Abstract
The green fluorescent protein (GFP) gene offers many advantages as a viability reporter for high-throughput antimicrobial drug screening. However, screening for antituberculosis compounds by using GFP driven by the heat shock promoter, hsp60, has been of limited utility due to the low signal-to-noise ratio. Therefore, an alternative promoter was evaluated for its enhanced fluorescence during microplate-based culture and its response to 18 established antimicrobial agents by using a green fluorescent protein microplate assay (GFPMA). Mycobacterium tuberculosis strains H37Rv, H37Ra, and Erdman were transformed with pFPCA1, which contains a red-shifted gfp gene driven by the acetamidase promoter of M. smegmatis mc2155. The pFPCA1 transformants achieved higher levels of GFP-mediated fluorescence than those carrying the hsp60 construct, with signal-to-noise ratios of 20.6 to 27.8 and 3.8 to 4.5, respectively. The MICs of 18 established antimicrobial agents for all strains carrying pFPCA1 in the GFPMA were within 1 to 2 twofold dilutions of those determined by either the fluorometric or the visual microplate Alamar Blue assay (MABA). No significant differences in MICs were observed between wild-type and pFPCA1 transformants by MABA. The optimized GFPMA is sufficiently simple, robust, and inexpensive (no reagent costs) to be used for routine high-throughput screening for antituberculosis compounds.
Tuberculosis continues to be a major global public health problem. In addition, the increasing prevalence of multidrug-resistant Mycobacterium tuberculosis is of concern (20) and has fostered a sense of urgency with regard to the need to acquire new drugs. Several rapid methods such as the microplate Alamar Blue assay (MABA) (3, 5), the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide-based assay (9), and the luciferase assay (1, 2, 7, 21) have been established for the screening of the antimycobacterial activities of compounds. However, each has one or more drawbacks for high-throughput screening, including the need to add a dye or a substrate to test the viability of the bacteria, a step which both increases labor and decreases safety.
Fluorometric assays based on the Aequorea victoria gfp gene encoding a green fluorescent protein (GFP) or its mutants have been used widely in prokaryotes (8, 10, 11, 24) due to several properties of gfp that are advantageous for use as a reporter for bacterial viability and growth, including the low level of toxicity, continuous production during replication, and easy imaging and quantification (11, 25). Previous studies with mycobacteria exclusively used the hsp60 promoter, which has been fused to gfp in a variety of investigations (8, 11, 26).
Collins et al. (6) demonstrated that a recombinant M. tuberculosis strain carrying pFPV2, an hsp60::gfp construct, could be used to assess the MICs of known antimycobacterial compounds. However, the low level of the fluorescence signal and the relatively high background autofluorescence of the medium precluded the establishment of a robust assay that could be used with confidence for high-throughput screening.
Previous investigators (6, 13, 17, 18, 23) have identified a highly inducible promoter sequence which regulates the expression of the acetamidase gene in M. smegmatis NCTC 8159 and which demonstrated a high level of gene expression in the presence of amide inducers such as acetamide.
In this study we demonstrate that an acetamidase promoter from M. smegmatis strain mc2155 permits constitutive, high-level gfp expression in M. tuberculosis strains H37Ra, H37Rv, and Erdman, resulting in a fluorescence signal significantly higher than that which is achievable with the hsp60 promoter and facilitating the determination of MICs of antimicrobial agents without the addition of reagents postincubation.
MATERIALS AND METHODS
Bacterial strains.
M. tuberculosis H37Rv (ATCC 27294), M. tuberculosis H37Ra (ATCC 25177), and M. tuberculosis Erdman (ATCC 35801) were obtained from the American Type Culture Collection (ATCC; Manassas, Va.). pFPV2 was originally obtained from R. H. Valdivia (25).
DNA manipulation, recombinant DNA methods, and analyses.
The chromosomal DNA of M. smegmatis mc2155 was purified as described previously (16). The acetamidase promoter of M. smegmatis mc2155 was amplified by PCR with primers AmiP1 (5′-CGTCTAGACGAGTA CGGCGCCCTGCTGACG-3′) and AmiP2 (5′-GAGGATCCTCCGCCGACGAAGAAGTTCAGC-3′), which annealed to sequences 0.6 and 2.9 kb upstream from the start codon of the acetamidase gene, respectively. The amplified product was subcloned into the BamHI and XbaI sites of plasmid pPFV2 to remove the hsp60 promoter, which was upstream of gfp. Colonies were selected on the basis of kanamycin resistance and detection of the acetamidase promoter by using primers AmiP1 and AmiP2. The plasmids were then purified and digested with BamHI and XbaI. A plasmid containing the expected fragment of 2.3 kb was selected for confirmatory sequencing and was designated pFPCA1.
