Abstract
This study aims to investigate the inhibitory effects of oridonin nanosuspension on human prostatic carcinoma PC-3 cell line in vitro. The PC-3 cells were incubated with increasing concentrations of oridonin solution and nanosuspensions for 12 hours, 24 hours, and 36 hours. MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] assay was performed to measure cellular viability and investigate the effect of oridonin on cell growth of PC-3. Annexin V-FITC/PI staining method was used to determine the effect of oridonin by fluorescence microscope and flow cytometry, respectively. Nanosuspension on early apoptosis of PC-3 cells was also evaluated. Oridonin significantly inhibited the growth of PC-3 cells after 12 hours, 24 hours, and 36 hours of treatment in a dose-dependent manner (P < 0.05). Compared with the same concentration of oridonin solution, oridonin nanosuspension enhanced the inhibition ratio of proliferation. The observation of propidium iodide fluorescence staining confirmed the MTT assay results. The cell proportion of PC-3 at the G2/M phase in the nanosuspension treatment group was upregulated compared with that of the control and oridonin solution groups. Both oridonin solution and nanosuspension promoted the early apoptosis of PC-3 cells. Furthermore, while improving the ratio of early apoptosis, oridonin nanosuspensions also enhanced growth suppression, and induced apoptosis of PC-3 cells. This shows great potential in the treatment of androgen-independent carcinoma of prostate by oridonin nanosuspensions.
Keywords: oridonin, nanosuspension, carcinoma of prostate, PC-3 cells, cell cycle, apoptosis
Introduction
Prostate cancer is the most common malignancy and is the second leading cause of cancer death in men.1,2 Apoptosis is the primary mechanism through which prostate cancer cells are killed when exposed to treatments such as chemotherapy or radiotherapy. 3 Phytochemicals represent a new class of compounds with anticarcinogenic properties that are gaining attention in the treatment of human cancers.4 However, many of these compounds suffer from sensitivity to degradative processes in the blood, or have limited water solubility that provides for minimal dissolution in the gastrointestinal tract and low bioavailability.5
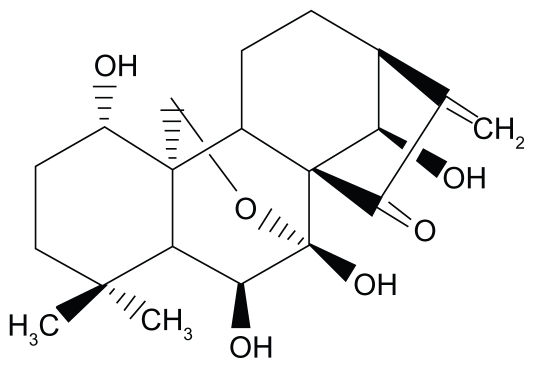
Oridonin (ORI) (Figure 1), extracted from Rabdosia rubescens, exhibits apoptosis- inducing activities on a variety of cancer cells.6 It has been reported that ORI suppresses the growth of MCF-7 cells through the induction of S cell cycle arrest and apoptosis and breast cancer cells cultured in vitro but does not affect normal human cells.7 Furthermore, it has been demonstrated that ORI effectively inhibits the proliferation of a wide variety of cancer cells, especially those from prostate (LNCaP, DU145, PC-3), with ED50s ranging from 1.8 to 7.5 μg/mL.8 In addition, the literature also describes the effects of ORI on cell proliferation, cell apoptosis, cell cycle parameters, androgen receptor, and prostate-specific antigen protein expression.9 Unfortunately, the poor solubility of ORI in water and physiologically acceptable organic solvent imposes some restraints to further pharmaceutical use.10 ORI is a poorly soluble drug; for example, the solubility in water is <1 mg/mL.11 The poor solubility may be overcome by particle size reduction using nanoscience approach.12 A nanoscience approach that has quickly gained a proven record within the pharmaceutical sciences is the formulation as nanosuspension.13 Nanosuspensions of drugs are submicron colloidal dispersions of pure particles of drug which are stabilized by surfactants.14 Currently, various delivery platforms have been explored to improve bioavailability by increasing dissolution rate and solubility.15 Nevertheless, several conventional approaches have encountered many limitations with regards to their application, such as increased toxicity. These conventional approaches often attempt to solubilize insoluble drugs with the use of excessive amounts of cosolvents, but this poses toxicity problems. Compared with these conventional approaches, nanosuspension technology may be an ultimate universal applicable technology to process these poor water-soluble drugs. The increase in dissolution velocity, saturation solubility, and bioavailability are the general advantages of nanosuspension formulations compared with microparticulate delivery systems, especially below 1–2 μm.12 As the nanosuspension was stabilized by a minimum of surfactants, and the drugs remained in a solid state, the formulation has low toxicity, higher mass per volume loading, and higher physiochemical stability.16
Figure 1.
