Abstract
Agar dilution MIC determination was used to compare the activity of DK-507k with those of ciprofloxacin, levofloxacin, gatifloxacin, moxifloxacin, sitafloxacin, amoxicillin, cefuroxime, erythromycin, azithromycin, and clarithromycin against 113 penicillin-susceptible, 81 penicillin-intermediate, and 67 penicillin-resistant pneumococci (all quinolone susceptible). DK-507k and sitafloxacin had the lowest MICs of all quinolones against quinolone-susceptible strains (MIC at which 50% of isolates were inhibited [MIC50] and MIC90 of both, 0.06 and 0.125 μg/ml, respectively), followed by moxifloxacin, gatifloxacin, levofloxacin, and ciprofloxacin. MICs of β-lactams and macrolides rose with those of penicillin G. Against 26 quinolone-resistant pneumococci with known resistance mechanisms, DK-507k and sitafloxacin were also the most active quinolones (MICs, 0.125 to 1.0 μg/ml), followed by moxifloxacin, gatifloxacin, levofloxacin, and ciprofloxacin. Mutations in quinolone resistance-determining regions of quinolone-resistant strains were in the usual regions of the parC and gyrA genes. Time-kill testing showed that both DK-507k and sitafloxacin were bactericidal against all 12 quinolone-susceptible and -resistant strains tested at twice the MIC at 24 h. Serial broth passages in subinhibitory concentrations of 10 strains for a minimum of 14 days showed that development of resistant mutants (fourfold or greater increase in the original MIC) occurred most rapidly for ciprofloxacin, followed by moxifloxacin, DK-507k, gatifloxacin, sitafloxacin, and levofloxacin. All parent strains demonstrated a fourfold or greater increase in initial MIC in <50 days. MICs of DK-507k against resistant mutants were lowest, followed by those of sitafloxacin, moxifloxacin, gatifloxacin, ciprofloxacin, and levofloxacin. Four strains were subcultured in subinhibitory concentrations of each drug for 50 days: MICs of DK-507k against resistant mutants were lowest, followed by those of sitafloxacin, moxifloxacin, gatifloxacin, levofloxacin, and ciprofloxacin. Exposure to DK-507k and sitafloxacin resulted in mutations, mostly in gyrA.
The incidence of pneumococci resistant to penicillin G and other β-lactam and non-β-lactam compounds has increased worldwide, including the United States, at an alarming rate. Major foci of infections currently include South Africa, Spain, and Central and eastern Europe (1, 14, 15). In the United States a recent survey has shown an increase in resistance to penicillin from <5% before 1989 (including <0.02% of isolates for which the MICs were ≥2.0 μg/ml) to 6.6% in 1991 to 1992 (including 1.3% of isolates for which MICs were ≥2.0 μg/ml) (3). In another more recent survey, 50.4% of 1,476 clinically significant pneumococcal isolates were not susceptible to penicillin (16). It is also important to note the high rates of isolation of penicillin-intermediate and -resistant pneumococci (approximately 30%) in middle ear fluids from patients with refractory otitis media, compared to the rates for other isolation sites (2). The problem of drug-resistant pneumococci is compounded by the ability of resistant clones to spread from country to country and from continent to continent (19, 20).
There is an urgent need for oral compounds for outpatient treatment of otitis media and respiratory tract infections caused by penicillin-intermediate and -resistant pneumococci (8, 9). Quinolones such as ciprofloxacin and ofloxacin yield moderate in vitro activity against pneumococci, with MICs clustering around the breakpoints. Newer quinolones such as levofloxacin, gatifloxacin, moxifloxacin, and gemifloxacin have greater antipneumococcal activity (6, 12, 17, 26, 28, 29, 31).
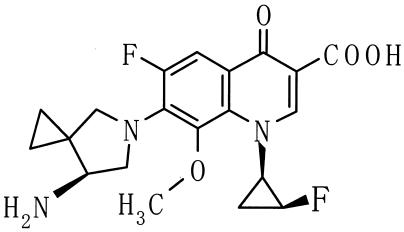
Several recent reports from Hong Kong (11), Canada (5), and Spain (18) have described a worrisome increase in the incidence of quinolone-resistant pneumococci. With the increasing use of broad-spectrum quinolones active against pneumococci for empirical therapy of community-acquired respiratory tract infections and prophylaxis use of older-generation quinolones (10, 13), the incidence of these strains is likely to increase. This report compared the antipneumococcal activities of two new quinolones, DK-507k (Fig. 1) and sitafloxacin (Daiichi Pharmaceuticals, Tokyo, Japan) to those of ciprofloxacin, levofloxacin, gatifloxacin, moxifloxacin, amoxicillin, cefuroxime, azithromycin, and clarithromycin by agar dilution testing of 261 quinolone-susceptible strains with differing susceptibilities to penicillin G and macrolides. Additionally, all quinolones were tested against 26 quinolone-resistant pneumococci with defined quinolone resistance mechanisms. Microdilution and time-kill studies of the activities of the above drugs against 12 pneumococcal strains and multistep studies to test the capability of DK-507k,sitafloxacin, ciprofloxacin, levofloxacin, gatifloxacin, and moxifloxacin to select for resistant clones of 10 pneumococcal strains were also performed.
FIG. 1.
Chemical structure of DK-507k.
MATERIALS AND METHODS
Bacteria.
For agar dilution MIC studies, 261 recently isolated (1998 to 2002) quinolone-susceptible pneumococcal strains (levofloxacin MICs ≤ 2.0 μg/ml), comprising 113 penicillin-susceptible (MICs ≤ 0.06 μg/ml), 81 penicillin-intermediate (MICs 0.125 to 1.0 μg/ml), and 67 penicillin-resistant (MICs = 2.0 to 16.0 μg/ml) strains, were tested. Of these, 147 were macrolide susceptible (MICs ≤ 0.5 μg/ml) and 114 were macrolide resistant (MICs ≥ 1.0 μg/ml). Additionally, 26 strains for which levofloxacin MICs were ≥4 μg/ml (1998 to 2002) from our collection were tested by agar dilution. These strains were also tested for mutations in parC, gyrA, parE, and gyrB and for efflux mechanisms. Twelve strains with differing susceptibilities to β-lactams, macrolides, and quinolones were selected for time-kill analyses. These strains comprised four penicillin G-susceptible, four penicillin G-intermediate, and four penicillin G-resistant strains; three strains were macrolide susceptible, and nine were macrolide resistant [all nine by the mefA mechanism]. Strains for which the macrolide MICs were higher because of the erm mechanism were not tested because of solubilization difficulty at the higher drug concentrations as well as lack of clinical significance of possible killing at such high concentrations. Additionally, macrolide-resistant strains in the United States usually contain the mefA gene. The three quinolone-resistant strains (see Table 4 and Results for their genotypes) were tested so as to test quinolones against clinical quinolone-resistant pneumococcal isolates which, although rare, do appear in the United States (16). Strains with higher-level quinolone resistance were not selected for time-kill experiments because of the doubtful significance of killing at such high MICs. Ten strains were selected for resistance selection studies (see Table 6). Strains in all studies were selected so as to include as large and representative a variety of drug-susceptible and drug-resistant strains as possible.
TABLE 4.
MICs for pneumococcal strains tested in time-kill experiments
| Drug | MIC (μg/ml) for strain:
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | |
| Penicillin G | 1.0 | 0.016 | 2.0 | 0.03 | 1.0 | 2.0 | 0.016 | 0.25 | 2.0 | 0.03 | 0.125 | 2.0 |
| DK-507k | 0.25 | 0.25 | 0.03 | 0.125 | 0.03 | 0.06 | 0.03 | 0.03 | 0.06 | 0.03 | 0.03 | 0.25 |
| Sitafloxacin | 0.25 | 0.125 | 0.03 | 0.06 | 0.03 | 0.06 | 0.03 | 0.03 | 0.06 | 0.06 | 0.03 | 0.25 |
| Ciprofloxacin | 16.0 | 16.0 | 0.5 | 2.0 | 0.5 | 1.0 | 0.5 | 1.0 | 1.0 | 1.0 | 0.5 | 8.0 |
| Levofloxacin | 8.0 | 8.0 | 0.5 | 1.0 | 0.5 | 0.5 | 0.5 | 0.5 | 1.0 | 1.0 | 0.5 | 8.0 |
| Gatifloxacin | 4.0 | 2.0 | 0.125 | 0.5 | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 | 4.0 |
| Moxifloxacin | 2.0 | 1.0 | 0.06 | 0.25 | 0.125 | 0.125 | 0.125 | 0.125 | 0.125 | 0.125 | 0.125 | 2.0 |
| Amoxicillin | 2.0 | 0.016 | 1.0 | 0.016 | 1.0 | 1.0 | 0.03 | 0.25 | 2.0 | 0.06 | 0.125 | 1.0 |
| Cefuroxime | 4.0 | 0.03 | 4.0 | 0.03 | 4.0 | 8.0 | 0.06 | 0.5 | 8.0 | 0.03 | 0.25 | 8.0 |
| Azithromycin | 0.03 | 0.03 | 4.0 | 4.0 | 4.0 | 8.0 | 4.0 | 8.0 | 2.0 | 4.0 | 2.0 | 0.016 |
| Clarithromycin | 0.016 | 0.016 | 2.0 | 1.0 | 2.0 | 4.0 | 2.0 | 2.0 | 0.5 | 2.0 | 2.0 | 0.008 |
TABLE 6.
