Abstract
The upsurge of multiple-drug-resistant microbes warrants the development and/or use of effective antibiotics. Triclosan, though used in cosmetic and dermatological preparations for several decades, has not been used as a systemic antibacterial agent due to problems of drug administration. Here we report the striking efficacy of triclosan in a mouse model of acute systemic bacterial infection. Triclosan not only significantly extends the survival time of the infected mice, it also restores blood parameters and checks liver damage induced by the bacterial infection. We believe that the excellent safety track record of triclosan in topical use coupled with our findings qualifies triclosan as a candidate drug or lead compound for exploring its potential in experimental systems for treating systemic bacterial infections.
Multiple-drug resistance is one of the major immediate threats to human health today. The upsurge of antibiotic-resistant bacteria in recent years has contributed much towards the increased mortality and morbidity associated with systemic infectious diseases like pneumonia, tuberculosis, and meningitis (42). It takes only a glance at the World Health Organization fact sheets for the gravity of the situation to sink in (42). Numerous antibiotics, which saved countless lives and dramatically reduced the duration of diseases in the mid-20th century, the golden age of chemotherapy, are currently all but ineffectual against weakly pathogenic microbes. The situation at hand, therefore, warrants the development and/or use of novel antibiotics to combat these resilient pathogens. While the quest for new antimicrobials, including peptides, goes on (7, 16, 24, 28, 35), there is at least one existing antimicrobial agent with as-yet-untapped potential—triclosan.
2,4,4′-Trichloro-2′-hydroxy-diphenyl ether, commonly referred to as triclosan, is a broad-spectrum hydrophobic antimicrobial agent. Because of its favorable safety profile, triclosan has been used for the past 2 decades in several dermatological preparations and oral hygiene products. Results of toxicology studies show that triclosan and its metabolites are well tolerated by a variety of species, including human beings (1, 5, 21, 37). Triclosan is not a carcinogen, mutagen, or teratogen and has been found to be safe in reproductive studies (5, 37). Though triclosan was suggested to have membranotropic effects, its recently discovered principal target is enoyl-acyl carrier protein (ACP) reductase, a key enzyme catalyzing the rate-determining step of the elongation cycle of the type II fatty acid biosynthesis pathway (14, 27, 39).
Fatty acids are essential components of phospholipids and sphingolipids that make up the plasma membrane and the membranes of intracellular organelles and are therefore indispensable to living systems. All organisms, therefore, have the ability to synthesize fatty acids from simple precursors, except the mycoplasmas, which scavenge them from their hosts. Fatty acid synthesis basically involves a succession of reduction and dehydration steps following the condensation of acetyl and malonyl moieties to build the fatty acid chain. This series of steps is brought about by distinct enzymes in the dissociative type of fatty acid synthase (FAS) or type II FAS (FASII) system present in bacteria and plastids of plants and algae but is brought about by a single multifunctional enzyme in the associative type of FAS (FASI) present in animal cells, yeasts, and fungi. These differences in the FAS systems of mammals, bacteria, and protozoans have given impetus to the development of the inhibitors of FASII as candidate drugs for treating infectious diseases (11, 25, 33). Among these inhibitors, none is as potent as triclosan (12, 33). The type I fatty acid biosynthesis pathway that operates in human beings is not inhibited by triclosan (33). The indispensability of fatty acid synthesis to life, coupled with the different systems of FAS operating in bacteria and its human hosts, makes triclosan a valuable antibacterial agent worthy of exploration for treating systemic bacterial infections.
The kinetics of inhibition of the bacterial enoyl-ACP reductases, as well as of the Plasmodium falciparum enoyl-ACP reductases by triclosan, has been well elucidated (10, 12, 13, 17, 30, 31, 32, 38). Moreover, in the span of the 2 decades of its use, there have been no reports of triclosan-resistant microbes in the wild, though Escherichia coli and Sphingosamine strains have been selected for resistance in vitro in the laboratory (9, 27). Though all these facts make triclosan a valuable antibiotic, its use has hitherto been limited to topical applications, principally due to problems of drug delivery. Toxicology studies performed with Sprague Dawley rats indicate that the oral, intravenous, and subcutaneous 50% lethal dose values for triclosan are approximately 4,000, 29, and 14,700 mg/kg of body weight, respectively (21), clearly ruling out intravenous delivery of triclosan. Here we report for the first time the efficacy of triclosan as an antibacterial agent in systemic bacterial infections. Our experiments demonstrate that triclosan, in a low-dose, galactosamine-sensitized mouse model of an acute E. coli O55:B5 infection, can increase the survival time by up to 48 h, compared to just 7 to 10 h for uninfected mice and 24 h with ampicillin and tetracycline. Our findings may have significant implications for treating systemic bacterial infections.
