Abstract
Dietary and endogenous nitrates are excreted in urine, and during infection with nitrate-reducing bacteria they are reduced to nitrite. At a low pH nitrite is converted to a variety of nitrogen oxides that are toxic to bacteria. We hypothesized that acidification of nitrite-rich infected urine would result in the killing of the nitrate-reducing bacteria. An Escherichia coli control strain and a mutant lacking nitrate reductase activity were preincubated in urine supplemented with sodium nitrate (0 to 10 mM) at pH 7.0. Then, the nitrite-containing bacterial culture was transferred (and diluted 1/10) to slightly acidic urine (pH 5 and 5.5) containing ascorbic acid (10 mM) and growth was monitored. The control strain produced nitrite in amounts related to the amount of nitrate added. This strain was killed when the culture was transferred to acidic urine. In contrast, the mutant that did not produce nitrite retained full viability. When control bacteria were grown in acidic urine with nitrate and ascorbic acid present from the start of the experiment, no inhibition of growth was noted. The MICs and minimal bactericidal concentrations of sodium nitrite-ascorbic acid in acidic urine were comparable to those of conventional antibiotics. Preincubation of nitrate-reducing E. coli in nitrate-rich urine leads to the accumulation of nitrite. Subsequent acidification of the urine results in generation of nitrogen oxides that are bactericidal. Killing, however, requires a sequential procedure in which the bacteria are first allowed to grow in a nitrate-rich neutral environment, later followed by acidification. We speculate that ingestion of nitrate followed some hours later by acidification of urine could be a new therapeutic strategy for the treatment of urinary tract infections.
Urinary tract infections are among the most common bacterial infections in humans, and Escherichia coli is the predominant (∼80%) causative species (20, 38). A wide variety of antimicrobial agents are used in the prophylaxis and treatment of urinary tract infections, and a growing problem of worldwide concern is the increasing resistance of pathogens to conventional antibiotics (42). In addition, oral administration of antimicrobial agents can disturb the normal intestinal microflora, resulting in diarrhea and opportunistic infections (13). Because of this, there is a continuous search for novel antibiotic regimens.
Nitric oxide (NO) is a lipophilic free radical with antimicrobial properties (10, 22, 40). When synthesized from l-arginine by inducible NO synthase, e.g., in white blood cells, this gas plays an important role in host defense (10, 17, 19, 33, 34). In 1994 a fundamentally different pathway for in vivo generation of NO was described (5, 29). This pathway involves nonenzymatic reduction of nitrite to NO and other nitrogen oxides, a reaction that requires acidic and reducing conditions. Nonenzymatic NO production was first described in the highly acidic stomach, with swallowed saliva being the source of nitrite (29). The nitrite is produced from salivary nitrate by the nitrate-reducing bacteria inhabiting the oral cavity (12). Similarly, during a lower urinary tract infection, the bacteria in urine may convert urinary nitrate to nitrite; and in the clinic a routine test for the detection of urinary tract infection is the test for nitrite in urine with a test strip. We have shown earlier that the growth of bacteria in slightly acidic urine is inhibited if exogenous nitrite is added (7, 28). This inhibition was potentiated in the presence of the reducing agent ascorbic acid. In parallel, large amounts of NO were generated. When this is translated into clinical terms, this could imply that acidification of urine during a urinary tract infection would result in generation of bactericidal nitrogen oxides from the infected nitrite-containing urine. Indeed, acidification of urine has been used in traditional medicine for the prevention and treatment of urinary tract infections (8), although convincing findings for this concept from clinical trials are lacking. In theory, an optimal strategy to generate maximal amounts of nitrogen oxides would be to first feed the bacteria with nitrate at neutral pH. Then, in a later step, when much nitrite has accumulated, a rapid lowering of the pH would result in maximal generation of the bactericidal compounds. The objective of this study was to test if such a sequential procedure would affect the growth of a nitrate-reducing urinary pathogen in vitro. For this we used different strains of E. coli, including a mutant lacking all three known nitrate reductase enzymes. In addition, to determine the potency of nitrite as an antimicrobial agent, we also determined the MICs and minimal bactericidal concentrations (MBCs) of exogenous sodium nitrite for an E. coli strain in acidic urine with and without the addition of ascorbic acid. The values obtained were compared to those obtained with conventional antibiotics used to treat urinary tract infections, nitrofurantoin and trimethoprim.
MATERIALS AND METHODS
Drugs.
Vitamin C (ascorbic acid), sodium nitrate, sodium nitrite, trimethoprim, and nitrofurantoin were supplied by Sigma (Stockholm, Sweden). Solutions were prepared on the day of the experiment.
Bacterial cultures and media.
