Abstract
The title compound, C13H16N2O2S, crystallizes in the thioamide form with an intramolecular hydrogen bond of type N—H⋯Obutyryl. Molecules are linked into chains parallel to [10 ] by a further hydrogen bond of type N—H⋯Oacetyl. C—H⋯O and C—H⋯S hydrogen bonds are also present.
] by a further hydrogen bond of type N—H⋯Oacetyl. C—H⋯O and C—H⋯S hydrogen bonds are also present.
Related literature
For related literature, see: D’hooghe et al. (2005 ▶); Glasser & Doughty (1964 ▶); Huebner et al. (1953 ▶); Jain & Rao (2003 ▶); Morales et al. (2000 ▶); Ru et al. (1994 ▶); Xu et al. (2004 ▶); Xue et al. (2003 ▶); Zeng et al. (2003 ▶); Zheng et al. (2004 ▶); Douglas & Dains (1934 ▶).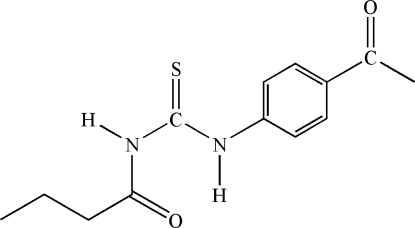
Experimental
Crystal data
C13H16N2O2S
M r = 264.34
Triclinic,
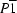
a = 7.5111 (5) Å
b = 9.7585 (8) Å
c = 10.5036 (5) Å
α = 65.283 (5)°
β = 76.245 (4)°
γ = 68.589 (5)°
V = 647.78 (8) Å3
Z = 2
Mo Kα radiation
μ = 0.25 mm−1
T = 100 (2) K
0.35 × 0.20 × 0.10 mm
Data collection
Oxford Diffraction Xcalibur S diffractometer
Absorption correction: multi-scan (CrysAlis RED; Oxford Diffraction, 2008 ▶) T min = 0.940, T max = 0.976
22401 measured reflections
3613 independent reflections
3036 reflections with I > 2σ(I)
R int = 0.030
Refinement
R[F 2 > 2σ(F 2)] = 0.031
wR(F 2) = 0.087
S = 1.06
3613 reflections
173 parameters
H atoms treated by a mixture of independent and constrained refinement
Δρmax = 0.45 e Å−3
Δρmin = −0.22 e Å−3
Data collection: CrysAlis CCD (Oxford Diffraction, 2008 ▶); cell refinement: CrysAlis RED (Oxford Diffraction, 2008 ▶); data reduction: CrysAlis RED; program(s) used to solve structure: SHELXS97 (Sheldrick, 2008 ▶); program(s) used to refine structure: SHELXL97 (Sheldrick, 2008 ▶); molecular graphics: XP (Siemens, 1994 ▶); software used to prepare material for publication: SHELXL97.
Supplementary Material
Crystal structure: contains datablocks I, global. DOI: 10.1107/S1600536808022095/pk2104sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536808022095/pk2104Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Table 1. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| N2—H02⋯O1 | 0.840 (16) | 1.874 (16) | 2.6211 (12) | 147.4 (16) |
| N1—H01⋯O2i | 0.835 (16) | 2.087 (16) | 2.9057 (12) | 166.7 (13) |
| C3—H3B⋯O2i | 0.99 | 2.54 | 3.1345 (13) | 118 |
| C1—H1C⋯Sii | 0.98 | 3.01 | 3.8996 (13) | 151 |
| C3—H3A⋯Sii | 0.99 | 2.92 | 3.8444 (11) | 155 |
Symmetry codes: (i) 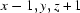 ; (ii)
; (ii) 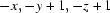 .
.
Acknowledgments
The authors are grateful to Allama Iqbal Open University, Islamabad, Pakistan, for the allocation of research and analytical laboratory facilities.