Results of multistep resistance selection by DK-507k, sitafloxacin, ciprofloxacin, levofloxacin, gatifloxacin, and moxifloxacin
| Strain | Initial MIC (μg/ml)c
|
Resistance selection
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| DK-507k | Lev | Gati | Moxi | Sita | Cipro | Selecting agent | No. of passages | MIC (μg/ml) | |
| 1 | 0.5 | 16 | 4 | 4 | 0.25 | 32 | DK-507k | 14 | 4 |
| Lev | 38 | 128 | |||||||
| Gati | 20 | 32 | |||||||
| Moxi | 24 | 16 | |||||||
| Sita | 22 | 4 | |||||||
| Cipro | 14 | 256 | |||||||
| 2 | 0.25 | 16 | 4 | 2 | 0.5 | 32 | DK-507k | 21 | 2 |
| Lev | 36 | 128 | |||||||
| Gati | 22 | 32 | |||||||
| Moxi | 19 | 32 | |||||||
| Sita | 14 | 2 | |||||||
| Cipro | 20 | 256 | |||||||
| 3 | 0.03 | 0.5 | 0.25 | 0.125 | 0.06 | 0.5 | DK-507k | 11 | 0.25 |
| Lev | 8 | 4 | |||||||
| Gati | 14 | 16 | |||||||
| Moxi | 9 | 1 | |||||||
| Sita | 12 | 0.5 | |||||||
| Cipro | 8 | 8 | |||||||
| 4 | 0.03 | 1 | 0.25 | 0.125 | 0.03 | 0.5 | DK-507k | 15 | 0.25 |
| Lev | 28 | 4 | |||||||
| Gati | 13 | 1 | |||||||
| Moxi | 9 | 1 | |||||||
| Sita | 24 | 0.5 | |||||||
| Cipro | 9 | 4 | |||||||
| 5 | 0.03 | 0.5 | 0.25 | 0.125 | 0.03 | 0.5 | DK-507k | 22 | 0.25 |
| Lev | 37 | 4 | |||||||
| Gati | 24 | 1 | |||||||
| Moxi | 12 | 0.5 | |||||||
| Sita | 18 | 0.5 | |||||||
| Cipro | 9 | 4 | |||||||
| 6 | 0.03 | 1 | 0.25 | 0.125 | 0.03 | 0.5 | DK-507k | 15 | 0.25 |
| Lev | 10 | 4 | |||||||
| Gati | 7 | 1 | |||||||
| Moxi | 16 | 1 | |||||||
| Sita | 17 | 1 | |||||||
| Cipro | 9 | 8 | |||||||
| 7 | 0.03 | 0.5 | 0.25 | 0.125 | 0.016 | 0.5 | DK-507k | 15 | 0.25 |
| Lev | 25 | 16 | |||||||
| Gati | 21 | 1 | |||||||
| Moxi | 20 | 0.5 | |||||||
| Sita | 28 | 0.5 | |||||||
| Cipro | 11 | 4 | |||||||
| 8 | 0.03 | 1 | 0.25 | 0.125 | 0.03 | 0.5 | DK-507k | 15 | 0.25 |
| Lev | 18 | 8 | |||||||
| Gati | 21 | 1 | |||||||
| Moxi | 12 | 2 | |||||||
| Sita | 18 | 0.5 | |||||||
| Cipro | 4 | 4 | |||||||
| 9 | 0.03 | 1 | 0.25 | 0.125 | 0.03 | 0.5 | DK-507k | 19 | 0.25 |
| Lev | 24 | 4 | |||||||
| Gati | 17 | 1 | |||||||
| Moxi | 10 | 0.5 | |||||||
| Sita | 14 | 0.5 | |||||||
| Cipro | 7 | 4 | |||||||
| 10 | 0.03 | 0.5 | 0.25 | 0.125 | 0.016 | 0.5 | DK-507k | 15 | 0.25 |
| Lev | 16 | 8 | |||||||
| Gati | 16 | 2 | |||||||
| Moxi | 16 | 0.5 | |||||||
| Sita | 18 | 0.5 | |||||||
| Cipro | 7 | 4 | |||||||
Initial parent mutations are in parentheses; mutations not in parentheses are new.
Parent and mutant strains for which the decrease in MIC in the presence of reserpine was fourfold or greater (bold face) are considered to have efflux mechanisms. Parent strains 6 and 10 had initial ciprofloxacin efflux mechanisms.
Drug abbreviations are as for Table 3.
Antimicrobials and MIC testing.
DK-507k and sitafloxacin susceptibility testing powders were obtained from Daiichi Pharmaceuticals, Tokyo, Japan. Other antimicrobials were obtained from their respective manufacturers. Amoxicillin was chosen to represent the β-lactam with the greatest potency against pneumococci for which penicillin G MICs are high, and cefuroxime was chosen as a representative of oral cephalosporins with good activity against penicillin-susceptible strains and reasonable activity against penicillin-intermediate (but not penicillin-resistant) strains. Azithromycin and clarithromycin represented drugs of the macrolide-lincosamide-streptogramin B group most widely used therapeutically against these strains (16). Agar dilution methodology was performed using Mueller-Hinton agar (BBL Microbiology Systems, Cockeysville, Md.) supplemented with 5% sheep blood (12, 27). Standard quality control strains, including Streptococcus pneumoniae ATCC 49619 (23), were included in each run of agar dilution MICs. For all 261 strains, agar dilution MICs on sheep blood agar plates with inocula of 104 CFU/spot were determined as previously described by our group (12, 27). MICs for the 12 strains subjected to time-kill testing were determined by inspection of broth macrodilutions.
Time-kill testing.
For time-kill studies, glass tubes containing 5 ml of cation-adjusted Mueller-Hinton broth (Difco) plus 5% lysed horse blood with doubling antibiotic concentrations were inoculated with 5 × 105 to 5 × 106 CFU/ml and incubated at 35°C in a shaking water bath. Antibiotic concentrations were chosen to comprise 3 doubling dilutions above and 2 dilutions below the agar dilution MIC. Growth controls with inoculum but no antibiotic were included with each experiment (25).
Lysed horse blood was prepared as described previously (25). The bacterial inoculum was prepared by suspending growth from an overnight blood agar plate in Mueller-Hinton broth until turbidity matched a no. 1 McFarland standard. Dilutions required to obtain the correct inoculum (5 × 105 to 5 × 106 CFU/ml) were determined by prior viability studies using each strain.
To inoculate each tube of serially diluted antibiotic, 50 μl of diluted inoculum was delivered by pipette beneath the surface of the broth. Tubes were then vortexed and plated for viability counts within 10 min (approximately 0.2 h). The original inoculum was determined by using the untreated growth control. Only tubes containing an initial inoculum within the range of 5 × 105 to 5 × 106 CFU/ml were acceptable.
Viability counts of antibiotic-containing suspensions were performed by plating 10-fold dilutions of 0.1-ml aliquots from each tube in sterile Mueller-Hinton broth onto Trypticase soy agar-5% sheep blood agar plates (BBL). Recovery plates were incubated for up to 72 h. Colony counts were performed on plates yielding 30 to 300 colonies. The lower limit of sensitivity of colony counts was 300 CFU/ml (25).
