Abstract
Peritoneal dialysate fluid (PDF) is a bacteriostatic medium that compromises the antibacterial activity of cell wall-active agents. By use of an in vitro static model, methicillin-resistant Staphylococcus aureus (MRSA), methicillin-susceptible S. aureus (MSSA), methicillin-susceptible Staphylococcus epidermidis (MSSE), and Streptococcus sanguis were exposed to daptomycin at concentrations of 10, 30, and 100 mg/liter, cefazolin at 125 mg/liter, and vancomycin at 25 mg/liter in cation-adjusted Mueller-Hinton Broth or Todd Hewitt Broth (for S. sanguis) and PDF at pHs of 5.5 and 7.4. The pH had no effect on antibacterial activity. Neither cefazolin nor vancomycin produced a bactericidal or a bacteriostatic effect versus MRSA, MSSA, MSSE, or S. sanguis in PDF, while all concentrations of daptomycin were bactericidal against all organisms in PDF. Daptomycin did not exhibit concentration-dependent activity in PDF. Daptomycin appears to be a promising agent for use in peritoneal dialysis-associated peritonitis, producing bacterial kill to a greater extent and at a higher rate than cefazolin or vancomycin in PDF.
Roughly 15% (∼20,000 to 30,000) of patients with end stage renal disease (ESRD) in the United States are managed with peritoneal dialysis (PD). This number is increasing by nearly 6,000 patients per year and is expected to increase even further in the face of increased economic pressure due to the lower cost of PD relative to hemodialysis (∼$32,000 versus $44,000/year) (18).Moreover, PD allows greater patient freedom and spares the body from large hemodynamic fluctuations when compared with hemodialysis (18).
The problem with PD has been, and continues to be, PD-associated peritonitis (PDAP). PDAP accounts for approximately 30 to 40% of all patient transfers to hemodialysis, represents the leading cause for hospitalization among patients on PD, and imposes a significant burden of morbidity and mortality (5, 6). Furthermore, several investigative groups have provided evidence suggesting that repeated or severe episodes of peritonitis may cause the peritoneal membrane to become unsuitable for dialysis, leading to technique failure, requiring either transfer to hemodialysis or transplantation to maintain life (4, 7, 13, 14).
The pathogens most commonly associated with peritonitis are gram positive. Approximately 28 to 32% of infections are caused by Staphylococcus epidermidis, 23 to 26% are caused by Staphylococcus aureus, and 20% are caused by other gram-positive species (5). Present empirical therapy for peritonitis typically includes administration of a cephalosporin, usually cefazolin, mixed in the peritoneal dialysate fluid (PDF). Vancomycin is used in the setting of known or suspected beta-lactam resistance (8). However, the bacteriostatic nature of PDF may compromise the antibacterial activity of such cell wall-active agents (2, 10-12, 15, 19, 21).
Daptomycin is a novel cyclic lipopeptide antibiotic with rapid, bactericidal activity against gram-positive pathogens (16). Daptomycin is under clinical investigation for the treatment of gram-positive bacterial infections, including those caused by isolates resistant to other antibacterial agents. Daptomycin potentially offers a novel strategy for use against PDAP due to a unique mechanism of action, low incidence of resistance, and lack of cross-resistance with other antibacterial classes.
The purpose of this investigation was to compare the activity of daptomycin with that of cefazolin and vancomycin against the most common pathogens in cases of PDAP in the context of PDF-induced growth suppression. By use of an in vitro model, methicillin-sensitive Staphylococcus aureus (MSSA), methicillin-resistant S. aureus (MRSA), methicillin-sensitive Staphylococcus epidermidis (MSSE), and Streptococcus sanguis were exposed to three concentrations of daptomycin and the standard concentrations of vancomycin and cefazolin used in the treatment of PDAP. Additionally, this study examined the effect of pH on antibiotic activity and the potential for growth suppression following exposure to PDF.