MATERIALS AND METHODS
Reagents.
Triclosan-5000 (minimum purity, 99%) was purchased from Kumar Products Ltd., Bangalore, India, and d-galactosamine and dimethyl sulfoxide (DMSO) were from Sigma. All other reagents used were of analytical grade.
Bacterial strain.
The bacterial strain used for the study was E. coli O55:B5. The inoculum used, which contained 107 CFU in 0.4 ml of pyrogen-free saline, was prepared by growing a single colony of the bacterium overnight in Luria-Bertani (LB) broth and washing the cells with 0.9% saline made in endotoxin-free water.
Animals.
BALB/c mice 6 to 9 weeks of age were used for the study. All animals were kept under pathogen-free conditions during the study.
Infection of animals.
The animals were injected intraperitoneally with 0.4 ml of pyrogen-free saline containing 107 CFU of bacteria and d-galactosamine (300 mg/kg of body weight) (4, 8, 19).
Drug administration.
A subcutaneous injection of triclosan (40 mg/kg) in sterile DMSO was given middorsally 2 h prior to bacterial infection and every 12 h after infection. Ampicillin (40 mg/kg), tetracycline (40 mg/kg), or pyrogen-free water alone was subcutaneously or intraperitoneally injected into other groups of mice. Each group consisted of six male mice, and the experiments were repeated at least three times. All drug stocks used were tested for their antibacterial efficacies by disk diffusion susceptibility tests before use in the animal experiments.
Histopathology.
Mice were sacrificed by cervical dislocation and dissected, and their livers, lungs, kidneys, and hearts were removed for histopathological analysis. For blood analysis, animals were bled from the retro-orbital complex, and the serum of each mouse was analyzed for urea, glucose, creatinine, serum glutamic pyruvic transaminase (SGPT) (serum alanine aminotransferase), and serum glutamic oxaloacetic transaminase (SGOT).
Assay for detection of TNF-α.
The tumor necrosis factor alpha (TNF-α) level in the serum was determined by a bioassay using the L2N2 macrophage cell line (15). TNF-α levels of uninfected mice, uninfected mice administered triclosan, uninfected mice administered 500 μg of lipopolysaccharide (LPS), and infected mice administered triclosan, ampicillin, and tetracycline were determined at 1, 2, and 3 h after infection or sham injection.
Quantitative assessment of viable bacteria in infected and treated mice.
Mice were subcutaneously injected with 40 mg of triclosan/kg in DMSO, plain DMSO, or 40 mg of ampicillin/kg, 3 h after which they were infected with 107 CFU bacteria injected intraperitoneally. Blood was drawn in heparin from the intraorbital vein at 3, 7, and 10 h after infection. The plasma was separated from the blood cells and plated at a dilution of 1:102 on LB agar. The number of colonies was assessed after overnight incubation at 37°C. The MICs and 50% inhibitory concentrations (IC50s) for these isolates were also determined by using the procedure outlined below.
Quantitation of triclosan in vivo after injection in mice.
Blood was collected in heparin from 6- to 8-week-old BALB/c mice at different time points (3, 7, 10, 20, and 36 h). Triclosan was subcutaneously injected into the mice at 0, 12, 24, and 36 h. The plasma was separated, and up to 1 ml of plasma in an equal volume of 0.2 M sodium acetate-acetic acid (pH 5.0) was added. To this, β-glucuronidase (100 U) was added, and the solution was mixed and incubated at 40°C for 10 to 15 h. Solid-phase extraction was done by using SEP-PAK columns (Millipore Waters). The columns were prepared by passing 3 ml of methanol and then 1 ml of water through them. After the sample was loaded, the column was rinsed with 1 ml of distilled water and dried under vacuum for 5 min. The samples were eluted with 700 μl of methanol. The eluate was diluted 1:1 in distilled water, loaded on a C18 reverse-phase column (4.6 mm by 25 cm), and eluted with an isocratic gradient of acetonitrile and 0.1% trifluoroacetic-acid-phosphate (60/40) by using FPLC-AKTA Basic (Amersham Pharmacia). The absorbance was measured at 230, 240, and 280 nm.
MIC and IC50 determinations.