Molecular structure of oridonin.
Compared with the previous publications in our lab and some other groups,11,12,17 the specialness of this present study is obvious. In previous publications, we mainly focused on the systematically investigated and constructed nanosuspension drug-delivery systems such as physicochemical properties and morphology analysis. Therefore, most of these works were carried out within colloidal and nanotechnology fields, and little research was performed on cell lines and pharmacodynamics.
It is self-evident that the physical properties of the drug’s vehicle could influence drug efficacy.18 In our previous studies, we developed and constructed ORI nanosuspension with acceptable ingredients, and we were able to formulate ORI nanosuspension that could be re-dispersed and diluted with water to any needed dilution without phase separation and without drug aggregation.11,19–22 It has been documented that the drug efficacy can be enhanced or degraded through nanoparticle,23,24 micelles,25 and liposomal formulations.26 Nevertheless, relatively few studies have been carried out concerning the influence of the nanosuspension of a drug on its antitumor efficacy.22,27 Thus, in the present study, we demonstrated that nanosuspension could influence the ORI efficacy on proliferation and apoptosis of PC-3 cells.
Materials and methods
Compound and materials
ORI (99% pure) was obtained from Shanxi Huike Plants Exploitation (Xian, China). ORI solution was prepared as our previous method described.11 Stock solutions of ORI were prepared by dissolving in dimethyl sulfoxide (DMSO) and the aliquots stored at −20°C prior to use. MTT [3-(4,5-dimethylthiazol- 2-yl)-2,5-diphenyltetrazolium bromide] and DMSO were purchased from Sigma Chemical (St. Louis, MO). Fetal bovine serum (FBS) was supplied by FMG Biotech Co., Ltd (Shanghai, China). Trypsin was obtained from GIBCO BRL (Gaithersburg, MD). Propidium iodide (PI), RNase A and annexin V-FITC were purchased from KeyGen Biotechnology (Nanjing, China). All the other chemicals and solvents were of chromatographic and pharmaceutical grade and used without additional treatments.
PC-3 cell culture
The human prostatic carcinoma PC-3 cells were grown in RPMI 1640 medium (GIBCO, Billings, MT) supplemented with 10% FBS and 1% antibiotic solution (penicillin 100 U/mL and streptomycin 100 μg/mL). Cells were maintained at 37°C in a humidified atmosphere of 5% CO2. The medium was changed every second day, and cells were subcultured when confluency reached 90% by 0.25% trypsin at 37°C.
Preparation of ORI nanosuspension
To produce the ORI nanosuspension, HPH technology was applied. Briefly, ORI coarse powder (1%, w/v) was dispersed in an aqueous surfactant solution containing 0.5% (w/v) lecithin, 0.1% (w/v) hydroxy-propyl methyl cellulose (HPMC), and 0.1% (w/v) polyvinylpyrrolidone (PVP) under magnetic stirring. The obtained mixture was firstly disintegrated into microparticles by high shear homogenizer using Ultra-Turrax® T25 (IKA, Germany) at 15,000 rpm for 5 minutes. The nanosuspension was then prepared using a piston-gap high pressure homogenizer EmulsiFlex-C3 (Avestin Inc., Ottawa, Canada) equipped with a heat exchanger. At first, 5 cycles at 500 bar and 10 cycles at 1000 bar were conducted as pre-milling step, and then 20 cycles at 1500 bar were run to obtain the nanosuspension.
For long-term stability, mannitlo was used as cryoprotectant for freezing the nanosuspension for 48 hours, and then freeze-dried with lyophilizer (FD5, SIM, USA). The ORI nanosuspensions were rapidly cooled down to −80°C for 48 hours (DW-HL218; Shanghai Huayan Co., Ltd, China), and then freeze-dried for 48 hours at −55°C and at a pressure of 15 mTorr. The mean particle size of the obtained nanosuspension was 912.5 ± 17.6 nm, as measured by Zetasizer (3000 SH; Malvern Instruments Ltd., UK).
Estimation of cell viability
The effect of ORI solution and ORI nanosuspension on cell viability was assessed by a colorimetric MTT assay. Briefly, PC-3 cells (1 × 106 cells) were seeded in 96-well culture plates and allowed to adhere overnight at 37°C in a humidified, 5% CO2 atmosphere. PC-3 cells were incubated with ORI nanosuspension and free ORI at the concentrations of 9.375, 18.75, 37.5, and 75 μmol/L for indicated time periods, respectively. The control group received drug-free medium with 0.05% v/v DMSO. Subsequently, MTT (5 mg/mL) was added to each well. After incubation at 37°C for 4 hours, the medium was discarded and 150 μL DMSO was added into each well. The optical density was measured by a microplate reader at a reference wavelength of 630 nm and a test wavelength of 570 nm. The cytotoxicity of the ORI nanosuspension was expressed as IC50 (concentration of 50% inhibition rate relative to controls, which was estimated from linear regression analysis of experimental data). The percentage of cell growth inhibition was calculated as follows: cell inhibitory ratio (%) = (A570control − A570sample)/A570control × 100%.