Time-kill assays were analyzed by determining the number of strains which yielded Δlog10 CFU/ml values of −1, −2, and −3 at 3, 6, 12, and 24 h, compared to counts at 0 h. Antimicrobials were considered bactericidal at the lowest concentration that reduced the original inoculum by ≥3 log10 CFU/ml (99.9%) at each of the time periods and bacteriostatic if the inoculum was reduced by 0 to <3 log10 CFU/ml. With the sensitivity threshold and inocula used in these studies, no problems were encountered in delineating 99.9% killing, when present. The problem of drug carryover was addressed by dilution as described previously (25). For macrolide time-kill testing, only strains for which macrolide MICs were ≤8.0 μg/ml were chosen, because of problems in solubilization at high concentrations and lack of clinical significance.
Multistep mutation analysis.
Glass tubes containing 1 ml of cation-adjusted Mueller-Hinton broth (Difco) supplemented with 5% lysed horse blood with doubling antibiotic dilutions were inoculated with approximately 5 × 105 CFU/ml at antibiotic concentrations from 4 doubling dilutions above to 3 doubling dilutions below the MIC of each agent for each strain. The initial inoculum was prepared by suspending growth from an overnight Trypticase soy blood agar plate (Difco) in Mueller-Hinton broth. Tubes were incubated at 35°C for 24 h. Daily passages were then performed for 50 days by taking a 10-μl inoculum from the tube nearest the MIC (usually 1 to 2 dilutions below) which had the same turbidity as the antibiotic-free controls. Periodically for some mutants, an aliquot from a tube used as an inoculum was frozen in double-strength skim milk at −70°C for later analysis. When an MIC for a strain increased fourfold, passaging was stopped and strains were subcultured in antibiotic-free medium for 10 serial passages. A minimum of 14 passages were made prior to termination. Four strains continued to be subcultured after reaching initial termination to finish the 50-day period regardless of MIC. A maximum of 50 serial passages in antibiotic were performed. The identities of parent and resistant clones were confirmed by pulsed-field gel electrophoresis as described previously (6, 7, 21).
Determination of quinolone resistance mechanism.
PCR was used to amplify parC, parE, gyrA, and gyrB with primers and cycling conditions described by Pan et al. (24). Template DNA for PCR was prepared with a Prep-A-Gene kit (Bio-Rad, Hercules, Calif.) as recommended by the manufacturer. After amplification, PCR products were purified from excess primers and nucleotides with a QIAquick PCR purification kit as recommended by the manufacturer (Qiagen, Valencia, Calif.) and sequenced directly with an Applied Biosystems model 373A DNA sequencer (7, 21, 24).
Presence of quinolone efflux mechanism.
MICs were determined in the presence and absence of 10 μg of reserpine (Sigma Chemicals, St. Louis, Mo.)/ml as described previously (4, 7, 21). By definition, an efflux mechanism existed when there was at least a fourfold-lower MIC in the presence of reserpine.
RESULTS
MICs for quinolone-susceptible and nonsusceptible clinical isolates.
Against 261 pneumococcal strains for which levofloxacin MICs were ≤2.0 μg/ml, DK-507k and sitafloxacin had the lowest MICs of all quinolones tested (MIC at which 50% of isolates were inhibited [MIC50] and MIC90 of both, 0.06 and 0.125 μg/ml, respectively), followed by moxifloxacin, gatifloxacin, levofloxacin, and ciprofloxacin. MICs of all β-lactams and macrolides rose with those of penicillin G (Table 1). Against 26 clinical isolates for which levofloxacin MICs were ≥4 μg/ml, DK-507k and sitafloxacin also had the lowest MICs (0.25 to 1.0 μg/ml; MIC90, 0.5 μg/ml). MICs of other quinolones ranged between 0.25 and >32 μg/ml (MIC90s, 4.0 to >32 μg/ml), with moxifloxacin, gatifloxacin, levofloxacin, and ciprofloxacin, in ascending order, giving the next-lowest MICs (Table 2).
TABLE 1.
Agar dilution MICs for 261 quinolone-susceptible strainsa
| Drug and susceptilityb | MICc (μg/ml)
|
||
|---|---|---|---|
| Range | 50 | 90 | |
| Penicillin | |||
| S | ≤0.008-0.06 | 0.016 | 0.03 |
| I | 0.125-1.0 | 0.5 | 1.0 |
| R | 2.0->8.0 | 2.0 | 8.0 |
| DK-507K | |||
| S | 0.03-0.25 | 0.06 | 0.125 |
| I | 0.03-0.125 | 0.06 | 0.125 |
| R | 0.03-0.125 | 0.06 | 0.125 |
| Sitafloxacin | |||
| S | 0.016-0.25 | 0.06 | 0.125 |
| I | ≤0.008-0.125 | 0.06 | 0.125 |
| R | 0.03-0.25 | 0.06 | 0.125 |
| Ciprofloxacin | |||
| S | 0.5-4.0 | 2.0 | 4.0 |
| I | 0.5-4.0 | 2.0 | 2.0 |
| R | 0.5-4.0 | 2.0 | 2.0 |
| Levofloxacin | |||
| S | 0.5-2.0 | 1.0 | 2.0 |
| I | 0.5-2.0 | 1.0 | 2.0 |
| R | 0.5-2.0 | 1.0 | 2.0 |
| Gatifloxacin | |||
| S | 0.125-0.5 | 0.25 | 0.5 |
| I | 0.125-0.5 | 0.25 | 0.5 |
| R | 0.25-0.5 | 0.25 | 0.5 |
| Moxifloxacin | |||
| S | 0.06-0.25 | 0.125 | 0.25 |
| I | 0.06-0.25 | 0.125 | 0.25 |
| R | 0.06-0.25 | 0.125 | 0.25 |
| Amoxicillin | |||
| S | ≤0.008-0.125 | 0.03 | 0.03 |
| I | 0.03-2.0 | 0.5 | 2.0 |
| R | 1.0-8.0 | 4.0 | 8.0 |
| Cefuroxime | |||
| S | ≤0.008-0.25 | 0.03 | 0.125 |
| I | 0.25-8.0 | 0.5 | 8.0 |
| R | 2.0-32.0 | 8.0 | 16.0 |
| Azithromycin | |||
| S | 0.06->64.0 | 0.125 | 8.0 |
| I | 0.06->64.0 | 0.125 | >64.0 |
| R | 0.06->64.0 | >64.0 | >64.0 |
| Clarithromycin | |||
| S | ≤0.016->64.0 | 0.03 | 4.0 |
| I | ≤0.016->64.0 | 0.06 | >64.0 |
| R | ≤0.016->64.0 | 32.0 | >64.0 |
Levofloxacin MICs, ≤2.0 μg/ml.
S, penicillin susceptible; I, penicillin intermediate; R, penicillin resistant.
50, MIC50; 90, MIC90.
TABLE 2.
Quinolone agar dilution MICs for 26 quinolone-resistant strainsa
| Quinolone | MICb (μg/ml)
|
||
|---|---|---|---|
| Range | 50 | 90 | |
| DK507K | 0.25-1.0 | 0.25 | 0.5 |
| Sitafloxacin | 0.25-1.0 | 0.5 | 0.5 |
| Ciprofloxacin | 4.0->32.0 | 16.0 | >32.0 |
| Levofloxacin | 8.0-32.0 | 16.0 | 32.0 |
| Gatifloxacin | 0.5-8.0 | 4.0 | 8.0 |
| Moxifloxacin | 0.25-4.0 | 2.0 | 4.0 |
Levofloxacin MICs, ≥4.0 μg/ml.
50, MIC50; 90, MIC90.
Mutations in the quinolone resistance-determining region (QRDR) for the 26 strains are presented in Table 3. As can be seen, quinolone resistance was associated with mutations in the QRDRs of parC, gyrA, parE, and/or gyrB. Quinolone nonsusceptibility was associated with amino acid substitution at position S79, D83, N91, R95, or K137 in ParC in 26 isolates (Table 3). Most of the strains had the S79F mutation alone (13 isolates) or double mutations S79F and K137N (7 isolates). Of 26 isolates, 24 had mutations in GyrA at S81, E85, or S114. Most strains had S81F (14 isolates) or S81Y (8 isolates) mutations. Mutation in ParE was found in 20 isolates, with substitution of V for I at position 460 in 18 strains. Mutations in GyrB were less common: one isolate had an E474K substitution (Table 3).