supplementary crystallographic information
Comment
Thiourea and its derivatives have found extensive applications in the fields of medicine, agriculture and analytical chemistry. Substituted thioureas are an important class of compounds, precursors or intermediates towards the synthesis of a variety of heterocyclic systems such as imidazole-2-thiones (Zeng et al., 2003), 2-imino-1, 3-thiazolines (D'hooghe et al., 2005) pyrimidines-2-thione (Jain & Rao, 2003) and (benzothiazolyl)-4-quinazolinones. N– (Substituted phenyl)-N-phenylthioureas and N– (substituted butanoyl)-N-phenylthioureas have been developed. Thioureas are also known to exhibit a wide range of biological activities including antiviral, antibacterial, antifungal, (Huebner et al., 1953) antitubercular, antithyroidal, herbicidal and insecticidal activities and as agrochemicals (Xu et al., 2004), e.g. 1-benzoyl-3-(4,5-disubstituted-pyrimidine-2-yl)- thioureas, which have excellent herbicidal activity (Zheng et al., 2004). Thioureas are also well known chelating agents for transition metals (Xue et al., 2003). N,N-Dialkyl-N'-benzoyl thioureas act as selective complexing agents for the enrichment of platinum metals even from strongly interfacing matrixes (Ru et al., 1994). The complexes of thiourea derivatives also show various biological activities (Glasser & Doughty, 1964). Thioureas and substituted thioureas are also known as epoxy resin curing agents.
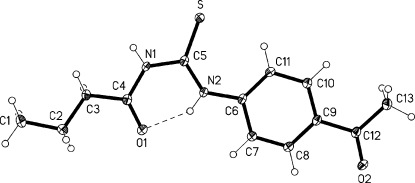
The title compound is a precursor for an attempt to synthesize imidazole derivatives and transition metal complexes as epoxy resin curing agents and accelerators. It crystallizes in the thioamide form (Fig. 1). The molecule is essentially planar (r.m.s. deviation of all non-H atoms 0.118 (1) Å), as reflected by the torsion angles O1—C4—N1—C5, C4—N1—C5—S and C4—N1—C5—N2 of 0.85 (17)°, 174.53 (8)° and -5.70 (15)°, respectively. The C4—O1, C5—S and C12—O2 bonds show a typical double bond character with bond lengths of 1.2246 (13), 1.6629 (11) and 1.2243 (13) Å, respectively. All the C—N bonds, C4—N1 = 1.3864 (13), C6—N2 = 1.4061 (12), C5—N2 = 1.3458 (13) and C5—N1 = 1.3948 (12) Å display a partial double bond character. Among the latter three C—N bonds, C4—N1 is the longest indicating a C(sp2)—N(sp2) single bond, while C5—N2 is the shortest bond with more double bond character. This demonstrates that there is π conjugation along S—C5—N2 but not along O1—C4—N1 and C4—N1—C5 as found in 1-(3-methoxybenzoyl)-3, 3-diethylthiourea (Morales et al., 2000). There is a strong intramolecular hydrogen bond N2—H02···O1, with H2···O1 = 1.874 (16) Å, forming a 6-membered ring.
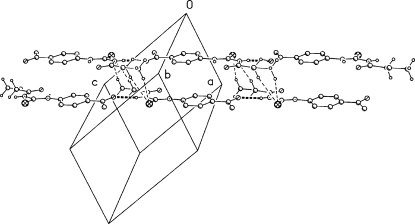
Molecules are connected in chains parallel to [101] by classical hydrogen bonds N1—H1···O2 and a weak bifurcated component C3—H3B···O2; the chains are further connected in an antiparallel sense by a bifurcated system of two C—H···S contacts (Table 2, Fig. 2).
Experimental
The title compound was synthesized by a slight modification of the published procedure (Douglas & Dains, 1934). A solution of butanoyl chloride (0.1 mol) in dry acetone (75 ml) was added dropwise to a suspension of ammonium thiocyanate (0.1 mol) in dry acetone (55 ml) and the reaction mixture was refluxed for 45 minutes. After cooling to room temperature, a solution of 4-aminoacetophenone (0.1 mol) in dry acetone (25 ml) was added and the resulting mixture refluxed for 1.5 hrs. The reaction mixture was poured into five times its volume of cold water whereupon the thiourea precipitated as a solid. The product was recrystallized from ethyl acetate as colourless crystals (2.85 g, 79%). m.p.458 K.
Refinement
H atoms of NH groups were refined freely. Methyl H atoms were included on the basis of idealized rigid groups (C—H 0.98 Å, H—C—H 109.5°) allowed to rotate but not tip. Other hydrogen atoms were included using a riding model with C—H 0.95 (aromatic) or 0.99 (methylene) Å. U(H) values were fixed at 1.5Uiso(C) of the parent C atom for methyl H, 1.2Uiso(C) for other H.
Figures
Fig. 1.
The molecule of the title compound in the crystal. Ellipsoids represent 50% probability levels.
Fig. 2.
