Abstract
Background: The objective of this retrospective study was to determine whether differences in survival exist between women with de novo stage IV and relapsed breast cancer.
Patients and methods: Three thousand five hundred and twenty-four women with de novo stage IV or relapsed breast cancer diagnosed from 1992 to 2007 were identified. Disease-free interval (DFI) was defined as the time from the diagnosis of primary nonmetastatic breast cancer to the date of the first distant metastases. Kaplan–Meier product limit method was used to estimate overall survival (OS). Cox proportional hazards model was fitted to determine the association between metastatic disease (relapsed versus de novo) and OS after controlling for other patient/tumor characteristics.
Results: Six hundred and forty-three (18.2%) women had de novo stage IV disease and 2881 (81.8%) had relapsed disease. Median follow-up was 19 months. Median OS among patients with de novo stage IV and relapsed disease was 39.2 and 27.2 months, respectively (P < 0.0001). In the multivariable model, women with relapsed disease had an increased risk of death compared with patients with de novo disease (HR = 1.75, 95% confidence interval 1.47–2.08, P < 0.0001). When the multivariable model was stratified by DFI, women with relapsed disease with DFI <6 months, ≥6 months to <2 years, or ≥2 to <5 years each had a significantly higher risk of death compared with women with de novo stage IV disease. The risk of death was not statistically different among patients with relapsed disease with DFI >5 years compared with those with de novo disease.
Conclusions: This large cohort study provides further insight into the natural history of relapsed and de novo stage IV breast cancer. DFI plays an important role in the prognosis for patients with relapsed breast cancer.
Keywords: breast cancer, de novo stage IV, metastatic
introduction
In 2009, an estimated 192 370 women in the United States will be diagnosed with breast cancer with an estimated 40 170 deaths attributed to this disease [1]. Approximately 6%–10% of women will present with metastatic disease at diagnosis (de novo stage IV disease) and depending on initial stage, tumor biology, and type of treatment received, ∼30% of women diagnosed with non-metastatic disease will recur (relapsed disease) [2, 3]. Once metastatic disease is diagnosed, treatment is palliative with reported median survivals ranging between 18 and 24 months [4, 5].
The last two decades have seen the introduction of a variety of new chemotherapeutic agents, hormone agents including aromatase inhibitors, and biological agents such as trastuzumab, lapatinib, and bevacizumab [5, 6]. Data from a variety of studies indicate that these drugs coupled with improved supportive care have probably positively impacted the survival of women with metastatic breast cancer [7–9]. Several studies have also reported a range of prognostic factors for women with metastatic breast cancer including factors such as age at diagnosis, hormone receptor status, human epidermal growth factor receptor 2 (HER2) status, and site of metastases for predicting survival from the time of metastases [5, 10]. Recent reports have also reported long-term survival among a subgroup of women with de novo stage IV breast cancer who undergo local treatment of their primary breast tumors [11, 12].
Clearly, women with metastatic breast cancer form a heterogeneous group with varying prognostic outcomes. Specifically, we hypothesize that women with de novo stage IV breast cancer represent a group that is distinct from that of women with relapsed breast cancer. As such, the primary goal of this retrospective study was to determine whether survival differences existed between patients with de novo stage IV breast cancer compared with those with relapsed disease. Furthermore, we also explored the significance of other potential prognostic factors among women with metastatic breast cancer.
patients and methods
study population
Data for this study population were obtained from a database maintained at the Department of Breast Medical Oncology of The University of Texas M. D. Anderson Cancer Center. We retrospectively identified a cohort of patients diagnosed from 1992 to 2007 with either de novo stage IV or relapsed breast cancer. Excluded from the analysis were male patients and those patients with more than one primary cancer. Furthermore, patients with relapsed disease had to have demonstrated distant metastases. Those with locoregional recurrence were excluded from the analyses. A number of variables were recorded including age at metastases, site of metastases, hormone receptor status, HER2 status, and disease-free interval (DFI) among patients with relapsed disease. All information obtained from the database was cross-checked with medical records to confirm the accuracy of the data obtained. This study was approved by the institutional review board.
