the AUC/MIC ratio needed for efficacy of garenoxacin varied among pathogens (including resistant strains). Mice had 105.9 to 107.2 CFU of 6 strains of S. aureus (2 methicillin resistant), 11 strains of S. pneumoniae (5 penicillin susceptible, 1 penicillin intermediate, and 5 penicillin resistant, and of the resistant strains, 3 were also ciprofloxacin resistant), and 4 gram-negative strains per thigh when treated for 24 h with 1 to 64 mg of garenoxacin per kg every 12 h. A sigmoid dose-response model was used to estimate the doses (mg/kg/24 h) required to achieve a net bacteriostatic effect over 24 h. MICs ranged from 0.008 to 4 μg/ml. The free drug 24-h AUC/MIC ratios for each static dose (2.8 to 128 mg/kg/day) varied from 8.2 to 145. The mean 24-h AUC/MIC ratios ± standard deviations for S. pneumoniae, S. aureus, and gram-negative strains were 33 ± 18, 81 ± 37, and 33 ± 30, respectively. Methicillin, penicillin, or ciprofloxacin resistance did not alter the magnitude of the AUC/MIC ratio required for efficacy.
Garenoxacin is a new des-F(6)-quinolone with broad-spectrum antimicrobial activity that is in development for the treatment of respiratory tract, skin, and intra-abdominal infections. Similar to the new generation of fluoroquinolones, garenoxacin has enhanced potency against gram-positive cocci including S. pneumoniae strains resistant to multiple drugs (12, 15, 18).
The goals of our experiments were to characterize the in vivo time course antimicrobial activity of garenoxacin and determine the pharmacokinetic-pharmacodynamic (PK-PD) parameters and parameter magnitude predictive of efficacy.
MATERIALS AND METHODS
Bacteria, media, and antibiotics.
Eleven strains of Streptococcus pneumoniae (five penicillin-susceptible, one penicillin-intermediate, and five penicillin-resistant strains, and of the resistant strains, three were also ciprofloxacin resistant), six strains of Staphylococcus aureus (four methicillin-susceptible and two methicillin-resistant S. aureus strains [MRSA]), and four gram-negative strains (two Escherichia coli, one Klebsiella pneumoniae, and one Pseudomonas aeruginosa) were used for these experiments. All organisms except S. pneumoniae were grown, subcultured, and quantified in Mueller-Hinton broth (Difco Laboratories, Detroit, Mich.) and Mueller-Hinton agar (Difco Laboratories). Sheep blood agar plates (Remel, Milwaukee, Wis.) were utilized for S. pneumoniae. The lower limit of organism quantification in these studies was 100 CFU/thigh. Garenoxacin (purity, 79.2%) was supplied by Bristol-Myers Squibb (Princeton, N.J.). Penicillin, ciprofloxacin, and methicillin were obtained from Sigma (St. Louis, Mo.).
In vitro susceptibility studies.
The MICs of garenoxacin, penicillin, methicillin, and ciprofloxacin for the various strains were determined by microdilution methods approved by the National Committee for Clinical Laboratory Standards.
Murine infection model.
Animals were maintained in accordance with criteria of the American Association for Accreditation of Laboratory Animal Care. All animal studies were approved by the Animal Research Committee of the William S. Middleton Memorial Veterans Hospital.
Six-week-old, specific-pathogen-free, female ICR/Swiss mice weighing 23 to 27 g were used for all studies (Harlan Sprague-Dawley, Indianapolis, Ind.). Mice were rendered neutropenic (neutrophils, <100/mm3) by injections of cyclophosphamide (Mead Johnson Pharmaceuticals, Evansville, Ind.) intraperitoneally 4 days (150 mg/kg of body weight) and 1 day (100 mg/kg) before thigh infection. Previous studies have shown that this regimen produces neutropenia in this model for 5 days (1). Broth cultures of freshly plated bacteria were grown to logarithmic phase overnight to an absorbance of 0.3 at 580 nm (Spectronic 88; Bausch and Lomb, Rochester, N.Y.). After a 1:10 dilution into fresh Mueller-Hinton broth, bacterial counts of the inoculum ranged from 106 to 107 CFU/ml. Thigh infections with each of the strains were produced by the injection of 0.1 ml of inoculum into the thighs of halothane-anesthetized mice 2 h before therapy with garenoxacin.
Drug pharmacokinetics.
