Abstract
Toidentify new leads for the treatment of Plasmodium falciparum malaria, we screened a panel of serotonin (5-hydroxytryptamine [5HT]) receptor agonists and antagonists and determined their effects on parasite growth. The 5HT1A receptor agonists 8-hydroxy-N-(di-n-propyl)-aminotetralin (8-OH-DPAT), 2,5-dimethoxy-4-iodoamphetamine, and 2,5-dimethoxy-4-bromophenylethylamine inhibited the growth of P. falciparum in vitro (50% inhibitory concentrations, 0.4, 0.7, and 1.5 μM, respectively). In further characterizing the antiparasitic effects of 8-OH-DPAT, we found that this serotonin receptor agonist did not affect the growth of Leishmania infantum, Trypanosoma cruzi, Trypanosoma brucei brucei, or Trichostrongylus colubriformis in vitro and did not demonstrate cytotoxicity against the human lung fibroblast cell line MRC-5. 8-OH-DPAT had similar levels of growth inhibition against several different P. falciparum isolates having distinct chemotherapeutic resistance phenotypes, and its antimalarial effect was additive when it was used in combination with chloroquine against a chloroquine-resistant isolate. In a patch clamp assay, 8-OH-DPAT blocked a P. falciparum surface membrane channel, suggesting that serotonin receptor agonists are a novel class of antimalarials that target a nutrient transport pathway. Since there may be neurological involvement with the use of 8-OH-DPAT and other serotonin receptor agonists in the treatment of falciparum malaria, new lead compounds derived from 8-OH-DPAT will need to be modified to prevent potential neurological side effects. Nevertheless, these results suggest that 8-OH-DPAT is a new lead compound with which to derive novel antimalarial agents and is a useful tool with which to characterize P. falciparum membrane channels.
Discovery of drugs for the treatment of malaria is essential because of the widespread resistance of Plasmodium falciparum to chloroquine and other drugs. Drug resistance contributes importantly to the 1 to 2 million deaths caused by malaria every year (25, 37). Natural products from traditional medicinal plants have formed the basis of new synthetic antimalarial analogues with potent activity, and numerous other lead compounds have been identified, including alkaloids, quinones, terpenes, and flavonoids (17, 26). During the screening of plant extracts used in traditional Polynesian medicine and other natural products for antiviral and antimicrobial activity (19), we found that some serotonin (5-hydroxytryptamine [5HT]) receptor agonists have antimalarial properties. The activity of these agonists was accompanied by the blocking of a membrane channel on parasitized erythrocytes. Serotonin agonists may therefore be useful for the characterization of the membrane transport properties of the malaria parasite and may provide new lead candidates for the treatment of malaria.
MATERIALS AND METHODS
Growth inhibition assays.
The P. falciparum isolates used were Uganda-Palo Alto (FUP; mefloquine resistant) (10), Falciparum Vietnam Oak Knoll (FVO; chloroquine resistant) (29), Indochina (chloroquine resistant) (31), Thailand (chloroquine, quinine, and mefloquine [multidrug] resistant) (32), GHA (sensitive to all tested drugs), and W-2 CDC Indochina III (resistant to chloroquine, quinine, and pyrimethamine but susceptible to mefloquine). Parasites were cultured by standard methods as previously described (30).
Inhibition of P. falciparum growth was assessed with two different assays. In the first assay, synchronous parasites at the schizont stage (0.1% parasitemia, 1% hematocrit) were plated onto 96-well tissue culture plates in triplicate at a final hematocrit of 1%. [3H]hypoxanthine incorporation was used to measure parasite metabolism (8). Inhibitor effects were determined over a 72-h period by comparing the hypoxanthine uptake in test cultures with cultures containing control compounds, and the 50% inhibitory concentration (IC50) was determined. In the second assay, antimalarial activity was determined by measuring levels of plasmodial lactate dehydrogenase in an assay using 1% parasitemia in a 2% hematocrit in 384-well microtiter plates as previously described (20). After 72 h of culture, microtiter plates were stored at −20°C. After thawing, 5 μl of culture was transferred to 25 μl of Malstat reagent and 5 ml of a 1:1 mixture of phenazine ethosulfate (2 mg/ml) and Nitro Blue Tetrazolium (grade III; 0.1 mg/ml). The plates were incubated in the dark for 2 h, and the resulting color was measured at 655 nm with a spectrophotometer. Artesunate and chloroquine were used as reference compounds. The results were expressed as the percent reduction in parasitemia compared with control samples, and the IC50 was determined.