The MICs of triclosan, tetracycline, and ampicillin for the infecting strain of E. coli used, O55:B5, were determined by growing the bacteria in the presence of various concentrations of the compound of interest in LB broth at 37°C with shaking. The absorbance at 600 nm was read after 12 h and plotted versus the log concentration of the antimicrobial used. The MIC was read as the lowest concentration that resulted in no bacterial growth after 12 h, and the IC50 was determined as the concentration of the antimicrobial at which there was a 50% reduction of the absorbance at 600 nm.
Statistical test.
The differences in the experimental values obtained for the different groups were tested for statistical significance by one-way analysis of variance (ANOVA). A P value less than 0.01 was considered significant.
RESULTS
Effect of triclosan on the survival time of infected mice.
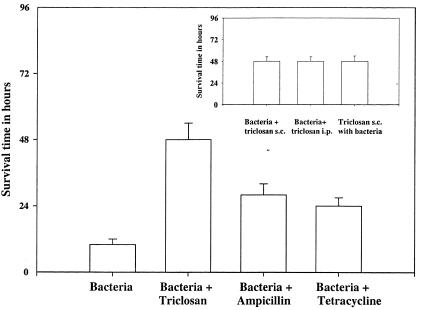
Four groups of at least five 6- to 8-week-old male BALB/c mice each were used for the study. The first group was injected intraperitoneally with 107 CFU of bacteria and d-galactosamine (300 mg/kg of body weight). The second group of mice was administered the drug alone—40 mg of triclosan, ampicillin, or tetracycline per kg. The third group was administered both bacteria and the drug, and the fourth group, which served as the sham infection control, was administered neither bacteria nor drug. Preliminary experiments demonstrated that death ensued within 7 to 10 h of infection in the untreated, low-dose, galactosamine-sensitized, bacterial-challenge mouse model as a result of hepatic necrosis. Also, triclosan injected intravenously or intramuscularly resulted in death or paralysis, rendering these two routes of drug delivery unsuitable. The subcutaneous administration of triclosan 2 h prior to, and every 12 h after, acute infection of the mice increased the survival time of the mice to 48 h, compared to a survival time of only 7 to 10 h for mice which were administered no drug following infection, and 24 to 30 h for infected mice which were administered ampicillin or tetracycline. Triclosan was effective in evoking a prolonged survival period of 48 h even on its administration along with, instead of prior to, the injection of bacteria. The route of administration (subcutaneous or intraperitoneal) did not evoke a significant difference in the results obtained with any of these drugs. Control mice as well as mice administered triclosan alone remained alive for several weeks after the completion of the experiment (Fig. 1). The differences in the survival times of the mice in the various groups were statistically significant (from five one-way ANOVA tests, P < 0.001)
FIG. 1.
Survival times based on a a low-dose, galactosamine-sensitized mouse model of an acute, systemic E. coli infection. The survival time of the mice was 48 h (±5 h) upon triclosan treatment compared to only 8 h (±1 h) for untreated and 28 h (±3 h) and 24 h (±2 h) for ampicillin- and tetracycline-treated mice, respectively. Statistical significance was tested by one-way ANOVA (P < 0.001, n = 5). (Inset) Triclosan administration along with, instead of prior to, infection as well as intraperitoneal administration instead of subcutaneous administration also increased the survival time to 48 h.
Histopathology studies.
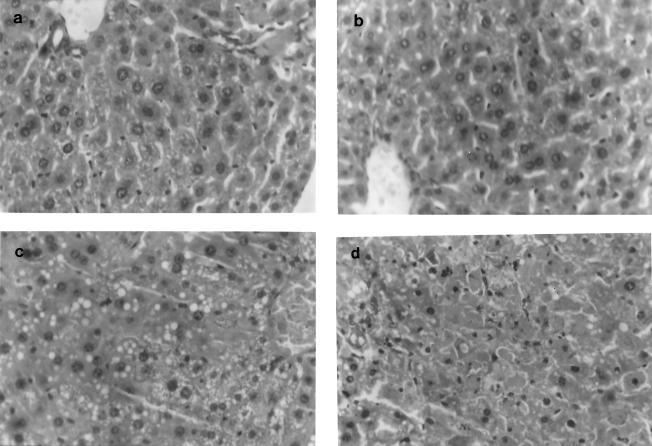
We conducted histopathological studies on each of these four groups of mice to study the effects of infection and administration of triclosan (Fig. 2). Following infection and/or administration of the drug, mice were killed by cervical dislocation and dissected, and major organs were removed for histopathological analysis. Sections of the lung, kidney, heart, and spleen did not show significant changes on infection or administration of the drug. While sections of liver from the control mice as well as from uninfected mice that were administered triclosan showed a normal pattern of hepatic and biliary parenchyma,sections of liver from infected mice that were not administered the drug showed hydropic and fatty changes, with areas of congestion and hemorrhage, i.e., hepatic necrosis. Sections of liver from infected mice administered triclosan showed significantly fewer areas of congestion, hemorrhages, and fatty changes. (The levels of subcutaneously and intraperitoneally administered triclosan at which there were no observed effects were found to be 700 and 900 mg/kg, respectively, as determined by assessing the liver histopathology for changes in the hepatic and biliary parenchyma.)