Fluorescent morphological assay
PC-3 cells were seeded into 6-well culture plates at a density of 1 × 105 cells per well and incubated with free ORI and ORI nanosuspension at a concentration of 25 μmol/L for 24 hours. Free ORI was prepared by dissolving ORI in 0.1% v/v DMSO. The control group received drug-free medium with 0.1% v/v DMSO. After cells were washed with PBS 3 times and collected, the cells obtained were stained with PI for 5 minutes at room temperature and then observed under reverse fluorescence microscopy (Nikon, Japan).
Flow cytometric analysis of cell cycle distribution
The analysis of cell cycle distribution was measured by staining DNA with PI as described by the manufacture’s protocol. Treated or untreated PC-3 cells were harvested and washed into conical tubes, fixed in cool 70% ethanol at 4°C overnight, and then incubated with 0.5 mL PI/Triton X-100 staining solution with ribonuclease A for 30 minutes at room temperature. For each cell population, at least 10,000 cells were analyzed by FACS. The distribution of different DNA contents was analyzed by FACScan flow cytometer (Becton Dickinson, Franklin Lakes, NJ).
Evaluation of apoptosis
The transversion of phosphatidyl serine from the inner to outer plasma membrane leaflet, an initial event in the apoptotic pathway, was assessed by dual dye staining using annexin V-FITC/PI.3,28 PC-3 cells were exposed to different concentrations of free ORI and ORI nanosuspension for 24 hours and allowed to adhere. The control group received drug-free medium with 0.05% v/v DMSO. At the end of the treatment period, the control (untreated) and treated cells were harvested and washed with cold PBS at 1200 rpm for 5 minutes. Then 1 × 105 cells were collected and gently resuspended in 500 μL binding buffer. 5 μL annexin V-FITC and 5 μL PI were added and incubated with cells in the dark for 10 minutes. At the end of incubation, analysis by FAC-Scan flow cytometer (Becton Dickinson) was carried out to discriminate between live and apoptotic cells.
Statistical analysis
Results were expressed as means ± SD of replicate analyses. Data analyses were performed (where appropriate) using ANOVA for a single factor, factorial treatment model. Differences with P-values less than 0.05 (P < 0.05) were considered statistically significant.
Results and discussion
Effects of ORI nanosuspension on growth inhibition of PC-3 cells
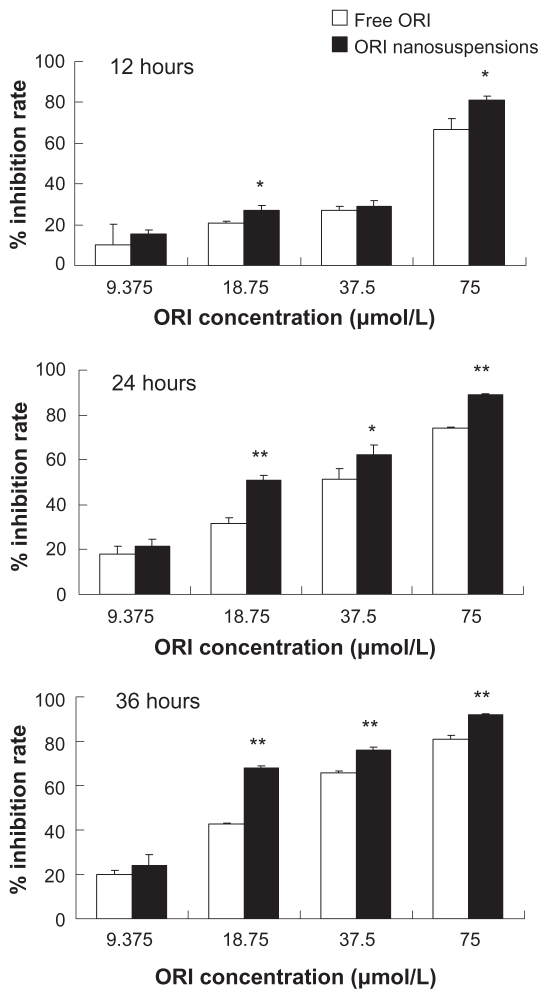
Cells were cultured with ORI nanosuspension and free ORI at the concentrations of 9.375, 18.75, 37.5, and 75 μmol/L for 12, 24, and 36 hours, respectively. The results showed that both ORI nanosuspension and solution can significantly inhibit PC-3 cell growth. Meanwhile, ORI nanosuspension and solution inhibited PC-3 cell growth in a concentration- and time-dependent manner (Figure 2). A significant increase in inhibition rates was shown in ORI nanosuspension-treated cells, when compared with ORI solution-treated cells under the same experimental conditions. Also, ORI nanosuspension induced a stronger growth inhibition in PC-3 cells after longer incubation. As we can see in Table 1, the IC50 value of ORI nanosuspension was apparently lower than that of free ORI after the same incubation time, accounting for 42.33 μmol/L to 58.71 μmol/L, 22.59 μmol/L to 37.83 μmol/L, and 17.53 μmol/L to 25.25 μmol/L after 12, 24, and 36 hours of treatment, respectively. These data suggest that ORI nanosuspension was much more effective at inhibiting the growth of PC-3 cells as compared with free ORI. Our MTT assay demonstrated that ORI nanosuspension had a remarkable inhibitory effect on the proliferation of PC-3 cells. The inhibitory effect was increased with concentration and incubation time.