Other parasite growth inhibition assays that were evaluated in accordance with standard World Health Organization drug screening protocols included Leishmania infantum [isolate MHOM/MA (BE)/67], Trypanosoma brucei brucei (Squib 427, a suramin-sensitive strain), Trypanosoma cruzi (Tulahen C2C4, a nifurtimox-sensitive strain), and Trichostrongylus colubriformis (Düwel, Hoechst; an albendazole-sensitive strain) (12). To evaluate mammalian cell cytotoxicity, the MRC-5 human lung fibroblast cell line was cultured in minimal essential medium Rega 3 medium supplemented with 20 mM glutamine, 16.5 mM NaHCO3, and 5% fetal calf serum. The test compounds were routinely tested at four concentrations (32, 8, 2, and 0.5 μM) in 384-well microtiter plates. If the IC50 was higher than 16 μM, the compound was classified as nontoxic, if it was between 16 and 1 μM, the compound was classified as moderately toxic, and if it was less than 1 μM, the compound was classified as highly toxic.
Serotonin receptor ligands.
The serotonin receptor agonists serotonin and 8-hydroxy-N-(di-n-propyl)-aminotetralin (8-OH-DPAT), buspirone, and dimethyltryptamine were purchased from Sigma (St. Louis, Mo.) and resuspended in water or dimethyl sulfoxide and then filter sterilized. The phenylalkylamine 2,5-dimethoxy-4-bromophenylethylamine (2-CB) was synthesized as previously described (13), while 2,5-dimethoxy-4-iodoamphetamine (DOI) was commercially available (Richland Biochemicals, Natick, Mass.). The serotonin receptor antagonists spiperone, ritanserin, ketanserin, and hydrobromide 1-(2-methoxyphenyl)-4-(4-[2-phthalimido]butyl)piperazine were purchased from Sigma, resuspended in dimethyl sulfoxide,and filter sterilized.
Serotonin receptor ligand binding assay.
The ability of ligands to bind to a serotonin receptor was determined by measuring the cyclic AMP (cAMP) induced by serotonin in synaptosomal membrane-enriched fractions (9). Briefly, samples extracted from the rat frontal cortex were incubated for 2 min at room temperature in 50 mM Tris-HCl buffer (pH 7.4) containing 0.5 mM EDTA, 0.4 mM ATP, 2.0 mM MgSO4, and 1.0 mM isobutyl methylxanthine. The cAMP reaction was stopped by boiling and ethanol (20%) addition. An equal volume of iodinated tracer (10,000 dpm) was added, and the samples were incubated overnight. Fifty microliters of 1% bovine serum albumin and 1 ml of cold (0°C) ethanol were then added, samples were centrifuged at 2,200 × g for 15 min, and cAMP was counted with a gamma spectrometer.
Patch clamp assay.
One-cell patch recordings were performed as previously described (14). Briefly, 10 MΩ patch pipettes were pulled (Sutter Instruments, Novato, Calif.) from aluminosilicate glass, coated with melted dental wax, fire polished, and filled with phosphate-buffered saline. Recordings were made with an EPC-9 patch clamp amplifier (Heka, Lambrecht, Germany) and digitized at 5 kHz via an ITC-16 interface (Instrutech, Great Neck, N.Y.). To focus patch clamp recordings on parasitized erythrocytes, P. falciparum-infected erythrocytes were treated with 0.1% saponin for 5 min and then washed twice with phosphate-buffered saline. It was previously demonstrated that the parasitophorous vacuole membrane remains intact upon saponin lysis (5). The voltage clamp and data acquisition were controlled via Pulse (Heka) running on a Macintosh Quadra computer. Data were low-pass filtered at 3.5 kHz. Experiments were performed at room temperature (23°C).
RESULTS
Serotonin receptor ligands inhibit growth of malaria parasites.
We first evaluated the antimalarial activity of test compounds with a standard [3H]hypoxanthine assay and cultured P. falciparum parasites. Three serotonin receptor agonists, 8-OH-DPAT, DOI, and 2-CB, markedly inhibited the growth of P. falciparum (FUP isolate) and had IC50s of 0.4, 0.7, and 1.5 μM, respectively (Table 1). The extent of P. falciparum growth inhibition correlated with the affinity of these serotonin receptor agonists to the 5HT1A receptor, as determined by measuring the cAMP induced by serotonin in synaptosomal membrane-enriched fractions. In contrast, serotonin receptor antagonists generally had substantially lower antimalarial activity and this activity was not correlated with serotonin receptor affinity (Table 1). Nevertheless, the growth-inhibitory activity of each of the serotonin receptor agonists was dose dependent for the P. falciparum FUP isolate.