Electroporation, plasmid transformation, and clone selection.
Plasmid DNA was isolated from Escherichia coli DH5α. Strains H37Rv, H37Ra, and Erdman were cultivated in 7H9 broth supplemented with glycerol and Tween 80 (7H9GTw), pelleted, and then suspended in 10% sterile glycerol. Electroporation and selection of transformants were performed as described previously (12). GFP-associated fluorescence was determined with a Victor2 D multilabel counter fluorometer (Perkin-Elmer Life Sciences Inc., Boston, Mass.) in the top-reading mode with excitation at 485 nm and emission at 535 nm. The selected transformants were cultured in 200 ml of 7H9GTw with kanamycin. The bacterial suspensions were then washed once, suspended in 20 ml of phosphate-buffered saline (PBS), and passaged through an 8-mm-pore-size filter; and aliquots were stored at −80°C.
Antimicrobial agents.
Capreomycin, chloramphenicol, clindamycin, clofazimine, cycloserine, ethambutol HCl, ethionamide, fusidic acid, isoniazid, minocycline, ofloxacin, p-aminosalicylic acid, streptomycin sulfate, and thiacetazone were purchased from Sigma Chemical Company. Amoxicillin, clavulanate lithium (2:2; wt/wt), and clarithromycin were purchased from the U.S. Pharmacopeia. Rifampin was purchased from Fisher Chemical Company. Ciprofloxacin HCl was purchased from Serologicals Corporation.
GFP microplate assay (GFPMA).
Antimycobacterial susceptibility testing was performed in black, clear-bottom, 96-well microplates (black view plates; Packard Instrument Company, Meriden, Conn.). Frozen H37Rv gfp, H37Ra gfp, and Erdman gfp were thawed, sonicated for 15 s, and cultured at 37°C with shaking in 200 ml of 7H9GTw-kanamycin until the turbidity reached 50 to 70 Klett units. The cells were pelleted, washed with PBS, and then suspended in 20 ml of PBS buffer. Aliquots were stored at −80°C for up to 2 to 3 months. Drug stock solutions were prepared in either dimethyl sulfoxide or distilled deionized water and were further diluted in 7H12 broth (7H9 broth supplemented with 4 μg of catalase per ml, 5 mg of bovine serum albumin per ml, 5.6 μg of palmitic acid per ml, and 1 mg of Casitone per ml) supplemented with 0.2% vol/vol glycerol (7H12G). Frozen inocula were diluted in 7H12G to make final bacterial densities of 5 × 104 to 5 × 105 CFU/ml. The remaining steps were done as previously reported by Collins et al. (6).
MABA.
Cell aliquots and serial dilution of drugs were prepared as described above for GFPMA. Frozen inocula were initially diluted in 7H12G to make final bacterial titers of 3.0 × 105 to 5.0 × 105 CFU/ml. The remaining steps were done as described previously (5).
RESULTS
GFP expression directed by the acetamidase promoter was continually detectable during cultivation without addition of acetamide, which is known to be an inducer of the acetamidase promoter (4, 13-15, 17, 19, 23). This was observed not only for H37Ra gfp but also for H37Rv gfp and Erdman gfp, whereas this construct lost promoter activity in M. bovis BCG (data not shown).