(This work was presented in part at the 43rd Interscience Conference on Antimicrobial Agents and Chemotherapy, Chicago, Ill., 14 to 17 September 2003.)
MATERIALS AND METHODS
Ninety-six concentration-time-kill curve experiments with cefazolin, vancomycin, or one of three concentrations of daptomycin against one strain each of MSSA, MRSA, MSSE, and S. sanguis were performed in duplicate.
In vitro model.
Concentration-time-kill curve experiments were conducted using a static in vitro model in order to emulate the conditions of PDF during an intraperitoneal dwell. The studies were performed using cation-adjusted Mueller-Hinton broth (CAMHB; Ca2+, 75 mg/liter; Mg2+, 25 mg/liter; Becton Dickinson Microbiology Systems, Sparks, Md.), or Todd Hewitt broth (THB; Becton Dickinson Microbiology Systems) for the Streptococcus isolate or commercially available 2.5% dextrose PDF (Baxter Dianeal PD-2; Baxter Healthcare Corp., Deerfield, Ill.) containing 25 g of dextrose/liter, 132 meq of Na+/liter, 3.5 meq of Ca2+/liter, 0.5 meq of Mg2+/liter, 96 meq of Cl−/liter, and 40 meq of lactate/liter, with no added amino acids, phosphate, or iron. Solution pH was adjusted, using sodium hydroxide or hydrogen chloride, to either 5.5, corresponding to the pH of freshly instilled PDF, or 7.4, corresponding to the pH of spent PDF following a 4-h intraperitoneal dwell. Forty milliliters of each solution was placed in a sterile, 50-ml, conical, screw-cap tube. Growth control experiments were conducted for each microorganism, at each pH, in both broth and PDF. In the experiments including antibiotic administration, an aliquot of bacteria was instilled into the tubes. Each experiment was run in duplicate for 24 h, and the models were placed in an incubator maintained at 37°C with CO2.
Bacteria.
American Type Culture Collection (ATCC) isolates of MSSA (ATCC 29213), MRSA (ATCC 33592), MSSE (ATCC 12228), and S. sanguis (ATCC 10556) were used in the experiments. Prior to the concentration-time-kill experiments, several colonies of each isolate were incubated overnight in 5 ml of CAMHB (or THB for S. sanguis). The overnight cultures were diluted 1:5 in fresh, warm CAMHB (or THB for S. sanguis) and incubated for approximately 30 min prior to the experiments in order to provide the organisms with optimal growth conditions. This bacterial suspension was then compared to a 0.5 McFarland standard to determine the amount necessary to add to the tube to achieve a starting inoculum of 105 to 106 CFU/ml, which is comparable to the bacterial burden associated with PDAP (11).
Susceptibility testing.
Susceptibilities to daptomycin, cefazolin, and vancomycin were tested in duplicate or triplicate for each isolate prior to concentration-time-kill experiments and for select isolates retrieved at 24 h postexposure. Susceptibility testing was performed by broth microdilution in CAMHB (plus 5% lysed horse blood for S. sanguis) according to NCCLS guidelines, with the exception of the calcium concentration (75 mg/liter). All isolates were subcultured onto fresh blood agar plates on at least three consecutive days prior to susceptibility testing.
Antibiotics.
Stock solutions of daptomycin (Cubist Pharmaceuticals, Lexington, Mass.), cefazolin (Eli Lilly & Co., Indianapolis, Ind.), and vancomycin (Eli Lilly & Company) were prepared using the appropriate amount of sterile distilled water and stored at −80°C until needed for individual experiments. Antibiotics were administered as bolus injections once during the 24-h period.
Pharmacokinetics.