TABLE 3.
Quinolone MICs for 26 strains with defined mutations in the QRDR
| Strain | HMCa no. | MIC (μg/ml)b
|
Detected mutation in QRDR of:
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| DK-507k | Sita | Cipro | Lev | Gati | Moxi | GyrA | GyrB | ParC | ParE | ||
| 1 | 1055 | 0.5 | 0.5 | 32 | 16 | 8 | 4 | S81F | S79F, K137N | 1460V | |
| 2 | 1059 | 0.25 | 0.25 | 16 | 8 | 2 | 2 | S81F | D83N | 1460N | |
| 3 | 1060 | 0.25 | 0.25 | 16 | 8 | 2 | 2 | S81F | S79F, K137N | 1460V | |
| 4 | 1066 | 0.5 | 0.25 | >32 | 16 | 4 | 2 | S81F | D83N | ||
| 5 | 1068 | 1 | 0.5 | 32 | 32 | 8 | 4 | S81F | S79F | 1460V | |
| 6 | 1070 | 0.25 | 0.25 | 16 | 16 | 2 | 2 | S81F | S79F, K137N | 1460V | |
| 7 | 1071 | 0.25 | 0.25 | 32 | 16 | 4 | 2 | S81A | S79Y | ||
| 8 | 1073 | 0.5 | 1 | 16 | 32 | 8 | 2 | S81C | S79F | 1460V | |
| 9 | 1074 | 0.5 | 0.25 | 32 | 16 | 4 | 2 | S81Y | S79F | ||
| 10 | 1076 | 0.5 | 0.25 | 32 | 16 | 4 | 2 | E474K | S79F, K137N | 1460V | |
| 11 | 1077 | 0.5 | 0.5 | >32 | 16 | 4 | 2 | S81F, S114G | D83G, N91D | ||
| 12 | 1078 | 0.5 | 0.5 | >32 | 16 | 4 | 2 | S81F | S79F, K137N | 1460V | |
| 13 | 1150 | 0.25 | 0.25 | 16 | 8 | 2 | 1 | S81F | S79F | 1460V | |
| 14 | 1156 | 0.5 | 1 | >32 | 32 | 8 | 4 | S79Y | |||
| 15 | 1062 | 1 | 1 | >32 | 32 | 8 | 4 | S81F | S79F | 1460V | |
| 16 | 1072 | 0.25 | 0.5 | 32 | 16 | 4 | 2 | S81F | S79F, K137N | 1460V | |
| 17 | 1146 | 0.25 | 0.5 | 32 | 8 | 2 | 1 | S81F | R95C | D435N | |
| 18 | 1147 | 0.5 | 0.5 | >32 | 16 | 4 | 2 | S81F | S79F, K137N | 1460V | |
| 19 | 2527 | 0.25 | 0.25 | 16 | 8 | 2 | 2 | S81Y | S79F | I460V | |
| 20 | 2529 | 0.25 | 0.25 | 16 | 8 | 2 | 1 | S81Y | S79F | I460V | |
| 21 | 2578 | 0.25 | 0.25 | 4 | 16 | 2 | 2 | S81Y | S79F | I460V | |
| 22 | 4026 | 0.5 | 0.5 | 32 | 16 | 4 | 1 | S81Y | S79F | I460V | |
| 23 | 5041 | 0.25 | 0.5 | 16 | 16 | 4 | 2 | S81F | S79F | ||
| 24 | 2536 | 0.25 | 0.5 | 4 | 8 | 1 | 2 | S81Y | S79F | I460V | |
| 25 | 2538 | 0.25 | 0.5 | 4 | 8 | 1 | 1 | S81Y | S79F | I460V | |
| 26 | 2542 | 0.25 | 0.5 | 4 | 8 | 2 | 1 | S81Y | S79F | I460V | |
HMC, Hershey Medical Center.
Sita, sitafloxacin; Cipro, ciprofloxacin; Lev, levofloxacin; Gati, gatifloxacin; Moxi, moxifloxacin.
In the presence of reserpine, ciprofloxacin MICs for 5 of 26 strains were lower (four- to eightfold), gatifloxacin MICs for 3 strains were lower (four- to eightfold), and moxifloxacin and sitafloxacin MICs for 1 strain were lower (fourfold). DK-507k and levofloxacin were not efflux substrates in the 26 strains tested. Strain 24 had efflux mechanisms against gatifloxacin, moxifloxacin, and sitafloxacin, and strain 22 had efflux mechanisms against ciprofloxacin and gatifloxacin.
Time-kill analyses.
MICs for the 12 strains subjected to time-kill analysis are represented in Table 4. As can be seen, three quinolone-resistant (levofloxacin MICs ≥ 8.0 μg/ml) and nine quinolone-susceptible strains were tested. Quinolone-resistant strains 1, 2, and 12 had S81F, S81F, and S81Y mutations, respectively, in GyrA and S79F plus K137N, S79F, and S79F mutations, respectively, in ParC. All three strains had an I460V substitution in ParE. Of 12 strains, four were penicillin susceptible, four were penicillin intermediate, and four were penicillin resistant. Three strains were susceptible to azithromycin and clarithromycin. Time-kill analyses are presented in Table 5. As can be seen, DK-507k and sitafloxacin, at twice the MIC, were bactericidal (99.9% killing) against all 12 strains after 24 h, with bactericidal activity against 11 of 12 strains after 12 h at twice the MIC. Significant killing was also observed at earlier time periods, with 90% killing of all strains after 3 h at twice the MIC. Similar kill kinetics relative to higher MICs were observed with other quinolones at 12 and 24 h, with slower killing at earlier time periods. Amoxicillin and cefuroxime gave similar kill kinetics, with bactericidal activity against all 12 strains at twice the MIC after 24 h. Azithromycin and clarithromycin both yielded slower killing at earlier time periods than quinolones and β-lactams. No difference in time-kill curves between drug-susceptible and -resistant strains for each class was observed.
TABLE 5.