Figure 2.
MTT assay showing that the treatment of ORI nanosuspension and free ORI solution inhibit growth of PC-3 cells in a time- and dose-dependent manner. Results are expressed as mean ± standard deviation (n = 6).
Notes: *P < 0.05, **P < 0.01, ***P < 0.005 versus the same dose of free ORI solution group.
Table 1.
The IC50 values (μmol/L) of oridonin (ORI) nanosuspension and ORI solution on PC-3 cells (n = 6)
| Formulation | 12 hours | 24 hours | 36 hours |
|---|---|---|---|
| ORI nanosuspension | 42.33 ± 3.21 | 22.59 ± 1.08 | 17.53 ± 1.53 |
| ORI solution | 58.71 ± 4.37 | 37.83 ± 4.64 | 25.25 ± 0.79 |
Previous reports demonstrate that ORI inhibited cell proliferation and induced apoptosis in cancer cells.29,30 In the present study, we demonstrated that ORI nanosuspension could inhibit the growth of PC-3 cells, which were more significant than the ORI solution. It is possible that, like some other nanoparticle drug-delivery systems, the small size of the nanosuspension facilitated its adhesion to the cells and increased the contact area and time between the drug and cells.31,32
ORI nanosuspension induced morphologic changes in PC-3 cells
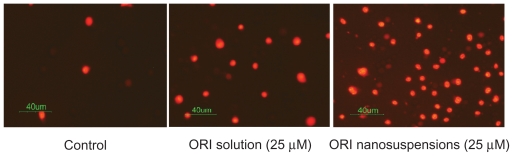
To investigate whether ORI inhibits the growth of PC-3 cells through inducing apoptosis in PC-3 cells, we examined the apoptotic morphological changes with ORI. After treatment with free ORI and ORI nanosuspension at the concentration of 25 μmol/L for 24 hours, PI assay was conducted. Observation under reverse fluorescence microscopy showed that PC-3 cells treated with ORI presented marked morphological changes. In Figure 3, the most obvious suggestion is that there are more dyed cells in the ORI nanosuspension than there are cells treated with free ORI and the controls. Typical morphologic changes of apoptosis including cell shrinkage, cell fragmentation, and cell irregularity in morphology were observed.33 The control cells showed normal morphology and only a thimbleful of cells were dyed. Meanwhile, as shown in Figure 3, the treatment of PC-3 cells with ORI nanosuspension resulted in more obvious changes in cell morphology in contrast to free ORI at the same dose. Results of PI staining demonstrated that ORI can induce apoptosis of PC-3 cells.34,35
Figure 3.
The effect of oridonin nanosuspension on apoptosis morphological change of PC-3 cells (X200).
Apoptosis is a form of self-regulated cell death, which differs from necrosis.36 Observation of morphological changes of ORI-treated cells suggested that ORI-induced PC-3 cell growth inhibition was involved in a mechanism of apoptosis.
