Abstract
In the title host–guest compound, C38H26O2·C4H8O2, the ethyl acetate molecule (guest), which adopts a fully extended conformation, and the biphenyl derivative (host) are connected via O—H⋯O hydrogen bonds [H⋯O = 1.90 (3) Å] into discrete assemblies. The hydrocarbon skeleton of the host molecule deviates only slightly from C2 symmetry. The OH groups of the host are involved in intramolecular O—H⋯O hydrogen bonding [H⋯O = 1.83 (3) Å].
Related literature
For related literature, see: Barbour et al. (1993 ▶); Ibragimov et al. (2001 ▶); Sardone (1996 ▶); Sumarna et al. (2003 ▶); Weber et al. (1993 ▶).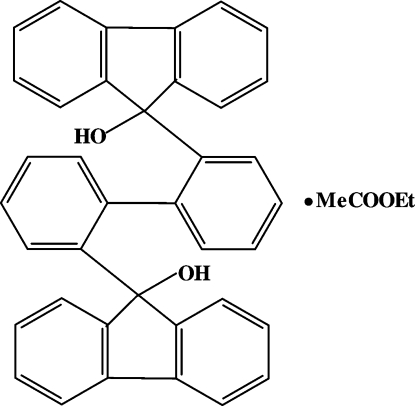
Experimental
Crystal data
C38H26O2·C4H8O2
M r = 602.69
Monoclinic,

a = 11.645 (2) Å
b = 16.364 (3) Å
c = 17.471 (3) Å
β = 97.72 (3)°
V = 3299.1 (10) Å3
Z = 4
Mo Kα radiation
μ = 0.08 mm−1
T = 293 (2) K
0.4 × 0.2 × 0.2 mm
Data collection
Stoe STADI4 diffractometer
Absorption correction: none
5807 measured reflections
5650 independent reflections
3654 reflections with I > 2σ(I)
3 standard reflections every 100 reflections intensity decay: 2.6%
Refinement
R[F 2 > 2σ(F 2)] = 0.068
wR(F 2) = 0.141
S = 1.20
5650 reflections
424 parameters
H atoms treated by a mixture of independent and constrained refinement
Δρmax = 0.24 e Å−3
Δρmin = −0.16 e Å−3
Data collection: STADI4 (Stoe & Cie, 1997 ▶); cell refinement: STADI4; data reduction: X-RED (Stoe & Cie, 1997 ▶); program(s) used to solve structure: SHELXS97 (Sheldrick, 2008 ▶); program(s) used to refine structure: SHELXL97 (Sheldrick, 2008 ▶); molecular graphics: XP (Siemens, 1994 ▶); software used to prepare material for publication: SHELXL97.
Supplementary Material
Crystal structure: contains datablocks I, global. DOI: 10.1107/S1600536808023271/gk2155sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536808023271/gk2155Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Table 1. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| O2—H1⋯O3i | 0.88 (4) | 1.90 (4) | 2.779 (3) | 173 (4) |
| O1—H2⋯O2 | 0.93 (4) | 1.84 (4) | 2.739 (3) | 161 (3) |
Symmetry code: (i)  .
.
supplementary crystallographic information
Comment
Crystalline inclusion compounds (clathrates, host–guest complexes) are of increasing importance in supramolecular chemistry because of their significant potential in addressing a variety of fundamental and practical issues. 2,2'-Bis(9-hydroxy-9-fluorenyl)biphenyl (I) is a host compound with good clathrate-forming ability and the crystal structures of its inclusion compounds with acetonitrile, cyclohexanone, n-propylamine (Barbour et al., 1993), acetone (three solvates) (Sardone, 1996; Ibragimov et al., 2001) and chloroform (two solvates) (Sumarna et al., 2003) were reported. Here, we report the crystal structure of a host–guest complex of (I) with ethyl acetate which resembles closely that of (I) with acetone (1/1) (Sardone, 1996). The molecule of (I) has three conformational degrees of freedom (rotation around the central aryl–aryl single bond and rotations around the aryl–fluorenyl bonds), however it exhibits considerable conformational rigidity due to the stabilizing effect of the intramolecular O—H···O hydrogen bond between the hydroxyl groups (Fig. 1, Table 1). The crystal packing is mainly stabilized by van der Waals forces (Fig.2).
Experimental
2,2'-Bis(9-hydroxy-9-fluorenyl)biphenyl was synthesized according to the procedure described by Weber et al., (1993). The stable in the air crystals were grown by slow evaporation from ethyl acetate solution.
