Abstract
DQ-113 is a new quinolone with potent activity against gram-positive pathogens. The in vivo activity of DQ-113 against Streptococcus pneumoniae was compared with those of gatifloxacin and ciprofloxacin in a mouse model. For this purpose, two strains of S. pneumoniae were used: penicillin-susceptible S. pneumoniae (PSSP) and penicillin-resistant S. pneumoniae (PRSP). The survival rates of mice infected with PSSP and PRSP at 14 days after infection were 80% in the DQ-113-treated group and 0 to 10% in the other three groups. In murine infections caused by PSSP, the 50% effective doses (ED50s) of DQ-113, gatifloxacin, and ciprofloxacin were 6.0, 41.3, and 131.6 mg/kg, respectively. Against PRSP-caused pneumonia in mice, the ED50s of DQ-113, gatifloxacin, and ciprofloxacin were 7.6, 64.7, and 125.9 mg/kg, respectively. Compared with the other drugs, DQ-113 showed excellent therapeutic efficacy and eradicated viable bacteria in both PSSP- and PRSP-infected mice. The means ± standard errors of the means of viable bacterium counts in the lungs of gatifloxacin-treated, ciprofloxacin-treated, and untreated control mice infected with PSSP were 2.91 ± 0.34, 3.13 ± 0.48, and 3.86 ± 0.80 log10CFU/ml, respectively. The same counts in mice infected with PRSP treated with the same three agents were 6.57 ± 0.99, 6.54 ± 0.40, and 7.17 ± 0.43 log10 CFU/ml, respectively. DQ-113 significantly decreased the number of viable bacteria in the lungs compared with gatifloxacin and ciprofloxacin. Of the drugs analyzed, the pharmacokinetic-pharmacodynamic parameter of area under the concentration-time curve (AUC)/MIC ratio for DQ-113 was significantly higher than those for gatifloxacin and ciprofloxacin. Our results suggest that DQ-113 has potent in vivo efficacy against both PSSP and PRSP.
Streptococcus pneumoniae is an important pathogen in many community-acquired respiratory infections, including community-acquired pneumonia, acute bacterial sinusitis, and acute otitis media, and in more-invasive infections, such as meningitis and bacteremia. S. pneumoniae is a leading cause of morbidity and mortality worldwide. Formerly, β-lactam antibiotics were very effective against S. pneumoniae; however, resistance to this class of antibiotic has become an increasing problem. The first isolate of penicillin-resistant S. pneumoniae (PRSP) of recognized clinical significance (MIC = 0.5 μg/ml) was recovered in Australia (9). Antimicrobial agent-resistant S. pneumoniae became widespread in many parts of the world during the 1980s (1). In Japan, rates of penicillin resistance among the pneumococci are reported to be 30 to 46% (12, 13, 17). They are as high as 60% in some parts of Latin America (14) and 80% in Korea (18). Unfortunately, S. pneumoniae is becoming increasingly resistant to a variety of antibiotics. Infections caused by PRSP may lead to clinical treatment failures. Concern over the emergence of penicillin-resistant and multidrug-resistant strains has led to the development of antipneumococcal fluoroquinolones, such as sparfloxacin, gatifloxacin, and moxifloxacin. These agents have high activities against S. pneumoniae and are now approved for first-line therapy of community-acquired pneumonia (2).
There is, however, growing concern about the development of quinolone-resistant S. pneumoniae (4, 10), with a recent survey revealing resistance to both the early fluoroquinolones and the newer quinolones, such as sparfloxacin, gatifloxacin, and moxifloxacin (11). Most troubling is the possibility of cross-resistance to the newer quinolones (11). Therefore, more-potent compounds must be developed to treat multidrug-resistant gram-positive bacteria, including S. pneumoniae.