staging and pathology
Staging of primary disease among women with relapsed disease and among women with de novo stage IV disease was on the basis of the staging guidelines set forth by the 6th edition of the American Joint Committee on Cancer Criteria [13]. Estrogen and progesterone receptor status was assessed using the dextran-coated charcoal ligand-binding method in patients for tumor specimens obtained before 1993. For tumor specimens obtained after 1993, immunohistochemistry staining of paraffin-embedded tissue sections with mAbs (6F11 for estrogen receptors and 1A6 for progesterone receptors) was used to determine hormone receptor status.
data analysis
Patient characteristics were tabulated and compared between groups with the chi-square test or the Wilcoxon's rank sum test, as appropriate. Median follow-up was calculated as the median observation time among all patients. Among women with relapsed breast cancer, DFI was defined as the time from the diagnosis of primary nonmetastatic breast cancer to the date of the first distant metastases and was divided into four groups: (i) <6 months, (ii) 6 months to <2 years, (iii) 2 to <5 years, and (iv) >5 years. Overall survival (OS) was measured from the date of the first distant metastases to the date of death from any cause and was estimated by the Kaplan–Meier product limit method and compared across groups using the log-rank statistic. Cox proportional hazards models were then fit to determine the association between de novo stage IV and relapsed disease and OS after adjusting for patient and tumor characteristics. The variables chosen to be included in the model were on the basis of clinical significance rather than statistical significance on univariate analysis. These variables included race, age at metastasis diagnosis, year of metastasis diagnosis, presence of lymphovascular invasion, hormone receptor status, grade of disease, and sites of metastases. Of note, HER2 status was not included in the final model as 33% of the data pertaining to this variable were missing. In addition to the variable describing whether the patient had de novo stage IV or relapsed disease, other variables included in the final model were race (black versus white, Hispanic/other versus white), age at metastases (continuous), year of diagnosis of metastatic disease (continuous variable), lymphovascular invasion (positive versus negative), hormone receptor status (positive versus negative), grade of disease (III versus I/II), and site of metastatic disease (visceral/other metastatic disease versus bone metastases, multiple/brain metastases versus bone metastases). All analyses were carried out using SAS 9.1 (SAS Institute, Cary, NC). All P values were two sided and values <0.05 were considered statistically significant.
results
baseline patient characteristics
Table 1 summarizes the patient and tumor characteristics stratified by de novo stage IV and relapsed disease. The final analyses included 3524 patients, of whom 643 (18.2%) had de novo stage IV disease and 2881 (81.8%) had relapsed disease. Compared with women with relapsed disease, women with de novo stage IV disease tended to be older, to have hormone receptor-positive disease, and to be more frequently of nonwhite race. Among women with relapsed disease, 65 (2.3%) had a DFI <6 months, 1064 (36.9%) had DFI of ≥6 months and <2 years, 1076 (37.3%) had DFI ≥ 2 years and <5 years, and 676 (23.5%) had DFI >5 years.