Refinement
H atoms from the OH groups were located from difference Fourier maps and fully refined. The remaining H atoms were positioned geometrically (C—H 0.93–0.98 Å) and refined as riding on their carrier atoms with Uiso(H) = 1.2Ueq(C), except the methyl groups where Uiso(H) = 1.5Ueq(C).
Figures
Fig. 1.
Perspective view of the title compound, showing 30% probability displacement ellipsoids for the non-H atoms. Dashed lines represent hydrogen bonds.
Fig. 2.
Packing diagram of the title compound (I) viewed down the a axis. H atoms have been ommited for clarity. Hydrogen bonds are shown as dashed lines.
Crystal data
| C38H26O2·C4H8O2 | F000 = 1272 |
| Mr = 602.69 | Dx = 1.213 Mg m−3 |
| Monoclinic, P21/c | Mo Kα radiation λ = 0.71073 Å |
| Hall symbol: -P 2ybc | Cell parameters from 25 reflections |
| a = 11.645 (2) Å | θ = 10–20º |
| b = 16.364 (3) Å | µ = 0.08 mm−1 |
| c = 17.471 (3) Å | T = 293 (2) K |
| β = 97.72 (3)º | Block, colourless |
| V = 3299.1 (10) Å3 | 0.4 × 0.2 × 0.2 mm |
| Z = 4 |
Data collection
| Stoe STADI4 diffractometer | Rint = 0.0000 |
| Radiation source: fine-focus sealed tube | θmax = 25.0º |
| Monochromator: graphite | θmin = 2.6º |
| T = 293(2) K | h = −13→11 |
| ω/2θ scans | k = 0→19 |
| Absorption correction: none | l = 0→20 |
| 5650 measured reflections | 3 standard reflections |
| 5807 independent reflections | every 100 reflections |
| 3654 reflections with I > 2σ(I) | intensity decay: 2.6% |
Refinement
| Refinement on F2 | Hydrogen site location: inferred from neighbouring sites |
| Least-squares matrix: full | H atoms treated by a mixture of independent and constrained refinement |
| R[F2 > 2σ(F2)] = 0.068 | w = 1/[σ2(Fo2) + (0.0281P)2 + 2.047P] where P = (Fo2 + 2Fc2)/3 |
| wR(F2) = 0.141 | (Δ/σ)max < 0.001 |
| S = 1.20 | Δρmax = 0.24 e Å−3 |
| 5650 reflections | Δρmin = −0.16 e Å−3 |
| 424 parameters | Extinction correction: SHELXL97 (Sheldrick, 2008), Fc*=kFc[1+0.001xFc2λ3/sin(2θ)]-1/4 |
| Primary atom site location: structure-invariant direct methods | Extinction coefficient: 0.0048 (4) |
| Secondary atom site location: difference Fourier map |
Special details
| Geometry. All e.s.d.'s (except the e.s.d. in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell e.s.d.'s are taken into account individually in the estimation of e.s.d.'s in distances, angles and torsion angles; correlations between e.s.d.'s in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell e.s.d.'s is used for estimating e.s.d.'s involving l.s. planes. |
| Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F2, conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > σ(F2) is used only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2 are statistically about twice as large as those based on F, and R- factors based on ALL data will be even larger. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| O1 | 0.70308 (18) | 0.69051 (14) | 0.20949 (13) | 0.0518 (6) | |
| O2 | 0.63649 (17) | 0.85042 (13) | 0.21819 (12) | 0.0470 (5) | |
| C1 | 0.8676 (3) | 0.62161 (19) | 0.1739 (2) | 0.0511 (8) | |
| C2 | 0.8325 (3) | 0.5837 (2) | 0.1044 (2) | 0.0716 (11) | |
| H2A | 0.7765 | 0.6073 | 0.0681 | 0.086* | |
| C3 | 0.8827 (4) | 0.5090 (3) | 0.0897 (3) | 0.0937 (14) | |
| H3A | 0.8596 | 0.4822 | 0.0432 | 0.112* | |
| C4 | 0.9657 (5) | 0.4747 (3) | 0.1430 (4) | 0.0995 (17) | |
| H4A | 0.9982 | 0.4248 | 0.1321 | 0.119* | |
| C5 | 1.0019 (3) | 0.5126 (3) | 0.2122 (3) | 0.0828 (13) | |
| H5A | 1.0588 | 0.4889 | 0.2478 | 0.099* | |
| C6 | 0.9523 (3) | 0.5867 (2) | 0.2280 (2) | 0.0616 (10) | |
| C7 | 0.9683 (3) | 0.6387 (2) | 0.2964 (2) | 0.0633 (10) | |
| C8 | 1.0404 (4) | 0.6306 (3) | 0.3666 (3) | 0.0891 (14) | |
| H8A | 1.0925 | 0.5874 | 0.3749 | 0.107* | |
| C9 | 1.0330 (5) | 0.6877 (4) | 0.4232 (3) | 0.1026 (18) | |
| H9A | 1.0814 | 0.6831 | 0.4698 | 0.123* | |
| C10 | 0.9559 (4) | 0.7516 (3) | 0.4126 (2) | 0.0903 (14) | |
| H10A | 0.9510 | 0.7885 | 0.4526 | 0.108* | |
| C11 | 0.8848 (3) | 0.7614 (2) | 0.3424 (2) | 0.0701 (10) | |
| H11A | 0.8334 | 0.8050 | 0.3343 | 0.084* | |
| C12 | 0.8930 (3) | 0.7041 (2) | 0.28501 (19) | 0.0545 (9) | |
| C13 | 0.8229 (3) | 0.70131 (18) | 0.20394 (17) | 0.0460 (7) | |
| C14 | 0.8464 (3) | 0.77519 (18) | 0.15426 (17) | 0.0434 (7) | |
| C15 | 0.9585 (3) | 0.8074 (2) | 0.16490 (18) | 0.0527 (8) | |
| H15A | 1.0139 | 0.7835 | 0.2013 | 0.063* | |
| C16 | 0.9899 (3) | 0.8735 (2) | 0.1234 (2) | 0.0592 (9) | |
| H16A | 1.0655 | 0.8931 | 0.1315 | 0.071* | |
| C17 | 0.9087 (3) | 0.9100 (2) | 0.07009 (19) | 0.0597 (9) | |
| H17A | 0.9284 | 0.9551 | 0.0422 | 0.072* | |
| C18 | 0.7978 (3) | 0.87899 (19) | 0.05842 (18) | 0.0524 (8) | |
| H18A | 0.7434 | 0.9038 | 0.0219 | 0.063* | |
| C19 | 0.7638 (3) | 0.81209 (18) | 0.09903 (16) | 0.0433 (7) | |
| C20 | 0.6441 (3) | 0.77988 (17) | 0.06964 (16) | 0.0423 (7) | |
| C21 | 0.6384 (3) | 0.73514 (19) | 0.00138 (17) | 0.0523 (8) | |
| H21A | 0.7072 | 0.7234 | −0.0180 | 0.063* | |
| C22 | 0.5355 (3) | 0.7074 (2) | −0.03899 (18) | 0.0584 (9) | |
| H22A | 0.5351 | 0.6771 | −0.0840 | 0.070* | |
| C23 | 0.4340 (3) | 0.7258 (2) | −0.01101 (18) | 0.0578 (9) | |
| H23A | 0.3637 | 0.7078 | −0.0371 | 0.069* | |
| C24 | 0.4363 (3) | 0.77059 (19) | 0.05528 (18) | 0.0514 (8) | |