DQ-113 is a new fluoroquinolone, which has the most-potent activity against gram-positive pathogens among the respiratory quinolones, such as gatifloxacin and moxifloxacin (20). In the present study, we examined the in vivo activity of DQ-113 against penicillin-susceptible S. pneumoniae (PSSP) and non-PSSP in a noncompromised mouse model of pneumonia and compared it with the activities of gatifloxacin and ciprofloxacin.
MATERIALS AND METHODS
Antimicrobial agents.
DQ-113 was provided by DAIICH Pharmaceutical Co. Gatifloxacin and Ciprofloxacin were extracted from commercial preparations purchased from KYORIN Pharmaceutical Co. and Bayer Pharmaceutical Co. DQ-113 was dissolved in 0.1 N NaOH and then reconstituted with 0.5% glucose and 0.1 N phosphate-buffered saline. Gatifloxacin and ciprofloxacin were dissolved in distilled water and normal saline, respectively.
Microorganisms.
Two strains of S. pneumoniae clinically isolated at Nagasaki University School of Medicine were used in the present study. One strain was the PSSP strain NU83127 (MIC of penicillin G, 0.03 μg/ml; serotype 4). The other was the PRSP strain NU187 (MIC of penicillin G, 2 μg/ml; serotype 19). Bacteria were stored at −80°C until use.
Laboratory animals.
Five-week-old male CBA/J specific-pathogen-free mice (body weight, 20 g) were purchased from Charles River Japan. The CBA/J mouse model of PRSP pneumonia has been described previously (19, 21). All mouse experiments were performed according to the guidelines of the Laboratory Animal Center for Biomedical Research, Nagasaki University School of Medicine.
Antibiotic susceptibility test.
MICs of antibiotics were determined by a broth dilution method with Mueller-Hinton broth (Difco Laboratories, Detroit, Mich.) supplemented with 5% lysed horse blood. Microtiter plates containing 5.0 × 104 CFU/well were incubated with antibiotic at 35°C for 18 h, and the lowest concentration of drug that prevented visible growth was considered the MIC.
Experimental murine model of pneumococcal pneumonia.
S. pneumoniae strains were cultured on a horse blood agar plate for 24 h at 37°C, then scraped and suspended in brain heart infusion broth mixed with horse serum, and cultured with shaking for 6 h at 37°C at 250 rpm. Bacteria were then harvested by centrifugation (800 × g, 3 min). The organisms were resuspended in normal saline, and final numbers of bacteria prepared were approximately 105 CFU of PSSP/ml and 108 CFU of PRSP/ml, as determined by turbidimetry. Infection was induced by intranasal inoculation of 0.05 ml of bacterial suspension, containing about 1 × 105 CFU of PSSP/ml (5 × 103 CFU/mouse), and 1 × 108 CFU of PRSP/ml (5 × 106 CFU/mouse), into anesthetized mice.
Survival studies.
Forty mice were allocated into four treatment groups: DQ-113, gatifloxacin, ciprofloxacin, and normal saline (controls). The doses of test drugs were adjusted to 10 mg/kg of body weight, and drugs were injected intraperitoneally twice daily for 14 days (20 mg/kg/day). Each antibiotic treatment was commenced 24 h after inoculation.
In addition, various doses of test drugs were administered to mice twice daily. Mortality was recorded for 14 days, and the 50% effective dose (ED50) of each drug was calculated by the probit method (3).
Bacteriological and histopathological examinations.
The drugs were injected intraperitoneally into the mice twice daily (20 mg/kg/day) beginning 24 h after inoculation. Mice (n = 10 for each group, DQ-113, gatifloxacin, ciprofloxacin, and normal saline [controls]) were sacrificed by cervical dislocation on day 3 (12 h after the fourth administration). For bacteriological examination, the lungs (n = 7 for each group) were dissected under aseptic conditions and suspended in saline (1 ml). Organs were homogenized with a Polytron homogenizer, quantitatively inoculated onto blood agar plates by serial dilutions, and incubated at 37°C for 18 h. The lowest level of detectable CFU/milliliter is 50 CFU/ml. Lung tissue for histological examination (n = 3 for each group) was fixed in 10% buffered formalin and stained with hematoxylin-eosin.