ORI nanosuspension induced cycle arrest in PC-3 cells
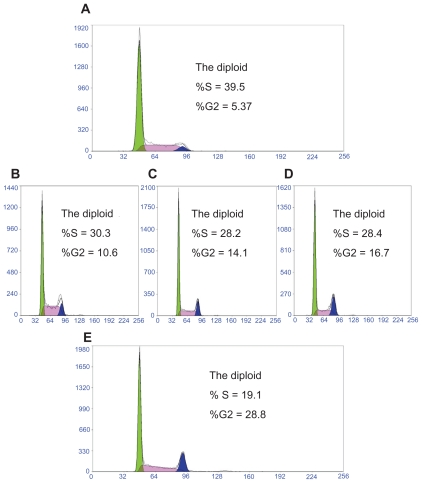
To confirm the effects of ORI on proliferation of PC-3 cell is mediated through inhibition of cell cycle progression, the cell cycle phases were analyzed by flow cytometry. Changes of cell cycle caused by ORI in PC-3 cells are shown in Figure 4. After treatment with free ORI for 24 hours, the percentage of S phase cells markedly decreased while the percentage of cells within the G2/M phase (10.6%–16.7%) markedly increased compared with that of the control cells. Further increases of ORI concentration had additional effects on the distribution of PC-3 cells in the cell cycle. As is shown in Figure 4, ORI nanosuspension could induce higher cell cycle arrest in contrast to the free ORI at the same concentration. Results indicated that ORI could arrest PC-3 cells in G2/M phase, and ORI nanosuspension induces a higher arrest compared with free ORI. Additionally, this arrest presents a concentration-dependent manner.2
Figure 4.
The effect of oridonin nanosuspension on the proliferation cycle of PC-3 cells. A) Control. B) 25 μmol/L ORI solution. C) 25 μmol/L ORI nanosuspension. D) 50 μmol/L ORI solution. E) 50 μmol/L ORI nanosuspension.
Judging from the result obtained by MTT experiments, 24 hours of ORI incubated with PC-3 was enough. In the 12-hour groups of the MTT experiments, the superiority of the nanosuspension formulation was not entirely displayed. Nevertheless, 36-hour cell incubation was not necessary as no new statistical difference appeared. Therefore, the incubation time of 24 hours was selected. Studies have shown that in addition to apoptosis, the growth inhibition induced by ORI treatment could partly be due to cell cycle arrest.37 Consistent with these results, our data showed the induction of growth inhibition of PC-3 cells by ORI could be of a significance of cell cycle arrest mechanism (together with a significance of apoptosis induction mechanism). Furthermore, we provided experimental evidence that the antitumor effect of ORI nanosuspension was associated with cell cycle arrest and morphological changes.37
Assessment of ORI nanosuspensioninduced apoptosis in PC-3 cells
One of the early physiological changes in a cell undergoing apoptosis is the redistribution of the phosphatidylserine (PS) from the inner to the outer leaflet of the plasma membrane.38 The binding of PS to annexin V conjugated to flourescein isothiocyanate (annexin V-FITC) was used to confirm apoptosis as described elsewhere.3,39
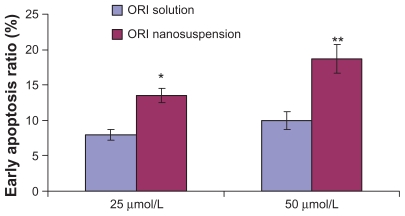
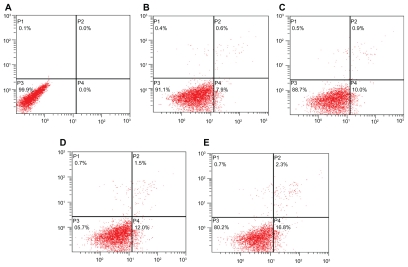
Annexin V-FITC/PI double-staining results showed that after treatment with ORI at different concentrations for 24 hours, the early apoptosis rate of PC-3 cells increased with ORI concentration, reaching 0.0%, 7.9%, 10.0%, at concentrations of 0 μmol/L, 25 μmol/L, and 50 μmol/L, respectively. That is to say, ORI treatment induces apoptosis in PC-3 cells in a concentration-dependent manner. To further investigate the effect of nanosuspension in inducing apoptosis in PC-3 cells, PC-3 cells were treated with ORI nanosuspension at the same concentration and experimental conditions. It was found that ORI nanosuspension-treated PC-3 cells revealed the expected increase in the early apoptosis rate, reaching 0.0%, 12.0%, 16.8%, at concentrations of 0 μmol/L, 25 μmol/L, and 50 μmol/L, respectively. As shown in Figure 5 and Figure 6, ORI nanosuspension presented a higher early apoptosis rate when compared with free ORI. Results showed that ORI could induce early apoptosis in PC-3 cells in a concentration-dependent manner, and there was a more prominent apoptosis rate in ORI nanosuspension-treated cells.
Figure 5.
The effect of oridonin nanosuspension on early apoptosis ratio of PC-3 cells (n = 3).
Notes: *P < 0.05, **P < 0.01, ***P < 0.005 versus the same dose of free ORI solution group.
Figure 6.
The effect of oridonin nanosuspensions on early apoptosis of PC-3 cells. A) Control. B) 25 μmol/L ORI solution. C) 50 μmol/L ORI solution. D) 25 μmol/L ORI nanosuspension. E) 50 μmol/L ORI nanosuspension.