| H24A | 0.3665 | 0.7831 | 0.0730 | 0.062* | |
| C25 | 0.5396 (3) | 0.79833 (17) | 0.09746 (16) | 0.0414 (7) | |
| C26 | 0.5264 (2) | 0.84892 (18) | 0.16933 (16) | 0.0424 (7) | |
| C27 | 0.4832 (3) | 0.93557 (18) | 0.14954 (17) | 0.0470 (8) | |
| C28 | 0.5316 (3) | 0.9954 (2) | 0.10912 (19) | 0.0614 (9) | |
| H28A | 0.5993 | 0.9855 | 0.0878 | 0.074* | |
| C29 | 0.4774 (4) | 1.0712 (2) | 0.1008 (2) | 0.0726 (11) | |
| H29A | 0.5088 | 1.1124 | 0.0733 | 0.087* | |
| C30 | 0.3779 (4) | 1.0858 (2) | 0.1327 (2) | 0.0762 (12) | |
| H30A | 0.3433 | 1.1370 | 0.1270 | 0.091* | |
| C31 | 0.3284 (3) | 1.0264 (2) | 0.1728 (2) | 0.0682 (10) | |
| H31A | 0.2605 | 1.0367 | 0.1938 | 0.082* | |
| C32 | 0.3816 (3) | 0.9507 (2) | 0.18140 (17) | 0.0511 (8) | |
| C33 | 0.3506 (3) | 0.8771 (2) | 0.22174 (17) | 0.0503 (8) | |
| C34 | 0.2593 (3) | 0.8615 (3) | 0.2634 (2) | 0.0678 (11) | |
| H34A | 0.2048 | 0.9018 | 0.2692 | 0.081* | |
| C35 | 0.2510 (3) | 0.7854 (3) | 0.2959 (2) | 0.0734 (12) | |
| H35A | 0.1899 | 0.7743 | 0.3235 | 0.088* | |
| C36 | 0.3315 (3) | 0.7256 (3) | 0.28821 (19) | 0.0673 (10) | |
| H36A | 0.3241 | 0.6746 | 0.3105 | 0.081* | |
| C37 | 0.4236 (3) | 0.7407 (2) | 0.24757 (18) | 0.0548 (9) | |
| H37A | 0.4785 | 0.7005 | 0.2425 | 0.066* | |
| C38 | 0.4321 (3) | 0.81679 (19) | 0.21478 (16) | 0.0451 (8) | |
| C39 | 0.5324 (3) | 0.5281 (3) | 0.1162 (2) | 0.0869 (13) | |
| H39A | 0.5772 | 0.4805 | 0.1328 | 0.130* | |
| H39B | 0.5655 | 0.5751 | 0.1437 | 0.130* | |
| H39C | 0.5330 | 0.5360 | 0.0618 | 0.130* | |
| C40 | 0.4118 (4) | 0.5169 (2) | 0.1319 (2) | 0.0696 (11) | |
| C41 | 0.2259 (3) | 0.5774 (3) | 0.1255 (3) | 0.0903 (13) | |
| H41A | 0.2232 | 0.5760 | 0.1807 | 0.108* | |
| H41B | 0.1883 | 0.5287 | 0.1026 | 0.108* | |
| C42 | 0.1664 (4) | 0.6520 (3) | 0.0910 (3) | 0.0979 (15) | |
| H42A | 0.0869 | 0.6516 | 0.1003 | 0.147* | |
| H42B | 0.1695 | 0.6527 | 0.0364 | 0.147* | |
| H42C | 0.2043 | 0.6997 | 0.1142 | 0.147* | |
| O3 | 0.3748 (3) | 0.45679 (18) | 0.15903 (17) | 0.0996 (10) | |
| O4 | 0.3455 (2) | 0.58069 (16) | 0.11015 (16) | 0.0795 (8) | |
| H2 | 0.677 (3) | 0.741 (2) | 0.223 (2) | 0.092 (14)* | |
| H1 | 0.627 (3) | 0.883 (2) | 0.257 (2) | 0.098 (15)* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| O1 | 0.0430 (13) | 0.0475 (14) | 0.0667 (15) | −0.0008 (11) | 0.0142 (11) | 0.0074 (12) |
| O2 | 0.0447 (13) | 0.0490 (13) | 0.0467 (13) | 0.0032 (10) | 0.0036 (10) | −0.0076 (11) |
| C1 | 0.0485 (19) | 0.0426 (19) | 0.065 (2) | −0.0026 (16) | 0.0176 (17) | 0.0049 (17) |
| C2 | 0.082 (3) | 0.051 (2) | 0.084 (3) | 0.002 (2) | 0.018 (2) | −0.008 (2) |