The strains used in the study were E. coli ATCC 25922, E. coli RK 4353, and an RK 4353 mutant that lacked all three known nitrate reductase enzymes (37). Strain ATCC 25922 was obtained from the Department of Clinical Microbiology,Uppsala, Sweden, and the RK 4353 strains were a generous gift from J. A. Cole, Birmingham, United Kingdom. Before each experiment the bacteria were grown aerobically in Mueller-Hinton broth for 6 h at 37°C, resulting in 2 × 108 to 5 × 108 CFU/ml.
All growth experiments described below were carried out in urine. Midstream urine was collected from 10 healthy subjects, pooled, divided into batches, and immediately frozen (−20°C) until use. The osmolality of the pooled urine was 372 mosmol/kg.
Experimental protocol.
The strains were diluted to 2 × 106 to 5 × 10 6 CFU/ml in urine (pH 7.0) to which different amounts of nitrate (1, 3, and 10 mM) were added, and the culture was incubated aerobically for 20 h at 37°C. After 20 h, 200 μl of the urinary culture was transferred to acidified urine (1.8 ml; pH 5.0 or 5.5) containing 10 mM ascorbic acid and was again incubated for 20 h at 37°C. Before and after each incubation period, the viable counts were determined (see below). In one set of experiments the bacteria were grown in acidic (pH 5.0) urine containing both nitrate (1 to 10 mM) and ascorbic acid from the start of the experiment.
Determination of bacteriostatic activity of acidified sodium nitrite.
The bacteriostatic activity of acidified sodium nitrite was determined on disposable, flat-bottom microwell plates (96 wells, each with a volume of 300 μl). Nitrite solutions with final concentrations in urine of 20 to 1,280 μM and ascorbic acid solutions of 1.25 to 40 mM were prepared. The urinary pH was adjusted (pH 5.0 to 6.5) with hydrochloric acid or sodium hydroxide after the addition of ascorbic acid. The culture was diluted to a bacterial density of 2 × 106 to 5 × 106 CFU/ml in the urine in the microwells. Bacterial growth was measured continuously for 20 h at 37°C by vertical photometry (optical density) at a wavelength of 540 nm in a computerized incubator (Spectra Max 340; Molecular Devices, Sunnyvale, Calif.). The MIC was defined as the lowest concentration at which no visible growth had taken place after 20 h. The MICs of nitrite and ascorbic acid were determined. We also determined the MICs of nitrite in combination with a fixed concentration of ascorbic acid (10 mM). The MICs of nitrofurantoin and trimethoprim were also determined with the same urine. In all experiments eight microwells were filled only with urine and were monitored by vertical photometry, and in some cases the absence of bacterial growth in this urine was also confirmed by determination of viable counts (see below).
Determination of bactericidal activity of acidified sodium nitrite by measurement of viable counts.
After 20 h of bacterial growth in the microwell plates at 37°C, 10 μl of urine was serially diluted with phosphate-buffered saline (pH 7.3) and transferred to agar plates. The agar plates were incubated for 24 h, and then the viable counts (the number of CFU per milliliter) were determined. The MBC was defined as the concentration that killed at least 99.9% (>3 log CFU/ml) of the original inoculum. Different combinations of nitrite and/or ascorbic acid were used, as described above for MIC determinations.
Urinary nitrite and nitrate concentration measurements.
The nitrite and nitrate concentrations in the samples were determined by chemiluminescence after reductive cleavage and subsequent determination of the amount of NO released into the gas phase.
A gas-tight syringe was used to directly introduce the samples into a reduction solution of a microreaction purge vessel coupled with a condenser and a heating jacket unit (Sievers, Boulder, Colo.). The condenser jacket temperature was controlled with a continuous flow of cold water, while the temperature of the heating jacket was controlled with a flow of warm water regulated by a constant-temperature circulating bath (MGW Lauda M3). Nitrogen was used as the carrier gas for NO and was added at a flow rate of 192 ml/min. The flow could be adjusted with a needle valve integrated with the purge vessel, and the outlet of the gas stream was passed through a scrubbing bottle containing sodium hydroxide (1 M, 0°C) in order to trap traces of acid before transfer into the NO analyzer.
A software system (Aerocrine AB, Stockholm, Sweden) was used to display the NO signals and collect the data, which were further manipulated with the Origin for Windows program (version 7.0; Microcal, Northampton, Mass.) and reported as the area under the curve.
The nitrite concentration was determined as described by Feelisch et al. (15). The reducing mixture, which consisted of 45 mmol of potassium iodide per liter and 10 mmol of iodine per liter in glacial acetic acid, was kept at a constant temperature of 56°C; and nitrogen was continuously bubbled into the mixture. The amount of nitrite in a given sample was quantified by simple subtraction of the peak areas of sample aliquots pretreated with sulfanilamide from those of untreated aliquots (10% [vol/vol] of a 5% solution of sulfanilamide in 1 N HCl was added to the biological sample [final concentration, 29 mmol/liter], and the samples were incubated for 15 min at room temperature). Under these conditions, nitrite reacts with sulfanilamide to form a stable diazonium ion that is not converted to NO.