Three concentrations of daptomycin, 10, 30, and 100 mg/liter, were used in this study for the purpose of exploring whether concentration-dependent activity would be evident in PDF. Typical intraperitoneal doses of cefazolin and vancomycin yield concentrations of 125 mg/liter and 20 to 50 mg/liter, respectively (8). Therefore, concentrations of 125 mg/liter for cefazolin and 25 mg/liter for vancomycin were simulated in the studies. Antibiotic concentrations were not corrected for protein binding because the reported concentration of protein in spent PDF, albeit in the absence of peritonitis, is between 0 and 3.5 g/dl (2, 11).
Pharmacodynamics.
At predetermined timed intervals, samples of CAMHB (or THB for S. sanguis) or PDF were removed from the models for quantification of the bacterial density by a spiral plating technique (Autoplate 4000; Spiral Biotech, Inc., Norwood, Mass.). In the experiments requiring antibiotic administration, antibiotic was not added until the 2-h time point in order to allow the PDF to exert its growth-suppressive effect on the organisms. Antibiotic carryover was minimized by saline dilution and/or spiral plating. Six samples, at 0, 2, 4, 6, 8, and 24 h, were removed for each 24-h experiment. Bacterial counts were determined by spiral plating 50 μl of the bacterial suspension onto Trypticase soy agar supplemented with 5% sheep blood.
After incubation for 18 to 36 h at 37°C with CO2, the numbers of CFU on each plate were counted visually. The theoretical lower limit of bacterial counting accuracy was 300 CFU/ml. Concentration-time-kill curves were constructed by plotting the log10 CFU per milliliter versus time, and estimates of the log10CFU per milliliter below our theoretical lower limit were included. Bactericidal activity was defined as a ≥3-log10 reduction in the bacterial burden. Time to 3-log10 kill (T3K) was obtained by visual inspection.
Statistical analysis.
Regression analysis could not be performed because a consistent method of analysis could not be applied to all three antibiotics. T3K was analyzed via two-tailed paired t test for statistical differences between the different solutions, pHs, and antibiotics. Significance was defined as a P value of <0.05. All graphing and statistical analyses were performed using Microsoft Excel 2000 (Microsoft Corp., Redmond, Wash.).
Post-PDF-exposure studies.
We conducted post-PDF-exposure studies in an attempt to elucidate the duration of growth suppression induced by PDF exposure after the microorganisms were transferred to growth-supporting media. These studies were modeled after the procedures commonly used to determine the presence of a postantibiotic effect (3). Specifically, 10 ml of CAMHB (or THB for S. sanguis) were inoculated with several colonies of each organism. Five serial 10-fold dilutions were made, followed by overnight incubation at 37°C with CO2. The overnight cultures were compared to a 0.5 McFarland standard, and 100 μl of the overnight culture that most closely approximated a 0.5 McFarland standard was inoculated into each 9.9-ml portion of CAMHB (or THB for S. sanguis) and PDF to achieve a starting inoculum of 106 CFU/ml. The bacterial suspensions were then incubated for 2 h at 37°C with CO2. Following incubation, 40 ml of fresh, warm CAMHB (or THB for S. sanguis) was inoculated with an appropriate amount of the incubated suspension, as determined via comparison to a 0.5 McFarland standard, to achieve an inoculum of 104 to 105 CFU/ml. Four samples were withdrawn at predetermined time intervals for bacterial density quantification by serial saline dilution. Bacterial counts were determined by a 1:10 serial dilution of 100 μl of medium into saline that was plated onto Trypticase soy agar supplemented with 5% sheep blood. After incubation for 18 to 36 h at 37°C with CO2, the colonies on each plate were counted visually, using the previously mentioned theoretical lower limit of bacterial counting accuracy. We defined the post-PDF-exposure effect (P-PDF-E) as T − C, where T is the time required for the CFU in the post-PDF-exposure culture to increase 1 log10 above the count at time zero and C is the time required for the CFU in the CAMHB (or THB for S. sanguis) culture to increase 1 log10 above the count at time zero. After equalization of the starting inocula, the times were calculated using a linear equation with the slope and the starting inoculum being the known variables.