TABLE 1.
Inhibitory effects of serotonin receptor agonists and antagonists on P. falciparum growtha
| Serotonin receptor ligand | Serotonin receptor ligand specificity | Ligand function | Serotonin receptor Kd (nM) | IC50 (μM) |
|---|---|---|---|---|
| 8-OH-DPAT | 5HT1A | Agonist | 2.0 | 0.38 |
| DOI | 5HT2 | Agonist | 10.0 | 0.70 |
| 2-CB | 5HT2 | Agonist | 90.0 | 1.52 |
| Spiperone | 5HT1A | Antagonist | 1,000 | 3.16 |
| Ritanserin | 5HT2 | Antagonist | 1.0-2.0 | 6.32 |
| Ketanserin | 5HT2 | Antagonist | 0.8-1.0 | 12.65 |
Percent inhibition of P. falciparum (Falciparum Uganda Palo Alto, FUP, isolate) growth was determined by measuring [3H]hypoxanthine incorporation (counts per minute) in vitro. Infected erythrocytes at the schizont stage (0.1% parasitemia) were plated onto 96-well tissue culture plates in triplicate at a final hematocrit of 1%, and [3H]hypoxanthine incorporation was used to measure parasite growth. Growth inhibition was determined over a 72-h period by comparing the parasitemia change in test cultures with cultures containing cell culture medium as follows: % growth inhibition = cpm of receptor ligand − cpm of background/cpm of culture medium control − cpm of background.
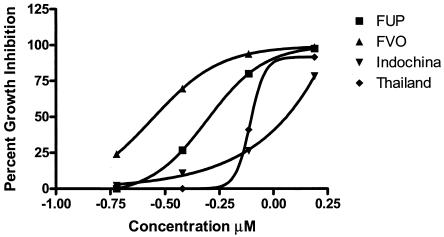
Since P. falciparum isolates are known to differ in sensitivity to chemotherapeutic compounds, we next determined the IC50 of 8-OH-DPAT against four other P. falciparum isolates with different antimalarial resistance patterns. We found that the chloroquine-resistant parasite isolate from Indochina and a multidrug-resistant parasite isolate from Thailand were less sensitive to growth inhibition by 8-OH-DPAT than were the chloroquine-resistant isolate, FVO, and the mefloquine resistant parasite isolate, FUP (Fig. 1).
FIG. 1.
Dose-dependent inhibition of P. falciparum growth by 8-OH-DPAT. FUP (chloroquine-sensitive), FVO, (chloroquine-resistant), Indochina (chloroquine-resistant), and Thailand (multidrug-resistant) isolates were used to demonstrate the growth-inhibitory effects of 8-OH-DPAT. Percent inhibition was determined with a [3H]hypoxanthine incorporation assay.
Serotonin receptor ligands are specific for the human malaria parasite P. falciparum.
We next evaluated the activities of serotonin receptor agonists and antagonists against other parasites. As shown in Table 2, theantiparasitic activity of 8-OH-DPAT was specific for P. falciparum (with a chloroquine-sensitive isolate from Ghana, the IC50 was 0.36 μM). Marginal antimalarial activity was also observed with the 5HT1A receptor agonist buspirone (an IC50 of 22 μM). No activity was observed against the protozoan parasite Trypanosoma brucei brucei, Trypanosoma cruzi, or Leishmania infantum or against the nematode Trichostrongylus colubriformis. Moreover, no cytotoxicity of any of these 5HT1A receptor agonists and antagonists for MRC-5 human lung fibroblast cells was observed.
TABLE 2.