Packing diagram of the title compound showing classical and "weak" H bonds as thick and thin dashed bonds respectively. H atoms not involved in H bonds are omitted for clarity.
Crystal data
| C13H16N2O2S | Z = 2 |
| Mr = 264.34 | F000 = 280 |
| Triclinic, P1 | Dx = 1.355 Mg m−3 |
| Hall symbol: -P 1 | Melting point: 458 K |
| a = 7.5111 (5) Å | Mo Kα radiation λ = 0.71073 Å |
| b = 9.7585 (8) Å | Cell parameters from 13985 reflections |
| c = 10.5036 (5) Å | θ = 2.6–30.6º |
| α = 65.283 (5)º | µ = 0.25 mm−1 |
| β = 76.245 (4)º | T = 100 (2) K |
| γ = 68.589 (5)º | Tablet, pale yellow |
| V = 647.78 (8) Å3 | 0.35 × 0.20 × 0.10 mm |
Data collection
| Oxford Diffraction Xcalibur S diffractometer | 3613 independent reflections |
| Radiation source: Enhance (Mo) X-ray Source | 3036 reflections with I > 2σ(I) |
| Monochromator: graphite | Rint = 0.030 |
| Detector resolution: 16 pixels mm-1 | θmax = 30.7º |
| T = 100(2) K | θmin = 2.6º |
| ω scans | h = −10→10 |
| Absorption correction: multi-scan(CrysAlis RED; Oxford Diffraction, 2008) | k = −13→13 |
| Tmin = 0.940, Tmax = 0.976 | l = −15→15 |
| 22401 measured reflections |
Refinement
| Refinement on F2 | Secondary atom site location: difference Fourier map |
| Least-squares matrix: full | Hydrogen site location: inferred from neighbouring sites |
| R[F2 > 2σ(F2)] = 0.031 | H atoms treated by a mixture of independent and constrained refinement |
| wR(F2) = 0.087 | w = 1/[σ2(Fo2) + (0.0536P)2 + 0.0789P] where P = (Fo2 + 2Fc2)/3 |
| S = 1.06 | (Δ/σ)max = 0.003 |
| 3613 reflections | Δρmax = 0.45 e Å−3 |
| 173 parameters | Δρmin = −0.22 e Å−3 |
| Primary atom site location: structure-invariant direct methods | Extinction correction: none |
Special details
| Geometry. All e.s.d.'s (except the e.s.d. in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell e.s.d.'s are taken into account individually in the estimation of e.s.d.'s in distances, angles and torsion angles; correlations between e.s.d.'s in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell e.s.d.'s is used for estimating e.s.d.'s involving l.s. planes. |
| Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F2, conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > σ(F2) is used only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2 are statistically about twice as large as those based on F, and R- factors based on ALL data will be even larger. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| S | 0.29224 (4) | 0.76793 (3) | 0.30824 (3) | 0.01606 (9) | |
| O1 | 0.44460 (12) | 0.23350 (9) | 0.43763 (8) | 0.01882 (17) | |
| O2 | 1.01149 (11) | 0.57384 (9) | −0.32905 (8) | 0.01832 (17) | |
| N1 | 0.30196 (13) | 0.47376 (10) | 0.46308 (9) | 0.01281 (18) | |