Time-kill result
| Drug and concn | No. of strains with indicated level of killinga at:
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 3 h
|
6 h
|
12 h
|
24 h
|
|||||||||
| −1 | −2 | −3 | −1 | −2 | −3 | −1 | −2 | −3 | −1 | −2 | −3 | |
| DK-507k | ||||||||||||
| 4 × MIC | 12 | 11 | 6 | 12 | 12 | 8 | 12 | 12 | 11 | 12 | 12 | 12 |
| 2 × MIC | 12 | 10 | 3 | 12 | 11 | 7 | 12 | 12 | 11 | 12 | 12 | 12 |
| MIC | 12 | 6 | 1 | 12 | 9 | 3 | 12 | 10 | 5 | 10 | 8 | 4 |
| Sitafloxacin | ||||||||||||
| 4 × MIC | 12 | 10 | 6 | 12 | 11 | 10 | 12 | 12 | 11 | 12 | 12 | 12 |
| 2 × MIC | 12 | 10 | 3 | 12 | 10 | 8 | 12 | 12 | 11 | 12 | 12 | 12 |
| MIC | 10 | 5 | 0 | 12 | 9 | 1 | 12 | 9 | 7 | 9 | 7 | 5 |
| Ciprofloxacin | ||||||||||||
| 4 × MIC | 11 | 5 | 1 | 12 | 9 | 6 | 12 | 12 | 8 | 12 | 12 | 12 |
| 2 × MIC | 10 | 5 | 1 | 12 | 7 | 5 | 12 | 12 | 8 | 12 | 12 | 11 |
| MIC | 8 | 4 | 1 | 11 | 4 | 0 | 12 | 10 | 4 | 6 | 5 | 4 |
| Levofloxacin | ||||||||||||
| 4 × MIC | 12 | 6 | 1 | 12 | 11 | 5 | 12 | 12 | 9 | 12 | 12 | 12 |
| 2 × MIC | 12 | 5 | 1 | 12 | 10 | 4 | 12 | 12 | 9 | 12 | 12 | 12 |
| MIC | 7 | 3 | 0 | 11 | 5 | 2 | 12 | 8 | 5 | 8 | 6 | 5 |
| Gatifloxacin | ||||||||||||
| 4 × MIC | 12 | 8 | 3 | 12 | 11 | 6 | 12 | 12 | 9 | 12 | 12 | 12 |
| 2 × MIC | 12 | 4 | 1 | 12 | 10 | 4 | 12 | 12 | 8 | 12 | 12 | 11 |
| MIC | 8 | 2 | 1 | 11 | 5 | 1 | 12 | 10 | 4 | 10 | 8 | 6 |
| Moxifloxacin | ||||||||||||
| 4 × MIC | 12 | 7 | 1 | 12 | 11 | 5 | 12 | 12 | 10 | 12 | 12 | 12 |
| 2 × MIC | 11 | 5 | 1 | 12 | 11 | 4 | 12 | 12 | 9 | 12 | 12 | 11 |
| MIC | 10 | 1 | 0 | 10 | 5 | 1 | 11 | 8 | 5 | 9 | 8 | 7 |
| Amoxicillin | ||||||||||||
| 4 × MIC | 11 | 8 | 0 | 12 | 11 | 8 | 12 | 12 | 12 | 12 | 12 | 12 |
| 2 × MIC | 10 | 8 | 0 | 12 | 11 | 7 | 12 | 12 | 11 | 12 | 12 | 12 |
| MIC | 8 | 5 | 0 | 11 | 9 | 4 | 11 | 11 | 8 | 10 | 8 | 7 |
| Cefuroxime | ||||||||||||
| 4 × MIC | 11 | 4 | 0 | 12 | 10 | 4 | 12 | 12 | 12 | 12 | 12 | 12 |
| 2 × MIC | 10 | 4 | 0 | 12 | 9 | 4 | 12 | 12 | 12 | 12 | 12 | 12 |
| MIC | 10 | 2 | 0 | 12 | 8 | 4 | 12 | 11 | 7 | 9 | 7 | 6 |
| Azithromycin | ||||||||||||
| 4 × MIC | 7 | 2 | 0 | 11 | 7 | 4 | 12 | 11 | 8 | 12 | 12 | 12 |
| 2 × MIC | 7 | 2 | 0 | 9 | 7 | 2 | 12 | 10 | 7 | 12 | 12 | 12 |
| MIC | 5 | 1 | 0 | 8 | 6 | 2 | 12 | 8 | 5 | 9 | 5 | 3 |
| Clarithromycin | ||||||||||||
| 4 × MIC | 8 | 3 | 0 | 11 | 7 | 3 | 12 | 11 | 7 | 12 | 12 | 12 |
| 2 × MIC | 5 | 0 | 0 | 9 | 6 | 1 | 12 | 10 | 6 | 12 | 12 | 11 |
| MIC | 5 | 0 | 0 | 8 | 3 | 1 | 10 | 7 | 2 | 7 | 3 | 3 |
−1, 90% killing; −2, 99% killing; −3, 99.9% killing.
Multistep resistance selection studies.
Initial MICs for parent strains and resistant mutants resulting from serial daily subculturing in subinhibitory concentrations of antimicrobials are summarized in Table 6. The four strains subcultured for 50 days are summarized in Table 7. Of the quinolones tested, DK-507k, ciprofloxacin, sitafloxacin, levofloxacin, moxifloxacin, and gatifloxacin all showed fourfold increases in initial MICs in less than 50 days. All selected mutants had the same pulsed-field electrophoresis patterns as their parent strains. The average times necessary for mutant selection were 10 days for ciprofloxacin, 15 days for moxifloxacin, 16 days for DK-507k, 17 days for gatifloxacin, 18 days for sitafloxacin, and 24 days for levofloxacin. The MICs of DK-507k against resistant mutants selected were the lowest, followed by those of sitafloxacin, moxifloxacin, gatifloxacin, ciprofloxacin, and levofloxacin.
TABLE 7.
Four multistep selection strains subcultured for 50 days
| Strain | Initial MIC(s) (μg/ml)b
|
Resistance selection
|
MIC (μg/ml) of strains resistant to:
|
Mutation(s) in QRDR of resistant mutanta
|
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DK-507k | Lev | Gati | Moxi | Sita | Cipro | Selecting agent | No. of passages | MIC (μg/ml) | DK-507k | Lev | Gati | Moxi | Sita | Cipro | GyrA | GyrB | ParC | ParE | |
| 1 | 0.5 | 16 | 4 | 4 | 0.25 | 32 | DK-507k | 50 | 8 | 8 | 128 | 16 | 16 | 4 | 256 | (E85K) | (S79F) + (K137N) | (I460V) | |
| Lev | 50 | 128 | 2 | 128 | 32 | 8 | 2 | 128 | (E85K) | (S79F) + (K137N) | (I460V) | ||||||||
| Gati | 50 | 128 | 32 | 256 | 128 | 125 | 16 | 256 | S81Y + (E85K) | (S79F) + D83G + (K137N) | (I460V) | ||||||||
| Moxi | 50 | 64 | 8 | 256 | 8 | 64 | 16 | 128 | (E85K) | (S79F) + (K137N) | D435N + (I460V) | ||||||||
| Sita | 50 | 8 | 8 | 64 | 16 | 16 | 8 | 64 | S81F + (E85K) | (S79F) + (K137N) | (I460V) | ||||||||
| Cipro | 50 | 1,024 | 2 | 64 | 2 | 8 | 2 | 1,024 | (E85K) | (S79F) + (K137N) | (I460V) | ||||||||
| 2 | 0.25 | 16 | 4 | 2 | 0.5 | 32 | DK-507k | 50 | 4 | 4 | 64 | 16 | 8 | 2 | 128 | (S81F) | R445S | (S79F) | (I460V) |
| Lev | 50 | 64 | 0.5 | 64 | 16 | 2 | 0.5 | 128 | (S81F) | (S79F) | (I460V) | ||||||||
| Gati | 50 | 16 | 2 | 64 | 16 | 16 | 2 | 64 | S81L | G406V | (S79F) | (I460V) | |||||||
| Moxi | 50 | 16 | 2 | 64 | 8 | 16 | 2 | 32 | S81V | A482G | (S79F) | (I460V) | |||||||
| Sita | 50 | 32 | 2 | 64 | 32 | 32 | 32 | 128 | E52D + S81V | (S79F) | (I460V) | ||||||||
| Cipro | 50 | 512 | 1 | 64 | 1 | 8 | 1 | 512 | E52D + (S81F) | (S79F) | (I460V) | ||||||||
| 4 | 0.03 | 1 | 0.25 | 0.12, 5 | 0.03 | 0.5 | DK-507k | 50 | 8 | 8 | 128 | 16 | 16 | 4 | 256 | (S81F) + G54A + V55N + A117S | E474K | (I460V) | |
| Lev | 50 | 16 | 0.125 | 16 | 4 | 2 | 0.25 | 32 | E85G | D83N | (I460V) + R447C | ||||||||
| Gati | 50 | 1 | 0.25 | 1 | 1 | 0.5 | 0.25 | 4 | (I460V) | ||||||||||
| Moxi | 50 | 4 | 0.5 | 32 | 1 | 4 | 1 | 64 | S81F | D83N | R447S + (I460V) | ||||||||
| Sita | 50 | 4 | 2 | 64 | 4 | 16 | 4 | 32 | S81Y | G486E | S79Y | (I460V) | |||||||
| Cipro | 50 | 512 | 2 | 64 | 1 | 8 | 2 | 512 | E85K | S79Y | (I460V) | ||||||||
| 5 | 0.03 | 0.5 | 0.25 | 0.125 | 0.03 | 0.5 | DK-507k | 50 | 1 | 1 | 16 | 0.5 | 4 | 1 | 32 | S81F | P454S | ||
| Lev | 50 | 4 | 0.125 | 4 | 0.5 | 0.5 | 0.25 | 8 | D83G | ||||||||||
| Gati | 50 | 0.5 | 0.25 | 8 | 0.5 | 0.5 | 0.25 | 8 | S81F | ||||||||||
| Moxi | 50 | 4 | 0.25 | 16 | 2 | 4 | 0.25 | 32 | E52D + S81Y | G406R | D83N | ||||||||
| Sita | 50 | 8 | 4 | 128 | 16 | 64 | 8 | 256 | S81Y + E85A | S79Y | |||||||||
| Cipro | 50 | 128 | 1 | 32 | 2 | 4 | 1 | 128 | S81F + G108A | S79Y | |||||||||
Mutations detected in parent strains are in parentheses; newly generated mutations are without parentheses.
Drug abbreviations are as for Table 3.