| C3 | 0.119 (4) | 0.057 (3) | 0.112 (4) | 0.005 (3) | 0.040 (3) | −0.018 (3) |
| C4 | 0.107 (4) | 0.050 (3) | 0.155 (5) | 0.011 (3) | 0.068 (4) | 0.007 (3) |
| C5 | 0.063 (3) | 0.059 (3) | 0.132 (4) | 0.013 (2) | 0.037 (3) | 0.035 (3) |
| C6 | 0.050 (2) | 0.048 (2) | 0.089 (3) | 0.0038 (17) | 0.021 (2) | 0.021 (2) |
| C7 | 0.049 (2) | 0.066 (2) | 0.074 (3) | −0.0098 (19) | 0.0057 (19) | 0.030 (2) |
| C8 | 0.071 (3) | 0.097 (4) | 0.094 (3) | −0.016 (3) | −0.007 (3) | 0.050 (3) |
| C9 | 0.104 (4) | 0.126 (5) | 0.071 (3) | −0.048 (4) | −0.016 (3) | 0.042 (3) |
| C10 | 0.099 (4) | 0.116 (4) | 0.054 (3) | −0.047 (3) | 0.007 (2) | 0.004 (3) |
| C11 | 0.071 (3) | 0.079 (3) | 0.061 (2) | −0.021 (2) | 0.011 (2) | 0.002 (2) |
| C12 | 0.053 (2) | 0.057 (2) | 0.053 (2) | −0.0129 (18) | 0.0085 (16) | 0.0087 (17) |
| C13 | 0.0390 (18) | 0.0460 (18) | 0.0540 (19) | −0.0014 (14) | 0.0100 (14) | 0.0024 (15) |
| C14 | 0.0471 (19) | 0.0391 (17) | 0.0466 (18) | −0.0027 (14) | 0.0155 (15) | −0.0051 (14) |
| C15 | 0.047 (2) | 0.056 (2) | 0.055 (2) | −0.0029 (17) | 0.0090 (16) | −0.0008 (17) |
| C16 | 0.056 (2) | 0.063 (2) | 0.063 (2) | −0.0176 (18) | 0.0205 (18) | −0.0044 (19) |
| C17 | 0.073 (3) | 0.053 (2) | 0.056 (2) | −0.0111 (19) | 0.0217 (19) | 0.0012 (17) |
| C18 | 0.061 (2) | 0.048 (2) | 0.0491 (19) | −0.0008 (17) | 0.0138 (16) | 0.0026 (16) |
| C19 | 0.0537 (19) | 0.0388 (17) | 0.0392 (16) | 0.0013 (15) | 0.0131 (14) | −0.0020 (14) |
| C20 | 0.0490 (19) | 0.0391 (17) | 0.0389 (16) | 0.0011 (14) | 0.0066 (14) | 0.0019 (13) |
| C21 | 0.063 (2) | 0.050 (2) | 0.0453 (18) | 0.0062 (17) | 0.0116 (16) | −0.0004 (15) |
| C22 | 0.083 (3) | 0.051 (2) | 0.0395 (18) | −0.0038 (19) | 0.0034 (18) | −0.0082 (16) |
| C23 | 0.066 (2) | 0.059 (2) | 0.0458 (19) | −0.0139 (18) | −0.0034 (17) | −0.0015 (17) |
| C24 | 0.051 (2) | 0.054 (2) | 0.0489 (19) | −0.0045 (16) | 0.0051 (16) | 0.0017 (16) |
| C25 | 0.0466 (19) | 0.0363 (16) | 0.0411 (16) | −0.0015 (14) | 0.0054 (14) | 0.0038 (13) |
| C26 | 0.0416 (18) | 0.0440 (18) | 0.0418 (17) | 0.0010 (14) | 0.0059 (14) | −0.0029 (14) |
| C27 | 0.055 (2) | 0.0431 (18) | 0.0416 (17) | 0.0042 (15) | 0.0021 (15) | −0.0021 (15) |
| C28 | 0.078 (3) | 0.051 (2) | 0.057 (2) | 0.0031 (19) | 0.0170 (19) | −0.0005 (17) |
| C29 | 0.110 (3) | 0.046 (2) | 0.061 (2) | 0.006 (2) | 0.008 (2) | 0.0046 (18) |
| C30 | 0.113 (4) | 0.055 (2) | 0.058 (2) | 0.033 (2) | 0.003 (2) | −0.0008 (19) |
| C31 | 0.077 (3) | 0.072 (3) | 0.056 (2) | 0.029 (2) | 0.0095 (19) | 0.002 (2) |
| C32 | 0.053 (2) | 0.055 (2) | 0.0449 (18) | 0.0139 (17) | 0.0031 (15) | −0.0025 (16) |
| C33 | 0.0410 (19) | 0.067 (2) | 0.0424 (17) | 0.0075 (17) | 0.0030 (14) | −0.0042 (16) |