Pharmacokinetic studies.
Studies were undertaken to determine the pharmacokinetic profiles of DQ-113, gatifloxacin, and ciprofloxacin in mice infected with PRSP. Groups of 5 mice each were administered DQ-113 at a dose of 10 mg/kg, gatifloxacin at a dose of 10 or 50 mg/kg, and ciprofloxacin at a dose of 10 or 100 mg/kg. Animals were sacrificed by cervical dislocation, and serum samples and lungs were collected from mice at 0.25, 0.5, 1, 2, 4, and 6 h after treatment. These samples were immediately frozen and stored at −80°C until assay. The concentrations of DQ-113 and gatifloxacin were determined by microbiological agar diffusion assay with Bacillus subtilis ATCC 6633, and ciprofloxacin concentrations were determined with Escherichia coli Kp. Pharmacokinetic parameters were calculated from the arithmetic means of serum and lung tissue concentrations.
Statistical analysis.
Data are expressed as means ± standard errors of the means (SEM). Survival analysis was performed by the log-rank test, and survival rates were calculated by the Kaplan-Meier method. Differences between numbers of viable bacteria in lungs were evaluated by the nonparametric multiple comparison test and the Steel-Dwass test following the Kruskal-Wallis test. P values of less than 0.05 were considered statistically significant.
RESULTS
In vitro susceptibility.
For the PSSP and PRSP strains used, the MICs of DQ-113, gatifloxacin, and ciprofloxacin were 0.008 and 0.004 μg/ml, 0.25 and 0.25 μg/ml, and 1.0 and 0.5 μg/ml, respectively.
In vivo efficacy against PSSP.
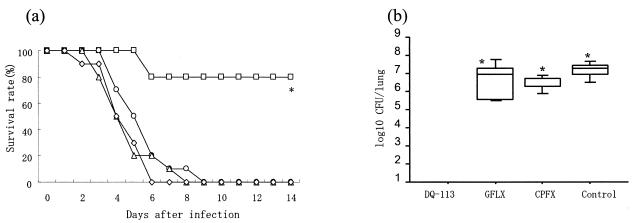
In the survival study, as shown in Fig. 1a, DQ-113-treated mice survived longer than gatifloxacin-treated, ciprofloxacin-treated, and untreated mice. The survival rate at 14 days after infection was 80% in the DQ-113-treated group and 0 to 10% in the other three groups (gatifloxacin treated, ciprofloxacin treated, and untreated). DQ-113 significantly improved survival in the PSSP-infected mouse model (80% versus 0 or 10%; P < 0.001). In the bacteriological study, DQ-113 (n = 7) eradicated viable bacteria in the lungs. The mean viable bacterium counts in the lungs of gatifloxacin-treated mice, ciprofloxacin-treated mice, and untreated controls (n = 7) were 2.91 ± 0.34, 3.13 ± 0.48, and 3.86 ± 0.80 log10 CFU/ml, respectively. The number of viable bacteria in the lungs of DQ-113-treated mice was significantly less than that in gatifloxacin-treated, ciprofloxacin-treated, and untreated mice (P < 0.01) (Fig. 1b). As shown in Table 1, in murine infections caused by PSSP, the ED50s of DQ-113, gatifloxacin, and ciprofloxacin were 6.0, 41.3, and 131.6 mg/kg, respectively. The therapeutic efficacies of DQ-113 were 6.8- and 21.3-fold superior to those of gatifloxacin and ciprofloxacin.
FIG. 1.