| H01 | 0.223 (2) | 0.5166 (17) | 0.5163 (15) | 0.020 (3)* | |
| N2 | 0.50761 (13) | 0.49957 (11) | 0.26018 (9) | 0.01395 (18) | |
| H02 | 0.522 (2) | 0.4018 (19) | 0.2950 (17) | 0.032 (4)* | |
| C1 | 0.19337 (17) | 0.00779 (13) | 0.85964 (12) | 0.0201 (2) | |
| H1A | 0.2003 | 0.0630 | 0.9169 | 0.030* | |
| H1B | 0.2504 | −0.1064 | 0.9066 | 0.030* | |
| H1C | 0.0587 | 0.0302 | 0.8482 | 0.030* | |
| C2 | 0.30349 (17) | 0.06471 (12) | 0.71538 (12) | 0.0192 (2) | |
| H2A | 0.4416 | 0.0343 | 0.7261 | 0.023* | |
| H2B | 0.2907 | 0.0134 | 0.6556 | 0.023* | |
| C3 | 0.22642 (15) | 0.24316 (12) | 0.64468 (11) | 0.0149 (2) | |
| H3A | 0.0909 | 0.2712 | 0.6285 | 0.018* | |
| H3B | 0.2281 | 0.2925 | 0.7096 | 0.018* | |
| C4 | 0.33675 (14) | 0.31196 (12) | 0.50663 (11) | 0.0134 (2) | |
| C5 | 0.37498 (14) | 0.57496 (12) | 0.33954 (10) | 0.01180 (19) | |
| C6 | 0.60770 (14) | 0.55630 (12) | 0.12737 (10) | 0.01210 (19) | |
| C7 | 0.70848 (15) | 0.44211 (12) | 0.06708 (11) | 0.0145 (2) | |
| H7 | 0.7064 | 0.3359 | 0.1171 | 0.017* | |
| C8 | 0.81080 (15) | 0.48184 (12) | −0.06408 (11) | 0.0145 (2) | |
| H8 | 0.8775 | 0.4033 | −0.1039 | 0.017* | |
| C9 | 0.81661 (14) | 0.63750 (12) | −0.13858 (11) | 0.0126 (2) | |
| C10 | 0.71839 (15) | 0.74982 (12) | −0.07705 (11) | 0.0148 (2) | |
| H10 | 0.7227 | 0.8555 | −0.1264 | 0.018* | |
| C11 | 0.61396 (15) | 0.71148 (12) | 0.05482 (11) | 0.0148 (2) | |
| H11 | 0.5478 | 0.7900 | 0.0949 | 0.018* | |
| C12 | 0.92871 (14) | 0.67675 (12) | −0.27915 (11) | 0.0143 (2) | |
| C13 | 0.93843 (19) | 0.84247 (14) | −0.35931 (12) | 0.0241 (3) | |
| H13A | 1.0270 | 0.8463 | −0.4451 | 0.036* | |
| H13B | 0.9845 | 0.8759 | −0.3005 | 0.036* | |
| H13C | 0.8102 | 0.9136 | −0.3848 | 0.036* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| S | 0.01598 (13) | 0.01249 (13) | 0.01624 (14) | −0.00382 (9) | 0.00445 (9) | −0.00563 (10) |
| O1 | 0.0236 (4) | 0.0161 (4) | 0.0159 (4) | −0.0068 (3) | 0.0050 (3) | −0.0081 (3) |
| O2 | 0.0209 (4) | 0.0178 (4) | 0.0143 (4) | −0.0047 (3) | 0.0052 (3) | −0.0086 (3) |
| N1 | 0.0140 (4) | 0.0132 (4) | 0.0103 (4) | −0.0044 (3) | 0.0036 (3) | −0.0058 (3) |
| N2 | 0.0168 (4) | 0.0127 (4) | 0.0110 (4) | −0.0054 (3) | 0.0035 (3) | −0.0050 (3) |
| C1 | 0.0255 (5) | 0.0160 (5) | 0.0161 (5) | −0.0089 (4) | 0.0005 (4) | −0.0023 (4) |
| C2 | 0.0232 (5) | 0.0127 (5) | 0.0181 (5) | −0.0055 (4) | 0.0031 (4) | −0.0050 (4) |
| C3 | 0.0153 (5) | 0.0142 (5) | 0.0127 (5) | −0.0054 (4) | 0.0024 (4) | −0.0037 (4) |
| C4 | 0.0137 (4) | 0.0148 (5) | 0.0120 (5) | −0.0052 (4) | −0.0007 (4) | −0.0048 (4) |
| C5 | 0.0112 (4) | 0.0149 (5) | 0.0100 (5) | −0.0049 (3) | −0.0002 (3) | −0.0048 (4) |
| C6 | 0.0117 (4) | 0.0153 (5) | 0.0095 (4) | −0.0050 (4) | 0.0010 (3) | −0.0051 (4) |