Nitrate was reduced to NO with a solution of vanadium(III) chloride in 1 N hydrochloric acid (saturated solution) at 95°C. Since vanadium(III)-HCl also converts nitrite to NO, the amount of nitrate was quantified by subtraction of the nitrite concentration calculated before.
Nitrate ingestion by healthy volunteers.
Eight healthy subjects (mean age, 37 years; age range, 25 to 47 years) volunteered for the study. The subjects fasted overnight. Basal urine samples were collected, and then the subjects ingested sodium nitrate (10 mg/kg of body weight) dissolved in 150 ml of water. Urine samples were collected at 1, 2, and 3 h and analyzed by the chemiluminescent method described above.
The study was approved by the local ethics committee at the Karolinska Institute.
RESULTS
Nitrate preincubation assay. (i) Urine nitrite levels.
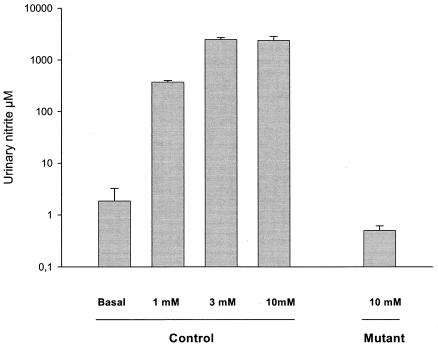
When E. coli ATCC 25922 was incubated for 20 h in basal urine (nitrate concentration, 0.66 mM), 2 μM nitrite was generated (Fig. 1). When the basal urine was supplemented with 1 mM sodium nitrate, the amount of nitrite formed increased to 370 μM. The maximum formation of nitrite was seen after supplementation with 3 and 10 mM sodium nitrate (2,470 and 2,400 μM nitrite, respectively). When the mutant lacking all three nitrate reductases was incubated for 20 h at 37°C with maximum substitution of sodium nitrate (10 mM), the amount of nitrite formed was only 0.5 μM. Note that the nitrite concentrations obtained after the cultures were transferred to the acidic urine in the second part of the experiment were 1/10 of the values given above.
FIG. 1.
Nitrite accumulation in urine after incubation of bacteria for 20 h with the addition of different amounts of sodium nitrate (1, 3, 10 mM). Basal urine contained 0.66 mM nitrate. The strains were E. coli ATCC 25922 (control) and an E. coli RK 4353 mutant lacking nitrate reductases (mutant). Data are means ± standard deviations (n = 6).
(ii) Growth monitoring.
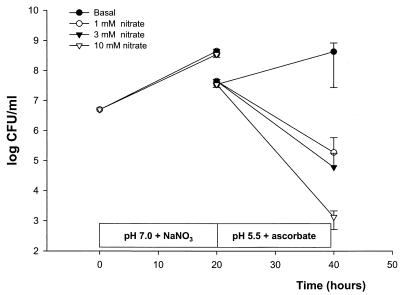
Incubation of E. coli ATCC 25922 in urine at pH 7.0 to which 0 to 10 mM sodium nitrate was added resulted in 3 × 108 to 4 × 108 CFU/ml (Fig. 2). Transfer of the nitrite-rich culture to acidic urine containing ascorbic acid resulted in a marked decrease in viable counts. This effect was dose dependent, with more effective killing occurring when the levels of sodium nitrate used in the preincubation step were higher. In the urine samples to which 10 mM sodium nitrate was added, more than 3 log CFU of the original inoculum per ml was killed, with a mean of 1.3 × 103 CFU/ml remaining (Fig. 2). If the bacteria were preincubated in basal urine without the addition of sodium nitrate, no inhibition was noted when the culture was transferred to the acidic urine.
FIG. 2.
Growth of E. coli ATCC 25922 determined by monitoring viable counts in urine. The bacteria were first grown at pH 7 with different amounts of sodium nitrate (1 to 10 mM NaNO3). After 20 h, the culture containing nitrite and bacteria was transferred (and thereby diluted 1/10) to slightly acidic urine (pH 5.5) containing ascorbic acid (10 mM), and bacterial growth was again monitored. Data are means ± standard deviations (n = 6).
When E. coli ATCC 25922 was incubated in acidic (pH 5.0) urine in which ascorbic acid (10 mM) and sodium nitrate (1 to 10 mM) were present from the start of the experiment, no inhibition of growth was noted (data not shown).
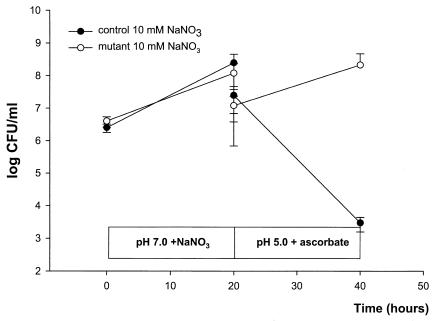
In experiments with strain RK 4353 and the RK 4353 mutant strain lacking nitrate reductases, both strains grew similarly well at pH 7.0 with 10 mM sodium nitrate. When the cultures were transferred to acidic urine containing ascorbic acid, the control strain was effectively killed, while the mutant retained full viability (Fig. 3).