Of the 60 multistep mutant strains, cross-resistance was seen in the majority of mutants selected; however MICs for 18 of 60 selected strains were unchanged from the initial MICs for the parents. Moxifloxacin MICs for 10 strains selected with DK-507k, levofloxacin, gatifloxacin, sitafloxacin, and ciprofloxacin showed no change from the initial MICs for the parents. Levofloxacin MICs for three strains selected with DK-507k, gatifloxacin, and moxifloxacin showed no change from initial MICs for the parents. Gatifloxacin MICs for two strains selected with moxifloxacin and ciprofloxacin showed no change from the initial MICs for the parent. Sitafloxacin MICs for two strains selected with gatifloxacin and moxifloxacin showed no change from initial MICs for the parent. Ciprofloxacin MICs for one strain selected with DK-507k showed no change from the initial MIC for the parent (Table 6).
Alterations in the QRDRs of parC, parE, gyrA, and gyrB genes of mutant strains are shown in Table 6. Exposure to DK-507k, levofloxacin, sitafloxacin, and moxifloxacin resulted in mutations, mostly in gyrA. The majority of mutations selected by ciprofloxacin were in ParC. Gatifloxacin selected equal numbers of mutants with alterations in parC and gyrB. Selection by moxifloxacin and levofloxacin caused the highest number of new mutations (17), followed by selection by sitafloxacin (9), gatifloxacin (8), ciprofloxacin (7), and DK-507k (5). Of the five new mutations generated by DK-507k selection, 80% were in gyrA. For strains 1 and 2, which already had one gyrA mutation, DK-507k exposure caused alteration of GyrB (strain 2) and induction or generation of an efflux mechanism (strain 1) (Table 6). For sitafloxacin, levofloxacin, ciprofloxacin, moxifloxacin, and gatifloxacin, rates of gyrA mutations were 67, 47, 43, 35, and 25%, respectively, of the new mutations generated by these quinolones. Fifty-seven percent of the new mutations selected by ciprofloxacin were in parC.
Treatment with the efflux inhibitor reserpine lowered ciprofloxacin MICs for 2 of 10 parent strains; in contrast, no efflux mechanism for DK-507k, levofloxacin, gatifloxacin, moxifloxacin, and sitafloxacin was detected among parent strains. The contributions of efflux mechanisms to MIC levels differed with selective agents among mutant strains. Four of 10 mutant strains selected in DK-507k demonstrated an efflux mechanism for resistance to DK-507k. The 10 DK-507k-selected mutants all had a reserpine-inhibited efflux mechanism for ciprofloxacin, three had an efflux mechanism for levofloxacin, and one had an efflux mechanism for gatifloxacin. DK-507k selected the most strains with an efflux mechanism (10), followed by ciprofloxacin (9), gatifloxacin (8), levofloxacin (7), sitafloxacin (7), and moxifloxacin (3) (Table 6).
The four strains selected and subcultured for a total of 50 days are summarized in Table 7. For these strains, DK-507k MICs were the lowest, followed by those of sitafloxacin, moxifloxacin, gatifloxacin, levofloxacin, and ciprofloxacin.
Of the 24 multistep mutant strains selected in 50 days, cross-resistance can be seen in the majority of mutants selected; however for 6 of the 24 selected strains the MICs showed no change from initial MICs for the parent. Gatifloxacin MICs for two strains selected with ciprofloxacin showed no change from the initial MICs for the parent. Levofloxacin MICs for one strain selected with gatifloxacin showed no change from the initial MIC for the parent. Moxifloxacin MICs for one strain selected with levofloxacin showed no change from the initial MIC for the parent. Sitafloxacin MICs for one strain selected with levofloxacin showed no change from the initial MIC for the parent. Ciprofloxacin MICs for one strain selected with moxifloxacin showed no change from the initial MIC for the parent (Table 7).
Alterations in the QRDRs of parC, parE, gyrA, and gyrB genes of 50-day mutant strains are shown in Table 7. Exposure to gatifloxacin and sitafloxacin resulted in new mutations, mostly in gyrA. Exposure to ciprofloxacin resulted in a twofold increase in the initial MIC when a single new mutation occurred in GyrA (strain 2); however, a greater-than-fourfold dilution increase could be seen for strains with mutations in both GyrA and ParC (strains 4 and 5). DK-507k and moxifloxacin selected equal numbers of mutants with alteration in gyrA and parC and in gyrB and parC, respectively. Exposure to levofloxacin resulted in mutations mostly in parC. Selection by moxifloxacin and sitafloxacin caused the highest number of new mutations (nine), followed by selection by DK-507k (seven), ciprofloxacin (six), gatifloxacin (five), and levofloxacin (four). Mutations in GyrA were S81F, -Y, -V, and -L; E85K, -G, and -A; and E52D, G54A, V55N, G108A, and A117S. These mutations in gyrA comprised the majority of new mutations due to 50 days of subculturing (Table 7).
DISCUSSION
DK-507k is a new 8-methoxyquinolone with a broad spectrum of in vitro activity against gram-positive and -negative bacteria (K. Kawakami, H. Ohki, K. Kimura, H. Takahashi, M. Tanaka, M. Takemura, and I. Hayakawa, Abstr. 41st Intersci. Conf. Antimicrob. Agents Chemother., abstr. F-546, 2001; T. Otani, M. Tanaka, T. Akasaka, Y. Kurosaka, I. Hayakawa, and K. Sato, Abstr. 41st Intersci. Conf. Antimicrob. Agents Chemother., abstr. F-547, 2001; M. Tanaka, M. Tachibana, H. Seki, and Y. Ohzone, Abstr. 41st Intersci. Conf. Antimicrob. Agents Chemother., abstr. F-548, 2001; T. Jindo, K. Shimoda, S. Itoh, T. Hagiwara, and K. Furuhama, Abstr. 41st Intersci. Conf. Antimicrob. Agents Chemother., abstr. F-549, 2001). MICs obtained in the present study are similar to those found in a preliminary study by Otani and coworkers (41st ICAAC). Sitafloxacin, another experimental quinolone, has similarly low MICs against quinolone-susceptible and -resistant pneumococci (22, 26, 30)
In our study, DK-507k and sitafloxacin had the lowest quinolone MICs against all pneumococcal strains tested, followed by moxifloxacin, gatifloxacin, levofloxacin, and ciprofloxacin. DK-507k and sitafloxacin also had the lowest MICs against resistant strains obtained by selection studies. MICs of all quinolones were similar to those described previously (12, 17, 22, 26, 28-31; Otani et al., 41st ICAAC). Additionally, DK-507k and sitafloxacin had significantly lower MICs against highly quinolone-resistant pneumococci, irrespective of quinolone resistance mechanisms. This was the case for double mutants with mutations in both parC and gyrA, strains which have previously been shown to be highly resistant to other quinolones, as well as for strains with an efflux mechanism. MICs of nonquinolone agents were similar to those described previously, with higher β-lactam and macrolide MICs in strains with raised penicillin MICs (14, 15, 25-31).
Time-kill results with sitafloxacin, ciprofloxacin, levofloxacin, gatifloxacin, and moxifloxacin were similar to those published previously (6, 12, 25, 30, 31). DK-507k gave kill kinetics which were similar to those of sitafloxacin. Both DK-507k and sitafloxacin gave the best kill kinetics relative to MICs of all quinolones tested against both quinolone-susceptible and -resistant pneumococci.
Alterations in GyrA, GyrB, ParC, and ParE were detected among resistant mutants selected by quinolone exposure. Eighty percent of DK-507k mutants had modifications in GyrA, showing the importance of this protein in the action of this quinolone. Most mutant strains for which quinolone MICs were elevated had mutations in GyrA at S81 or in ParC at D83 or S79 as previously reported (6, 7, 21, 24). All ciprofloxacin-selected mutants had S79F or -Y mutations after 50 days, with additional mutations in gyrA.
The contribution of efflux mechanisms to higher MICs of quinolones has been found to be important for ciprofloxacin (4). However, moxifloxacin, gatifloxacin, and levofloxacin are less affected by the efflux mechanism (7, 21). In our data, clinically resistant isolates did not have a DK-507k efflux mechanism. However, DK-507k shows a propensity to select or induce an efflux mechanism in multistep selection studies, compared to the other quinolones tested.