FIG. 2.
Liver histopathology (methylene blue and eosin yellow staining) indicates normal biliary parenchyma in control (a) and triclosan-administered (b) mice. Liver sections of infected mice (c) show hydropic and fatty changes with areas of hemorrhage, indicative of severe liver damage, which is considerably reduced in infected mice administered triclosan (d).
Effect of triclosan on blood parameters.
We also analyzed the levels of serum urea (urease enzymatic method) (6, 41), blood glucose (glucose oxidase and peroxidase enzymatic method) (36), serum creatinine (Mos Jaffe's kinetic method) (2, 3), and serum SGPT (Reitman and Frankel's method) and SGOT (Reitman and Frankel's method) (18, 26). All these blood parameters remained within the normal range (23) upon administration of triclosan. Levels in the blood of glucose and urea decreased and increased, respectively, in the infected mice to 35.1 and 88.75 mg/dl, respectively, compared to levels in their uninfected (67.5 and 54 mg/dl) and triclosan-administered (70.4 and 50.25 mg/dl) counterparts. Blood glucose and urea levels in triclosan-administered infected mice were 32.4 and 72 mg/dl, with the urea levels tending to be similar to the levels seen in the control mice. (Urea levels of triclosan-administered infected mice were significantly lower than those of the untreated infected mice as determined by the t test [P = 0.058].) The levels of SGOT and SGPT in uninfected control mice (57.5 and 73.3 U/ml) as well as those receiving triclosan alone (63.1 and 81.3 U/ml) were similar. Levels in serum of SGOT and SGPT increased upon bacterial infection to 130 and 200 U/ml, respectively, but were nearly normal in triclosan-administered infected mice at 92.6 and 110 U/ml (Table 1).
TABLE 1.
Analysis of blood samples of all four groups of micea
| Mouse groupb | Urea (mg/dl) | Glucose (mg/dl) | Creatinine (mg/dl) | SGPT (U/ml) | SGOT (U/ml) |
|---|---|---|---|---|---|
| Control | 54.0 (±6.3) | 67.5 (±5.3) | 1.7 (±0.1) | 73.3 (±5.1) | 57.5 (±4.0) |
| Administered drug only | 50.25 (±4.1) | 70.4 (±7.2) | 1.8 (±0.15) | 81.3 (±6.1) | 63.1 (±6.8) |
| Infected with bacteria | 88.75 (±7.8) | 35.1 (±3.2) | 2.3 (±0.18) | 200.0 (±12.3) | 130.0 (±11.1) |
| Administered drug and infected with bacteria | 72.0 (±6.6) | 32.4 (±2.8) | 1.6 (±0.16) | 110.0 (±9.1) | 92.6 (±8.7) |
The data reported are the mean values from three independent sets of experiments conducted each time with six mice in each group. Numbers in parentheses indicate values of standard deviation.
The group of uninfected mice administered triclosan only was bled after 5 days of continual drug administration, the group of mice with bacterial infection was bled after 6 h of bacterial infection, and the group of infected mice administered triclosan was bled after 2 days of bacterial infection.
Effect of triclosan on TNF-α levels.
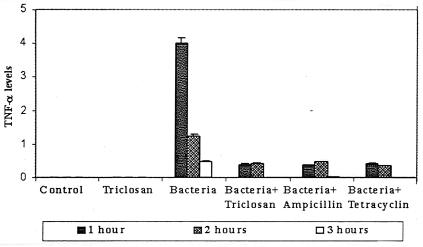
A bioassay to mark the presence of serum TNF-α demonstrated that TNF-α levels were not detectable in the control group or in uninfected mice receiving triclosan; however, the serum TNF-α level was elevated in mice with acute bacterial infection. It reached a maximum at around 1 h after infection and declined during the next 2 h. Significantly, TNF-α was substantially reduced 1 h after infection in mice treated with triclosan prior to infection and was undetectable by 3 h after infection (Fig. 3). The antibiotics ampicillin and tetracycline also modulated TNF-α levels in a similar manner (Fig. 3). Triclosan did not lower TNF-α levels in mice administered 500 μg of LPS alone, suggesting that triclosan exerts its effects on TNF-α production by virtue of its antibacterial effect only.