Single-dose serum pharmacokinetic studies were performed in thigh-infected mice given subcutaneous doses (0.2 ml/dose) of garenoxacin (16, 64, and 256 mg/kg). For each of the doses examined, four groups of three mice were sampled by retro-orbital puncture at 0.5- to 1-h intervals over 6 h (sample times included 0.5, 1, 2, 3, 4, 5, and 6 h). Individual animals were sampled three or four times. The total volume collected from individual animals was less than 10% of the total blood volume. Samples were then centrifuged for 5 min at 10,000 × g and serum was removed. Serum garenoxacin concentrations were determined by standard microbiologic assays with S. aureus ATCC 6538p as the test organism and antibiotic medium 1 as the agar diffusion medium. The lower limit of detection of the assays was 0.20 μg/ml. Intraday variation was less than 4%. All pharmacokinetic studies were performed on the same day. Pharmacokinetic constants, including elimination half-life, area under the concentration-time curve (AUC), and peak level, were calculated by a noncompartmental model. For doses used in treatments for which actual kinetic measurements were not made, estimates were based upon linear extrapolation from the three studied dose levels. Protein binding in the serum of infected neutropenic mice was performed with ultrafiltration methods (2). The degree of binding was measured by using garenoxacin concentrations of 10 and 100 μg/ml.
Treatment protocols. (i) In vivo PAE.
Two hours after infection with S. pneumoniae ATCC 10813, S. aureus ATCC 25923, or K. pneumoniae ATCC 43816, neutropenic mice were treated with single subcutaneous doses of garenoxacin (16 or 64 mg/kg). Groups of two treated and untreated control mice were sacrificed at sampling intervals ranging from 1 to 6 h. Control growth was determined at four sampling times over 12 h (at 0, 2, 6, and 12 h). The treated groups were sampled nine times over 24 h (at 0, 1, 2, 4, 6, 12, 18, and 24 h). The thighs were removed at each time point and processed immediately for CFU determination. The times that the levels of garenoxacin (total and free drug) in the serum remained above the MICs of the drug for the organisms were calculated from the pharmacokinetic studies based upon a linear extrapolation from the peak serum drug level. The postantibiotic effect (PAE) was calculated by subtracting the time it took for organisms to increase 1 log in the thighs of saline-treated animals from the time it took organisms to grow the same amount in treated animals after serum drug levels fell below the MIC of the drug for the infecting organism (7): PAE = T − C, where C is the time for 1 log10 control growth and T is the time for 1 log10 treatment growth after levels have fallen below the MIC.
(ii) PK-PD parameter determination.
Neutropenic mice were infected with either penicillin-susceptible S. pneumoniae ATCC 10813 or MRSA ATCC 33591. Treatment with garenoxacin was initiated 2 h after infection. Groups of two mice were treated for 24 h with 24 different dosing regimens and twofold-increasing total doses divided into 1, 2, 4, or 8 doses. The range of total doses of garenoxacin was from 4 to 128 mg/kg/24 h, a range of 32-fold. Drug doses were administered subcutaneously in 0.2-ml volumes. The mice were sacrificed after 24 h of therapy, and the thighs were removed and processed for CFU determination. Untreated control mice were sacrificed just before treatment and after 24 h.
(iii) PK-PD parameter magnitude studies.
Similar dosing studies with six twofold-increasing garenoxacin doses administered every 12 h (q12h) were utilized to treat thigh-infected neutropenic animals with 11 strains of S. pneumoniae (5 penicillin-susceptible, 1 penicillin-intermediate, and 5 penicillin-resistant strains, and of the resistant strains, 3 were also ciprofloxacin resistant), 6 strains of S. aureus (4 methicillin-susceptible S. aureus and 2 MRSA), and 4 gram-negative strains (2 E. coli, 1 K. pneumoniae, and 1 P. aeruginosa). The garenoxacin MICs for the organisms studied varied by 125-fold. The total daily dose of garenoxacin used in these studies varied from 2 to 128 mg/kg. In treatment against the organisms with higher MICs (≥0.5 μg/ml), the starting dose level was 4 mg/kg. For all other organisms the starting dose level was 2 mg/kg.
Data analysis.
The results of these studies were analyzed according to the sigmoid dose effect model. The model is derived from the Hill equation: E = (Emax × DN)/(ED50N + DN), where E is the effect or, in this case, the log change in CFU per thigh between treated mice and untreated controls after the 24-hour period of study, Emax is the maximum effect, D is the 24-hour total dose, ED50 is the dose required to achieve 50% of Emax, and N is the slope of the dose-effect curve. The indices Emax, ED50, and N were calculated by using nonlinear least-squares regression. The correlation between efficacy and each of the three PK-PD parameters (T>MIC, AUC/MIC, and peak/MIC) studied was determined by nonlinear least-squares multivariate regression (Sigma Stat; Jandel Scientific Software, San Rafael, Calif.). The coefficient of determination, or R2, was used to estimate the variance that could be due to regression with each of the PK-PD parameters.