Activities of 5HT1A serotonin receptor ligands against different parasitic organismsa
| Ligand | Concn (μM) showing activity against
|
Concn (μM) showing cytotoxicityb | ||||
|---|---|---|---|---|---|---|
| Trypanosoma brucei bracei | Trypanosoma cruzi | Trichostrongylus colubriformis | Leishmania infantum | Plasmodium falciparum | ||
| 8-OH-DPAT | >32 | >32 | >32 | >32 | 0.36 | >32 |
| Buspirone | >32 | >32 | >32 | >32 | 22.0 | >32 |
| NAN-190c | >32 | >32 | >32 | >32 | >32 | >32 |
Parasite growth inhibition was determined in colorimetric assays or by direct staining of parasite cultures as described in Materials and Methods. Each compound was first tested in fourfold titrations ranging from 32 to 0.5 μM, and the IC50 was determined by comparison with control cultures. If the IC50 was below 0.5 μM, the compound was classified as highly active and was evaluated in a secondary screen. Values shown are means of results from assays performed in triplicate. Positive control compounds had the followings IC50 (micromolar) in each assay: suramin, 0.09 for T. bruce brucei; nifurtimox, 0.25 for T. cruzi; albendazole, 0.02 for T. colubriformis; pentostam, 8.0 for L. infantum; chloroquine, 0.02 for P. falciparum.
Cytotoxicity was determined with the MRC-5 human lung fibroblast cell line.
NAN-190, hydrobromide 1-(2-methoxyphenyl)-4-(4-[2-phthalimido]buty1)piperazine.
The antimalarial activity of 8-OH-DPAT is additive in combination with that of chloroquine.
Since calcium channel blockers are known to reverse chloroquine resistance in P. falciparum, we determined whether 8-OH-DPAT also reverses chloroquine resistance. We conducted an isobologram analysis with a chloroquine-resistant P. falciparum isolate (W-2 CDC Indochina III strain). We found that the effects of 8-OH-DPAT and chloroquine were additive. Thus, 8-OH-DPAT did not act as a chloroquine resistance reversal agent, but when combined, the two drugs were active at nanomolar concentrations against chloroquine-resistant parasites (data not shown).
8-OH-DPAT blocks a malaria parasite membrane channel.
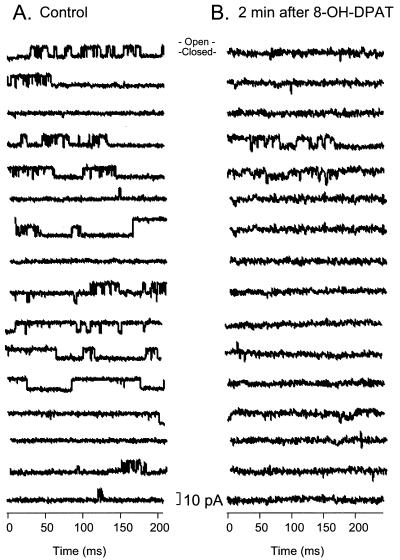
To determine if 8-OH-DPAT targets a membrane channel on the malaria parasite, a patch clamp assay was performed with erythrocytes infected with the FUP isolate. Patch recordings in phosphate-buffered saline revealed channel transitions between (at least) two states. When 8-OH-DPAT was added to the bath, these transitions were no longer recorded after about 2 min, indicating blocking of the channel (Fig. 2). Those few transitions that were observed were in the same direction as the ion control, suggesting that these are channel openings. Since the patch clamp recordings were performed in the cell-attached mode, blocking of the membrane channel may be mediated by a secondary messenger cascade.
FIG. 2.
Blocking of an ion channel on the parasitophorous vacuole membrane of P. falciparum by a 5HT1A agonist, as shown by patch clamp recordings of parasitized erythrocytes. (A) Control baseline recordings showing noncontinuous sweeps of current recorded with the pipette potential clamped at −100 mV (transmembrane potential not known). Each line shows every third sweep from a series of 50 sweeps. The channel is open when the amplitude of the recording is increased and closed (blocked) when the recording is flat. (B) Recordings 2 min after the 5HT1A agonist 8-OH-DPAT (0.1 mg/ml) was added to the bath under the same conditions as the control (A).
DISCUSSION
This study shows that the 5HT1A receptor agonist 8-OH-DPAT has activity against the human malaria parasite P. falciparum. This study also demonstrates that 8-OH-DPAT targets a membrane channel on P. falciparum-infected erythrocytes. 8-OH-DPAT inhibited P. falciparum growth at about 10-fold lower concentrations than did the serotonin receptor antagonists tested (Table 1) and was specific for P. falciparum and not cytotoxic (Table 2). The lack of correlation between the growth inhibition activity and the serotonin receptor affinity of agonists and antagonists suggests that the serotonin-like receptor of P. falciparum differs from the receptor found on neurons.