FIG. 3.
Growth of E. coli RK 4353 and an E. coli RK 4353 mutant lacking all three known nitrate reductase enzymes determined by monitoring viable counts. The bacteria were first grown at pH 7 with sodium nitrate (10 mM NaNO3). After 20 h the culture was transferred (and thereby diluted 1/10) to acidified urine (pH 5.0) containing 10 mM ascorbic acid, and bacterial growth was again monitored. Data are means ± standard deviations (n = 5).
MICs of sodium nitrite.
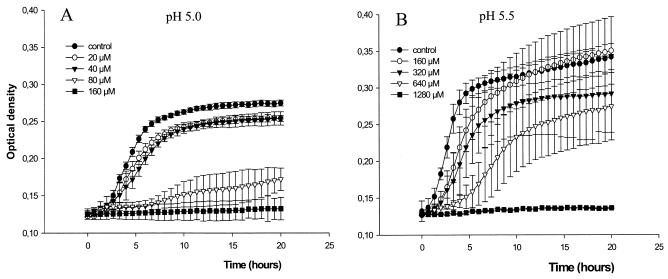
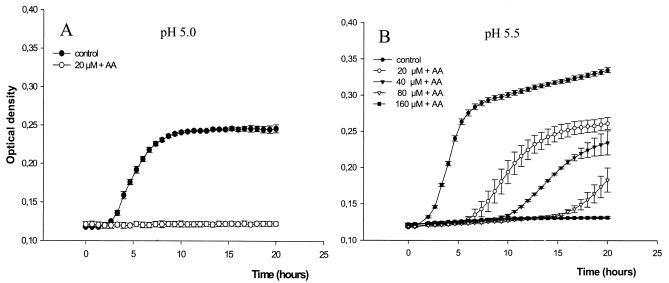
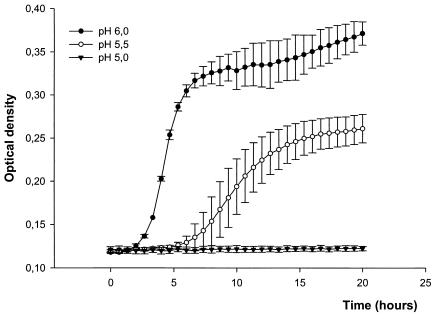
The growth of E. coli ATCC 25922 was inhibited by sodium nitrite in acidified urine in a dose-dependent manner (Fig. 4). The MICs of sodium nitrite at pH 5 and pH 5.5 were 160 and 1,280 μM, respectively (Table 1). Addition of ascorbic acid (10 mM) further enhanced the inhibition of bacterial growth by sodium nitrite (Fig. 5). The MICs of sodium nitrite-ascorbic acid at pH 5 and pH 5.5 were 20 and 160 μM, respectively. Ascorbic acid alone showed poor antibacterial effects, with MICs >40 mM (Table 1). When a fixed concentration of 20 μM sodium nitrite plus 10 mM ascorbic acid was used, the growth-inhibitory effects were highly pH dependent (Fig. 6). The MICs of trimethoprim and nitrofurantoin are shown in Table 1. The MICs of sodium nitrite for E. coli RK 4353 and the E. coli RK 4353 mutant were similar to the MICs for E. coli ATCC 25922 (data not shown).
FIG. 4.
Effects of sodium nitrite (0 to 1,280 μM) on the growth of E. coli ATCC 25922 in urine at pH 5.0 (A) and pH 5.5 (B). Growth was monitored by spectrophotometry. Data are means ± standard deviations (n = 4).
TABLE 1.
MICs and MBCs of sodium nitrite, ascorbic acid, and two conventional antibiotics for E. coli ATCC 25922 in urine at different aciditiesa
| Compound | MIC (μM)
|
MBC (μM)
|
||||||
|---|---|---|---|---|---|---|---|---|
| pH 5.0 | pH 5.5 | pH 6.0 | pH 6.5 | pH 5.0 | pH 5.5 | pH 6.0 | pH 6.5 | |
| Sodium nitrite | 160 | 1,280 | >1,280 | >1,280 | 320 | >1,280 | >1,280 | >1,280 |
| Ascorbic acid | >40,000 | >40,000 | >40,000 | >40,000 | >40,000 | >40,000 | >40,000 | >40,000 |
| Sodium nitrite + ascorbic acid (10 mM) | 20 | 160 | >1,280 | >1,280 | 40 | 320 | >1,280 | >1,280 |
| Trimethoprim | 110 | 55 | 14 | 14 | ||||
| Nitrofurantoin | 67 | 67 | 67 | 134 | ||||
Each experiment was done in quadruplicate.