We utilized the 24-h static dose as well as the doses necessary to achieve the ED25, ED50, ED75, and 1 log10 reduction in colony counts compared to numbers at the start of therapy to compare the impact of the dosing interval on treatment efficacy. If these dose values remained similar among each of the dosing intervals, this result would support the 24-h AUC/MIC ratio as the predictive parameter. If the dose values increased as the dosing interval was lengthened, this increase would suggest that T>MIC is the predictive parameter. Lastly, if the dose values decreased as the dosing interval was increased, this result would support the peak/MIC ratio as the pharmacodynamically important parameter.
To allow a comparison of the potency of garenoxacin against a variety of organisms, we utilized the 24-h static dose. The magnitude of the PK-PD parameter associated with each endpoint dose was calculated from the following equation: log10D = {log10[E/(Emax − E)]}/N + logED50, where E is the control growth when D is dose; E is the control growth plus 1 log when D is 1 log kill; and E is the control growth plus 2 log when D is 2 log kill. The significance of the differences among the various dosing endpoints was determined by analysis of variance on ranks.
RESULTS
In vitro susceptibility testing.
The MICs of garenoxacin, penicillin, methicillin, or ciprofloxacin for the 21 study strains are shown in Table 1. Garenoxacin MICs varied 125-fold (range, 0.008 to 1.0 μg/ml).
TABLE 1.
Comparative efficacy of garenoxacin against both gram-positive and gram-negative pathogens
| Organism | MIC (mg/liter)
|
Static dose (mg/kg) | AUC/MIC
|
||||
|---|---|---|---|---|---|---|---|
| Garenoxacin | Penicillin | Methicillin | Ciprofloxacin | 24 h | Free drug 24 h | ||
| S. pneumoniae strain | |||||||
| 10813 | 0.03 | 0.008 | 17.9 | 207 | 45.5 | ||
| 131 | 0.06 | 0.25 | 10.4 | 72.3 | 16.0 | ||
| 141 | 0.06 | 0.06 | 44.5 | 220 | 48.4 | ||
| 1325 | 0.06 | 1.0 | 25.9 | 142 | 31.2 | ||
| 1293 | 0.03 | 1.0 | 20.5 | 247 | 54.5 | ||
| T6 | 0.25 | 2.0 | 57.8 | 72.4 | 16.1 | ||
| 6303 | 0.06 | 0.06 | 30.6 | 158 | 34.8 | ||
| 203120 | 1.0 | 64.0 | 123 | 37.0 | 8.20 | ||
| 402123 | 0.50 | 64.0 | 86.2 | 52.0 | 11.5 | ||
| 145 | 0.03 | 4.0 | 10.1 | 140 | 30.9 | ||
| 146 | 0.03 | 1.0 | 24.6 | 273 | 60.1 | ||
| Mean ± SD | 32.5 ± 18 | ||||||
| S. aureus strain | |||||||
| 33591 | 0.016 | >8.0 | 5.64 | 150 | 33 | ||
| MRSA | 0.016 | >8.0 | 13.2 | 344 | 75 | ||
| 25923 | 0.016 | 0.12 | 12.0 | 313 | 68 | ||
| 29213 | 0.016 | 0.12 | 11.9 | 313 | 68 | ||
| 6538 | 0.016 | 0.06 | 35.5 | 662 | 145 | ||
| SM | 0.008 | 0.12 | 8.35 | 435 | 96 | ||
| Mean ± SD | 80.8 ± 37 | ||||||
| P. aeruginosa 27854 | 1.0 | 0.50 | >128 | >40 | >9 | ||
| E. coli 148 | 0.12 | 0.008 | 25.2 | 69.3 | 15.2 | ||
| E. coli 25922 | 0.016 | 0.008 | 2.82 | 75.0 | 16.5 | ||
| K. pneumoniae 43816 | 0.06 | 0.008 | 58.7 | 308 | 67.8 | ||
| Mean ± SD | 33.2 ± 30a | ||||||
The data from P. aeruginosa were not used to calculate the mean.
Pharmacokinetics.
The time course of serum levels of garenoxacin in infected neutropenic mice following subcutaneous doses of 16, 64, and 256 mg/kg are shown in Fig. 1. Over the dose range studied, kinetics were nonlinear with the elimination half-life increasing 2.2-fold with dose escalation. The elimination half-life ranged from 0.72 to 1.6 h. The AUC/dose and peak/dose values for the escalating single doses ranged from 0.1 to 0.5 and 0.2 to 0.3, respectively. Garenoxacin binding in mouse serum was 78% at drug concentrations of 10 and 100 μg/ml. This percentage is similar to the degree of binding in other animal species and in human serum (Paul Hale [Bristol-Myers Squibb], personal communication). Both free and total drug levels were considered in pharmacokinetic calculations throughout the study.