Most of the present anticancer drugs mediate their effect via apoptosis induction in cancer cells, and apoptosis is suggested as one of the major mechanisms for the targeted therapy of various cancers including prostate cancer.40 We demonstrated that ORI could inhibit the growth of MCF-7 cells by apoptosis induction and arresting cell-cycle progression. Moreover, ORI nanosuspension was more significant than ORI solution.
Conclusion
In the present study, our data indicate an increased apoptosis in the ORI nanosuspension treatment group as compared with the solution group or the untreated control. In summary, ORI nanosuspension suppressed the PC-3 proliferation and enhanced the apoptosis of human prostate cancer PC-3 cells. These data suggest that nanosuspension may be an optimized ORI delivery formulation for prostate cancer therapy.
Acknowledgment
This work was supported by the Science Innovations of Shandong University (2009272).
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Scott SL, Gumerlock PH, Beckett L, Li Y, Goldberg Z. Survival and cell cycle kinetics of human prostate cancer cell lines after single- and multifraction exposures to ionizing radiation. Int J Radiat Oncol Biol Phys. 2004;59(1):219–227. doi: 10.1016/j.ijrobp.2004.01.027. [DOI] [PubMed] [Google Scholar]
- 2.Liou SF, Lin HH, Liang JC, Chen IJ, Yeh JL. Inhibition of human prostate cancer cells proliferation by a selective alpha1-adrenoceptor antagonist labedipinedilol-A involves cell cycle arrest and apoptosis. Toxicology. 2009;256:1–2. 13–24. doi: 10.1016/j.tox.2008.10.025. [DOI] [PubMed] [Google Scholar]
- 3.Ezekwudo D, Shashidharamurthy R, Devineni D, Bozeman E, Palaniappan R, Selvaraj P. Inhibition of expression of anti-apoptotic protein Bcl-2 and induction of cell death in radioresistant human prostate adenocarcinoma cell line (PC-3) by methyl jasmonate. Cancer Lett. 2008;270(2):277–285. doi: 10.1016/j.canlet.2008.05.022. [DOI] [PubMed] [Google Scholar]
- 4.Zhou JR, Yu L, Zhong Y, Blackburn GL. Soy phytochemicals and tea bioactive components synergistically inhibit androgen-sensitive human prostate tumors in mice. J Nutr. 2003;133(2):516–521. doi: 10.1093/jn/133.2.516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abeylath SC, Turos E, Dickey S, Lim DV. Glyconanobiotics: novel carbohydrated nanoparticle antibiotics for MRSA and Bacillus anthracis. Bioorg Med Chem. 2008;16(5):2412–2418. doi: 10.1016/j.bmc.2007.11.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang H, Ye Y, Chui JH, et al. Oridonin induces G2/M cell cycle arrest and apoptosis through MAPK and p53 signaling pathways in HepG2 cells. Oncol Rep. 2010;24(3):647–651. [PubMed] [Google Scholar]
- 7.Hsieh TC, Wijeratne EK, Liang JY, Gunatilaka AL, Wu JM. Differential control of growth, cell cycle progression, and expression of NF-kappaB in human breast cancer cells MCF-7, MCF-10A, and MDA-MB-231 by ponicidin and oridonin, diterpenoids from the chinese herb Rabdosia rubescens. Biochem Biophys Res Commun. 2005;337(1):224–231. doi: 10.1016/j.bbrc.2005.09.040. [DOI] [PubMed] [Google Scholar]
- 8.Ikezoe T, Chen SS, Tong XJ, Heber D, Taguchi H, Koeffler HP. Oridonin induces growth inhibition and apoptosis of a variety of human cancer cells. Int J Oncol. 2003;23(4):1187–1193. [PubMed] [Google Scholar]
- 9.Chen S, Gao J, Halicka HD, Traganos F, Darzynkiewicz Z. Downregulation of androgen-receptor and PSA by phytochemicals. Int J Oncol. 2008;32(2):405–411. [PMC free article] [PubMed] [Google Scholar]
- 10.Angelloz-Nicoud P, Lalou C, Binoux M. Prostate carcinoma (PC-3) cell proliferation is stimulated by the 22-25-kDa proteolytic fragment (1-160) and inhibited by the 16-kDa fragment (1-95) of recombinant human insulin-like growth factor binding protein-3. Growth Horm IGF Res. 1998;8(1):71–75. doi: 10.1016/s1096-6374(98)80324-9. [DOI] [PubMed] [Google Scholar]