| C7 | 0.0163 (5) | 0.0132 (5) | 0.0138 (5) | −0.0052 (4) | 0.0017 (4) | −0.0059 (4) |
| C8 | 0.0148 (4) | 0.0148 (5) | 0.0139 (5) | −0.0041 (4) | 0.0013 (4) | −0.0072 (4) |
| C9 | 0.0124 (4) | 0.0155 (5) | 0.0099 (5) | −0.0043 (4) | 0.0001 (4) | −0.0051 (4) |
| C10 | 0.0171 (5) | 0.0139 (5) | 0.0126 (5) | −0.0054 (4) | 0.0017 (4) | −0.0052 (4) |
| C11 | 0.0172 (5) | 0.0141 (5) | 0.0131 (5) | −0.0044 (4) | 0.0024 (4) | −0.0073 (4) |
| C12 | 0.0143 (4) | 0.0165 (5) | 0.0112 (5) | −0.0048 (4) | 0.0008 (4) | −0.0054 (4) |
| C13 | 0.0345 (6) | 0.0192 (5) | 0.0167 (5) | −0.0126 (5) | 0.0104 (5) | −0.0077 (4) |
Geometric parameters (Å, °)
| S—C5 | 1.6629 (11) | C12—C13 | 1.4993 (15) |
| O1—C4 | 1.2246 (13) | N1—H01 | 0.835 (16) |
| O2—C12 | 1.2243 (13) | N2—H02 | 0.840 (16) |
| N1—C4 | 1.3864 (13) | C1—H1A | 0.9800 |
| N1—C5 | 1.3948 (12) | C1—H1B | 0.9800 |
| N2—C5 | 1.3458 (13) | C1—H1C | 0.9800 |
| N2—C6 | 1.4061 (12) | C2—H2A | 0.9900 |
| C1—C2 | 1.5245 (15) | C2—H2B | 0.9900 |
| C2—C3 | 1.5185 (14) | C3—H3A | 0.9900 |
| C3—C4 | 1.5036 (14) | C3—H3B | 0.9900 |
| C6—C11 | 1.3949 (14) | C7—H7 | 0.9500 |
| C6—C7 | 1.4008 (14) | C8—H8 | 0.9500 |
| C7—C8 | 1.3807 (14) | C10—H10 | 0.9500 |
| C8—C9 | 1.3999 (14) | C11—H11 | 0.9500 |
| C9—C10 | 1.3923 (14) | C13—H13A | 0.9800 |
| C9—C12 | 1.4856 (14) | C13—H13B | 0.9800 |
| C10—C11 | 1.3921 (14) | C13—H13C | 0.9800 |
| C4—N1—C5 | 128.62 (9) | H1A—C1—H1B | 109.5 |
| C5—N2—C6 | 131.77 (9) | C2—C1—H1C | 109.5 |
| C3—C2—C1 | 110.45 (9) | H1A—C1—H1C | 109.5 |
| C4—C3—C2 | 114.32 (8) | H1B—C1—H1C | 109.5 |
| O1—C4—N1 | 122.98 (9) | C3—C2—H2A | 109.6 |
| O1—C4—C3 | 123.40 (9) | C1—C2—H2A | 109.6 |
| N1—C4—C3 | 113.60 (9) | C3—C2—H2B | 109.6 |
| N2—C5—N1 | 113.62 (9) | C1—C2—H2B | 109.6 |
| N2—C5—S | 128.35 (8) | H2A—C2—H2B | 108.1 |
| N1—C5—S | 118.03 (7) | C4—C3—H3A | 108.7 |
| C11—C6—C7 | 119.43 (9) | C2—C3—H3A | 108.7 |
| C11—C6—N2 | 125.91 (9) | C4—C3—H3B | 108.7 |
| C7—C6—N2 | 114.66 (9) | C2—C3—H3B | 108.7 |
| C8—C7—C6 | 120.86 (9) | H3A—C3—H3B | 107.6 |
| C7—C8—C9 | 120.25 (10) | C8—C7—H7 | 119.6 |
| C10—C9—C8 | 118.54 (9) | C6—C7—H7 | 119.6 |
| C10—C9—C12 | 122.35 (9) | C7—C8—H8 | 119.9 |
| C8—C9—C12 | 119.11 (9) | C9—C8—H8 | 119.9 |
| C11—C10—C9 | 121.78 (9) | C11—C10—H10 | 119.1 |
| C10—C11—C6 | 119.12 (9) | C9—C10—H10 | 119.1 |
| O2—C12—C9 | 119.80 (9) | C10—C11—H11 | 120.4 |
| O2—C12—C13 | 120.49 (9) | C6—C11—H11 | 120.4 |
| C9—C12—C13 | 119.71 (9) | C12—C13—H13A | 109.5 |
| C4—N1—H01 | 115.4 (10) | C12—C13—H13B | 109.5 |
| C5—N1—H01 | 115.9 (10) | H13A—C13—H13B | 109.5 |
| C5—N2—H02 | 111.5 (11) | C12—C13—H13C | 109.5 |
| C6—N2—H02 | 116.4 (11) | H13A—C13—H13C | 109.5 |
| C2—C1—H1A | 109.5 | H13B—C13—H13C | 109.5 |
| C2—C1—H1B | 109.5 | ||
| C1—C2—C3—C4 | 175.26 (9) | C6—C7—C8—C9 | 0.55 (16) |