FIG. 3.
The serum TNF-α level, undetectable in the untreated mice, was elevated in mice with bacterial infection. Triclosan, ampicillin, or tetracycline administration prior to infection resulted in a significantly lower level of TNF-α. Animals not infected with bacteria but treated with triclosan showed no TNF-α in their sera. Animals treated with 500 μg of LPS/kg had TNF-α levels of 5.2, 6.0, and 4.6 at 1, 2, and 3 h, respectively.
Effect of triclosan on bacteremia.
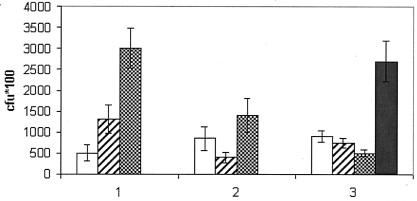
While the TNF-α levels gave an idea of the efficacy of the triclosan treatment, in order to assess the antibacterial effect of triclosan, a more direct method was required. Therefore, we assessed the effect of triclosan by assessing bacteremia after infection with and without treatment. Counts of viable bacteria present in the blood after infection with and without treatment with triclosan showed that while the bacteremia in the control mice increased rapidly from around 5 × 104 CFU at 3 h up to 3 × 105 CFU at 10 h after infection, the counts of viable bacteria in the ampicillin- and triclosan-treated mice were much lower. In the triclosan-treated mice, the bacteremia steadily decreased to less than 5 × 104 CFU at 10 h after infection but reached around 3 × 105 CFU at 45 h. In the ampicillin-treated mice, the viable-cell counts decreased to less than 5 × 104 CFU at 7 h after infection but rose to around 1.5 × 105 CFU at 10 h after infection (Fig. 4). Neither the IC50s nor the MICs of ampicillin and triclosan were altered for the bacterial strains isolated at 10 and 45 h, respectively, from the ampicillin- and triclosan-treated mice.
FIG. 4.
Counts of viable bacteria in the blood of infected control mice (bars labeled “1”), ampicillin-treated mice (bars labeled “2”), or triclosan-treated mice (bars labeled “3”) after 3 h (open bars), 7 h (hatched bars), 10 h (checkerboard bars), and 45 h (solid bar). Triclosan or ampicillin administration resulted in lower bacteremia.
Levels of triclosan in blood.
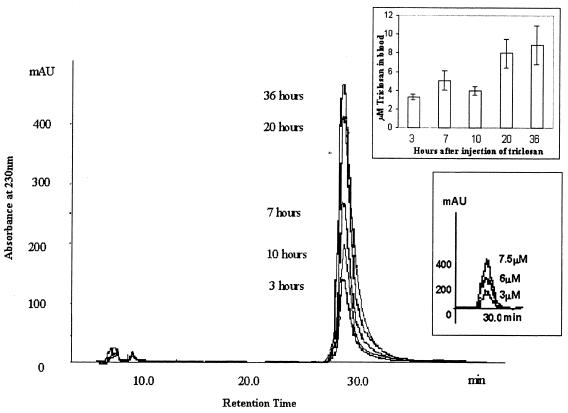
Since it was clear that the bacterial counts in the triclosan-treated mice were definitely lower than in the control infected mice, we needed a method of verifying that the concentration of triclosan in the blood at the dosage used was sufficient to invoke a clinical response. We developed a solid-phase extraction procedure coupled with a fast-performance liquid chromatography (FPLC) method to determine the levels of triclosan in the blood of mice after administration of triclosan subcutaneously. The triclosan level in the blood was detectable (3.3 μM, i.e., 950 ng/ml) 3 h after administration and was further elevated at 7 h (5.1 μM, i.e., 1,468 ng/ml), but declined at 10 h (4 μM, i.e., 1,152 ng/ml). Following repeated triclosan dosages at 12 and 24 h, the triclosan levels at 20 and 36 h were also elevated (8 and 8.9 μM, i.e., 2,304 and 2,563 ng/ml, respectively) (Fig. 5).