Efficacy against PSSP. (a) Effects of DQ-113, gatifloxacin, and ciprofloxacin on survival rates of mice. DQ-113 (□), gatifloxacin (○), ciprofloxacin (▵), or saline (⋄) was administered intraperitoneally twice daily for 14 days starting 24 h after infection. Each antibiotic was administered at a dose of 20 mg/kg/day. The survival rate at 14 days after infection was 80% in the DQ-113-treated group and 0 to 10% in the other three groups (gatifloxacin treated, ciprofloxacin treated, and untreated). DQ-113 significantly improved the survival rate. *, P < 0.001, compared with other groups. (b) Number of viable bacteria in lungs after four drug doses. Each bar represents the mean ± SEM of the results from 7 mice. In the bacteriological study, DQ-113 (n = 7) eradicated viable bacteria in the lung. The mean viable bacterium counts in the lungs of gatifloxacin (GFLX)-treated mice, ciprofloxacin (CPFX)-treated mice, and untreated controls (n = 7) were 2.91 ± 0.34, 3.13 ± 0.48, and 3.86 ± 0.80 log10 CFU/ml, respectively. DQ-113 significantly reduced the number of viable bacteria compared with gatifloxacin, ciprofloxacin, and saline (control). *, P < 0.01.
TABLE 1.
Protective effects of DQ-113, gatifloxacin, and ciprofloxacin against mice infected with S. pneumoniae
| Infection | Drug | MIC (μg/ml) | ED50 (mg/kg/day) (95% confidence interval) |
|---|---|---|---|
| PSSPa | DQ-113 | 0.008 | 6.010 (1.989-9.895) |
| Gatifloxacin | 0.25 | 41.27 (27.91-59.46) | |
| Ciprofloxacin | 1.0 | 131.6 (95.61-173.2) | |
| PRSPb | DQ-113 | 0.004 | 7.601 (3.061-11.90) |
| Gatifloxacin | 0.25 | 64.69 (45.30-90.52) | |
| Ciprofloxacin | 0.5 | 125.9 (83.55-178.3) |
Groups of 10 mice each were infected intranasally with 0.05 ml of PSSP bacterial suspension (5 × 103 CFU/mouse).
Groups of 10 mice each were infected intranasally with 0.05 ml of PRSP bacterial suspension (5 × 106 CFU/mouse).
In vivo efficacy against PRSP.
In the survival study, gatifloxacin-treated, ciprofloxacin-treated, and untreated mice died between 6 and 9 days after infection. In contrast, in the DQ-113-treated group, the survival rate at 14 days after infection was 80% (Fig. 2a). DQ-113 significantly improved the survival rate (P < 0.001). In the bacteriological study, DQ-113 (n = 7) eradicated viable bacteria in the lungs. The means ± SEM of viable bacteria in the lungs of gatifloxacin-treated, ciprofloxacin-treated, and untreated mice (n = 7) were 6.57 ± 0.99, 6.54 ± 0.40, and 7.17 ± 0.43 log10 CFU/ml, respectively. DQ-113 significantly reduced the number of viable bacteria in the lungs compared to gatifloxacin, ciprofloxacin, and normal saline (control) (P < 0.01) (Fig. 2b). As shown in Table 1, against PRSP-caused pneumonia in mice, the ED50s of DQ-113, gatifloxacin, and ciprofloxacin were 7.6, 64.7, and 125.9 mg/kg, respectively. DQ-113 had activities 8.5- and 16.6-fold greater than those of gatifloxacin and ciprofloxacin.
FIG. 2.
Efficacy against PRSP. (a) Effect of DQ-113, gatifloxacin, and ciprofloxacin on survival rates of mice. DQ-113 (□), gatifloxacin (○), ciprofloxacin (▵), or saline (⋄) was administered intraperitoneally twice daily for 14 days starting 24 h after infection. Each antibiotic was administered at a dose of 20 mg/kg/day. In the survival study, gatifloxacin-treated, ciprofloxacin-treated, and untreated mice died between 6 and 9 days after infection. In contrast, in the DQ-113-treated group, the survival rate at 14 days after infection was 80%. DQ-113 significantly improved the survival rate. *, P < 0.001, compared with other groups. (b) Number of viable bacteria in lungs after four doses. Each bar represents the mean ± SEM of the results from 7 mice. In the bacteriological study, DQ-113 (n = 7) eradicated viable bacteria in the lung. The means ± SEM of viable bacteria in the lungs of gatifloxacin (GFLX)-treated, ciprofloxacin (CPFX)-treated, and untreated mice (n = 7) were 6.57 ± 0.99, 6.54 ± 0.40, and 7.17 ± 0.43 log10 CFU/ml, respectively. DQ-113 significantly reduced the number of viable bacteria in the lungs compared to gatifloxacin, ciprofloxacin, and saline (control). *, P < 0.01.