RESULTS
MICs.
The preexposure MICs for MRSA, MSSA, MSSE, and S. sanguis were as follows: daptomycin, 1, 0.5, 0.5, and 1 mg/liter, respectively; cefazolin, 32, 0.25, 0.5, and 0.25 mg/liter, respectively; and vancomycin, 1, 1, 2, and 0.5 mg/liter, respectively. An eight-well increase (to 64 mg/liter) and a two-well increase (to 1 mg/liter) in cefazolin MICs were observed following exposure to cefazolin against MSSA in PDF and against S. sanguis in THB, respectively. No other changes in postexposure MICs were noted.
Post-PDF-exposure studies.
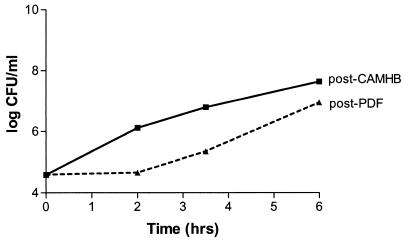
The P-PDF-E was most pronounced with MSSA, with a calculated value of 2.6 h (Fig. 1). The P-PDF-Es were 1.9 h for MRSA, 1.8 h for MSSE, and 0.8 h for S. sanguis. Since the most common exchange duration with PD is ∼4 h, this effect on bacterial growth may be of clinical significance.
FIG. 1.
Post-PDF-exposure growth (growth in CAMHB after a 2-h exposure to PDF) of MSSA compared to post-CAMHB-exposure growth (growth in CAMHB after a 2-h exposure to CAMHB).
Time-kill curves.
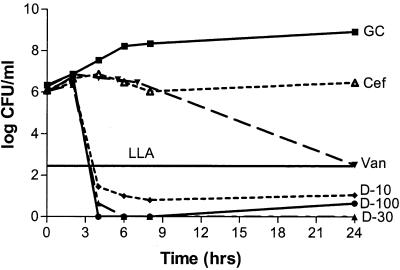
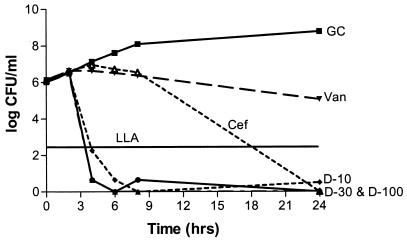
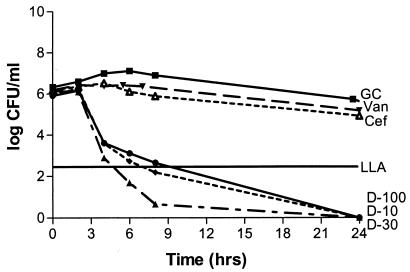
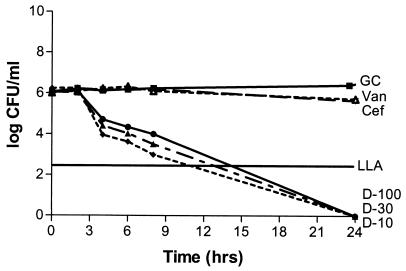
Solution pH did not produce a statistically significant effect on antibiotic activity (P, 0.07-to 0.634). Antibacterial activities were not statistically different for any of the three daptomycin concentrations with regard to the type of solution (P, 0.07-to 0.21), while a significant difference was noted with both vancomycin and cefazolin (P, 0.012 and 0.038, respectively). Cefazolin was bactericidal versus MSSA in CAMHB and S. sanguis in THB but not versus MRSA or MSSE in CAMHB. Vancomycin was bactericidal versus MRSA and MSSE in CAMHB but not against MSSA in CAMHB or S. sanguis in THB. All three concentrations of daptomycin were bactericidal against the four organisms in CAMHB (or THB for S. sanguis) at a higher rate than what was seen for cefazolin or vancomycin, except for the 10-mg/liter concentration versus S. sanguis, which resulted in a 2-log reduction. Regrowth occurred with daptomycin versus S. sanguis, possibly due to a lack of calcium supplementation in the THB, although the calcium concentration was above the manufacturer's recommended concentration of 50 mg/liter (Fig. 2 and 3). Neither cefazolin nor vancomycin produced a significant decrease in the starting inoculum of MRSA, MSSA, MSSE, or S. sanguis in PDF, while all three concentrations of daptomycin were bactericidal against all four organisms in PDF. Daptomycin did not appear to exhibit concentration-dependent activity in PDF (Fig. 4 and 5).