FIG. 1.
Serum garenoxacin concentrations after administration of single doses of 16, 64, and 256 mg/kg of body weight in infected neutropenic mice. Each symbol represents the mean ± standard deviation of the levels in the sera of three mice. t1/2, serum elimination half-life in hours; Cmax, peak serum level; AUC, serum area under the concentration time curve.
In vivo PAE.
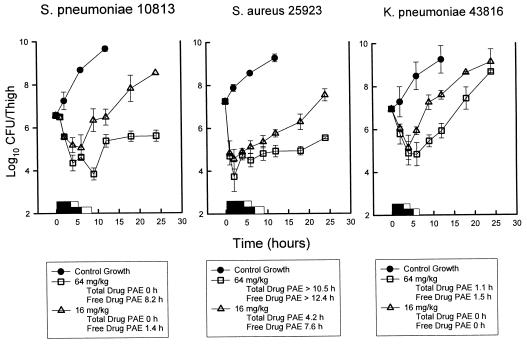
At the start of therapy, mice had 106.6 to 107.2 CFU of S. pneumoniae, S. aureus, or K. pneumoniae per thigh. The growth of organisms in the thighs of saline-treated mice ranged from 2.0 ± 0.2 to 3.1 ± 0.1 log10 CFU/thigh over 12 h. Growth of 1 log10 CFU/thigh in saline-treated animals occurred in 3.0, 4.2, and 4.3 h, in animals infected with S. pneumoniae, S. aureus, and K. pneumoniae, respectively. Based upon the serum pharmacokinetic determinations, serum garenoxacin levels following the single doses of 16 and 64 mg/kg remained above the MIC for S. pneumoniae ATCC 10813 (MIC, 0.03 mg/liter) for 5.8 and 8.5 h (4.2 and 6.2 h, based on free drug levels), respectively. For these doses, T>MIC against S. aureus ATCC 25923 (MIC, 0.016 mg/liter) was 6.5 and 9.3 h (4.9 and 7.4 h, based on free drug levels). Against K. pneumoniae, serum drug levels exceed the MIC (0.06 mg/liter) for 5.1 and 7.7 h (3.5 and 5.8 h, based on free drug levels), respectively. These garenoxacin doses produced reductions of 1.5 ± 0.4 and 2.7 ± 0.2 log10 CFU/thigh at time points of 6 to 9 h, respectively, for S. pneumoniae compared to values at the start of therapy. These doses also produced concentration-dependent killing of S. aureus organisms of 2.7 ± 0.6 and 3.5 ± 0.2 log10 CFU/thigh in 2 to 4 h. There was a lesser degree of killing against K. pneumoniae organisms (range, 1.8 to 2.1 log10 CFU/thigh). The growth curves for each of the three studies are shown in Fig. 2. Against S. pneumoniae, escalating doses produced free drug PAEs ranging from 1.4 to 8.2 h. Study with S. aureus produced free drug PAEs ranging from 7.6 to >12.4 h. With a dose of 64 mg/kg, we did not observe a growth of 1 log10 after drug levels fell below the MIC for S. aureus during the period of study. However, against K. pneumoniae, no remarkable PAEs were observed, even when free drug levels were considered. No detectable drug carryover was observed in any of the treatment groups.
FIG. 2.
In vivo PAE of garenoxacin after administration of single doses of 16 and 64 mg/kg against S. pneumoniae ATCC 10813, S. aureus ATCC 6538p, and K. pneumoniae ATCC 43816. Each symbol represents the mean ± standard deviation for two mice. The widths of hollow bars represent the duration of time total serum levels exceeded the MIC for the infecting pathogen. The widths of the solid bars represent the duration of time free drug serum levels exceeded the MIC for the infecting pathogen.
PK-PD parameter determination.
At the start of therapy, mice had, per thigh, 6.7 ± 0.1 and 6.5 ± 0.2 log10 CFU of S. pneumoniae ATCC 10813 and S. aureus ATCC 33591, respectively. The organisms grew 3.1 ± 0.2 and 2.6 ± 0.3 log10 CFU/thigh, respectively, after 24 h in untreated control mice. Escalating doses of garenoxacin again resulted in concentration-dependent killing of both strains. The highest doses studied reduced organism burden from 4.0 ± 0.1 to 4.5 ± 0.01 log10 CFU/thigh. The dose-response relationship for the four dosing intervals against S. pneumoniae and S. aureus are shown in Fig. 3a and b, respectively. The curves were fairly similar among the three shortest dosing intervals (q3h, q6h, and q12h) against both organisms. Similar to results of studies of other quinolones, the once-daily interval was less active due to the short elimination half-life in small rodents (2, 5, 9). The dosing endpoints (ED25, ED50, ED75, static dose, and 1 log kill) are presented in Table 2. At each of these endpoints we did not observe a significant difference as the dosing interval was lengthened from q3h to q12h. However, there was a trend toward lower dose values for the regimen of q3h in the treatment against S. aureus near the maximal effect (ED75 and 1 log kill). These analyses suggest that treatment efficacy was dependent upon dose level and independent of the dosing intervals studied.