FIG. 5.
Growth curves (monitored by spectrophotometry) of E. coli ATCC 25922 in urine at pH 5.0 (A) and pH 5.5 (B) with different amounts of sodium nitrite (20 to 160 μM) and a fixed concentration of 10 mM ascorbic acid (AA). Data are means ± standard deviations (n = 4).
FIG. 6.
Growth curves (monitored by spectrophotometry) of E. coli ATCC 25922 in urine in the presence of sodium nitrite (20 μM) and ascorbic acid (10 mM) at different urinary pHs. Data are means ± standard deviations (n = 4).
MBCs.
The bactericidal effects of sodium nitrite with or without ascorbic acid are summarized in Table 1. The MBCs of nitrite-ascorbic acid for E. coli ATCC 25922 were 40 μM at pH 5.0 and 320 μM at pH 5.5.
Urine nitrate levels after ingestion of sodium nitrate.
Basal nitrate levels in urine were 0.7 ± 0.16 mM in fasting individuals, and these levels increased about 10-fold to 7.0 ± 8.8 mM 1 h after ingestion of sodium nitrate. At 2 and 3 h after ingestion the nitrate levels were 9.7 ± 8.7 and 8.6 ± 4.8 mM, respectively.
DISCUSSION
We have shown here that the urinary pathogen E. coli is effectively killed when it is transferred from nitrate-rich urine to mildly acidified urine. Larger amounts of nitrate in the first medium resulted in more nitrite accumulation and more effective killing. In contrast, a mutant lacking a nitrate-reducing capacity retained full viability by this procedure. Notably, the nitrite that accumulated in the first medium was sufficient to kill the bacteria, despite the 10-fold dilution during transfer of the culture. Nitrite is rapidly converted to toxic nitrogen oxides when the pH is lowered (5, 41), which most likely explains the present results. The exact chemical nature of the toxic nitrite-derived compound is not known, nor is the exact mechanism by which killing occurs. The chemistry of acidified nitrite is very complex, and a variety of nitrogen oxides are generated directly or after reactions with other compounds (30, 34). These include NO, N2O3, N2O4, NO+, HNO2, NO2, ONOO−, and S-nitrosothiols, many of which have antimicrobial activities (9, 11, 18, 25, 26, 43). It is possible that several of these compounds act together. NO and related nitrogen oxides affect bacteria in several ways, including by direct damage of the DNA or inhibition of key proteins involved in DNA synthesis, respiration, and other vital cell functions (14).
The fact that ascorbic acid potentiates the bactericidal effects of nitrite in urine suggests that the production of NO at some stage is important to achieve the antibacterial effects observed. Thus, ascorbic acid greatly increases the level of production of NO from nitrite (41), at the expense of most of the other nitrogen oxides mentioned above (4, 27).
Interestingly, when the control strain was exposed to a low pH, ascorbate, and nitrate from the start of the experiment, no effect on growth was observed. Thus, effective killing required a sequential procedure. There are several possible reasons for this. The optimum pH for the nitrate reductase is higher (about pH 8), and it probably operates less effectively at a low pH (24). Obviously, it would be of value for the bacteria not to reduce nitrate to nitrite under acidic conditions, as nitrite will automatically convert to toxic nitrogen oxides, thereby leading to self-destruction. However, if the process is slow, it is possible that bacteria have time to adapt and up-regulate defensive pathways that protect them from nitrite-dependent nitrosative stress. One such mechanism includes flavohemoglobins, an ancient family of proteins found in many bacteria, including E. coli (35, 36). These proteins effectively bind to, e.g., NO, thereby protecting the bacteria from nitrosative stress. Rapid up-regulation of these proteins would allow nitrate reduction to occur even in acidic medium. Other compounds that can help bacteria detoxify NO or related compounds include glutathione, homocysteine, and superoxide dismutase (13). Alternatively, the bacteria may further reduce NO to other less toxic nitrogen metabolites (40).
At pH 5, the MIC of exogenous sodium nitrite was 160 μM without ascorbic acid, and the MIC was as low as 20 μM when ascorbic acid was present. Acidified nitrite seems to be at least as potent as the conventional bacteriostatic antibiotics (trimethoprim, nitrofurantoin) tested in this model. In this study we used pooled urine from 10 healthy volunteers on a normal Western diet. It is likely that the antimicrobial activity of acidified nitrite in urine will vary because of the different compositions of urine, e.g., in relation to diet. Also, urinary osmolality may influence the antibacterial effects of nitrite in acidified urine (2).