FIG. 2.
Daptomycin, vancomycin, and cefazolin against MRSA in CAMHB at a pH of 5.5. GC, growth control; D-10, daptomycin at 10 mg/liter; D-30, daptomycin at 30 mg/liter; D-100, daptomycin at 100 mg/liter; Van, vancomycin; Cef, cefazolin; LLA, lower limit of accuracy. Antibiotic was not added until the 2-h time point. Plotted data represent the means for duplicate simulations.
FIG. 3.
Daptomycin, vancomycin, and cefazolin against MSSA in CAMHB at a pH of 7.4. Antibiotic was not added until the 2-h time point. Plotted data represent the means for duplicate simulations. For abbreviations, see the legend to Fig. 2.
FIG. 4.
Daptomycin, vancomycin, and cefazolin against MRSA in PDF at a pH of 5.5. Antibiotic was not added until the 2-h time point. Plotted data represent the means for duplicate simulations. For abbreviations, see the legend to Fig. 2.
FIG. 5.
Daptomycin, vancomycin, and cefazolin against MSSA in PDF at a pH of 7.4. Antibiotic was not added until the 2-h time point. Plotted data represent the means for duplicate simulations. For abbreviations, see the legend to Fig. 2.
DISCUSSION
The United States healthcare system confronts a growing number of patients who require maintenance dialysis to sustain life. Presently, the dialysis population in the United States includes approximately 340,000 patients, and this number is anticipated to nearly double over the next 8 years (18). An aging population, a rising incidence of diabetes, and a longer life span on dialysis all contribute to both the recently observed and the future anticipated expansion of the dialysis population.
An important issue regarding the use of agents such as beta-lactams or vancomycin in the setting of PDAP relates to the growth pattern of bacteria in PDF. These cell wall-active agents require actively growing bacteria to exert their influence (1, 17). However, PDF has been shown to compromise the efficacy of cell wall-active agents, suggesting a bacteriostatic effect of PDF on the microorganisms, which may be clinically significant (2, 10-12, 15, 19, 21). The results of the post-PDF-exposure studies suggest that some of the difficulties encountered when treating PDAP may be due to the combination of the mechanisms of action of the antibiotics typically used to treat these infections, namely cefazolin and vancomycin, and bacterial growth suppression by PDF.
PDAP may represent an excellent use for daptomycin. Daptomycin exhibits rapid, bactericidal activity against most gram-positive organisms as well as a prolonged postantibiotic effect (16). Moreover, the results of this study suggest that daptomycin is active against bacteria in both a stationary or exponential growth phase. Although the protein binding of daptomycin in serum is high (∼90%) (16), drug sequestration due to albumin binding is likely less important in the PD setting. The intraperitoneal albumin concentration immediately after placement in the peritoneal cavity is extremely low (near zero) because the fresh dialysate does not contain albumin. The albumin concentration rises slowly during the dwell within the peritoneal cavity, a process that may be accelerated by peritonitis, but the concentration remains relatively low. Since antibiotic administration for PDAP is usually intraperitoneal, the low protein concentration in PDF increases the free concentration of the drug. Furthermore, intraperitoneal administration decreases the exposure of the extraperitoneal space to the drug, reducing the likelihood of adverse effects. Moreover, the antibacterial activity of daptomycin is dependent on the concentration of free calcium ions (9; N. S. Oliver, V. Laganas, M. Bouchard, I. B. Parr, and J. A. Silverman, Abstr. 41st Intersci. Conf. Antimicrob. Agents Chemother., abstr. 1801, 2001), and the concentration of calcium in PDF is high (∼70 mg/liter), which augments antimicrobial efficacy.