| C34 | 0.044 (2) | 0.102 (3) | 0.058 (2) | 0.012 (2) | 0.0087 (17) | 0.001 (2) |
| C35 | 0.052 (2) | 0.117 (4) | 0.052 (2) | −0.015 (2) | 0.0106 (18) | 0.007 (2) |
| C36 | 0.067 (2) | 0.083 (3) | 0.051 (2) | −0.018 (2) | 0.0041 (19) | 0.0135 (19) |
| C37 | 0.056 (2) | 0.055 (2) | 0.053 (2) | −0.0053 (17) | 0.0055 (17) | 0.0032 (16) |
| C38 | 0.0438 (18) | 0.052 (2) | 0.0393 (17) | −0.0020 (15) | 0.0046 (14) | −0.0004 (15) |
| C39 | 0.072 (3) | 0.086 (3) | 0.099 (3) | 0.003 (2) | −0.003 (2) | 0.013 (3) |
| C40 | 0.086 (3) | 0.062 (3) | 0.057 (2) | −0.009 (2) | −0.004 (2) | 0.015 (2) |
| C41 | 0.072 (3) | 0.103 (4) | 0.100 (3) | −0.019 (3) | 0.027 (3) | 0.007 (3) |
| C42 | 0.071 (3) | 0.095 (3) | 0.130 (4) | 0.000 (3) | 0.024 (3) | −0.001 (3) |
| O3 | 0.119 (2) | 0.086 (2) | 0.091 (2) | −0.0147 (19) | 0.0023 (18) | 0.0400 (18) |
| O4 | 0.0705 (18) | 0.0692 (18) | 0.101 (2) | −0.0052 (14) | 0.0190 (15) | 0.0229 (15) |
Geometric parameters (Å, °)
| O1—C13 | 1.423 (3) | C22—C23 | 1.372 (5) |
| O1—H2 | 0.93 (4) | C22—H22A | 0.9300 |
| O2—C26 | 1.442 (3) | C23—C24 | 1.368 (4) |
| O2—H1 | 0.88 (4) | C23—H23A | 0.9300 |
| C1—C2 | 1.375 (5) | C24—C25 | 1.399 (4) |
| C1—C6 | 1.394 (4) | C24—H24A | 0.9300 |
| C1—C13 | 1.524 (4) | C25—C26 | 1.529 (4) |
| C2—C3 | 1.394 (5) | C26—C27 | 1.528 (4) |
| C2—H2A | 0.9300 | C26—C38 | 1.532 (4) |
| C3—C4 | 1.369 (6) | C27—C28 | 1.372 (4) |
| C3—H3A | 0.9300 | C27—C32 | 1.396 (4) |
| C4—C5 | 1.373 (6) | C28—C29 | 1.391 (5) |
| C4—H4A | 0.9300 | C28—H28A | 0.9300 |
| C5—C6 | 1.388 (5) | C29—C30 | 1.372 (5) |
| C5—H5A | 0.9300 | C29—H29A | 0.9300 |
| C6—C7 | 1.457 (5) | C30—C31 | 1.370 (5) |
| C7—C12 | 1.382 (5) | C30—H30A | 0.9300 |
| C7—C8 | 1.397 (5) | C31—C32 | 1.384 (4) |
| C8—C9 | 1.370 (6) | C31—H31A | 0.9300 |
| C8—H8A | 0.9300 | C32—C33 | 1.464 (4) |
| C9—C10 | 1.375 (7) | C33—C38 | 1.386 (4) |
| C9—H9A | 0.9300 | C33—C34 | 1.390 (4) |
| C10—C11 | 1.394 (5) | C34—C35 | 1.378 (5) |
| C10—H10A | 0.9300 | C34—H34A | 0.9300 |
| C11—C12 | 1.385 (5) | C35—C36 | 1.374 (5) |
| C11—H11A | 0.9300 | C35—H35A | 0.9300 |
| C12—C13 | 1.537 (4) | C36—C37 | 1.386 (5) |
| C13—C14 | 1.534 (4) | C36—H36A | 0.9300 |
| C14—C15 | 1.396 (4) | C37—C38 | 1.379 (4) |
| C14—C19 | 1.404 (4) | C37—H37A | 0.9300 |
| C15—C16 | 1.378 (4) | C39—C40 | 1.478 (5) |
| C15—H15A | 0.9300 | C39—H39A | 0.9600 |
| C16—C17 | 1.372 (5) | C39—H39B | 0.9600 |
| C16—H16A | 0.9300 | C39—H39C | 0.9600 |
| C17—C18 | 1.377 (4) | C40—O3 | 1.197 (4) |
| C17—H17A | 0.9300 | C40—O4 | 1.323 (4) |
| C18—C19 | 1.391 (4) | C41—O4 | 1.454 (4) |