The question then remains if the amounts of nitrate-nitrite and ascorbic acid needed for bactericidal effects are physiologically achievable without troublesome side effects and if urinary acidity can reach levels as low as pH 5 to 5.5 for a sufficient period of time. At 1 h after ingestion of sodium nitrate (10 mg/kg), the nitrate levels in urine had already increased to 7 mM, which is well above the concentration needed to generate enough nitrite in this study. Also, the ascorbic acid levels used here (10 mM) seem easily achievable, as shown in an earlier study (6), in which ingestion of 1 to 2 g of vitamin C daily gave similar levels in urine. Nitrate is a natural constituent of green leafy vegetables. For comparison, the amount of nitrate ingested here corresponds to the amount found in about 300 g of spinach.
Several methods for the reduction of urinary pH have been described. Ascorbic acid itself has been used, although the results have varied (3, 31, 32). Other compounds include ammonium chloride (21, 23, 39), memantine (16), methenamine hippurate (39), and furosemide (1). Use of the acidifiers mentioned above achieves urinary pHs between 4.6 and 5.5. Even if the pH can be sufficiently lowered, it will likely be important that this process be rather rapid so that bacteria will not have time to up-regulate defense mechanisms. Another potential problem is that infected urine often has a higher pH, and therefore, acidification is probably more difficult (31). On the other hand, considering the potent antibacterial effect of acidified nitrite, a short transient decrease in the urinary pH below a critical level is probably sufficient to kill most bacteria if nitrite levels are sufficiently high. One great advantage of the concept described here compared to treatment with many traditional antibiotics is that the bacteria from the gastrointestinal tract are probably much less affected, if they are affected at all. In this study we have studied only the effects of acidified nitrite on E. coli, the pathogen that is dominant in the lower urinary tract. Naturally, the sequential procedure described here will work only against bacteria with nitrate reductases. Bacteria that do not reduce nitrate, e.g., Staphylococcus saphrophyticus, will not be sensitive to this treatment, similar to the E. coli nitrate reductase mutant used in this study.
Several issues need to be carefully addressed in the design of a clinical study to test the concept described here. The dose of nitrate ingested and the time interval between nitrate intake and acidification of the urine will probably be critical. In this study we used a preincubation period of 20 h in nitrate-rich urine. Maximum amounts of nitrite are generated with this long incubation time. For practical reasons a shorter incubation time will be necessary in a clinical setting, which may result in the generation of less nitrite. A potential problem is therefore that nitrite levels will be too low to effectively kill the bacteria when the urine is acidified. The timing of the two therapeutic interventions (nitrate intake and urinary acidification) needs to be carefully planned, especially in relation to efficacy and patient acceptance. Food and fluid intake, sleep, voiding, etc., are factors that also need to be taken into consideration.
Conclusion.
We have done an in vitro evaluation of a new concept for the treatment of urinary tract infections caused by nitrate-reducing bacteria. This method involves a two-step procedure in which bacteria are first fed nitrate, followed later by acidification of the urine. Nitrate-reducing E. coli was effectively killed by this sequential procedure, whereas a mutant lacking nitrate reductase activity retained full viability. Controlled in vivo studies are needed to evaluate the clinical potential of this novel antibiotic regimen.
Acknowledgments
We thank J. A. Cole for generously providing us with the mutant strain used in this study. We also thank Carina Nihlén for expert technical assistance.
This study was supported by grants from the Ekhaga Foundation, the Swedish Research Council (grants 12585, 12586, and 14285), the Jeanssen Foundation, The Swedish Cancer Society (grant 010623), and the Johanna Hagstrand and Sigfrid Linnérs Foundation.