In summary, DK-507k and sitafloxacin were the most potent quinolones tested against both quinolone-susceptible and -resistant pneumococci. The incidence of quinolone-resistant pneumococci is currently very low. However, this situation may change with the introduction of broad-spectrum quinolones into clinical practice, and in particular in the pediatric population, leading to selection of quinolone-resistant strains. Both DK-507k and sitafloxacin are promising new antipneumococcal agents, irrespective of the susceptibility of pneumococcal strains to quinolones and other agents. Pharmacokinetic and pharmacodynamic studies (free area under the curve/MIC or maximum concentration of a drug in serum/MIC), followed by toxicity and animal studies, are required to see whether these compounds will be promising clinically.
Table 6b.
| MIC(s) (μg/ml)b of strains resistant to:
|
Mutation(s) in QRDR of resistant mutanta
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| DK-507k | Lev | Gati | Moxi | Sita | Cipro | GyrA | GyrB | ParC | ParE |
| 4 | 64 | 16 | 8 | 2 | 128 | (E85K) | (S79F) + (K137N) | (I460V) | |
| 4 | 128 | 32 | 8 | 2 | 128 | (E85K) | (S79F) + (K137N) | (I460V) | |
| 2 | 64 | 16 | 8 | 2 | 128 | (E85K) | (S79F) + (K137N) | (I460V) | |
| 2 | 64 | 16 | 16 | 2 | 64 | (E85K) | (S79F) + (K137N) | D435N + (I460V) | |
| 2 | 32 | 16 | 4 | 4 | 64 | (E85K) | (S79F) + (K137N) | (I460V) | |
| 1 | 32 | 8 | 4 | 0.5 | 256 | (E85K) | (S79F) + (K137N) | (I460V) | |
| 2 | 64 | 16 | 8 | 2 | 128 | (S81F) | R445S | (S79F) | (I460V) |
| 1 | 128 | 16 | 4 | 1 | 128 | E85G | (S79F) | (I460V) | |
| 1 | 32 | 32 | 2 | 0.5 | 64 | (S81F) | (S79F) | (I460V) | |
| 4 | 64 | 16 | 8 | 2 | 64 | S81V | (S79F) | (I460V) | |
| 2 | 32 | 16 | 4 | 2 | 128 | (S81F) | (I460V) | ||
| 1 | 32 | 16 | 4 | 1 | 256 | (S81F) | (S79F) | (I460V) | |
| 0.5 | 8 | 2 | 1 | 0.5 | 8 | S81F | (I460V) | ||
| 1 | 64 | 16 | 4 | 1 | 64 | S81F | E474K | D435N + P454S + (I460V) | |
| 2 | 64 | 32 | 8 | 2 | 64 | S81F | S79A + D83N | (I460V) | |
| 0.5 | 16 | 8 | 4 | 0.5 | 32 | S81F | D83N | D435N + (I460V) | |
| 2 | 16 | 8 | 4 | 1 | 32 | S81F | D435N + (I460V) | ||
| 1 | 16 | 8 | 4 | 0.5 | 128 | S81F | D83N | (I460V) | |
| 0.25 | 1 | 1 | 0.5 | 0.125 | 0.5 | S81F | (I460V) | ||
| 0.25 | 4 | 1 | 0.5 | 0.125 | 4 | E85K | S79Y | (I460V) | |
| 0.25 | 1 | 1 | 0.125 | 0.06 | 1 | D435E | (I460V) | ||
| 0.25 | 2 | 1 | 1 | 0.125 | 4 | S81F | R447S + (I460V) | ||
| 0.5 | 2 | 1 | 1 | 0.5 | 4 | S81Y | G486E | (I460V) | |
| 0.125 | 2 | 0.5 | 0.25 | 0.125 | 8 | (I460V) | |||
| 0.25 | 2 | 0.5 | 0.125 | 0.125 | 8 | ||||
| 0.25 | 4 | 1 | 0.125 | 0.25 | 16 | D80G | D83Y | ||
| 0.25 | 1 | 1 | 0.25 | 0.125 | 4 | S81F | |||
| 0.25 | 8 | 2 | 2 | 0.25 | 16 | S81Y | |||
| 0.5 | 2 | 4 | 1 | 0.5 | 8 | S81Y | |||
| 0.125 | 2 | 0.5 | 0.25 | 0.125 | 8 | ||||
| 0.25 | 2 | 1 | 0.25 | 0.25 | 8 | (I460V) | |||
| 1 | 4 | 8 | 2 | 1 | 64 | S81Y | D83Y | (I460V) | |
| 0.25 | 4 | 2 | 0.125 | 0.25 | 16 | (I460V) | |||
| 0.125 | 2 | 1 | 1 | 0.125 | 8 | P413L | D78A | (I460V) | |
| 1 | 4 | 2 | 1 | 1 | 8 | E85G | (I460V) | ||
| 0.25 | 4 | 1 | 0.5 | 0.125 | 32 | D83N | (I460V) | ||
| 0.25 | 2 | 0.5 | 0.5 | 0.125 | 2 | E85K | |||
| 0.25 | 16 | 2 | 1 | 0.25 | 32 | E85G | |||
| 0.25 | 2 | 1 | 0.25 | 0.25 | 4 | P454S | |||
| 0.06 | 1 | 0.25 | 0.25 | 0.06 | 2 | K137N | R447H + I460V | ||
| 0.25 | 2 | 1 | 0.5 | 0.25 | 2 | S81F | |||
| 0.125 | 1 | 0.25 | 0.125 | 0.06 | 8 | ||||
| 0.25 | 2 | 1 | 0.25 | 0.25 | 4 | (K137N) | (I460V) | ||
| 0.5 | 8 | 4 | 1 | 0.5 | 16 | E85G | (K137N) | D435N + (I460V) | |
| 0.125 | 4 | 1 | 0.25 | 0.125 | 16 | D83N + (K137N) | (I460V) | ||
| 1 | 64 | 16 | 8 | 1 | 64 | E85K | D435V | S79Y + (K137N) | |
| 0.5 | 16 | 4 | 2 | 0.5 | 64 | S81Y | D83N + (K137N) | (I460V) | |
| 0.5 | 16 | 4 | 2 | 0.5 | 128 | S81F | D79Y + (K137N) | (I460V) | |
| 0.25 | 2 | 1 | 0.125 | 0.125 | 8 | ||||
| 0.25 | 4 | 1 | 0.5 | 0.125 | 8 | D435A | |||
| 0.25 | 2 | 1 | 0.125 | 0.125 | 8 | ||||
| 0.06 | 1 | 0.5 | 0.5 | 0.03 | 2 | R406G | |||
| 0.25 | 2 | 1 | 0.25 | 0.5 | 2 | D80S | |||
| 0.25 | 2 | 4 | 0.25 | 0.25 | 16 | A419T + A465V | |||
| 0.25 | 2 | 1 | 0.5 | 0.25 | 4 | S81Y | |||
| 0.5 | 8 | 4 | 1 | 0.25 | 8 | E85K | D435N | ||
| 0.125 | 2 | 1 | 0.25 | 0.125 | 4 | D435E | |||
| 0.25 | 8 | 2 | 2 | 0.25 | 16 | S81Y | S405F | ||
| 0.5 | 2 | 2 | 1 | 0.5 | 8 | ||||
| 0.25 | 16 | 4 | 2 | 0.125 | 64 | S81F | S79Y | ||
Acknowledgments
This study was supported by a grant from Daiichi Pharmaceutical Co., Tokyo, Japan.