Table 1.
Baseline patient and tumor characteristics stratified by de novo stage IV versus relapsed disease
|
De novo |
Relapsed |
P value | |||
| n | % | n | % | ||
| N | 643 | – | 2881 | ||
| Median age at metastasis (range) | 52 (17–91) | – | 50 (21–91) | <0.0001 | |
| Race | |||||
| Black | 109 | 17.0 | 328 | 11.4 | |
| Hispanic | 0 | 0.0 | 315 | 10.9 | |
| Other | 86 | 13.4 | 85 | 3.0 | |
| White | 448 | 69.7 | 2153 | 74.7 | <0.0001 |
| Menopausal status | |||||
| Premenopausal | 237 | 37.6 | 1373 | 48.5 | |
| Postmenopausal | 393 | 62.4 | 1457 | 51.5 | <0.0001 |
| Histology | |||||
| Other | 104 | 16.9 | 355 | 12.5 | |
| Ductal | 511 | 83.1 | 2474 | 87.5 | 0.004 |
| Hormone receptor status | |||||
| Negative | 173 | 30.0 | 940 | 37.5 | |
| Positive | 404 | 70.0 | 1570 | 62.5 | 0.001 |
| HER2 | |||||
| Negative | 397 | 75.3 | 1389 | 76.2 | |
| Positive | 130 | 24.7 | 433 | 23.8 | 0.669 |
| First site of metastasis | |||||
| Multiple | 68 | 10.6 | 409 | 14.2 | |
| Visceral only | 165 | 25.7 | 737 | 25.6 | |
| Visceral + bone | 132 | 20.5 | 415 | 14.4 | |
| Bone only | 206 | 32.0 | 757 | 26.3 | |
| Brain only | 6 | 0.9 | 89 | 3.1 | |
| Other | 66 | 10.3 | 473 | 16.4 | <0.0001 |
| Nuclear grade | |||||
| I | 26 | 4.6 | 72 | 2.8 | |
| II | 195 | 34.3 | 771 | 29.8 | |
| III | 347 | 61.1 | 1740 | 67.4 | 0.005 |
| LVI | |||||
| Negative | 156 | 50.5 | 1624 | 59.4 | |
| Positive | 153 | 49.5 | 1108 | 40.6 | 0.002 |
| Surgery type | |||||
| BCS | 109 | 42.4 | 778 | 27.5 | |
| Mastectomy | 148 | 57.6 | 2046 | 72.5 | <0.0001 |
| Any chemotherapy | |||||
| No | – | 549 | 19.1 | ||
| Yes | – | 2332 | 80.9 | – | |
| DFI | |||||
| <6 months | – | 65 | 2.3 | ||
| 6 months to 2 years | – | 1064 | 36.9 | ||
| 2–5 years | – | 1076 | 37.3 | ||
| >5 years | – | 676 | 23.5 | – | |
DFI, disease-free interval; LVI, lymphovascular invasion; BCS, breast conservative surgery.
survival estimates
At the time of the analyses, 1737 (60.3%) patients with relapsed disease and 359 (55.8%) patients with de novo stage IV disease had died. Median follow-up for the whole cohort was 19.0 months (range 0–161.8 months), and among those with relapsed and de novo stage IV disease, it was 18.1 months (0–152.1 months) and 24.4 months (range 0.1–161.8 months), respectively.
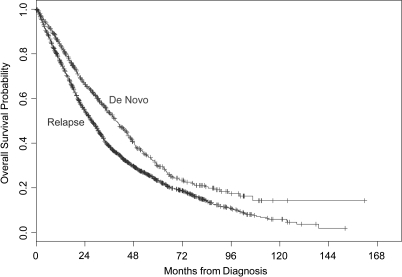
Table 2 summarizes OS estimates. Median survival among women with relapsed and de novo stage IV disease was observed to be 27.2 and 39.2 months, respectively, with this difference being statistically significant (P < 0.0001) (Figure 1). Table 3 summarizes the results of the multivariable Cox proportional hazards model with hazard ratios >1.0 indicating an increased risk of death. Women with relapsed disease had 1.75 times the risk of death compared with women with de novo disease [95% confidence interval (CI) 1.47–2.08, P < 0.0001].
Table 2.
Overall survival estimates in de novo stage IV and relapsed patients
| Relapsed |
De novo |
|||||