MICs of triclosan, ampicillin, and tetracycline.
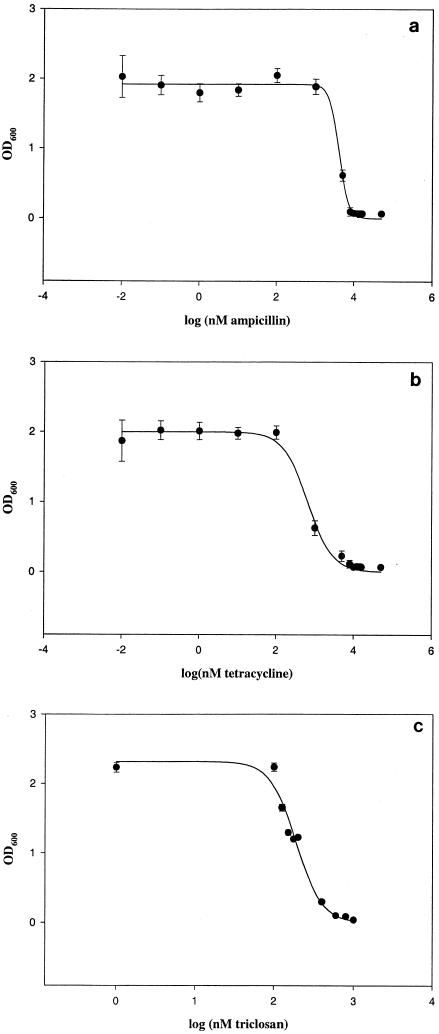
The MICs of triclosan, ampicillin, and tetracycline were determined by standard techniques as described above (Fig. 6). The results confirmed that the strain of E. coli which we used for the experiments, O55:B5, was indeed sensitive to triclosan, ampicillin, and tetracycline. The MICs of triclosan, ampicillin, and tetracycline were 600 nM (i.e., 172 ng/ml), 8 μM (i.e., 2,971 ng/ml), and 5 μM (i.e., 2,222 ng/ml), respectively (the range of MIC breakpoints of ampicillin and tetracycline for E. coli-susceptible strains are 5.4 to 21.5 μM, i.e., 2,005 to 7,985 ng/ml, and 2.25 to 9 μM, i.e., 1,000 to 4,000 ng/ml, respectively).
FIG. 6.
Inhibition curves of ampicillin (MIC, 8 μM) (a), tetracycline (MIC, 5 μM) (b), and triclosan (MIC, 600 nM) (c). The IC50 of triclosan was found to be around 150 nM, and the MIC was 600 nM. OD600, optical density at 600 nm.
DISCUSSION
In order to avoid immune system-related complications associated with chronic infections, we chose a low-dose, galactosamine-sensitized mouse model of an acute E. coli O55:B5 infection akin to that used in endotoxin-related studies (4, 8, 19). d-Galactosamine acts by increasing the susceptibilities of mice by several thousandfold to the lethal effects of TNF, a monokine produced on stimulation with LPS. Such a model typically results in death at around 7 to 10 h following infection, thereby allowing quick assessment of the treatment undertaken by monitoring the survival times of the mice. As shown in Fig. 1, the survival time of the infected mice administered triclosan subcutaneously or intraperitoneally prior to or during infection was significantly prolonged (∼48 h) compared to that of mice administered ampicillin or tetracycline (∼24 to 30 h). The longer survival time achieved with triclosan clearly indicates the superiority of triclosan as an antibacterial agent for systemic infections compared to ampicillin and tetracycline (20, 22, 29, 40, 43). Moreover, at the dosage of 40 mg/kg, the concentration of triclosan used during this study, the levels of triclosan in the blood were around 3 to 9 μM (864 to 2,592 ng/ml) (as determined by FPLC), which is greater than the MIC of triclosan. This explains why triclosan at this dosage is effective at reducing the bacteremia and increasing the survival time of the mice. Assuming a total volume in the blood of 3 ml per mouse (average weight, ∼20 g), the effective amount of triclosan in the blood coming from a dosage of 40 mg/kg, i.e., 800 μg/mouse, is 3 μg, which is therapeutic and yet comes from a dosage below the level at which no effects were observed.