Daptomycin appears to be a promising agent for use in PDAP, producing bacterial kill to a greater extent and at a higher rate than cefazolin or vancomycin in PDF. The activity of daptomycin does not appear to depend on the metabolic activity of the bacteria, allowing the drug to exert a bactericidal effect in the bacteriostatic environment of PDF. The potential for daptomycin to exert a similar effect on bacteria in the metabolically less active, yet clinically important, sessile state remains to be determined, but the ability to kill in the stationary phase is provocative in this context. The duration of present treatment regimens for PDAP is usually 10 to 14 days (21 days recommended for S. aureus), with many patients needing treatment extensions (8). Recent studies have revealed an ominous increase in the incidence of antibiotic resistance, most notably among gram-positive organisms, in PD patients (20). More efficient bacterial killing may allow for shorter courses of therapy for PDAP, may prevent further development of antimicrobial resistance, and may prolong the use of the peritoneal membrane for PD by diminishing the time-weighted exposure of the membrane to inflammatory mediators (4). Based on the findings of this study, we are pursuing clinical testing of daptomycin in patients afflicted with PDAP.
Acknowledgments
This study was funded by a grant from Cubist Pharmaceuticals.
REFERENCES
- 1.Cozens, R. M., E. Tuomanen, W. Tosch, O. Zak, J. Suter, and A. Tomasz. 1986. Evaluation of the bactericidal activity of β-lactam antibiotics on slowly growing bacteria cultured in the chemostat. Antimicrob. Agents Chemother. 29:797-802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Craddock, C. F., R. Edwards, and R. G. Finch. 1987. Pseudomonas peritonitis in continuous ambulatory peritoneal dialysis: laboratory predictors of treatment failure. J. Hosp. Infect. 10:179-186. [DOI] [PubMed] [Google Scholar]
- 3.Craig, W. A., and S. Gudmundsson. 1996. Postantibiotic effect, p. 296-329. In V. Lorian (ed.), Antibiotics in laboratory medicine, 4th ed. Williams & Wilkins, Baltimore, Md.
- 4.Davies, S. J., J. Bryan, L. Phillips, and G. I. Russell. 1996. Longitudinal changes in peritoneal kinetics: the effects of peritoneal dialysis and peritonitis. Nephrol. Dial. Transplant. 11:498-506. [PubMed] [Google Scholar]
- 5.Finkelstein, E. S., J. Jekel, L. Troidle, N. Gorban-Brennan, F. O. Finkelstein, and F. J. Bia. 2002. Patterns of infection in patients maintained on long-term peritoneal dialysis therapy with multiple episodes of peritonitis. Am. J. Kidney Dis. 39:1278-1286. [DOI] [PubMed] [Google Scholar]
- 6.Fried, L. F., J. Bernardini, J. R. Johnston, and B. Piraino. 1996. Peritonitis influences mortality in peritoneal dialysis patients. J. Am. Soc. Nephrol. 7:2176-2182. [DOI] [PubMed] [Google Scholar]
- 7.Huarte-Loza, E., R. Selgas, A. R. Carmona, M. E. Martinez, J. Munoz, M. P. Fontan, O. Ortega, F. Escuin, and L. S. Sicilia. 1987. Peritoneal membrane failure as a determinant of the CAPD future. Contrib. Nephrol. 57:219-229. [DOI] [PubMed] [Google Scholar]
- 8.Keane, W. F., G. R. Bailie, E. Boeschoten, R. Gokal, T. A. Golper, C. J. Holmes, Y. Kawaguchi, B. Piraino, M. Riella, and S. Vas. 2000. Adult peritoneal dialysis-related peritonitis treatment recommendations: 2000 update. Perit. Dial. Int. 20:396-411. [PubMed] [Google Scholar]