| Median | 95% confidence interval | P value | Median | 95% confidence interval | P value | |
| All | 27.2 | 25.9–29.1 | 39.2 | 35.3–44.2 | ||
| Age at metastases | ||||||
| <50 | 26.6 | 24.4–29.6 | 45.1 | 37.2–53.4 | ||
| ≥50 | 27.6 | 25.6–29.6 | 0.670 | 36.7 | 32.3–41.4 | 0.012 |
| Race | ||||||
| Black | 16.8 | 14.4–19.5 | 25.6 | 20.2–34.8 | ||
| Hispanic | 26.9 | 20.5–30.9 | – | – | ||
| Other | 46.0 | 28.6–80.5 | 46.7 | 37.2–56.7 | ||
| White | 29.4 | 27.4–30.8 | <0.0001 | 40.6 | 35.3–45.1 | 0.002 |
| Menopausal status | ||||||
| Premenopausal | 29.6 | 26.6–31.2 | 45.1 | 37.5–53.4 | ||
| Postmenopausal | 25.9 | 24.0–27.7 | 0.018 | 38.1 | 33.5–42.1 | 0.017 |
| Histology | ||||||
| Other | 28.8 | 25.6–33.1 | 45.2 | 38.1–53.0 | ||
| Ductal | 26.8 | 25.2–28.6 | 0.313 | 39.0 | 34.1–44.5 | 0.385 |
| Stage | ||||||
| I | 34.7 | 31.0–39.0 | ||||
| II | 29.1 | 26.1–30.5 | ||||
| III | 22.7 | 20.5–25.6 | <0.0001 | |||
| Hormone receptor status | ||||||
| Negative | 16.0 | 14.7–17.8 | 22.6 | 20.0–27.2 | ||
| Positive | 34.7 | 33.1–37.6 | <0.0001 | 45.9 | 42.7–51.3 | <0.0001 |
| HER2 | ||||||
| Negative | 24.4 | 21.8–27.5 | 42.7 | 37.6–48.5 | ||
| Positive | 29.6 | 26.8–33.5 | 0.003 | 41.4 | 34.5–47.5 | 0.885 |
| First site of metastasis | ||||||
| Multiple | 15.6 | 12.8–18.2 | 15.9 | 11.6–30.5 | ||
| Visceral only | 25.3 | 22.1–28.6 | 39.1 | 33.7–45.2 | ||
| Visceral + bone | 22.5 | 20.1–28.6 | 28.8 | 23.6–39.4 | ||
| Bone only | 41.5 | 35.5–46.2 | 52.2 | 44.5–60.2 | ||
| Brain only | 11.6 | 8.0–16.9 | 11.4 | 1.9–48.6 | ||
| Other | 25.9 | 23.8–28.6 | <0.0001 | 39.2 | 18.9–64.8 | <0.0001 |
| Nuclear grade | ||||||
| I | 33.1 | 21.0–50.3 | 47.1 | 43.5–59.1 | ||
| II | 41.6 | 36.8–45.9 | 48.5 | 40.5–58.4 | ||
| III | 20.2 | 19.3–21.6 | <0.0001 | 34.5 | 29.0–40.0 | 0.006 |
| LVI | ||||||
| Negative | 30.7 | 29.1–33.0 | 51.6 | 45.1–58.4 | ||
| Positive | 22.4 | 20.4–24.7 | <0.0001 | 37.5 | 26.2–48.5 | 0.074 |
| Any chemotherapy | ||||||
| No | 33.9 | 30.8–38.3 | ||||
| Yes | 26.0 | 24.1–27.4 | <0.0001 | |||
| DFI | ||||||
| <6 months | 17.4 | 13.1–26.7 | ||||
| 6 months to 2 years | 17.3 | 16.0–18.9 | ||||
| 2–5 years | 30.4 | 27.7–32.2 | ||||
| >5 years | 47.4 | 41.8–53.4 | <0.0001 | |||
DFI, disease-free interval; LVI, lymphovascular invasion.
Figure 1.
Kaplan–Meier curves illustrating overall survival for patients with de novo stage IV versus relapsed disease.
Table 3.
Multivariable model
| Variable | Hazard ratio | Lower 95% confidence interval | Upper 95% confidence interval | P value |
| Relapsed versus de novo | 1.75 | 1.47 | 2.08 | <0.0001 |
| Race (black versus white) | 1.38 | 1.18 | 1.61 | <0.0001 |
| Race (Hispanic and other versus white) | 0.88 | 0.75 | 1.03 | 0.112 |
| Age at metastasis | 1.01 | 1.00 | 1.01 | 0.0004 |
| Year of metastatic diagnosis | 1.01 | 0.98 | 1.03 | 0.606 |
| LVI (positive versus negative) | 1.19 | 1.08 | 1.32 | 0.001 |
| HR (positive versus negative) | 0.59 | 0.53 | 0.66 | <0.0001 |
| Grade (III versus I/II) | 1.51 | 1.34 | 1.71 | <0.0001 |
| Visceral or other metastases versus bone only | 1.39 | 1.24 | 1.56 | <0.0001 |
| Multiple or brain metastases versus bone only | 2.10 | 1.80 | 2.43 | <0.0001 |
LVI, lymphovascular invasion; HR, hormone receptor.