| C18—H18A | 0.9300 | C41—C42 | 1.491 (5) |
| C19—C20 | 1.514 (4) | C41—H41A | 0.9700 |
| C20—C21 | 1.393 (4) | C41—H41B | 0.9700 |
| C20—C25 | 1.403 (4) | C42—H42A | 0.9600 |
| C21—C22 | 1.383 (4) | C42—H42B | 0.9600 |
| C21—H21A | 0.9300 | C42—H42C | 0.9600 |
| C13—O1—H2 | 106 (2) | C24—C23—H23A | 120.0 |
| C26—O2—H1 | 106 (3) | C22—C23—H23A | 120.0 |
| C2—C1—C6 | 120.9 (3) | C23—C24—C25 | 122.5 (3) |
| C2—C1—C13 | 128.0 (3) | C23—C24—H24A | 118.7 |
| C6—C1—C13 | 111.1 (3) | C25—C24—H24A | 118.7 |
| C1—C2—C3 | 118.5 (4) | C24—C25—C20 | 118.2 (3) |
| C1—C2—H2A | 120.8 | C24—C25—C26 | 115.7 (3) |
| C3—C2—H2A | 120.8 | C20—C25—C26 | 126.1 (3) |
| C4—C3—C2 | 120.6 (5) | O2—C26—C27 | 110.9 (2) |
| C4—C3—H3A | 119.7 | O2—C26—C25 | 108.4 (2) |
| C2—C3—H3A | 119.7 | C27—C26—C25 | 112.5 (2) |
| C3—C4—C5 | 121.3 (4) | O2—C26—C38 | 110.0 (2) |
| C3—C4—H4A | 119.4 | C27—C26—C38 | 101.5 (2) |
| C5—C4—H4A | 119.4 | C25—C26—C38 | 113.4 (2) |
| C4—C5—C6 | 118.9 (4) | C28—C27—C32 | 120.5 (3) |
| C4—C5—H5A | 120.6 | C28—C27—C26 | 129.3 (3) |
| C6—C5—H5A | 120.6 | C32—C27—C26 | 110.2 (3) |
| C5—C6—C1 | 119.9 (4) | C27—C28—C29 | 118.6 (3) |
| C5—C6—C7 | 131.5 (4) | C27—C28—H28A | 120.7 |
| C1—C6—C7 | 108.6 (3) | C29—C28—H28A | 120.7 |
| C12—C7—C8 | 119.6 (4) | C30—C29—C28 | 120.5 (4) |
| C12—C7—C6 | 109.0 (3) | C30—C29—H29A | 119.7 |
| C8—C7—C6 | 131.4 (4) | C28—C29—H29A | 119.7 |
| C9—C8—C7 | 118.7 (5) | C31—C30—C29 | 121.4 (3) |
| C9—C8—H8A | 120.6 | C31—C30—H30A | 119.3 |
| C7—C8—H8A | 120.6 | C29—C30—H30A | 119.3 |
| C8—C9—C10 | 121.7 (5) | C30—C31—C32 | 118.5 (3) |
| C8—C9—H9A | 119.2 | C30—C31—H31A | 120.7 |
| C10—C9—H9A | 119.2 | C32—C31—H31A | 120.7 |
| C9—C10—C11 | 120.3 (5) | C31—C32—C27 | 120.4 (3) |
| C9—C10—H10A | 119.8 | C31—C32—C33 | 130.7 (3) |
| C11—C10—H10A | 119.8 | C27—C32—C33 | 108.8 (3) |
| C12—C11—C10 | 118.0 (4) | C38—C33—C34 | 119.7 (3) |
| C12—C11—H11A | 121.0 | C38—C33—C32 | 109.1 (3) |
| C10—C11—H11A | 121.0 | C34—C33—C32 | 131.1 (3) |
| C7—C12—C11 | 121.6 (3) | C35—C34—C33 | 118.9 (3) |
| C7—C12—C13 | 111.0 (3) | C35—C34—H34A | 120.6 |
| C11—C12—C13 | 127.4 (3) | C33—C34—H34A | 120.6 |
| O1—C13—C1 | 107.5 (2) | C36—C35—C34 | 121.1 (3) |
| O1—C13—C14 | 112.8 (2) | C36—C35—H35A | 119.4 |
| C1—C13—C14 | 112.6 (2) | C34—C35—H35A | 119.4 |
| O1—C13—C12 | 110.2 (2) | C35—C36—C37 | 120.5 (4) |
| C1—C13—C12 | 100.4 (3) | C35—C36—H36A | 119.7 |
| C14—C13—C12 | 112.7 (3) | C37—C36—H36A | 119.7 |
| C15—C14—C19 | 118.1 (3) | C38—C37—C36 | 118.5 (3) |
| C15—C14—C13 | 117.2 (3) | C38—C37—H37A | 120.7 |
| C19—C14—C13 | 124.8 (3) | C36—C37—H37A | 120.7 |
| C16—C15—C14 | 122.4 (3) | C37—C38—C33 | 121.2 (3) |