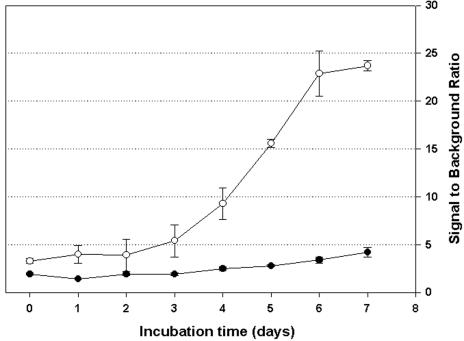
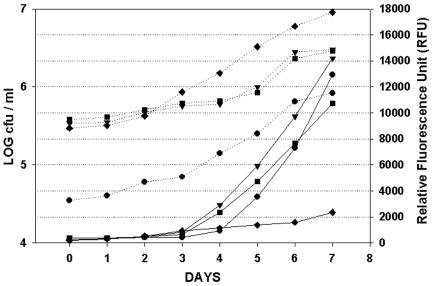
H37Rv harboring pFPCA1 produced a significantly higher net fluorescence signal (over that of the background) than the same strain harboring pFPV2 (Fig. 1). In addition, the fluorescence curve derived from the former construct (pFPCA1) was better correlated to cell growth (numbers of CFU) (Fig. 2), as determined by a better correlation (Pearson product-moment correlation coefficient; r value) between the fluorescence reading and cell growth [r values for Erdman(pFPCA1), H37Ra(pFPCA1), and H37Rv(pFPCA1), 0.9613, 0.9823, and 0.9661, respectively; r value for H37Rv(pFPV2), 0.9356]. Thus, the fluorescence reading could be used to monitor more efficiently the growth of the bacteria in culture. At day 7 the relative fluorescence units per CFU for M. tuberculosis Pacetamidase(Pace)::gfp strains H37Ra, Erdman, and H37Rv were approximately 1.56 × 10−2, 0.47 × 10−2, and 0.37 × 10−2, respectively, whereas that for M. tuberculosis Phsp60::gfp strain H37Rv was approximately 2.58 × 10−4.
FIG. 1.
Ratio of fluorescence signal to medium background signal during incubation of M. tuberculosis H37Rv gfp containing either pFPV2 (•; Phsp60::gfp) or pFPCA1 (○; Pace::gfp) in 7H12G. Data are means ± standard deviations for two independent experiments.
FIG. 2.
GFP expression (solid lines) and CFU (dotted lines) during growth of M. tuberculosis H37Ra Pace::gfp (•), H37Rv Pace::gfp (▪), Erdman Pace::gfp (▾), and H37Rv Phsp60::gfp (♦) in 7H12G. Data are from one representative experiment of two experiments.
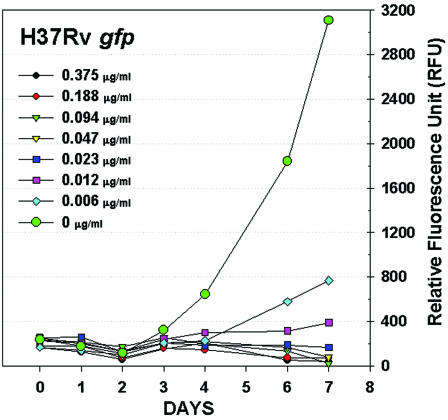
The utilities of these gfp strains for screening for antituberculosis activity were investigated by determining MICs in tests with a 96-well format. The kinetic response to serial twofold dilutions of antimycobacterial agents was easily detected by fluorescence reading of the microplate cultures for 1 week, an example of which is shown in Fig. 3 for H37Rv gfp and rifampin.
FIG. 3.
Rifampin dose-response curves for M. tuberculosis H37Rv gfp determined by fluorescence.
The MICs of 18 antimicrobial agents for H37Rv (Table 1), Erdman (Table 2), and H37Ra (Table 3) were determined by GFPMA and were compared with those determined by MABA after day 6 to 8 days of incubation. At least two independent experiments were conducted with each strain. The MICs of all drugs by GFPMA differed by ≤2 twofold dilutions with respect to the MICs obtained by both the fluorometric and the visual MABA, with most differences being ≤1 twofold dilution. For all three strains, no significant differences in MICs determined by the fluorometric or the visual MABA were found between the corresponding parent and gfp strains of M. tuberculosis.
TABLE 1.