Histopathology studies using sections of kidney, spleen, heart, and lung revealed no significant changes, whereas sections of the liver mirrored the pathological state of the mice (Fig. 2). A normal pattern of biliary parenchyma was seen in sections of liver from uninfected mice as well as those from infected mice administered only triclosan, demonstrating the excellent safety profile of triclosan. Sections of liver from infected mice were, however, punctuated by fatty and hydropic changes as well as by regions of hemorrhages, indicative of severe liver damage. The finding of considerably fewer areas of congestion and fatty changes in sections of liver from triclosan-administered infected mice clearly reiterates its efficacy as a systemic antibacterial agent. Thus, these histopathological studies confirm the safety of triclosan injected subcutaneously or intraperitoneally and also highlight its profound antibacterial effect.
Triclosan by itself did not alter any of the blood parameters (Table 1). However, its administration to infected mice brought most of these parameters closer to the values observed for the uninfected mice. These results thus highlight the potency of triclosan as a curative agent in systemic bacterial infections.
TNF-α, a polyfunctional, proinflammatory cytokine, is known to exhibit characteristic kinetics of appearance in the serum following infection with gram-negative bacteria and plays the role of a central mediator of the shock which develops in the course of bacterial infections (34). The elevated level of TNF-α, a hallmark of bacterial infections, is reduced by treatment with several antibiotics (22, 29). Tetracyclines, for instance, have been reported to significantly reduce the serum interleukin-1α and TNF-α levels at the times of peak production, i.e., around 4 and 2 h, respectively, after LPS challenge, and to maintain this effect even 12 h afterwards (22). In our study, the serum TNF-α level in mice infected with E. coli did shoot up rapidly within the first hour of infection and subsequently decreased to undetectable levels, as expected. Strikingly, however, the initial high level of TNF-α in infected mice was substantially lowered on administration of triclosan prior to infection, as it also was by administration of ampicillin and tetracycline (Fig. 3). Production of TNF-α was not seen in the sham-infected control mice or in the uninfected mice treated with triclosan. Triclosan had no effect on TNF-α levels of mice treated with LPS alone, ruling out any direct link between the production of the cytokine and the hydroxydiphenyl ether. This finding that triclosan, in fact, modulates serum TNF-α levels in the infected mice alone adds credence to our other results, confirming the efficacy of triclosan as a systemic antibacterial agent in the acute-bacterial-challenge mouse model.
We have also demonstrated that the effects seen in the experiments are antimicrobial in nature by quantitative assessment of viable bacteria in treated and control mice, again ruling out the theory that the effect is entirely due to triclosan-blocking cytokine effects, improving survival as a biological response modifier (Fig. 3 and 4). Moreover, the levels of triclosan in blood reached clinical levels during the time that the counts of viable bacteria in the blood were reduced in the triclosan-treated mice compared to counts in the controls, thus confirming the antimicrobial effect of triclosan (Fig. 4 and 5).
FIG. 5.
FPLC detection of triclosan levels in blood with a C18 reverse-phase column by monitoring absorbance at 230, 240, and 280 nm. Triclosan levels in blood were detectable by 3 h after intraperitoneal injection. Triclosan levels were detected at 3, 7, 10, 20, and 36 h after infection. Additional dosages of triclosan were given at 12, 24, and 36 h, respectively.
Triclosan, as has been described above, has an excellent safety profile for topical use in human beings. However, it has not been used to treat any systemic infections to date. As our experiments demonstrate, perhaps strikingly, triclosan outshines widely used potent antibiotics like ampicillin and tetracycline in systemic bacterial infections. Though our studies have been limited to a mouse model of an acute E. coli O55:B5 infection, the findings underscore the hitherto unexplored potential of triclosan for combating not only malarial systemic infections, as has already been demonstrated, but also rampant bacterial infections (33); these findings also pave the way for the development of triclosan as an antimicrobial for systemic use in experimental systems.
Acknowledgments
This work was supported by grants from the Department of Biotechnology, Government of India, to N.S. and A.S.