Lung and serum concentrations of DQ-113, gatifloxacin, and ciprofloxacin in mice.
Table 2 shows the pharmacokinetic-pharmacodynamic parameters of test drug in serum and lungs after administration of intraperitoneal injection into the mice infected with PRSP. Ratios of the area under the concentration-time curve (AUC) in the lungs to the AUC in serum for DQ-113, gatifloxacin, and ciprofloxacin (dose of 10 mg/kg) were 9.62, 3.24, and 3.52, respectively. The AUC/MIC ratio in the lungs for DQ-113 was 1,827 at a dose of 10 mg/kg; it was significantly higher than those of gatifloxacin and ciprofloxacin.
TABLE 2.
Pharmacokinetic-pharmacodynamic parameters of DQ-113, gatifloxacin, and ciprofloxacin in serum and lungs of mice infected with PRSP (n = 5)a
| Drug (MIC) | Dose (mg/kg) | Tissue | Cmax (μg/ml) | Tmax (h) | t1/2 (h) | AUC (mg · h/ml) | Lung/serum AUC ratio | AUC/MIC ratio |
|---|---|---|---|---|---|---|---|---|
| DQ-113 (0.004) | 10 | Serum | 1.05 | 0.25 | 0.96 | 0.76 | 9.62 | 191.18 |
| Lung | 3.82 | 0.5 | 1.19 | 7.31 | 1,826.94 | |||
| GAFX (0.25) | 10 | Serum | 2.00 | 0.25 | 0.58 | 1.71 | 3.24 | 6.82 |
| Lung | 6.30 | 0.25 | 0.57 | 5.54 | 22.16 | |||
| 50 | Serum | 10.39 | 0.25 | 0.93 | 15.13 | 2.76 | 60.54 | |
| Lung | 24.64 | 0.25 | 1.02 | 41.73 | 166.92 | |||
| CPFX (0.5) | 10 | Serum | 1.52 | 0.25 | 0.45 | 1.02 | 3.52 | 2.02 |
| Lung | 3.44 | 0.25 | 0.57 | 3.59 | 7.19 | |||
| 100 | Serum | 10.68 | 0.25 | 0.96 | 31.29 | 3.38 | 62.59 | |
| Lung | 35.37 | 1 | 1.19 | 105.87 | 211.75 |
Abbreviations: GAFX, gatifloxacin; CPFX, ciprofloxacin; Cmax, maximum concentration of drug in serum; Tmax, time to maximum concentration of drug in serum; t1/2, half-life.
Histopathological examination.
In the PRSP study, microscopic examination of lung specimens from mice sacrificed 2 days after treatment (day 3) showed features of acute bronchopneumonia. Acute inflammatory cells infiltrated around bronchi and exudates had collected in the alveolar spaces. Histopathological findings in the gatifloxacin-treated and ciprofloxacin-treated groups were almost the same as those in the untreated controls. However, a few inflammatory cells were observed in the DQ-113-treated group. Similar findings were observed in the PSSP study (data not shown.)