Comparative MICs of 18 established drugs for M. tuberculosis H37Rva
| Drug | MIC (μg/ml)
|
||||
|---|---|---|---|---|---|
| Wild-type H37Rv, MABA
|
H37Rv gfp
|
||||
| MABA
|
GFPMA | ||||
| Fluorometric | Visual | Fluorometric | Visual | ||
| INH | 0.023 | 0.023 | 0.023-0.047 | 0.023 | 0.023 |
| ETA | 0.313 | 0.156-0.313 | 0.313 | 0.313 | 0.156 |
| THA | 0.059 | 0.029 | 0.015-0.029 | 0.029 | 0.015 |
| EMB | 1.875-3.75 | 1.875-3.75 | 1.875-3.75 | 1.875-3.75 | 0.469-0.938 |
| AMOX/CLAV | 7.813 | 3.906-7.813 | 3.906 | 3.906-7.813 | 3.906-7.813 |
| CS | 3.125-12.5 | 3.125-12.5 | 12.5 | 12.5 | 12.5 |
| RIF | 0.023-0.047 | 0.012-0.023 | 0.012-0.023 | 0.023 | 0.023-0.047 |
| CF | 0.039-0.156 | 0.039-0.078 | 0.039 | 0.039 | 0.078 |
| SM | 0.313-0.625 | 0.156-0.625 | 0.313 | 0.313 | 0.156-0.625 |
| CAP | 0.938-3.75 | 0.938-3.75 | 0.469 | 0.469 | 0.938-1.875 |
| CLA | 0.977-1.953 | 0.977 | 0.488 | 0.488 | 0.488-0.977 |
| MIN | 0.977-1.953 | 0.977-1.953 | 0.488-0.977 | 0.488-0.977 | 0.977-1.953 |
| CP | 3.906-7.813 | 3.906-7.813 | 7.813 | 7.813 | 1.953-3.906 |
| CM | 1.953-7.813 | 1.953-7.813 | 7.813-15.625 | 7.813 | 1.953-7.813 |
| FUS | 1.563 | 0.781-1.563 | 0.391-0.781 | 0.391 | 0.391 |
| CIPRO | 0.469 | 0.469 | 0.469 | 0.469 | 0.469-0.938 |
| OFLOX | 0.781 | 0.781 | 0.781 | 0.781 | 0.391-0.781 |
| PAS | 0.156-0.625 | 0.156-0.313 | 0.313-0.625 | 0.313-0.625 | 0.313-0.625 |
Data are ranges from one representative experiment of duplicate experiments. Abbreviations: INH, isoniazid; ETA, ethionamide; THA, thiacetazone; EMB, ethambutol HCl; AMOX/CLAV, amoxicillin-clavulanate lithium; CS, cycloserine; RIF, rifampin; CF, clofazimine; SM, streptomycin sulfate; CAP, capreomycin; CLA, clarithromycin; MIN, minocycline; CP, chloramphenicol; CM, clindamycin; FUS, fusidic acid; CIPRO, ciprofloxacin HCl; OFLOX, ofloxacin; PAS, p-aminosalicylic acid.
TABLE 2.
Comparative MICs of 18 established drugs for M. tuberculosis Erdmana
| Drug | MIC (μg/ml)
|
||||
|---|---|---|---|---|---|
| Wild-type Erdman, MABA
|
Erdman gfp
|
||||
| MABA
|
GFPMA | ||||
| Fluorometric | Visual | Fluorometric | Visual | ||
| INH | 0.047-0.094 | 0.023 | 0.023 | 0.023 | 0.023 |
| ETA | 0.313 | 0.313-0.625 | 0.313 | 0.313 | 0.313 |
| THA | 0.059 | 0.059 | 0.059 | 0.059 | 0.059-0.234 |
| EMB | 3.75 | 0.938-1.875 | 0.938 | 0.938 | 1.875 |
| AMOX/CLAV | 7.813 | 3.906 | 3.906 | 3.906 | 3.906-7.813 |
| CS | 25 | 25 | 12.5 | 12.5 | 12.5 |
| RIF | 0.047 | 0.047 | 0.023 | 0.023-0.047 | 0.023 |
| CF | 0.078-0.156 | 0.039 | 0.039-0.156 | 0.078 | 0.078-0.156 |
| SM | 0.625-1.25 | 0.625 | 0.313 | 0.313 | 0.313 |
| CAP | 0.469 | 0.469 | 0.469-0.938 | 0.469-0.938 | 0.469-0.938 |
| CLA | 0.977 | 0.977 | 1.953 | 1.953 | 1.953-3.906 |
| MIN | 1.953 | 1.953 | 1.953-3.906 | 1.953 | 1.953-3.906 |
| CP | 3.906-7.813 | 3.906-7.813 | 3.906-7.813 | 3.906 | 3.906-7.813 |
| CM | 3.906-7.813 | 1.953-3.906 | 1.953-3.906 | 1.953 | 7.813 |
| FUS | 12.5 | 12.5 | 3.125-6.25 | 3.125 | 6.25 |
| CIPRO | 0.469 | 0.469 | 0.234 | 0.234 | 0.469 |
| OFLOX | 0.781 | 0.781 | 0.391 | 0.391 | 0.781 |
| PAS | 0.625-1.25 | 0.625 | 0.156-0.313 | 0.156-0.313 | 0.625-1.25 |
Data are ranges from one representative experiment of duplicate experiments. See footnote a of Table 1 for definition of drug abbreviations.