FIG. 3.
(a) The relationship between the garenoxacin dosing interval and efficacy against S. pneumoniae ATCC 10813 in a murine thigh infection model. Each symbol represents the mean data per mouse from two thighs. (b) The relationship between the garenoxacin dosing interval and efficacy against S. aureus ATCC 33591 in a murine thigh infection model. Each symbol represents the mean data per mouse from two thighs.
TABLE 2.
Impact of garenoxacin dosing interval on in vivo efficacy against S. pneumoniae 10813 and S. aureus 33591
| Dosing interval | ED25 (95% CI)a | ED50 (95% CI) | ED75 (95% CI) | Static dose (95% CI) | 1 log kill (95% CI) |
|---|---|---|---|---|---|
| S. pneumoniae | |||||
| q3h | 11.5 (8.2-14.8) | 23 (16.3-29.7) | 46.2 (32.8-59.6) | 18.2 (12.9-23.5) | 25.8 (18.3-33.3) |
| q6h | 8.3 (3.7-12.8) | 18.4 (8.2-28.6) | 41.1 (18.5-63.7) | 16 (7.2-24.8) | 25 (11.2-38.8) |
| q12h | 14.4 (0.6-28.2) | 31 (0.7-61.3) | 64 (3-125) | 31 (0.7-61.3) | 50.3 (23.3-78.3) |
| q24h | 12.9 (1.0-24.9) | 38.8 (1.2-76.4) | 115 (111-227) | 65.7 (1.7-130) | >128 |
| S. aureus | |||||
| q3h | 2.85 (2.6-3.1) | 3.40 (3.1-3.8) | 4.96 (4.5-5.5) | 4.33 (3.9-4.8) | 5.84 (5.3-6.4) |
| q6h | 1.05 (1.0-1.2) | 4.07 (2.9-5.2) | 15.9 (11.1-20.5) | 10.0 (7.0-13.0) | 30 (21.1-38.1) |
| q12h | 1.56 (1.4-1.8) | 3.44 (3.0-3.8) | 7.60 (6.7-8.5) | 5.95 (5.0-7.0) | 11.5 (10.1-12.9) |
| q24h | 2.9 (1.5-4.3) | 7.46 (3.8-11.1) | 19.1 (9.7-28.5) | 7.91 (4.0-11.8) | 26.6 (13.6-39.6) |
CI, confidence interval.
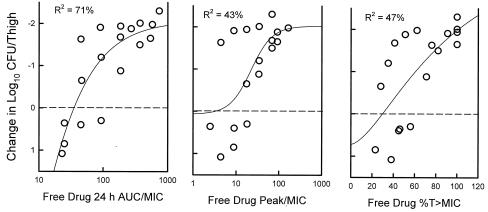
The relationships between microbiologic effect and each of the pharmacodynamic parameters, 24-h AUC/MIC, percent T>MIC, and peak/MIC, against S. pneumoniae ATCC 10813 are shown in Fig. 4. As with fluoroquinolone antibiotics, the strongest relationship was seen when results were correlated with the 24-h AUC/MIC ratio, with an R2 value of 90% (2, 3, 5, 6, 9, 10). Correlations with the percent T>MIC (free drug) and peak/MIC were similar with R2 values of 56 and 75%, respectively. Consideration of bound or unbound drug levels did not appreciably impact the relationship between efficacy and percent T>MIC (data not shown). Similar analysis of study with S. aureus is shown in Fig. 5 and also establishes the strength of the correlation of the 24-h AUC/MIC with efficacy (for 24-h AUC/MIC, R2 = 71%; for peak/MIC, R2 = 43; and for T>MIC, R2 = 47%). Here also consideration of the levels of both total and free drug in serum did not remarkably affect these relationships (data not shown).
FIG. 4.
The relationships of the garenoxacin free drug 24-h AUC/MIC ratio, the percentage of the dosing interval that levels in serum remained above the MIC, and the peak/MIC ratio for S. pneumoniae ATCC 10813 with the log10 CFU/thigh after 24 h of therapy. Each symbol represents the mean data per mouse from two thighs. R2, coefficient of determination.