DISCUSSION
Generally, quinolones inhibit bacterial DNA gyrase and topoisomerase IV, which hinders DNA supercoiling and relaxation, thereby causing bacterial cell death. The early fluoroquinolones, such as ciprofloxacin and norfloxacin, have a proven record in the treatment of gram-negative infections. However, they have only modest activity against gram-positive bacteria, particularly S. pneumoniae. Thus, agents with potent activities against gram-positive bacteria have been developed. Sparfloxacin, levofloxacin, and grepafloxacin have far better activities against gram-positive pathogens than ciprofloxacin and norfloxacin, but their potencies are still less than ideal. Increasing resistance to quinolones has been documented in Hong Kong, Canada, and Spain (4, 8, 10). Therefore, improvements in the activities of agents against gram-positive organisms was necessary. Newer quinolones, such as gatifloxacin, gemifloxacin, and moxifloxacin, have exhibited a marked improvement and have much better in vitro activities with lower MICs against S. pneumoniae (5, 6, 16). However, newer non-quinolone-susceptible S. pneumoniae strains were reported in Hong Kong (11). Therefore, compounds with more-potent activity against S. pneumoniae need to be developed.
In the present study, the in vivo activity of DQ-113, a new quinolone, against S. pneumoniae was compared with those of ciprofloxacin and gatifloxacin through evaluation of survival rate, bacteriological, pharmacological, and histopathological effects.
DQ-113 exhibited potent activity against both PRSP and PSSP. Data from the MIC study showed the antibacterial activity of DQ-113 to be 16- to 32-fold greater than that of gatifloxacin and 64-fold greater than that of ciprofloxacin. DQ-113 was reported to possess the most-potent activity against staphylococci, streptococci, and enterococci among the other new quinolones, such as gatifloxacin, moxifloxacin, vancomycin, and linezolid (20). DQ-113 treatment significantly decreased the number of viable bacteria compared with treatment with gatifloxacin and ciprofloxacin. DQ-113 also significantly improved survival rates in both the PRSP- and PSSP-infected mouse models. The protective efficacy (ED50) of DQ-113 was more potent than that of gatifloxacin and ciprofloxacin. The AUC/MIC ratio is an important pharmacodynamic parameter that influences the outcome of fluoroquinolone therapy (7, 15). The AUC/MIC ratio in the lungs for DQ-113 was significantly higher than those for gatifloxacin and ciprofloxacin. Moreover, the lung/serum AUC ratio for DQ-113 was about three times higher than those for gatifloxacin and ciprofloxacin (dose of 10 mg/kg). These profiles were consistent with the significant improvement in in vivo efficacy of DQ-113 against S. pneumoniae in a mouse model. Thus, DQ-113 showed good in vivo efficacy against both PSSP and PRSP. This is the first study of the in vivo efficacy of DQ-113 against S. pneumoniae. Our results are consistent with a previous report revealing the excellent antibacterial activity of DQ-113 in vitro (20).
DQ-113 was reported to have the most-potent activity against S. pneumoniae. The MICs at which 90% of the isolates tested are inhibited of DQ-113, gatifloxacin, and ciprofloxacin for PSSP and PRSP were 0.03 and 0.015, 0.5 and 0.25, and 4 and 2 μg/ml, respectively (20). Thus, it is quite likely that DQ-113 may be effective against quinolone-resistant S. pneumoniae.
In conclusion, a new quinolone, DQ-113, has high efficacies against both PSSP and PRSP in a mouse model of infection. In the future, DQ-113 may become a feasible first-line therapy for community-acquired respiratory infections, including those caused by S. pneumoniae.
REFERENCES
- 1.Appelbaum, P. C. 1992. Antimicrobial resistance in Streptococcus pneumoniae: an overview. Clin. Infect. Dis. 15:77-83. [DOI] [PubMed] [Google Scholar]
- 2.Bartlett, J. G., R. F. Breiman, L. A. Mandell, and T. M. File, Jr. 1998. Community-acquired pneumonia in adults: guideline for management. Clin. Infect. Dis. 26:811-838. [DOI] [PubMed] [Google Scholar]
- 3.Bliss, C. I. 1985. Statistics in bioassay. Academic Press, Inc., New York, N.Y.