REFERENCES
- 1.Appelbaum, P. C. 1992. Antimicrobial resistance in Streptococcus pneumoniae—an overview. Clin. Infect. Dis. 15:77-83. [DOI] [PubMed] [Google Scholar]
- 2.Block, S., C. J. Harrison, J. A. Hedrick, R. D. Tyler, R. A. Smith, E. Keegan, and S. A. Chartrand. 1995. Penicillin-resistant Streptococcus pneumoniae in acute otitis media: risk factors, susceptibility patterns and antimicrobial management. Pediatr. Infect. Dis. J. 14:751-759. [DOI] [PubMed] [Google Scholar]
- 3.Breiman, R. F., J. C. Butler, F. C. Tenover, J. A. Elliott, and R. R. Facklam. 1994. Emergence of drug-resistant pneumococcal infections in the United States. JAMA 271:1831-1835. [PubMed] [Google Scholar]
- 4.Brenwald, N. P., M. J. Gill, and R. Wise. 1998. Prevalence of a putative efflux mechanism among fluoroquinolone-resistant clinical isolates of Streptococcus pneumoniae. Antimicrob. Agents Chemother. 42:2032-2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen, D. K., A. McGeer, J. C. de Azavedo, and D. E. Low. 1999. Decreased susceptibility of Streptococcus pneumoniae to fluoroquinolones in Canada. N. Engl. J. Med. 22:233-239. [DOI] [PubMed] [Google Scholar]
- 6.Davies, T. A., L. M. Kelly, G. A. Pankuch, K. L. Credito, M. R. Jacobs, and P. C. Appelbaum. 1999. Antipneumococcal activities of gemifloxacin compared to those of nine other agents. Antimicrob. Agents Chemother. 44:304-310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davies, T. A., G. A. Pankuch, B. E. Dewasse, M. R. Jacobs, and P. C. Appelbaum. 1999. In-vitro development of resistance to five quinolones and amoxicillin/clavulanate in Streptococcus pneumoniae. Antimicrob. Agents Chemother. 43:1177-1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Friedland, I. R., and G. S. Istre. 1992. Management of penicillin-resistant pneumococcal infections. Pediatr. Infect. Dis. J. 11:433-435. [DOI] [PubMed] [Google Scholar]
- 9.Friedland, I. R., and G. H. McCracken, Jr. 1994. Management of infections caused by antibiotic-resistant Streptococcus pneumoniae. N. Engl. J. Med. 331:377-382. [DOI] [PubMed] [Google Scholar]
- 10.Friedlander, A. M. 2000. Anthrax: clinical features, pathogenesis, and potential biological warfare threat. Curr. Clin. Top. Infect. Dis. 20:335-349. [PubMed] [Google Scholar]
- 11.Ho, P.-L., T.-L. Que, D. N.-C. Tsang, T.-K. Ng, K.-H. Chow, and W.-H. Seto. 1999. Emergence of fluoroquinolone resistance among multiply resistant strains of Streptococcus pneumoniae in Hong Kong. Antimicrob. Agents Chemother. 43:1310-1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hoellman, D. B., G. Lin, M. R. Jacobs, and P. C. Appelbaum. 1999. Anti-pneumococcal activity of gatifloxacin compared with other quinolone and non-quinolone agents. J. Antimicrob. Chemother. 43:645-649. [DOI] [PubMed] [Google Scholar]
- 13.Hughes, W. T., D. Armstrong, G. P. Bodey, E. J. Bow, A. E. Brown, T. Calandra, R. Feld, P. A. Pizzo, K. V. I. Rolston, J. L. Shenep, and L. S. Young. 2002. Guidelines for the use of antimicrobial agents in neutropenic patients with cancer. Clin. Infect. Dis. 34:730-735. [DOI] [PubMed] [Google Scholar]
- 14.Jacobs, M. R. 1992. Treatment and diagnosis of infections caused by drug-resistant Streptococcus pneumoniae. Clin. Infect. Dis. 15:119-127. [DOI] [PubMed] [Google Scholar]
- 15.Jacobs, M. R., and P. C. Appelbaum. 1995. Antibiotic-resistant pneumococci. Rev. Med. Microbiol. 6:77-93. [Google Scholar]
- 16.Jacobs, M. R., S. Bajaksouzian, A. Zilles, G. Lin, G. A. Pankuch, and P. C. Appelbaum. 1999. Susceptibilities of Streptococcus pneumoniae and Haemophilus influenzae to 10 oral antimicrobial agents based on pharmacodynamic parameters: 1997 U.S. surveillance study. Antimicrob. Agents Chemother. 43:1901-1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jorgensen, J. H., L. M. Weigel, M. J. Ferraro, J. M. Swenson, and F. C. Tenover. 1999. Activities of newer fluoroquinolones against Streptococcus pneumoniae clinical isolates including those with mutations in the gyrA, parC, and parE loci. Antimicrob. Agents Chemother. 43:329-334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liñares, J., A. G. Campa, and R. Pallares. 1999. Fluoroquinolone-resistance in Streptococcus pneumoniae. N. Engl. J. Med. 20:1546-1548. [DOI] [PubMed] [Google Scholar]
- 19.McDougal, L. K., R. Facklam, M. Reeves, S. Hunter, J. M. Swenson, B. C. Hill, and F. C. Tenover. 1992. Analysis of multiply antimicrobial-resistant isolates of Streptococcus pneumoniae from the United States. Antimicrob. Agents Chemother. 36:2176-2184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Munoz, R., J. M. Musser, M. Crain, D. E. Briles, A. Marton, A. J. Parkinson, U. Sorensen, and A. Tomasz. 1992. Geographic distribution of penicillin-resistant clones of Streptococcus pneumoniae: characterization by penicillin-binding protein profile, surface protein A typing, and multilocus enzyme analysis. Clin. Infect. Dis. 15:112-118. [DOI] [PubMed] [Google Scholar]
- 21.Nagai, K., T. A. Davies, G. A. Pankuch, B. E. DeWasse, M. R. Jacobs, and P. C. Appelbaum. 2000. In vitro selection of resistance to clinafloxacin, ciprofloxacin, and trovafloxacin in Streptococcus pneumoniae. Antimicrob. Agents Chemother. 44:2740-2746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nakane, T., S. Iyobe, K. Sato, and S. Mitsuhashi. 1995. In vitro antibacterial activity of DU-6859a, a new fluoroquinolone. Antimicrob. Agents Chemother. 39:2822-2826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.National Committee for Clinical Laboratory Standards. 2003. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, 5th ed. Approved standard M7-A6. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 24.Pan, X. S., J. Ambler, S. Mehtar, and L. M. Fisher. 1996. Involvement of topoisomerase IV and DNA gyrase as ciprofloxacin targets in Streptococcus pneumoniae. Antimicrob. Agents Chemother. 40:2321-2326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pankuch, G. A., M. R. Jacobs, and P. C. Appelbaum. 1994. Study of comparative antipneumococcal activities of penicillin G, RP 59500, erythromycin, sparfloxacin, ciprofloxacin and vancomycin by using time-kill methodology. Antimicrob. Agents Chemother. 38:2065-2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pankuch, G. A., M. R. Jacobs, and P. C. Appelbaum. 1995. Activity of CP 99,219 compared to DU-6859a, ciprofloxacin, ofloxacin, levofloxacin, lomefloxacin, tosufloxacin, sparfloxacin and grepafloxacin against penicillin-susceptible and -resistant pneumococci. J. Antimicrob. Chemother. 35:230-232. [DOI] [PubMed] [Google Scholar]
- 27.Pankuch, G. A., M. R. Jacobs, and P. C. Appelbaum. 1995. Comparative activity of ampicillin, amoxycillin, amoxycillin/clavulanate and cefotaxime against 189 penicillin-susceptible and -resistant pneumococci. J. Antimicrob. Chemother. 35:883-888. [DOI] [PubMed] [Google Scholar]
- 28.Spangler, S. K., M. R. Jacobs, and P. C. Appelbaum. 1992. Susceptibilities of penicillin-susceptible and -resistant strains of Streptococcus pneumoniae to RP 59500, vancomycin, erythromycin, PD 131628, sparfloxacin, temafloxacin, Win 57273, ofloxacin, and ciprofloxacin. Antimicrob. Agents Chemother. 36:856-859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Spangler, S. K., M. R. Jacobs, G. A. Pankuch, and P. C. Appelbaum. 1993. Susceptibility of 170 penicillin-susceptible and -resistant pneumococci to six oral cephalosporins, four quinolones, desacetylcefotaxime, Ro 23-9424 and RP 67829. J. Antimicrob. Chemother. 31:273-280. [DOI] [PubMed] [Google Scholar]
- 30.Visalli, M. A., M. R. Jacobs, and P. C. Appelbaum. 1996. MIC and time-kill study of DU-6859a, ciprofloxacin, levofloxacin, sparfloxacin, cefotaxime, imipenem, and vancomycin against nine penicillin-susceptible and -resistant pneumococci. Antimicrob. Agents Chemother. 40:362-366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Visalli, M. A., M. R. Jacobs, and P. C. Appelbaum. 1997. Antipneumococcal activity of BAY 12-8039, a new quinolone, compared with activities of three other quinolones and four β-lactams, Antimicrob. Agents Chemother. 41:2786-2789. [DOI] [PMC free article] [PubMed] [Google Scholar]