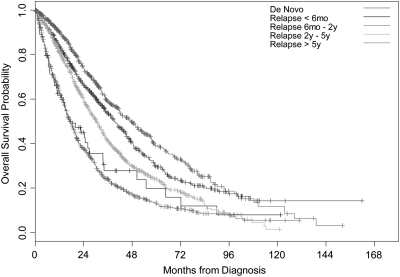
Among women with relapsed disease, those with DFIs <6 months, ≥6 months to <2 years, ≥ 2 to <5 years, and ≥5 years had median OS estimates of 17.4 months, 17.3 months, 30.4 months, and 47.4 months, respectively (P < 0.0001) (Figure 2). Compared with women with de novo stage IV disease, those with relapsed disease whose DFI was <6 months (P < 0.0001), ≥6 months and <2 years (P < 0.0001), and ≥ 2 and <5 years (P < 0.0001) had worse OS. However, women with relapsed disease whose DFI was >5 years had a better OS on univariate analyses compared with women with de novo stage IV disease (P = 0.005). In the multivariable model, compared with women with de novo stage IV disease, women with relapsed disease who had a DFI of <6 months (HR = 1.93, 95% CI 1.30–2.86, P = 0.001), ≥6 months and <2 years (HR = 2.07, 95% CI 1.72–2.49, P < 0.0001), ≥ 2 and <5 years (HR = 1.74, 95% CI 1.44–2.10, P < 0.0001) had an increased risk of death that was statistically significant. Compared with women with de novo stage IV disease, those with relapsed disease who had a DFI >5 years had a higher risk of death; however, this was not statistically significant (HR = 1.11, 95% CI 0.88–1.41, P = 0.38).
Figure 2.
Kaplan–Meier curves illustrating overall survival for patients with de novo stage IV disease and those with relapsed disease. Patients with relapsed disease are stratified by disease-free interval.
Among women with relapsed disease, 549 (19.1%) women had not received chemotherapy either in the adjuvant or in the neoadjuvant setting (chemotherapy-naive group). In the univariate analysis, these women had similar median OS compared with women with de novo stage IV disease (33.9 versus 39.2 months, P = 0.130). However, in the multivariable model, adjusted for patient and tumor characteristics, women with relapsed disease who were chemotherapy naive had 1.68 times the risk of death compared with women with de novo stage IV disease (95% CI 1.33–2.12, P < 0.0001).
discussion
The primary objective of this study was to determine whether women with de novo stage IV breast cancer represented a group of patients with a different prognostic outcome compared with women with relapsed breast cancer. In this study, we observed that the median survival of women with de novo stage IV breast cancer was 12 months longer than that of women with relapsed disease, with the difference being statistically significant on both univariate and multivariate analyses.
Several studies have reported improvement in survival of women with metastatic breast cancer [7–9]. Using a cohort of 834 women with breast cancer treated with adjuvant anthracycline-based chemotherapy at The University of Texas M. D. Anderson Cancer Center and who developed a recurrence from 1974 to 2000, Giordano et al. [8] reported a median survival of 15 months among women diagnosed with metastatic disease from 1974 to 1979 and 58 months among those diagnosed from 1995 to 2000. In the multivariable model, this translated into a reduction of 1% in the risk of death with each increasing year of diagnosis of metastatic disease. In a study that looked specifically at a cohort of 724 women with de novo stage IV disease, Andre et al. [9] reported a median survival of 23 months among women diagnosed from 1987 to 1993 and 29 months among those diagnosed from 1994 to 2000 (P < 0.0001).
Data from these studies leave little room to doubt that there has been a significant improvement in the survival of women with both relapsed and de novo stage IV disease over time, which is presumably attributed to the introduction of newer therapeutic agents and improved supportive care. However, the question that we asked here was whether the prognostic outcome of women with de novo stage IV disease was different compared with those women with relapsed disease. Unadjusted median survival was significantly superior in the de novo stage IV group compared with the relapsed group (39.2 versus 27.2 months, P < 0.0001). When adjusted for a number of patient and tumor characteristics, the risk of death among women with relapsed disease was 1.75 times that of women with de novo stage IV disease and this was statistically significant (95% CI 1.47–2.08, P < 0.0001). Recognizing that DFI is an important prognostic variable among women with relapsed disease [6], with longer DFI associated with superior prognostic outcome compared with those with shorter DFI, we further stratified our analysis according to DFI. Using this stratification, we made two important observations. First, we observed increasing median survival with increasing DFI among women with relapsed disease, thereby concurring with previously published studies [6]. Specifically, we observed a median survival of 17 months among women with DFI of <6 months and a median survival of 47 months among those whose DFI was >5 years. Second, in the multivariable model, the risk of death among women with relapsed disease whose DFI was <5 years was significantly greater compared with that of women with de novo stage IV. Although a trend for increased risk of death was observed among women with relapsed disease whose DFI >5 years compared with women with de novo stage IV disease, this association was not significantly different. These results are provocative, indicating the prognostic superiority of de novo stage IV compared with relapsed disease. A possible hypothesis to explain this phenomenon may be that among women with relapsed disease, the absence of the primary tumor, removed as part of treatment of early-stage disease, may result in more aggressive metastatic disease compared with that of women with de novo stage IV disease. Indeed, there are data from experimental studies that show an increase in the growth kinetics of distant metastases following removal of the primary tumor [14, 15]. This, however, would appear to contradict some of the recent data that indicate long-term survival among a subset of women with de novo stage IV disease who undergo local treatment of their primary tumors [11, 12]. Another hypothesis may be that the disease among women with relapsed disease is more resistant to chemotherapeutic agents due to exposure to adjuvant treatment compared with the chemonaive women with de novo stage IV disease. Regardless, these are issues that should be the subject of future studies.