- 11.Gao L, Zhang D, Chen M, Zheng T, Wang S. Preparation and characterization of an oridonin nanosuspension for solubility and dissolution velocity enhancement. Drug Dev Ind Pharm. 2007;33(12):1332–1339. doi: 10.1080/03639040701741810. [DOI] [PubMed] [Google Scholar]
- 12.Wang Y, Zhang D, Liu Z, et al. In vitro and in vivo evaluation of silybin nanosuspensions for oral and intravenous delivery. Nanotechnology. 2010;21(15):155104. doi: 10.1088/0957-4484/21/15/155104. [DOI] [PubMed] [Google Scholar]
- 13.Wong J, Brugger A, Khare A, et al. Suspensions for intravenous (IV) injection: A review of development, preclinical and clinical aspects. Adv Drug Delivery Rev. 2008;60(8):939–954. doi: 10.1016/j.addr.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 14.Rabinow BE. Nanosuspensions in drug delivery. Nat Rev Drug Discovery. 2004;3(9):785–796. doi: 10.1038/nrd1494. [DOI] [PubMed] [Google Scholar]
- 15.Muller RH, Keck CM. Challenges and solutions for the delivery of biotech drugs – a review of drug nanocrystal technology and lipid nanoparticles. J Biotechnol. 2004;113:1–3. 151–170. doi: 10.1016/j.jbiotec.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 16.Keck CM, Muller RH. Drug nanocrystals of poorly soluble drugs produced by high pressure homogenisation. Eur J Pharm Biopharm. 2006;62(1):3–16. doi: 10.1016/j.ejpb.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 17.Gao L, Zhang D, Chen M, et al. Studies on pharmacokinetics and tissue distribution of oridonin nanosuspensions. Int J Pharm. 2008;355:1–2. 321–327. doi: 10.1016/j.ijpharm.2007.12.016. [DOI] [PubMed] [Google Scholar]
- 18.Spernath A, Aserin A, Ziserman L, Danino D, Garti N. Phosphatidylcholine embedded microemulsions: physical properties and improved Caco-2 cell permeability. J Control Release. 2007;119(3):279–290. doi: 10.1016/j.jconrel.2007.02.014. [DOI] [PubMed] [Google Scholar]
- 19.Gao L, Zhang DR, Chen MH, et al. Studies on pharmacokinetics and tissue distribution of oridonin nanosuspensions. Int J Pharm. 2008;355:1–2. 321–327. doi: 10.1016/j.ijpharm.2007.12.016. [DOI] [PubMed] [Google Scholar]
- 20.Zhang DR, Ren TC, Lou HX, Xing J. The tissue distribution in mice and pharmacokinetics in rabbits of oridonin-solid lipid nanoparticles. Yao Xue Xue Bao. 2005;40(6):573–576. [PubMed] [Google Scholar]
- 21.Dai W, Zhang D, Duan C, et al. Preparation and characteristics of oridonin-loaded nanostructured lipid carriers as a controlled-release delivery system. J Microencapsul. 2010;27(3):234–241. doi: 10.3109/02652040903079526. [DOI] [PubMed] [Google Scholar]
- 22.Lou H, Zhang X, Gao L, et al. In vitro and in vivo antitumor activity of oridonin nanosuspension. Int J Pharm. 2009;379(1):181–186. doi: 10.1016/j.ijpharm.2009.06.022. [DOI] [PubMed] [Google Scholar]
- 23.Beck RCR, Pohlmann AR, Hoffmeister C, et al. Dexamethasone-loaded nanoparticle-coated microparticles: correlation between in vitro drug release and drug transport across Caco-2 cell monolayers. Eur J Pharm Biopharm. 2007;67(1):18–30. doi: 10.1016/j.ejpb.2007.01.007. [DOI] [PubMed] [Google Scholar]
- 24.Liao J, Anchun M, Zhu Z, Quan Y. Antibacterial titanium plate deposited by silver nanoparticles exhibits cell compatibility. Int J Nanomedicine. 2010;5:337–342. doi: 10.2147/ijn.s9518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mathot F, van Beijsterveldt L, Preat V, Brewster M, Arien A. Intestinal uptake and biodistribution of novel polymeric micelles after oral administration. J Control Release. 2006;111:1–2. 47–55. doi: 10.1016/j.jconrel.2005.11.012. [DOI] [PubMed] [Google Scholar]
- 26.Degim Z, Unal N, Essiz D, Abbasoglu U. The effect of various liposome formulations on insulin penetration across Caco-2 cell monolayer. Life Sci. 2004;75(23):2819–2827. doi: 10.1016/j.lfs.2004.05.027. [DOI] [PubMed] [Google Scholar]