8-OH-DPAT inhibited an ion membrane channel on the surface of P. falciparum, as demonstrated with a patch clamp assay (Fig. 2). The ion membrane channel characterized in this study may be identical to the nutrient channel that was described with a patch clamp assay (6, 7). P. falciparum modifies the membrane permeability of its host erythrocyte (16) and controls calcium levels with a plasma membrane transport pump (1, 33). 8-OH-DPAT is known to reduce the influx of sodium and calcium into rat synaptosomes (22) and disrupt noncognitive performance in rats (27). In addition, there is evidence that 8-OH-DPAT may function as an antidepressant (15). Other antidepressants, such as desipramine and imipramine (23), have modest antimalarial activity alone and have also been found to facilitate the reversal of chloroquine resistance (3, 4, 18, 34). Nevertheless, since desipramine has not had success in clinical trials for the treatment of chloroquine-resistant malaria (35), new leads and structure-function relationships need to be explored (2).
We conclude that 8-OH-DPAT may be a valuable lead compound with which to build new combinatorial libraries for antimalarials. 8-OH-DPAT is structurally similar to thebaine-like molecules and pain management pharmaceuticals such as morphine; therefore, selective compounds lacking neurological activity would need to be selected. The use of 8-OH-DPAT directly for the treatment of falciparum malaria is unlikely because of possible neurological side effects, such as the serotonin syndrome (11, 21). The avoidance of neurological effects would need to be addressed in the design and evaluation of related compounds. For example, compounds that are predicted to have neurological effects could be screened in animal models of the serotonin syndrome (24, 28).
These data indicate that 8-OH-DPAT may target a 5HT1A-like receptor present in P. falciparum and thus may be used to identify this membrane channel by affinity purification or as a biotinylated probe for quantitative proteomic analysis (36). This receptor may be a nutrient channel critical for parasite development. Thus, this P. falciparum receptor may be an important target for malaria chemotherapeutic intervention.
Acknowledgments
We thank J. Richard Pink of the World Health Organization for facilitating the antiparasite screening in vitro in the laboratory of Louis Maes of Tibotec, Mechelen, Belgium; Bruce McConnell, Kevan Shokat, David Julius, and James McKerrow for helpful discussions; Wasim Siddiqui for providing malaria parasite reagents; Kay Lynn Peter and Ann Murai for secretarial assistance; and Mimi Zeiger for helpful suggestions about the manuscript.
Funding for this study was provided by the UNDP/World Bank/WHO Special Program for Research and Training in Tropical Diseases (TDR).
REFERENCES
- 1.Alleva, L. M., and K. Kirk. 2001. Calcium regulation in the intraerythrocytic malaria parasite Plasmodium falciparum. Mol. Biochem. Parasitol. 117:121-128. [DOI] [PubMed] [Google Scholar]
- 2.Bhattacharjee, A. K., D. E. Kyle, and J. L. Vennerstrom. 2001. Structural analysis of chloroquine resistance reversal by imipramine analogs. Antimicrob. Agents Chemother. 45:2655-2657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bitonti, A. J., A. Sjoerdsma, P. P. McCann, D. E. Kyle, A. M. Oduola, R. N. Rossan, W. K. Milhous, and D. E. Davidson, Jr. 1988. Reversal of chloroquine resistance in malaria parasite Plasmodium falciparum by desipramine. Science 242:1301-1303. [DOI] [PubMed] [Google Scholar]
- 4.Coutaux, A. F., J. J. Mooney, and D. F. Wirth. 1994. Neuronal monoamine reuptake inhibitors enhance in vitro susceptibility to chloroquine in resistant Plasmodium falciparum. Antimicrob. Agents Chemother. 38:1419-1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Delplace, P., B. Fortier, G. Tronchin, J. F. Dubremetz, and A. Vernes. 1987. Localization, biosynthesis, processing and isolation of a major 126 kDa antigen of the parasitophorous vacuole of Plasmodium falciparum. Mol. Biochem. Parasitol. 23:193-201. [DOI] [PubMed] [Google Scholar]