FIG. 5.
The relationships of the garenoxacin free drug 24-h AUC/MIC ratio, the percentage of the dosing interval that levels in serum remained above the MIC, and the peak/MIC ratio for S. aureus ATCC 33591 with the log10 CFU/thigh after 24 h of therapy. Each symbol represents the mean data per mouse from two thighs. R2, coefficient of determination.
24-hour static dose determination.
Calculations of the doses necessary to achieve a static effect against multiple organisms are shown in Table 1. The growth curves of the 11 pneumococcal, 6 staphylococcal, and 4 gram-negative strains in thighs of control animals were relatively similar. At the start of therapy the number of pneumococci was 5.9 to 7.2 log10 (mean, 6.8 ± 0.3 log10) CFU per thigh. In untreated control mice, the number of organisms grew 1.5 to 3.1 log10 (mean, 2.3 ± 0.5 log10) CFU per thigh.The maximal reduction in S. pneumoniae with garenoxacin-treated mice compared to untreated controls ranged from 1.3 ± 0.3 to 6.4 ± 0.4 log10 (mean, 3.0 ± 1.5 log10) CFU per thigh. Less killing was observed against the quinolone-resistant strains. A similar degree of killing was observed in those animals infected with S. aureus (mean, 4.1 ± 0.9 log10 CFU/thigh). Organism reductions against gram-negative bacilli ranged from 1.4 ± 0.3 to 2.7 ± 0.4 log10 CFU/thigh (mean, 2.3 ± 0.6 log10 CFU/thigh).
Table 1 shows the 24-hour dose and free drug 24-h AUC/MIC ratios necessary to achieve a net static effect. Against the 21 organisms studied, static doses ranged from 2.8 to >128 mg/kg/day, a range of more than 45-fold. However, the free drug 24-h AUC/MIC ratio corresponding with these static doses varied only 17-fold (8.2 to 145; mean ± standard deviation of 47 ± 33). The 24-h AUC/MIC ratio associated with a static effect was relatively similar among all of the organisms studied (mean 24-h AUC/MIC ratio for S. pneumoniae, 33; for S. aureus, 81; and for gram-negative strains, 33) and similar to that observed with fluoroquinolone antibiotics (2, 5, 6). Penicillin, methicillin, and ciprofloxacin resistance did not alter the magnitude of the 24-h AUC/MIC ratio necessary for efficacy.
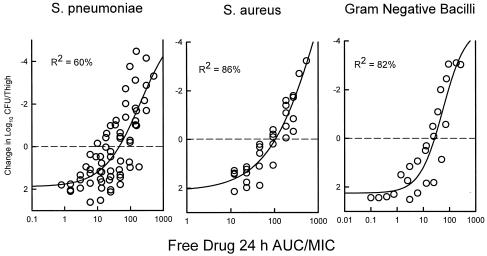
The relationship between the 24-h free drug AUC/MIC ratio and efficacy against the three organism groups is demonstrated graphically in Fig. 6. The dose-response relationships were relatively strong, with R2 values ranging from 60 to 86%.
FIG. 6.
The relationship between the garenoxacin free drug 24 h AUC/MIC and efficacy against 11 S. pneumoniae, 5 S. aureus, and 4 gram-negative bacilli. Each symbol represents the mean data per mouse from two thighs. R2, coefficient of determination.
DISCUSSION
A variety of in vitro and in vivo studies have demonstrated that the quinolones exhibit concentration-dependent killing and produce prolonged PAEs with susceptible gram-positive and gram-negative pathogens (4, 5, 6, 14). The efficacy of antibiotics characterized by this pattern of activity is best correlated with one of the concentration-dependent PK-PD parameters. Several animal infection models have identified the AUC/MIC ratio as the principal PK-PD parameter predictive of efficacy, while the peak/MIC ratio has been suggested to be important for preventing the selection of resistant mutants during therapy (4, 9).
The present studies characterized the in vivo pharmacodynamic activity of a new des-F(6)-quinolone, garenoxacin. Penicillin resistance in S. pneumoniae and methicillin resistance in S. aureus had no impact upon the in vitro and in vivo potency of garenoxacin. The three pneumococcal organisms resistant to ciprofloxacin also demonstrated elevated garenoxacin MICs. However, the relative increase in the MIC did not preclude reaching the therapeutic study endpoints. The quinolone resistance mechanism of these organisms has not been determined. Similar to studies with numerous quinolones, the antimicrobial activity of the des-fluoroquinolone derivative was enhanced by escalating drug concentrations (2, 5, 9, 10, 13, 14, 17). The in vivo PAEs were of moderate duration against the S. pneumoniae and S. aureus strains studied. However, a similar study against a gram-negative strain failed to demonstrate any prolonged growth suppression. One would predict that either the AUC/MIC or peak/MIC ratio would be the PK-PD parameter that most strongly correlated with the efficacy of garenoxacin given this pattern of antimicrobial activity. Data from the present studies of multiple dosing regimens confirmed that the 24-h AUC/MIC is the best PK-PD predictor of efficacy of this new des-fluoroquinolone.