- 9.Lamp, K. C., and M. J. Rybak. 1993. Teicoplanin and daptomycin bactericidal activities in the presence of albumin or serum under controlled conditions of pH and ionized calcium. Antimicrob. Agents Chemother. 37:605-609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ludlam, H., L. Johnston, and P. Hopkins. 1992. Susceptibility testing of bacteria recovered from patients with peritonitis complicating continuous ambulatory peritoneal dialysis. Antimicrob. Agents Chemother. 36:1097-1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.MacDonald, W. A., J. Watts, and M. I. Bowmer. 1986. Factors affecting Staphylococcus epidermidis growth in peritoneal dialysis solutions. J. Clin. Microbiol. 24:104-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morton, A. R., G. Evans, A. Shannon, and D. Zoutman. 1994. Growth of coagulase negative staphylococci in peritoneal dialysate fluid. Effect of calcium concentration. ASAIO J. 40:M431-M434. [DOI] [PubMed] [Google Scholar]
- 13.Ota, K., M. Mineshima, N. Watanabe, and S. Naganuma. 1987. Functional deterioration of the peritoneum: does it occur in the absence of peritonitis? Nephrol. Dial. Transplant. 2:30-33. [PubMed] [Google Scholar]
- 14.Selgas, R., M. J. Fernandez-Reyes, E. Bosque, M. A. Bajo, F. Borrego, C. Jimenez, G. Del Peso, and F. De Alvaro. 1994. Functional longevity of the human peritoneum: how long is continuous peritoneal dialysis possible? Results of a prospective medium long-term study. Am. J. Kidney Dis. 23:64-73. [DOI] [PubMed] [Google Scholar]
- 15.Shalit, I., D. F. Welch, V. H. San Joaquin, and M. I. Marks. 1985. In vitro antibacterial activities of antibiotics against Pseudomonas aeruginosa in peritoneal dialysis fluid. Antimicrob. Agents Chemother. 27:908-911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tally, F. P., and M. F. DeBruin. 2000. Development of daptomycin for gram-positive infections. J. Antimicrob. Chemother. 46:523-526. [DOI] [PubMed] [Google Scholar]
- 17.Tuomanen, E., R. Cozens, W. Tosch, O. Zak, and A. Tomasz. 1986. The rate of killing of Escherichia coli by beta-lactam antibiotics is strictly proportional to the rate of bacterial growth. J. Gen. Microbiol. 132:1297-1304. [DOI] [PubMed] [Google Scholar]
- 18.United States Renal Data System Coordinating Center. 2002. USRDS annual data report. United States Renal Data System Coordinating Center, Minneapolis, Minn. [Online.]
- 19.Wilcox, M. H., I. Geary, and R. C. Spencer. 1992. In-vitro activity of imipenem, in comparison with cefuroxime and ciprofloxacin, against coagulase-negative staphylococci in broth and peritoneal dialysis fluid. J. Antimicrob. Chemother. 29:49-55. [DOI] [PubMed] [Google Scholar]
- 20.Zelenitsky, S., L. Barns, I. Findlay, M. Alfa, R. Ariano, A. Fine, and G. Harding. 2000. Analysis of microbiological trends in peritoneal dialysis-related peritonitis from 1991 to 1998. Am. J. Kidney Dis. 36:1009-1013. [DOI] [PubMed] [Google Scholar]
- 21.Zelenitsky, S., C. Franczuk, A. Fine, R. Ariano, and G. Harding. 2002. Antibiotic tolerance of peritoneal bacterial isolates in dialysis fluids. J. Antimicrob. Chemother. 49:863-866. [DOI] [PubMed] [Google Scholar]