- 6.Desai, S. A., D. J. Krogstad, and E. W. McCleskey. 1993. A nutrient-permeable channel on the intraerythrocytic malaria parasite. Nature 362:643-646. [DOI] [PubMed] [Google Scholar]
- 7.Desai, S. A., S. M. Bezrukov, and J. Zimmerberg. 2000. A voltage-dependent channel involved in nutrient uptake by red blood cells infected with the malaria parasite. Nature 406:1001-1005. [DOI] [PubMed] [Google Scholar]
- 8.Desjardins, R. E., C. J. Canfield, J. D. Haynes, and J. D. Chulay. 1979. Quantitative assessment of antimalarial activity in vitro by a semiautomated microdilution technique. Antimicrob. Agents Chemother. 16:710-718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fillion, G., J. C. Rousselle, D. Beaudoin, P. Pradelles, M. Goiny, F. Dray, and J. Jacob. 1979. Serotonin sensitive adenylate cyclase in horse brain synaptosomal membranes. Life Sci. 24:1813-1822. [DOI] [PubMed] [Google Scholar]
- 10.Geiman, Q. M., and M. J. Meagher. 1967. Susceptibility of a New World monkey to Plasmodium falciparum from man. Nature 215:437-439. [DOI] [PubMed] [Google Scholar]
- 11.Gillman, P. K. 1999. The serotonin syndrome and its treatment. J. Psychopharmacol. 13:100-109. [DOI] [PubMed] [Google Scholar]
- 12.Girault, S., P. Grellier, A. Berecibar, L. Maes, E. Mouray, P. Lemiere, M. A. Debreu, E. Davioud-Charvet, and C. Sergheraert. 2000. Antimalarial, antitrypanosomal, and antileishmanial activities and cytotoxicity of bis(9-amino-6-chloro-2-methoxyacridines): influence of the linker. J. Med. Chem. 43:2646-2654. [DOI] [PubMed] [Google Scholar]
- 13.Glennon, R. A. 1987. Central serotonin receptors as targets for drug research. J. Med. Chem. 30:1-12. [DOI] [PubMed] [Google Scholar]
- 14.Hamill, O. P., A. Marty, E. Neher, B. Sakmann, and F. J. Sigworth. 1981. Improved patch clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pfluegers Arch. 391:85-100. [DOI] [PubMed] [Google Scholar]
- 15.Harkin, A., J. P. Kelly, M. McNamara, T. J. Connor, K. Dredge, A. Redmond, and B. E. Leonard. 1999. Activity and onset of action of reboxetine and effect of combination with sertraline in an animal model of depression. Eur. J. Pharmacol. 364:123-132. [DOI] [PubMed] [Google Scholar]
- 16.Kirk, K. 2001. Membrane transport in the malaria infected erythrocyte. Physiol. Rev. 81:495-537. [DOI] [PubMed] [Google Scholar]
- 17.Krettli, A. U., V. F. Andrade-Neto, M. G. Brandao, and W. M. Ferrari. 2001. The search for new antimalarial drugs from plants used to treat fever and malaria or plants randomly selected: a review. Mem. Inst. Oswaldo Cruz 96:1033-1042. [DOI] [PubMed] [Google Scholar]
- 18.Kyle, D. E., A. M. Oduola, S. K. Martin, and W. K. Milhous. 1990. Plasmodium falciparum: modulation by calcium antagonists of resistance to chloroquine, desethylchloroquine, quinine, and quinidine in vitro. Trans. R. Soc. Trop. Med. Hyg. 84:474-478. [DOI] [PubMed] [Google Scholar]
- 19.Locher, C. P., M. T. Burch, H. F. Mower, J. Berestecky, H. Davis, B. Van Poel, A. Lasure, D. A. Vanden Berghe, and A. J. Vlietinck. 1995. Anti-microbial activity and anti-complement activity of extracts obtained from selected Hawaiian medicinal plants. J. Ethnopharmacol. 49:23-32. [DOI] [PubMed] [Google Scholar]
- 20.Makler, M. T., and D. J. Hinrichs. 1993. Measurement of the lactate dehydrogenase activity of Plasmodium falciparum as an assessment of parasitemia. Am. J. Trop. Med. Hyg. 48:205-210. [DOI] [PubMed] [Google Scholar]
- 21.Mason, P. J., V. A. Morris, and T. J. Balcezak. 2000. Serotonin syndrome. Presentation of 2 cases and review of the literature. Medicine 79:201-209. [DOI] [PubMed] [Google Scholar]