Numerous in vivo and in vitro models and clinical trials have suggested that the magnitude of the 24-h AUC/MIC ratio predictive of quinolone efficacy ranges from a value of 25 to 50 against pneumococci and near 100 against other pathogens (2, 5, 6, 11, 14, 16). Studies have demonstrated that this parameter value is independent of the dosing interval, the fluoroquinolone used, the animal species, and the site of infection (3, 5, 6, 8, 9).
The magnitude of the 24-hour AUC/MIC ratio required for efficacy of this novel quinolone derivative in these studies is similar to that observed with compounds from the fluoroquinolone class. In addition, this parameter magnitude was not affected by drug resistance. In studies with pneumococci, a 24-h AUC/MIC ratio near 30 was associated with a net bacteriostatic effect. Similarly, a 24-h AUC/MIC ratio of 30 to 40 was found to predict maximal survival with this pneumococcal infection model (M. A. Banevicius, H. M. Mattoes, D. P. Nicolau, and D. Xuan, Abstr. 40th Intersci. Conf. Antimicrob. Agents Chemother., abstr. 290, 2000). However, the 24-h AUC/MIC ratios required to achieve this endpoint in a study with S. aureus were more than twofold higher than with pneumococci. These higher values may be related to a difference between the MIC and minimal bactericidal concentration against S. aureus, which was more than twofold for five of the six staphylococcal organisms studied (data not shown). Another possible explanation for the higher 24-h AUC/MIC ratios may be related to the inaccuracy of MIC measurements at very low concentrations. It is also possible that pharmacokinetic estimates at concentrations (lowest MIC, 0.006 μg/ml) more than 30-fold lower than the lower limit of assay detection (0.2 μg/ml) are inaccurate. This problem is difficult to account for in studies with compounds of this potency. The MIC range for S. aureus in this study was 0.006 to 0.016 μg/ml. Similar results were described with the fluoroquinolone sitafloxacin in a study with S. aureus (D. Andes and W. A. Craig, Abstr. 39th Intersci. Conf. Antimicrob. Agents Chemother., abstr. 28, 1999). In a similar study with S. aureus, Tam et al. reported a 24-h AUC/MIC ratio of more than 100 to achieve a bacteriostatic effect in a murine thigh infection model (V. Tam, M. Deziel, W. Liu, R. Bachhawat, D. Gajjar, D. Grasela, and G. Drusano, Abstr. 40th Infect. Dis. Soc. Am. Annu. Meet., abstr. 48, 2002). The higher values reported in this investigation may be related to the 24-h dosing regimen utilized. We found reduced in vivo activity with 24-h dosing in this model due to the rapid elimination half-life in mice. Because of this, we chose to utilize data from the 6-h dosing intervals.
Relative to other fluorinated quinolones, the lack of the fluorine at position 6 does not appear to dramatically alter the PK-PD properties of this quinolone derivative. The pharmacokinetics of a 600-mg oral or intravenous dose of garenoxacin in humans would produce a free drug 24-h AUC of 33 (D. Gajjar, R. Russo, A. Bello, L. Christopher, M. Geraldes, and D. Grasela, 41st Intersci. Conf. Antimicrob. Agents Chemother., abstr. 44, 2001). Based upon a pharmacodynamic goal of an AUC/MIC ratio of 50 to 100, this model would predict that garenoxacin would be successful in the treatment of organisms for which the MICs are as high as 0.25 to 0.5 μg/ml. If one considers garenoxacin pharmacokinetics in relation to the MIC at which 90% of the S. pneumoniae strains are inhibited (0.06 μg/ml), the current dosing regimen would achieve a 24-h AUC/MIC ratio of 550 based on free drug levels. These pharmacodynamic studies support the current once-daily garenoxacin dosing regimen for use in empirical therapy.