TABLE 3.
Comparative MICs of 18 established drugs for M. tuberculosis H37Raa
| Drug | MIC (μg/ml)
|
||||
|---|---|---|---|---|---|
| Wild-type H37Ra, MABA
|
H37Ra gfp
|
||||
| MABA
|
GFPMA | ||||
| Fluorometric | Visual | Fluorometric | Visual | ||
| INH | 0.023 | 0.023 | 0.023 | 0.023 | 0.023 |
| ETA | 0.313 | 0.313 | 0.313 | 0.313 | 0.156 |
| THA | 0.029 | 0.029 | 0.029 | 0.015-0.029 | 0.029 |
| EMB | 0.938 | 0.938 | 0.469 | 0.469 | 0.469 |
| AMOX/CLAV | 7.813-15.625 | 7.813-15.625 | 1.953-7.813 | 1.953-7.813 | 1.953-3.906 |
| CS | 12.5 | 12.5 | 6.25 | 12.5 | 6.25 |
| RIF | 0.003-0.006 | 0.003-0.006 | 0.003 | 0.003 | 0.003-0.012 |
| CF | 0.01 | 0.01 | 0.01 | 0.01 | 0.01-0.02 |
| SM | 0.313 | 0.313 | 0.156 | 0.156 | 0.156-0.313 |
| CAP | 0.938 | 0.938 | 0.469 | 0.469 | 0.938 |
| CLA | 0.977 | 0.488-0.977 | 0.244 | 0.244 | 0.244-0.488 |
| MIN | 0.488 | 0.488 | 0.244-0.488 | 0.244-0.488 | 0.488 |
| CP | 7.813 | 3.906 | 3.906 | 3.906 | 1.953 |
| CM | 3.906 | 1.953 | 1.953 | 1.953 | 0.977-1.953 |
| FUS | 0.781-1.563 | 0.781-1.563 | 0.391 | 0.391 | 0.391-0.781 |
| CIPRO | 0.469 | 0.469 | 0.234 | 0.234 | 0.234 |
| OFLOX | 0.781 | 0.781 | 0.391 | 0.391 | 0.391 |
| PAS | 0.625-1.25 | 0.625 | 0.625 | 0.625 | 0.313 |
Data are ranges from one representative experiment of duplicate experiments. See footnote a of Table 1 for definition of drug abbreviations.
DISCUSSION
Previously, GFP coupled with a widely used hsp60 promoter has been used to screen drugs for their activities against M. aurum both in vitro and in macrophages (22), as well as to screen germicides for their activities against M. terrae (27). The hsp60 promoter was also used to regulate GFP expression in M. tuberculosis H37Ra to determine the MICs of established anti-TB drugs (6). However, neither the level of the absolute fluorescence signal nor the signal-to-noise (background) ratio was sufficiently high to make this construct a candidate for routine high-throughput screening.
In this study we replaced the hsp60 promoter in pFPV2 (25) with the 2.3-kb DNA segment containing the acetamidase promoter from M. smegmatis mc2155. The resulting recombinant M. tuberculosis strain produced strong fluorescence regardless of the presence of acetamide.
The genetic variation within the promoter region between two different strains of M. smegmatis (NCTC 8159 versus mc2155) might account for the different promoter activities.
Evidently, the resulting constitutive property of pFPCA1 is more favorable for a general viability reporter. It gave a higher relative fluorescence unit per CFU compared with that obtained with the previous gfp construct (pFPV2) as well as emitted a fluorescence signal that correlated well with the increase in cell growth.
We demonstrated here that by using an improved gfp recombinant M. tuberculosis strain with a high signal-to-noise ratio, a good correlation between cell growth and fluorescence activity during incubation can be obtained and that the MICs of antimycobacterial agents determined by GFPMA are consistent with those obtained by MABA. The GFPMA described here appears to be sufficiently robust for use for routine high-throughput screening for antituberculosis agents.