| C16—C15—H15A | 118.8 | C37—C38—C26 | 128.4 (3) |
| C14—C15—H15A | 118.8 | C33—C38—C26 | 110.3 (3) |
| C17—C16—C15 | 119.4 (3) | C40—C39—H39A | 109.5 |
| C17—C16—H16A | 120.3 | C40—C39—H39B | 109.5 |
| C15—C16—H16A | 120.3 | H39A—C39—H39B | 109.5 |
| C16—C17—C18 | 119.2 (3) | C40—C39—H39C | 109.5 |
| C16—C17—H17A | 120.4 | H39A—C39—H39C | 109.5 |
| C18—C17—H17A | 120.4 | H39B—C39—H39C | 109.5 |
| C17—C18—C19 | 122.7 (3) | O3—C40—O4 | 122.3 (4) |
| C17—C18—H18A | 118.7 | O3—C40—C39 | 125.3 (4) |
| C19—C18—H18A | 118.7 | O4—C40—C39 | 112.4 (3) |
| C18—C19—C14 | 118.3 (3) | O4—C41—C42 | 107.5 (3) |
| C18—C19—C20 | 114.4 (3) | O4—C41—H41A | 110.2 |
| C14—C19—C20 | 126.7 (3) | C42—C41—H41A | 110.2 |
| C21—C20—C25 | 117.8 (3) | O4—C41—H41B | 110.2 |
| C21—C20—C19 | 114.2 (3) | C42—C41—H41B | 110.2 |
| C25—C20—C19 | 127.6 (3) | H41A—C41—H41B | 108.5 |
| C22—C21—C20 | 123.2 (3) | C41—C42—H42A | 109.5 |
| C22—C21—H21A | 118.4 | C41—C42—H42B | 109.5 |
| C20—C21—H21A | 118.4 | H42A—C42—H42B | 109.5 |
| C23—C22—C21 | 118.4 (3) | C41—C42—H42C | 109.5 |
| C23—C22—H22A | 120.8 | H42A—C42—H42C | 109.5 |
| C21—C22—H22A | 120.8 | H42B—C42—H42C | 109.5 |
| C24—C23—C22 | 120.0 (3) | C40—O4—C41 | 117.0 (3) |
Hydrogen-bond geometry (Å, °)
| D—H···A | D—H | H···A | D···A | D—H···A |
| O2—H1···O3i | 0.88 (4) | 1.90 (4) | 2.779 (3) | 173 (4) |
| O1—H2···O2 | 0.93 (4) | 1.84 (4) | 2.739 (3) | 161 (3) |
Symmetry codes: (i) −x+1, y+1/2, −z+1/2.
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: GK2155).
References
- Barbour, L. J., Bourne, S. A., Caira, M. R., Nassimbeni, L. R., Weber, E., Skobridis, E. K. & Wierig, A. (1993). Supramol. Chem.1, 331–336.
- Ibragimov, B. T., Beketov, K. M., Weber, E., Seidel, J., Sumarna, O., Makhkamov, K. K. & Kohnke, K. (2001). J. Phys. Org. Chem.14, 697–703.
- Sardone, N. (1996). Private communication (refcode NABNIN). CCDC, Union Road, Cambridge, England.
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Siemens (1994). XP Siemens Analytical X-ray Instruments Inc., Madison, Wisconsin, USA.
- Stoe & Cie (1997). STADI4 and X-RED Stoe & Cie, Darmstadt, Germany.
- Sumarna, O., Seidel, J., Weber, E., Seichter, W., Ibragimov, B. T. & Beketov, K. M. (2003). Cryst. Growth Des.3, 541–546.
- Weber, E., Skobridis, K., Wierig, A., Stathi, S., Nassimbeni, L. R. & Niven, M. L. (1993). Angew. Chem. Int. Ed. Engl.32, 606–608.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablocks I, global. DOI: 10.1107/S1600536808023271/gk2155sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536808023271/gk2155Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report