- 4.Chen, D. K., A. McGeer, J. C. DeAzavedo, D. E. Low, and The Canadian Bacterial Surveillance Network. 1999. Decreased susceptibility of Streptococcus pneumoniae to fluoroquinolones in Canada. N. Engl. J. Med. 341:233-239. [DOI] [PubMed] [Google Scholar]
- 5.Coyle, E. A., G. W. Kaatz, and M. J. Rybak. 2001. Activity of newer fluoroquinolones against ciprofloxacin-resistant Streptococcus pneumoniae. Antimicrob. Agents Chemother. 45:1654-1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davies, T. A., L. M. Kelly, G. A. Pankucii, K. L. Credito, M. R. Jacobs, and P. C. Appelbaum. 2000. Antipneumococcal activities of gemofloxacin compared to those of nine other agents. Antimicrob. Agents Chemother. 44:304-331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Forrest, A., D. E. Nix, C. H. Ballow, T. F. Goss, M. C. Birmingham, and J. J. Schentag. 1993. Pharmacodynamics of intravenous ciprofloxacin in seriously ill patients. Antimicrob. Agents Chemother. 37:1073-1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Garcia-Rey, C., L. Aguillar, and F. Baquero. 2000. Influences of different factors on prevalence of ciprofloxacin resistance in Streptococcus pneumoniae in Spain. Antimicrob. Agents Chemother. 44:3381-3382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hansman, D., and M. M. Bullen. 1967. A resistant pneumococcus. Lancet ii:264-265. [DOI] [PubMed] [Google Scholar]
- 10.Ho, P. L., T. L. Que, D. N. C. Tsang, T. K. Ng, K. H. Chow, and W. H. Seto. 1999. Emergence of fluoroquinolone resistance among multiply resistant stains of streptococcus pneumoniae in Hong Kong. Antimicrob. Agents Chemother. 43:1310-1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ho, P. L., R. W. H. Yung, D. N. C. Tsang, T. L. Que, M. Ho, W. H. Seto, T. K. Ng, W. C. Yam, and W. W. S. Ng. 2001. Increasing resistance of Streptococcus pneumoniae to fluoroquinolones: result of a Hong Kong multicenter study in 2000. J. Antimicrob. Chemother. 48:659-665. [DOI] [PubMed] [Google Scholar]
- 12.Ikemoto, H., C. Ito, T. Yoshida, K. Watanabe, T. Mori, I. Ohno, S. Okada, J. Igari, M. Arakawa, K. Igarashi, T. Oguri, M. Okada, K. Ozaki, T. Terai, N. Aoki, H. Inoue, T. Nagatake, N. Kitamura, O. Sekine, Y. Suzaki, M. Ando, M. Suga, K. Sato, K. Nakata, and N. Kusano. 1999. Susceptibilities of bacteria isolated from patients with lower respiratory infectious diseases to antibiotics. Jpn. J. Antibiot. 52:353-397. [PubMed] [Google Scholar]
- 13.Ikemoto, H., M. Arakawa, F. Gejyo, K. Igarashi, T. Miri, M. Okada, K. Ozaki, J. Igari, N. Aoki, T. Oguri, N. Kitamura, T. Terai, O. Sekine, Y. Suzuki, H. Inoue, T. Nakadake, Y. Karasawa, C. Ito, T. Yoshida, K. Nakata, T. Nakatani, I. Ohno, S. Okada, H. Inagawa, K. Kudo, N. Kobayashi, M. Ando, M. Suga, K. Sato, T. Kondo, M. Tosaka, H. Kobayashi, S. Kawai, S. Takayasu, S. Kohno, K. Tomono, K. Shimada, K. Nakano, Y. Miyazaki, K. Izumikawa, T. Yamaguti, C. Mochida, H. Yokouchi, A. Ito, M. Sumitomo, M. Nasu, H. Nagai, T. Yamasaki, T. Matsushima, and T. Nakano. 2000. Susceptibilities of bacteria isolated from patients with lower respiratory infectious diseases to antibiotics. Jpn. J. Antibiot. 53:261-298. [PubMed] [Google Scholar]