Acknowledgments
We thank Chanpen Wiwat, Saradee Warit, Sanghyun Cho, Fangqiu Zhang, Yuehong Wang, Kamolchanok Rakseree, Arunee Thong-On, and Nantawan Thong-On for technical assistance.
This study was supported by the Thailand Research Fund, the National Center for Genetic Engineering and Biotechnology, and contract NIH/NIAID/DAIDS-01-13 from the National Institutes of Health. Chartchai Changsen was supported by the Mahidol University Medical Scholar Program.
REFERENCES
- 1.Arain, T. M., A. E. Resconi, M. J. Hickey, and C. K. Stover. 1996. Bioluminescence screening in vitro (Bio-Siv) assays for high-volume antimycobacterial drug discovery. Antimicrob. Agents Chemother. 40:1536-1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arain, T. M., A. E. Resconi, D. C. Singh, and C. K. Stover. 1996. Reporter gene technology to assess activity of antimycobacterial agents in macrophages. Antimicrob. Agents Chemother. 40:1542-1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boonphong, S., P. Kittakoop, M. Isaka, P. Palittapongarnpim, A. Jaturapat, K. Danwisetkanjana, M. Tanticharoen, and Y. Thebtaranonth. 2001. A new antimycobacterial, 3-beta-acetoxy-15 alpha,22-dihydroxyhopane, from the insect pathogenic fungus Aschersonia tubulata. Planta Med. 67:279-281. [DOI] [PubMed] [Google Scholar]
- 4.Chebrou, H., F. Bigey, A. Arnaud, and P. Galzy. 1996. Amide metabolism: a putative ABC transporter in Rhodococcus sp. R312. Gene 182:215-218. [DOI] [PubMed] [Google Scholar]
- 5.Collins, L., and S. G. Franzblau. 1997. Microplate Alamar Blue assay versus BACTEC 460 system for high-throughput screening of compounds against Mycobacterium tuberculosis and Mycobacterium avium. Antimicrob. Agents Chemother. 41:1004-1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Collins, L. A., M. N. Torrero, and S. G. Franzblau. 1998. Green fluorescent protein reporter microplate assay for high-throughput screening of compounds against Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 42:344-347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Deb, D. K., K. K. Srivastava, R. Srivastava, and B. S. Srivastava. 2000. Bioluminescent Mycobacterium aurum expressing firefly luciferase for rapid and high throughput screening of antimycobacterial drugs in vitro and in infected macrophages. Biochem. Biophys. Res. Commun. 279:457-461. [DOI] [PubMed] [Google Scholar]
- 8.Dhandayuthapani, S., L. E. Via, C. A. Thomas, P. M. Horowitz, D. Deretic, and V. Deretic. 1995. Green fluorescent protein as a marker for gene expression and cell biology of mycobacterial interactions with macrophages. Mol. Microbiol. 17:901-912. [DOI] [PubMed] [Google Scholar]
- 9.Foongladda, S., D. Roengsanthia, W. Arjrattanakool, C. Chuchottaworn, A. Chaiprasert, and S. G. Franzblau. 2002. Rapid and simple MTT method for rifampicin and isoniazid susceptibility testing of Mycobacterium tuberculosis. Int. J. Tuberc. Lung Dis. 6:1118-1122. [PubMed] [Google Scholar]
- 10.Kain, S. R., M. Adams, A. Kondepudi, T. T. Yang, W. W. Ward, and P. Kitts. 1995. Green fluorescent protein as a reporter of gene expression and protein localization. BioTechniques 19:650-655. [PubMed] [Google Scholar]
- 11.Kremer, L., A. Baulard, J. Estaquier, O. Poulain-Godefroy, and C. Locht. 1995. Green fluorescent protein as a new expression marker in mycobacteria. Mol. Microbiol. 17:913-922. [DOI] [PubMed] [Google Scholar]
- 12.Larsen, M. H. 2000. Some common methods in mycobacterial genetics: transformation, p. 319-320. In G. F. Hatfull and W. R. Jacobs, Jr. (ed.), Molecular genetics of mycobacteria. American Society for Microbiology, Washington D.C.