- 27.Ajana I, Astier A, Gibaud S. Arsthinol nanosuspensions: pharmacokinetics and anti-leukaemic activity on NB4 promyelocytic leukaemia cells. J Pharm Pharmacol. 2009;61(10):1295–1301. doi: 10.1211/jpp/61.10.0004. [DOI] [PubMed] [Google Scholar]
- 28.Martin SJ, Reutelingsperger CP, McGahon AJ, et al. Early redistribution of plasma membrane phosphatidylserine is a general feature of apoptosis regardless of the initiating stimulus: inhibition by overexpression of Bcl-2 and Abl. J Exp Med. 1995;182(5):1545–1556. doi: 10.1084/jem.182.5.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xu W, Sun J, Zhang T, et al. A rapid HPLC/ESI-MS method for the quantitative determination of oridonin in rat plasma. Pharmazie. 2006;61(9):757–759. [PubMed] [Google Scholar]
- 30.Cheng Y, Qiu F, Ye YC, Tashiro S, Onodera S, Ikejima T. Oridonin induces G2/M arrest and apoptosis via activating ERK-p53 apoptotic pathway and inhibiting PTK-Ras-Raf-JNK survival pathway in murine fibrosarcoma L929 cells. Arch Biochem Biophys. 2009;490(1):70–75. doi: 10.1016/j.abb.2009.08.011. [DOI] [PubMed] [Google Scholar]
- 31.Lamprecht A. IBD: selective nanoparticle adhesion can enhance colitis therapy. Nat Rev Gastroenterol Hepatol. 2010;7(6):311–312. doi: 10.1038/nrgastro.2010.66. [DOI] [PubMed] [Google Scholar]
- 32.Du D, Liu S, Chen J, Ju H, Lian H, Li J. Colloidal gold nanoparticle modified carbon paste interface for studies of tumor cell adhesion and viability. Biomaterials. 2005;26(33):6487–6495. doi: 10.1016/j.biomaterials.2005.03.048. [DOI] [PubMed] [Google Scholar]
- 33.Xu WL, Liu JR, Liu HK, et al. Inhibition of proliferation and induction of apoptosis by gamma-tocotrienol in human colon carcinoma HT-29 cells. Nutrition. 2009;25(5):555–566. doi: 10.1016/j.nut.2008.10.019. [DOI] [PubMed] [Google Scholar]
- 34.Cheng Y, Qiu F, Ye YC, Tashiro S, Onodera S, Ikejima T. Oridonin induces G2/M arrest and apoptosis via activating ERK-p53 apoptotic pathway and inhibiting PTK-Ras-Raf-JNK survival pathway in murine fibrosarcoma L929 cells. Arch Biochem Biophys. 2009;490(1):70–75. doi: 10.1016/j.abb.2009.08.011. [DOI] [PubMed] [Google Scholar]
- 35.Zhang JF, Liu JJ, Liu PQ, Lin DJ, Li XD, Chen GH. Oridonin inhibits cell growth by induction of apoptosis on human hepatocelluar carcinoma BEL-7402 cells. Hepatol Res. 2006;35(2):104–110. doi: 10.1016/j.hepres.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 36.Song G, Luo Q, Qin J, Wang L, Shi Y, Sun C. Effects of oxymatrine on proliferation and apoptosis in human hepatoma cells. Colloids Surf B Biointerfaces. 2006;48(1):1–5. doi: 10.1016/j.colsurfb.2005.12.012. [DOI] [PubMed] [Google Scholar]
- 37.Ren KK, Wang HZ, Xie LP, et al. The effects of oridonin on cell growth, cell cycle, cell migration and differentiation in melanoma cells. J Ethnopharmacol. 2006;103(2):176–180. doi: 10.1016/j.jep.2005.07.020. [DOI] [PubMed] [Google Scholar]
- 38.Rucker-Martin C, Henaff M, Hatem SN, Delpy E, Mercadier JJ. Early redistribution of plasma membrane phosphatidylserine during apoptosis of adult rat ventricular myocytes in vitro. Basic Res Cardiol. 1999;94(3):171–179. doi: 10.1007/s003950050140. [DOI] [PubMed] [Google Scholar]
- 39.Ernst JD, Yang L, Rosales JL, Broaddus VC. Preparation and characterization of an endogenously fluorescent annexin for detection of apoptotic cells. Anal Biochem. 1998;260(1):18–23. doi: 10.1006/abio.1998.2677. [DOI] [PubMed] [Google Scholar]
- 40.Huang X, Zhu D, Lou Y. A novel anticancer agent, icaritin, induced cell growth inhibition, G1 arrest and mitochondrial transmembrane potential drop in human prostate carcinoma PC-3 cells. Eur J Pharmacol. 2007;564:1–3. 26–36. doi: 10.1016/j.ejphar.2007.02.039. [DOI] [PubMed] [Google Scholar]