- 14.Jones, R. N. 1999. The impact of antimicrobial resistance: changing epidemiology of community-acquired respiratory-tract infections. Am. J. Health Syst. Pharm. 56(Suppl. 3):S4-S11. [DOI] [PubMed] [Google Scholar]
- 15.Preston, S. L., G. L. Drusano, A. L. Berman, C. L. Fowler, A. T. Chow, B. Dornsief, V. Reichl, J. Natarajan, and M. Corrado. 1998. Pharmacodynamics of levofloxacin: a new paradigm for early clinical trials. JAMA 279:125-129. [DOI] [PubMed] [Google Scholar]
- 16.Saravolatz, L., O. Manzor, C. Check, J. Pawlak, and B. Belian. 2001. Antimicrobial activity of moxifloxacin, gatifloxacin and six fluoroquinolones against Streptococcus pneumoniae. J. Antimicrob. Chemother. 47:875-877. [DOI] [PubMed] [Google Scholar]
- 17.Shimada, K., K. Nakano, I. Ohno, S. Okada, K. Hayashi, H. Yokouchi, M. Arakawa, F. Gejyo, K. Igarashi, H. Ikemoto, T. Mori, M. Okada, K. Ozaki, J. Igari, N. Aoki, T. Oguri, N. Kitamura, T. Terai, Y. Suzuki, H. Inoue, T. Nakadake, Y. Karasawa, C. Ito, T. Yoshida, K. Nakata, T. Nakatani, H. Inagawa, M. Ando, M. Suga, K. Sato, K. Kudo, N. Kobayashi, M. Tosaka, M. Hasegawa, S. Kohno, K. Tomono, Y. Miyazaki, H. Kobayashi, S. Kawai, S. Takayasu, Y. Hirakata, J. Matsuda, C. Mochida, A. Ito, M. Sumitomo, M. Nasu, H. Nagai, T. Matsushima, Y. Niki, K. Hiramatsu, and T. Nakano. 2001. Susceptibilities of bacteria isolated from patients with lower respiratory infectious diseases to antibiotics. Jpn. J. Antibiot. 54:331-364. [PubMed] [Google Scholar]
- 18.Song, J. H., N. Y. Lee, S. Ichiyama, R. Yoshida, Y. Hirakata, W. Fu, A. Chongthaleong, N. Aswapokee, C. H. Chiu, M. K. Lalitha, K. Thomas, J. Perera, T. T. Yee, F. Jamal, U. C. Warsa, B. X. Vinh, M. R. Jacobs, P. C. Appelbaum, C. H. Pai. 1999. Spread of drug-resistant Streptococcus pneumoniae in Asian countries: Asian Network for Surveillance of Resistant Pathogens (ANSORP) Study. Clin. Infect. Dis. 26:1206-1211. [DOI] [PubMed] [Google Scholar]
- 19.Takashima, K., K. Terada, T. Matsumoto, T. Ito, Y. Iizawa, M. Nakao, and K. Yamaguchi. 1996. Establishment of a model of penicillin-resistant Streptococcus pneumoniae pneumonia in healthy CBA/J mice. J. Med. Microbiol. 45:319-322. [DOI] [PubMed] [Google Scholar]
- 20.Tanaka, M., E. Yamazaki, M. Chiba, K. Yoshihara, T. Akasaka, M. Takemura, and K. Sato. 2002. In vitro antibacterial activities of DQ-113, a potent quinolone, against clinical isolates. Antimicrob. Agents Chemother. 46:904-908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tateda, K., K. Takashima, H. Miyazaki, T. Matsumoto, T. Hatori, and K. Yamaguchi. 1996. Noncompromised penicillin-resistant pneumococcal pneumonia CBA/J mouse model and comparative efficacies of antibiotics in this model. Antimicrob. Agents Chemother. 44:1520-1525. [DOI] [PMC free article] [PubMed] [Google Scholar]