- 13.Mahenthiralingam, E., P. Draper, E. O. Davis, and M. J. Colston. 1993. Cloning and sequencing of the gene which encodes the highly inducible acetamidase of Mycobacterium smegmatis. J. Gen. Microbiol 139(Pt 3):575-583. [DOI] [PubMed] [Google Scholar]
- 14.Manabe, Y. C., J. M. Chen, C. G. Ko, P. Chen, and W. R. Bishai. 1999. Conditional sigma factor expression, using the inducible acetamidase promoter, reveals that the Mycobacterium tuberculosis sigF gene modulates expression of the 16-kilodalton alpha-crystallin homologue. J. Bacteriol. 181:7629-7633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Narayanan, S., S. Selvakumar, R. Aarati, S. K. Vasan, and P. R. Narayanan. 2000. Transcriptional analysis of inducible acetamidase gene of Mycobacterium smegmatis. FEMS Microbiol. Lett. 192:263-268. [DOI] [PubMed] [Google Scholar]
- 16.Palittapongarnpim, P., P. Luangsook, S. Tansuphaswadikul, C. Chuchottaworn, R. Prachaktam, and B. Sathapatayavongs. 1997. Restriction fragment length polymorphism study of Mycobacterium tuberculosis in Thailand using IS6110 as probe. Int. J. Tuberc. Lung Dis. 1:370-376. [PubMed] [Google Scholar]
- 17.Parish, T., E. Mahenthiralingam, P. Draper, E. O. Davis, and M. J. Colston. 1997. Regulation of the inducible acetamidase gene of Mycobacterium smegmatis. Microbiology 143(Pt 7):2267-2276. [DOI] [PubMed] [Google Scholar]
- 18.Parish, T., and N. G. Stoker. 1997. Development and use of a conditional antisense mutagenesis system in mycobacteria. FEMS Microbiol. Lett. 154:151-157. [DOI] [PubMed] [Google Scholar]
- 19.Parish, T., J. Turner, and N. G. Stoker. 2001. amiA is a negative regulator of acetamidase expression in Mycobacterium smegmatis. BioMed Central Microbiol. 1:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schluger, N. W. 2000. The impact of drug resistance on the global tuberculosis epidemic. Int. J. Tuberc. Lung Dis. 4:S71-S75. [PubMed] [Google Scholar]
- 21.Shawar, R. M., D. J. Humble, J. M. Van Dalfsen, C. K. Stover, M. J. Hickey, S. Steele, L. A. Mitscher, and W. Baker. 1997. Rapid screening of natural products for antimycobacterial activity by using luciferase-expressing strains of Mycobacterium bovis BCG and Mycobacterium intracellulare. Antimicrob. Agents Chemother. 41:570-574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Srivastava, R., D. K. Deb, K. K. Srivastava, C. Locht, and B. S. Srivastava. 1998. Green fluorescent protein as a reporter in rapid screening of antituberculosis compounds in vitro and in macrophages. Biochem. Biophys. Res. Commun. 253:431-436. [DOI] [PubMed] [Google Scholar]
- 23.Triccas, J. A., T. Parish, W. J. Britton, and B. Gicquel. 1998. An inducible expression system permitting the efficient purification of a recombinant antigen from Mycobacterium smegmatis. FEMS Microbiol. Lett. 167:151-156. [DOI] [PubMed] [Google Scholar]
- 24.Valdivia, R. H., and S. Falkow. 1996. Bacterial genetics by flow cytometry: rapid isolation of Salmonella typhimurium acid-inducible promoters by differential fluorescence induction. Mol. Microbiol. 22:367-378. [DOI] [PubMed] [Google Scholar]
- 25.Valdivia, R. H., A. E. Hromockyj, D. Monack, L. Ramakrishnan, and S. Falkow. 1996. Applications for green fluorescent protein (GFP) in the study of host-pathogen interactions. Gene 173:47-52. [DOI] [PubMed] [Google Scholar]
- 26.Via, L. E., R. Curcic, M. H. Mudd, S. Dhandayuthapani, R. J. Ulmer, and V. Deretic. 1996. Elements of signal transduction in Mycobacterium tuberculosis: in vitro phosphorylation and in vivo expression of the response regulator MtrA. J. Bacteriol. 178:3314-3321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zafer, A. A., Y. E. Taylor, and S. A. Sattar. 2001. Rapid screening method for mycobactericidal activity of chemical germicides that uses Mycobacterium terrae expressing a green fluorescent protein gene. Appl. Environ. Microbiol. 67:1239-1245. [DOI] [PMC free article] [PubMed] [Google Scholar]