Several groups have reported a number of prognostic factors for women with metastatic breast cancer [6, 8, 10]. In a recent study, Largillier et al. [10] reported on prognostic factors in a cohort of 1038 women with relapsed breast cancer treated at a single institution. In a multivariate analysis, the authors reported that age at initial diagnosis, hormone receptor status, and site of metastases were independent prognostic factors able to predict for survival following development of first metastatic recurrence. In the study by Andre et al. [9] that looked at de novo stage IV breast cancer, similar prognostic factors were reported. In our present study, we observed that factors that were significantly associated with improved survival for the whole cohort included younger age at diagnosis of metastases, white race, positive hormone receptor status, absence of lymphovascular invasion, lower grade of disease, and absence of visceral metastases. Among women with relapsed disease, DFI was found to be an important prognostic factor. Of note, unlike previous studies, our multivariate models were adjusted for whether women had relapsed or de novo stage IV disease.
We acknowledge that our study has a number of important limitations that need to be considered when interpreting the data presented. First, as a retrospective study, it is subject to all the inherent biases associated with this type of study design. Secondly, due to large amounts of missing data, we were unable to adjust for HER2 status in our models. Trastuzumab has been shown in randomized clinical trials to have significantly improved the survival of women with HER2-positive metastatic disease [16] and recent data also indicate that the introduction of trastuzumab into standard therapy may have changed the natural history of HER2-positive breast tumors such that HER2-positive status in the trastuzumab era may be associated with a superior prognostic outcome compared with women whose tumors are HER2 negative [17]. Furthermore, we were unable to assess whether breast tumor subtypes had differing prognostic outcomes among women with de novo stage IV disease compared with those with recurrent breast cancer. This would certainly be an interesting question to explore in future studies.
In conclusion, the results of our study indicate that women with de novo stage IV breast cancer have superior outcomes compared with women with relapsed breast cancer. Despite the limitations of the study, the results are provocative enough to indicate that de novo stage IV breast cancer should be considered as a separate entity entirely. This would certainly have implications on how future clinical trials looking at women with metastatic breast cancer are designed. Further supporting this notion is the fact that a number of retrospective data have shown that subgroups of women with de novo stage IV disease can attain long-term survival when their distant disease is controlled and their primary tumors are treated [11, 12]. If we were to accept de novo stage IV breast cancer as being a separate entity, the next question that would be important to answer would be whether intrinsic breast tumor subtypes which have been shown to be of prognostic significance in early-stage breast cancer [18, 19] have the same impact on survival outcome among women with de novo stage IV breast cancer. We believe the data that we present here will encourage further research that will serve to answer these important questions.
funding
Nellie B. Connally Breast Cancer Fund; National Institutes of Health 1K07 CA 109064 to SHG.
Acknowledgments
Presented in part as a poster presentation at the San Antonio Breast Cancer Symposium 2008.