REFERENCES
- 1.Alvarado, L. C., L. E. Voyer, G. Bortolazzo, and M. A. Costa. 1991. Urinary acidification by furosemide test. Medicina (Buenos Aires) 51:338-342. [PubMed] [Google Scholar]
- 2.Asscher, A. W., M. Sussman, W. E. Waters, R. H. Davis, and S. Chick. 1966. Urine as a medium for bacterial growth. Lancet ii:1037-1041. [DOI] [PubMed] [Google Scholar]
- 3.Barton, C. H., M. L. Sterling, R. Thomas, N. D. Vaziri, C. Byrne, and G. Ryan. 1981. Ineffectiveness of intravenous ascorbic acid as an acidifying agent in man. Arch. Intern. Med. 141:211-212. [PubMed] [Google Scholar]
- 4.Bartsch, H., H. Ohshima, and B. Pignatelli. 1988. Inhibitors of endogenous nitrosation. Mechanisms and implications in human cancer prevention. Mutat. Res. 202:307-324. [DOI] [PubMed] [Google Scholar]
- 5.Benjamin, N., F. O'Driscoll, H. Dougall, C. Duncan, L. Smith, M. Golden, and H. McKenzie. 1994. Stomach NO synthesis. Nature 368:502. [DOI] [PubMed] [Google Scholar]
- 6.Brandt, R., K. E. Guyer, and W. L. Banks, Jr. 1977. Urinary glucose and vitamin C. Am. J. Clin. Pathol. 68:592-594. [DOI] [PubMed] [Google Scholar]
- 7.Carlsson, S., N. P. Wiklund, L. Engstrand, E. Weitzberg, and J. O. Lundberg. 2001. Effects of pH, nitrite, and ascorbic acid on nonenzymatic nitric oxide generation and bacterial growth in urine. Nitric Oxide 5:580-586. [DOI] [PubMed] [Google Scholar]
- 8.Clark, A. 1931. Escherichia coli bacilluria under ketogenic treatment. Proc. Staff Meet. Mayo Clin. 6:605-608. [Google Scholar]
- 9.Cui, X., C. L. Joannou, M. N. Hughes, and R. Cammack. 1992. The bacteriocidal effects of transition metal complexes containing the NO+ group on the food-spoilage bacterium Clostridium sporogenes. FEMS Microbiol. Lett. 77:67-70. [DOI] [PubMed] [Google Scholar]
- 10.De Groote, M. A., and F. C. Fang. 1995. NO inhibitions: antimicrobial properties of nitric oxide. Clin. Infect. Dis. 21(Suppl. 2):S162-S165. [DOI] [PubMed] [Google Scholar]
- 11.De Groote, M. A., D. Granger, Y. Xu, G. Campbell, R. Prince, and F. C. Fang. 1995. Genetic and redox determinants of nitric oxide cytotoxicity in a Salmonella typhimurium model. Proc. Natl. Acad. Sci. USA 92:6399-6403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Duncan, C., H. Dougall, P. Johnston, S. Green, R. Brogan, C. Leifert, L. Smith, M. Golden, and N. Benjamin. 1995. Chemical generation of nitric oxide in the mouth from the enterosalivary circulation of dietary nitrate. Nat. Med. 1:546-551. [DOI] [PubMed] [Google Scholar]
- 13.Edlund, C., and C. E. Nord. 2000. Effect on the human normal microflora of oral antibiotics for treatment of urinary tract infections. J. Antimicrob. Chemother. 46(Suppl. A):41-48. [PubMed] [Google Scholar]
- 14.Fang, F. C. 1997. Perspectives series: host/pathogen interactions. Mechanisms of nitric oxide-related antimicrobial activity. J. Clin. Investig. 99:2818-2825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Feelisch, M., T. Rassaf, S. Mnaimneh, N. Singh, N. S. Bryan, D. Jourd'Heuil, and M. Kelm. 2002. Concomitant S-, N-, and heme-nitros(yl)ation in biological tissues and fluids: implications for the fate of NO in vivo. FASEB J. 16:1775-1785. [DOI] [PubMed] [Google Scholar]
- 16.Freudenthaler, S., I. Meineke, K. H. Schreeb, E. Boakye, U. Gundert-Remy, and C. H. Gleiter. 1998. Influence of urine pH and urinary flow on the renal excretion of memantine. Br. J. Clin. Pharmacol. 46:541-546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Granger, D. L., J. B. Hibbs, Jr., J. R. Perfect, and D. T. Durack. 1988. Specific amino acid (l-arginine) requirement for the microbiostatic activity of murine macrophages. J. Clin. Investig. 81:1129-1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hurst, J. K., and S. V. Lymar. 1997. Toxicity of peroxynitrite and related reactive nitrogen species toward Escherichia coli. Chem. Res. Toxicol. 10:802-810. [DOI] [PubMed] [Google Scholar]
- 19.James, S. L. 1995. Role of nitric oxide in parasitic infections. Microbiol. Rev. 59:533-547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kahlmeter, G. 2000. The ECO.SENS Project: a prospective, multinational, multicentre epidemiological survey of the prevalence and antimicrobial susceptibility of urinary tract pathogens—interim report. J. Antimicrob. Chemother. 46(Suppl. 1):15-22. [PubMed] [Google Scholar]