REFERENCES
- 1.Andes, D., and W. A. Craig. 1998. In vivo activities of amoxicillin and amoxicillin-clavulanate against Streptococcus pneumoniae: application to breakpoint determinations. Antimicrob. Agents Chemother. 42:2375-2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andes, D., and W. A. Craig. 2002. Pharmacodynamics of the new fluoroquinolone gatifloxacin in murine thigh and lung infection models. Antimicrob. Agents Chemother. 46:1665-1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andes, D. R., and W. A. Craig. 1998. Pharmacodynamics of fluoroquinolones in experimental models of endocarditis. Clin. Infect. Dis. 27:47-50. [DOI] [PubMed] [Google Scholar]
- 4.Blaser, J., B. B. Stone, M. C. Groner, and S. H. Zinner. 1987. Comparative study with enoxacin and netilmicin in a pharmacodynamic model to determine importance of ratio of antibiotic peak concentration to MIC for bacterial activity and emergence of resistance. Antimicrob. Agents Chemother. 31:1054-1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Craig, W., and A. Dalhoff. 1998. Pharmacodynamics of fluoroquinolones in experimental animals, p. 208-232. In J. Kuhlman, A. Dalhoff, and H. J. Zeiller (ed.), Handbook of experimental pharmacology, vol. 127. Quinolone antibacterials. Springer-Verlag, Berlin, Germany.
- 6.Craig, W. A. 1998. Pharmacokinetics and pharmacodynamics of antibiotics in mice and men. Clin. Infect. Dis. 26:1-12. [DOI] [PubMed] [Google Scholar]
- 7.Craig, W. A., and S. Gudmundsson. 1996. Postantibiotic effect, p. 296-329. In V. Lorian (ed.), Antibiotics in laboratory medicine, 4th ed. Lippincott, Williams & Wilkins, Baltimore, Md.
- 8.Craig, W. A. 2001. Does the dose matter? Clin. Infect. Dis. 33(Suppl. 3):233-237. [DOI] [PubMed] [Google Scholar]
- 9.Drusano, G. L., D. E. Johnson, M. Rosen, and H. C. Standiford. 1993. Pharmacodynamics of a fluoroquinolone antimicrobial agent in a neutropenic rat model of Pseudomonas sepsis. Antimicrob. Agents Chemother. 37:483-490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dudley, M. N. 1991. Pharmacodynamics and pharmacokinetics of antibiotics with special reference to the fluoroquinolones. Am. J. Med. 91(Suppl. 6A):45-50. [DOI] [PubMed] [Google Scholar]
- 11.Forrest, A., D. E. Nix, C. H. Ballow, T. F. Goss, M. C. Birmingham, and J. J. Schentag. 1993. Pharmacodynamics of intravenous ciprofloxacin in seriously ill patients. Antimicrob. Agents Chemother. 37:1073-1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fung-Tomc, J. C., B. Minassian, B. Kolek, E. Huczko, L. Aleksunes, T. Stickle, T. Washo, E. Gradelski, L. Valera, and D. P. Bonner. 2000. Antibacterial spectrum of a novel des-fluoro(6) quinolone, BMS-284756. Antimicrob. Agents Chemother. 44:3351-3356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leggett, J. E., B. Fantin, S. Ebert, K. Totsuka, B. Vogelman, W. Calamae, H. Mattie, and W. A. Craig. 1989. Comparative antibiotic dose-effect relationships at several dosing intervals in murine pneumonitis and thigh-infection models. J. Infect. Dis. 159:281-292. [DOI] [PubMed] [Google Scholar]
- 14.Lister, P. D., and C. C. Sanders. 1999. Pharmacodynamics of trovafloxacin, ofloxacin, and ciprofloxacin against Streptococcus pneumoniae in an in vitro pharmacokinetic model. Antimicrob. Agents Chemother. 43:1118-1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Low, D. E., J. de Azavedo, K. Weiss, T. Mazzulli, M. Kuhn, D. Church, K. Forward, G. Zhand, A. Simor, and A. McGeer. 2002. Antimicrobial resistance among clinical isolates of Streptococcus pneumoniae in Canada during 2000. Antimicrob. Agents Chemother. 46:1295-1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Preston, S. L., G. L. Drusano, A. L. Berman, L. Adam, C. L. Fowler, A. T. Chow, B. Dornseif, V. Reichl, J. Natarajan, and M. Corrado. 1998. Pharmacodynamics of levofloxacin. A new paradigm for early clinical trials. JAMA 279:125-129. [DOI] [PubMed] [Google Scholar]
- 17.Vogelman, B., S. Gudmundsson, J. Leggett, J. Turnidge, S. Ebert, and W. A. Craig. 1988. Correlation of antimicrobial pharmacokinetic parameters with therapeutic efficacy in an animal model. J. Infect. Dis. 158:831-847. [DOI] [PubMed] [Google Scholar]
- 18.Weller, T. M. A., J. M. Andrews, G. Jevons, and R. Wise. 2002. The in vitro activity of garenoxacin, a new des-fluorinated quinolone. J. Antimicrob. Chemother. 49:177-184. [DOI] [PubMed] [Google Scholar]