References
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2009. CA Cancer J Clin. 2009;59(4):225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Harris JR, Morrow M, Bonadonna G. Cancer of the breast. In: De Vitta VT Jr, Hellman S, Rosenberg SA, editors. Cancer. Principles and Practice in Oncology. 4th edition. Philadelphia, PA: JB Lippincott; 1993. pp. 1264–1332. [Google Scholar]
- 3.Miller KD, Sledge GW., Jr The role of chemotherapy for metastatic breast cancer. Hematol Oncol Clin North Am. 1999;13:415–434. doi: 10.1016/s0889-8588(05)70063-0. [DOI] [PubMed] [Google Scholar]
- 4.Norton L. Metastatic breast cancer: length and quality of life. N Engl J Med. 1991;325:1370–1371. doi: 10.1056/NEJM199111073251909. [DOI] [PubMed] [Google Scholar]
- 5.Cardoso F, Di LA, Lohrisch C, et al. Second and subsequent lines of chemotherapy for metastatic breast cancer: what did we learn in the last two decades? Ann Oncol. 2002;13:197–207. doi: 10.1093/annonc/mdf101. [DOI] [PubMed] [Google Scholar]
- 6.Beslija S, Bonneterre J, Burstein HJ, et al. Third consensus on medical treatment of metastatic breast cancer. Ann Oncol. 2009;20(11):1771–1785. doi: 10.1093/annonc/mdp261. [DOI] [PubMed] [Google Scholar]
- 7.Dawood S, Broglio K, Gonzalez-Angulo AM, et al. Trends in survival over the past two decades among white and black patients with newly diagnosed stage IV breast cancer. J Clin Oncol. 2008;26(30):4891–4898. doi: 10.1200/JCO.2007.14.1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Giordano SH, Buzdar AU, Smith TL, et al. Is breast cancer survival improving? Cancer. 2004;100(1):44–52. doi: 10.1002/cncr.11859. [DOI] [PubMed] [Google Scholar]
- 9.Andre F, Slimane K, Bachelot T, et al. Breast cancer with synchronous metastases: trends in survival during a 14-year period. J Clin Oncol. 2004;22(16):3302–3308. doi: 10.1200/JCO.2004.08.095. [DOI] [PubMed] [Google Scholar]
- 10.Largillier R, Ferrero JM, Doyen J, et al. Prognostic factors in 1,038 women with metastatic breast cancer. Ann Oncol. 2008;19(12):2012–2019. doi: 10.1093/annonc/mdn424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Blanchard DK, Shetty PB, Hilsenbeck SG, Elledge RM. Association of surgery with improved survival in stage IV breast cancer patients. Ann Surg. 2008;247(5):732–738. doi: 10.1097/SLA.0b013e3181656d32. [DOI] [PubMed] [Google Scholar]
- 12.Rao R, Feng L, Kuerer HM, et al. Timing of surgical intervention for the intact primary in stage IV breast cancer patients. Ann Surg Oncol. 2008;15(6):1696–1702. doi: 10.1245/s10434-008-9830-4. [DOI] [PubMed] [Google Scholar]
- 13.Singletary SE, Allred C, Ashley P, et al. Staging system for breast cancer: revisions for the 6th edition of the AJCC Cancer Staging Manual. Surg Clin North Am. 2003;83:803–819. doi: 10.1016/S0039-6109(03)00034-3. [DOI] [PubMed] [Google Scholar]
- 14.Gunduz N, Fisher B, Saffer EA. Effect of surgical removal on the growth and kinetics of residual tumor. Cancer Res. 1979;39:3861–3865. [PubMed] [Google Scholar]
- 15.Fisher B, Saffer EA, Rudock C, et al. Presence of a growth-stimulating factor in serum following primary tumor removal in mice. Cancer Res. 1989;49:1996–2001. [PubMed] [Google Scholar]
- 16.Slamon DJ, Leyland-Jones B, Shak S, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344(11):783–792. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 17.Dawood S, Kristine B, Hortobagyi GN, Giordano S. Prognosis of women with stage IV breast cancer by HER2 status and trastuzumab treatment: an institutional based review. J Clin Oncol. 2008;26(Suppl) doi: 10.1200/JCO.2008.19.9844. (Abstr 1018) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Perou CM, Sorlie T, Eisen MB, et al. Molecular portraits of human breast tumors. Nature. 2000;406:747–752. doi: 10.1038/35021093. [DOI] [PubMed] [Google Scholar]
- 19.Sorlie T, Perou CM, Tibshirani R, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci U S A. 2001;98:10869–10874. doi: 10.1073/pnas.191367098. [DOI] [PMC free article] [PubMed] [Google Scholar]