- 22.Melena, J., G. Chidlow, and N. N. Osborne. 2000. Blockade of voltage-sensitive Na+ channels by the 5-HT(1A) receptor agonist 8-OH-DPAT: possible significance for neuroprotection. Eur. J. Pharmacol. 406:319-324. [DOI] [PubMed] [Google Scholar]
- 23.Menezes, C. M., K. Kirchgatter, S. M. Di Santi, C. Savalli, F. G. Monteiro, G. A. Paula, and E. I. Ferreira. 1997. Antimalarial effect in vitro and lack of modulating effect of desipramine and imipramine. Trans. R. Soc. Trop. Med. Hyg. 91:697-700. [DOI] [PubMed] [Google Scholar]
- 24.Nisijima, K., K. Shioda, T. Yoshino, K. Takano, and S. Kato. 2003. Diazepam and chlormethiazole attenuate the development of hyperthermia in an animal model of the serotonin syndrome. Neurochem. Int. 43:155-164. [DOI] [PubMed] [Google Scholar]
- 25.Olliaro, P. L., and Y. Yuthavong. 1999. An overview of chemotherapeutic targets for antimalarial drug discovery. Pharmacol. Ther. 81:91-110. [DOI] [PubMed] [Google Scholar]
- 26.Phillipson, J. D., and C. W. Wright. 1991. Can ethnopharmacology contribute to the development of antimalarial agents? J. Ethnopharmacol. 32:155-165. [DOI] [PubMed] [Google Scholar]
- 27.Ruotsalainen, S., E. MacDonald, E. Koivisto, R. Stefanski, A. Haapalinna, P. Riekkinen, Jr., and J. Sirvio. 1998. 5-HT1A receptor agonist (8-OH-DPAT) and 5-HT2 receptor agonist (DOI) disrupt the non-cognitive performance of rats in a working memory task. J. Psychopharmacol. 12:177-185. [DOI] [PubMed] [Google Scholar]
- 28.Ryan, P. M., J. P. Kelly, P. L. Chambers, and B. E. Leonard. 2001. The toxicity profile of a single dose of paroxetine: an alternative approach to acute toxicity testing in the rat. Pharmacol. Toxicol. 88:59-66. [DOI] [PubMed] [Google Scholar]
- 29.Siddiqui, W. A., J. V. Schnell, and Q. M. Geiman. 1972. A model in vitro system to test the susceptibility of human malarial parasites to antimalarial drugs. Am. J. Trop. Med. Hyg. 21:393-399. [PubMed] [Google Scholar]
- 30.Siddiqui, W. A., and K. L. Palmer. 1981. Propagation of malaria parasites in vitro, p. 183-212. In J. Maramorosch (ed.), Advances in cell culture, vol. 1. Academic Press, Inc., New York, N.Y.
- 31.Stanley, H. A., R. F. Howard, and R. T. Reese. 1985. Recognition of a Mr 56K glycoprotein on the surface of Plasmodium falciparum merozoites by mouse monoclonal antibodies. J. Immunol. 134:3439-3444. [PubMed] [Google Scholar]
- 32.Thaithong, S., G. H. Beale, and M. Chutmongkonkul. 1983. Susceptibility of Plasmodium falciparum to five drugs: an in vitro study of isolates mainly from Thailand. Trans. R. Soc. Trop. Med. Hyg. 77:228-231. [DOI] [PubMed] [Google Scholar]
- 33.Tiffert, T., H. M. Staines, J. C. Ellory, and V. L. Lew. 2000. Functional state of the plasma membrane Ca2+ pump in Plasmodium falciparum-infected human red blood cells. J. Physiol. 525(Pt. 1):125-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.van Schalkwyk, D. A., J. C. Walden, and P. J. Smith. 2001. Reversal of chloroquine resistance in Plasmodium falciparum using combinations of chemosensitizers. Antimicrob. Agents Chemother. 45:3171-3174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Warsame, M., W. H. Wernsdorfer, and A. Bjorkman. 1992. Lack of effect of desipramine on the response to chloroquine of patients with chloroquine-resistant falciparum malaria. Trans. R. Soc. Trop. Med. Hyg. 86:235-236. [DOI] [PubMed] [Google Scholar]
- 36.Wolters, D. A., M. P. Washburn, and J. R. Yates III. 2001. An automated multidimensional protein identification technology for shotgun proteomics. Anal. Chem. 73:5683-5690. [DOI] [PubMed] [Google Scholar]
- 37.Wongsrichanalai, C., A. L. Pickard, W. H. Wernsdorfer, and S. R. Meshnick. 2002. Epidemiology of drug-resistant malaria. Lancet Infect. Dis. 2:209-218. [DOI] [PubMed] [Google Scholar]