| C5—N1—C4—O1 | 0.85 (17) | C7—C8—C9—C10 | 0.39 (15) |
| C5—N1—C4—C3 | −177.92 (9) | C7—C8—C9—C12 | 179.66 (9) |
| C2—C3—C4—O1 | 18.45 (15) | C8—C9—C10—C11 | −0.70 (16) |
| C2—C3—C4—N1 | −162.79 (9) | C12—C9—C10—C11 | −179.95 (10) |
| C6—N2—C5—N1 | 176.36 (10) | C9—C10—C11—C6 | 0.07 (16) |
| C6—N2—C5—S | −3.91 (17) | C7—C6—C11—C10 | 0.87 (15) |
| C4—N1—C5—N2 | −5.70 (15) | N2—C6—C11—C10 | −179.38 (10) |
| C4—N1—C5—S | 174.53 (8) | C10—C9—C12—O2 | −179.50 (10) |
| C5—N2—C6—C11 | 11.68 (18) | C8—C9—C12—O2 | 1.26 (15) |
| C5—N2—C6—C7 | −168.56 (11) | C10—C9—C12—C13 | 0.06 (16) |
| C11—C6—C7—C8 | −1.19 (16) | C8—C9—C12—C13 | −179.18 (10) |
| N2—C6—C7—C8 | 179.03 (9) |
Hydrogen-bond geometry (Å, °)
| D—H···A | D—H | H···A | D···A | D—H···A |
| N2—H02···O1 | 0.840 (16) | 1.874 (16) | 2.6211 (12) | 147.4 (16) |
| N1—H01···O2i | 0.835 (16) | 2.087 (16) | 2.9057 (12) | 166.7 (13) |
| C3—H3B···O2i | 0.99 | 2.54 | 3.1345 (13) | 118 |
| C1—H1C···Sii | 0.98 | 3.01 | 3.8996 (13) | 151 |
| C3—H3A···Sii | 0.99 | 2.92 | 3.8444 (11) | 155 |
Symmetry codes: (i) x−1, y, z+1; (ii) −x, −y+1, −z+1.
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: PK2104).
References
- D’hooghe, M., Waterinckx, A. & De Kimpe, N. (2005). J. Org. Chem.70, 227–232. [DOI] [PubMed]
- Douglas, I. B. & Dains, F. B. (1934). J. Am. Chem. Soc.56, 719–721.
- Glasser, A. C. & Doughty, R. M. (1964). J. Pharm. Sci.53, 40–42. [DOI] [PubMed]
- Huebner, O. F., Marsh, J. L., Mizzoni, R. H., Mull, R. P., Schro, D. C. & Troxell, H. A. (1953). J. Am. Chem. Soc.75, 2274–2275.
- Jain, V. K. & Rao, J. T. (2003). J. Inst. Chem.75, 24–26.
- Morales, A. D., Novoa de Armas, H., Blaton, N. M., Peeters, O. M., De Ranter, C. J., Márquez, H. & Pomés Hernández, R. (2000). Acta Cryst. C56, 503–504. [DOI] [PubMed]
- Oxford Diffraction (2008). CrysAlis CCD and CrysAlis RED Oxford Diffraction Ltd, Abingdon, Oxfordshire, England.
- Ru, C., Wang, Y., Li, J., Ma, D., Gong, F., Liu, Y. & Lu, P. (1994). Yingyong Huaxue, 11, 82–85.
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Siemens (1994). XP Siemens Analytical X-ray Instruments Inc., Madison, Wisconsin, USA.
- Xu, Y., Hua, W., Liu, X. & Zhu, D. (2004). Chin. J. Org. Chem.24, 1217–1222.
- Xue, S., Duan, L., Ke, S. & Jia, L. (2003). Chem. Mag.5, 67–70.
- Zeng, R.-S., Zou, J.-P., Zhi, S.-J., Chen, J. & Shen, Q. (2003). Org. Lett.5, 1657–1659. [DOI] [PubMed]
- Zheng, W., Yates, S. R., Papiernik, S. K. & Guo, M. (2004). Environ. Sci. Technol.38, 6855–6860. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablocks I, global. DOI: 10.1107/S1600536808022095/pk2104sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536808022095/pk2104Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report