- 21.Kamberi, M., K. Tsutsumi, T. Kotegawa, K. Kawano, K. Nakamura, Y. Niki, and S. Nakano. 1999. Influences of urinary pH on ciprofloxacin pharmacokinetics in humans and antimicrobial activity in vitro versus those of sparfloxacin. Antimicrob. Agents Chemother. 43:525-529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kaplan, S. S., J. R. Lancaster, Jr., R. E. Basford, and R. L. Simmons. 1996. Effect of nitric oxide on staphylococcal killing and interactive effect with superoxide. Infect. Immun. 64:69-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Karkkainen, S., and P. J. Neuvonen. 1986. Pharmacokinetics of amitriptyline influenced by oral charcoal and urine pH. Int. J. Clin. Pharmacol. Ther. Toxicol. 24:326-332. [PubMed] [Google Scholar]
- 24.Kay, C. J., and M. J. Barber. 1986. Assimilatory nitrate reductase from Chlorella. Effect of ionic strength and pH on catalytic activity. J. Biol. Chem. 261:14125-14129. [PubMed] [Google Scholar]
- 25.Klebanoff, S. J. 1993. Reactive nitrogen intermediates and antimicrobial activity: role of nitrite. Free Radic. Biol. Med. 14:351-360. [DOI] [PubMed] [Google Scholar]
- 26.Kono, Y., H. Shibata, K. Adachi, and K. Tanaka. 1994. Lactate-dependent killing of Escherichia coli by nitrite plus hydrogen peroxide: a possible role of nitrogen dioxide. Arch. Biochem. Biophys. 311:153-159. [DOI] [PubMed] [Google Scholar]
- 27.Licht, W. R., S. R. Tannenbaum, and W. M. Deen. 1988. Use of ascorbic acid to inhibit nitrosation: kinetic and mass transfer considerations for an in vitro system. Carcinogenesis 9:365-372. [DOI] [PubMed] [Google Scholar]
- 28.Lundberg, J. O., S. Carlsson, L. Engstrand, E. Morcos, N. P. Wiklund, and E. Weitzberg. 1997. Urinary nitrite: more than a marker of infection. Urology 50:189-191. [DOI] [PubMed] [Google Scholar]
- 29.Lundberg, J. O., E. Weitzberg, J. M. Lundberg, and K. Alving. 1994. Intragastric nitric oxide production in humans: measurements in expelled air. Gut 35:1543-1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McKnight, G. M., C. W. Duncan, C. Leifert, and M. H. Golden. 1999. Dietary nitrate in man: friend or foe? Br. J. Nutr. 81:349-358. [DOI] [PubMed] [Google Scholar]
- 31.Murphy, F. J., S. Zelman, and W. Mau. 1965. Ascorbic acid as a urinary acidifying agent. 2. Its adjunctive role in chronic urinary infection. J. Urol. 94:300-303. [DOI] [PubMed] [Google Scholar]
- 32.Nahata, M. C., L. Shrimp, T. Lampman, and D. C. McLeod. 1977. Effect of ascorbic acid on urine pH in man. Am. J. Hosp. Pharm. 34:1234-1237. [PubMed] [Google Scholar]
- 33.Nathan, C. 1997. Inducible nitric oxide synthase: what difference does it make? J. Clin. Investig. 100:2417-2423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nathan, C., and M. U. Shiloh. 2000. Reactive oxygen and nitrogen intermediates in the relationship between mammalian hosts and microbial pathogens. Proc. Natl. Acad. Sci. USA 97:8841-8848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Poole, R. K., M. F. Anjum, J. Membrillo-Hernandez, S. O. Kim, M. N. Hughes, and V. Stewart. 1996. Nitric oxide, nitrite, and Fnr regulation of hmp (flavohemoglobin) gene expression in Escherichia coli K-12. J. Bacteriol. 178:5487-5492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Poole, R. K., and M. N. Hughes. 2000. New functions for the ancient globin family: bacterial responses to nitric oxide and nitrosative stress. Mol. Microbiol. 36:775-783. [DOI] [PubMed] [Google Scholar]
- 37.Potter, L. C., P. D. Millington, G. H. Thomas, R. A. Rothery, G. Giordano, and J. A. Cole. 2000. Novel growth characteristics and high rates of nitrate reduction of an Escherichia coli strain, LCB2048, that expresses only a periplasmic nitrate reductase. FEMS Microbiol. Lett. 185:51-57. [DOI] [PubMed] [Google Scholar]
- 38.Ronald, A. 2002. The etiology of urinary tract infection: traditional and emerging pathogens. Am. J. Med. 113(Suppl. 1A):14S-19S. [DOI] [PubMed] [Google Scholar]
- 39.Wall, I., and H. G. Tiselius. 1990. Long-term acidification of urine in patients treated for infected renal stones. Urol. Int. 45:336-341. [DOI] [PubMed] [Google Scholar]
- 40.Watmough, N. J., G. Butland, M. R. Cheesman, J. W. Moir, D. J. Richardson, and S. Spiro. 1999. Nitric oxide in bacteria: synthesis and consumption. Biochim. Biophys. Acta 1411:456-474. [DOI] [PubMed] [Google Scholar]
- 41.Weitzberg, E., and J. O. Lundberg. 1998. Nonenzymatic nitric oxide production in humans. Nitric Oxide 2:1-7. [DOI] [PubMed] [Google Scholar]
- 42.Wise, R., T. Hart, O. Cars, M. Streulens, R. Helmuth, P. Huovinen, and M. Sprenger. 1998. Antimicrobial resistance. Is a major threat to public health. BMJ 317:609-610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhu, L., C. Gunn, and J. S. Beckman. 1992. Bactericidal activity of peroxynitrite. Arch. Biochem. Biophys. 298:452-457. [DOI] [PubMed] [Google Scholar]