REFERENCES
- 1.Bhargava, H. N., and P. A. Leonard. 1996. Triclosan: applications and safety. Am. J. Infect. Control 24:209-218. [DOI] [PubMed] [Google Scholar]
- 2.Bowers, L. D. 1980. Kinetic serum creatinine assays. I. The role of various factors in determining specificity. Clin. Chem. 26:551-554. [PubMed] [Google Scholar]
- 3.Bowers, L. D., and E. T. Wong. 1980. Kinetic serum creatinine assays. II. A critical evaluation and review. Clin. Chem. 26:555-561. [PubMed] [Google Scholar]
- 4.Decker, K., and D. Keppler. 1974. Galactosamine hepatitis: key role of the nucleotide deficiency period in the pathogenesis of cell injury and cell death. Rev. Physiol. Biochem. Pharmacol. 71:77-106. [DOI] [PubMed] [Google Scholar]
- 5.DeSalva, S. J., B. M. Kong, and Y. J. Lin. 1989. Triclosan: a safety profile. Am. J. Dent. 2:185-196. [PubMed] [Google Scholar]
- 6.Dumas, B. T., W. A. Watson, and H. G. Biggs. 1971. Albumin standards and the measurement of serum albumin with bromocresol green. Clin. Chim. Acta 258:21-30. [DOI] [PubMed] [Google Scholar]
- 7.Dyatkina, N. B., C. D. Roberts, J. D. Keicher, Y. Dai, J. P. Nadherny, W. Zhang, U. Schmitz, A. Kongpachith, K. Fung, A. A. Novikov, L. Lou, M. Velligan, A. A. Khorlin, and M. S. Chen. 2002. Minor groove DNA binders as antimicrobial agents. 1. Pyrrole tetraamides are potent antibacterials against vancomycin resistant Enterococci [corrected] and methicillin resistant Staphylococcus aureus. J. Med. Chem. 45:805-817. [DOI] [PubMed] [Google Scholar]
- 8.Galanos, C., M. A. Freudenberg, and W. Reutter. 1979. Galactosamine-induced sensitization to the lethal effects of endotoxin. Proc. Natl. Acad. Sci. USA 76:5939-5943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hay, A. G., P. M. Dees, and G. S. Sayler. 2001. Growth of a bacterial consortium on triclosan. FEMS Microbiol. Ecol. 36:105-112. [DOI] [PubMed] [Google Scholar]
- 10.Heath, R. J., S. W. White, and C. O. Rock. 2002. Inhibitors of fatty acid synthesis as antimicrobial chemotherapeutics. Appl. Microbiol. Biotechnol. 58:695-703. [DOI] [PubMed] [Google Scholar]
- 11.Heath, R. J. 2001. Bacterial fatty-acid biosynthesis: an antibacterial drug target waiting to be exploited. Drug Discov. Today 6:715. [DOI] [PubMed] [Google Scholar]
- 12.Heath, R. J., L. Jing, G. E. Roland, and C. O. Rock. 2000. Inhibition of the Staphylococcus aureus NADPH-dependent enoyl-acyl carrier protein reductase by triclosan and hexachlorophene. J. Biol. Chem. 275:4654-4659. [DOI] [PubMed] [Google Scholar]
- 13.Heath, R. J., J. R. Ronald, D. R. Holland, E. Zhang, M. E. Snow, and C. O. Rock. 1999. Mechanism of triclosan inhibition of bacterial fatty acid synthesis. J. Biol. Chem. 274:11110-11114. [DOI] [PubMed] [Google Scholar]
- 14.Heath, R. J., Y. Yuen-Tsu, M. A. Shapiro, E. Olson, and C. O. Rock. 1998. Broad spectrum antimicrobial biocides target the FabI component of fatty acid synthesis. J. Biol. Chem. 273:30316-30320. [DOI] [PubMed] [Google Scholar]
- 15.Hogan, M. M., and S. N. Vogel. 1990. Measurement of TNF-alpha and beta, unit 6.10. In J. E. Coligan et al. (ed.), Current protocols in immunology, vol. 1. John Wiley & Sons, New York, N.Y.
- 16.Kanamaru, T., Y. Nakano, Y. Toyoda, K.-I. Miyagawa, M. Tada, T. Kaisho, and M. Nakao. 2001. In vitro and in vivo antibacterial activities of TAK-083, an agent for treatment of Helicobacter pylori infection. Antimicrob. Agents Chemother. 45:2455-2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kapoor, M., M. J. Dar, A. Surolia, and N. Surolia. 2001. Kinetic determinants of the interaction of enoyl-ACP reductase from Plasmodium falciparum with its substrates and inhibitors. Biochem. Biophys. Res. Commun. 289:832-837. [DOI] [PubMed] [Google Scholar]
- 18.Karmen, A., F. Wroblewski, and J. S. Ladue. 1954. Transaminase activity in human blood. J. Clin. Investig. 33:131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Keppler, D., R. Lesch, W. Reutter, and K. Decker. 1968. Experimental hepatitis induced by d-galactosamine. Exp. Mol. Pathol. 9:279-290. [DOI] [PubMed] [Google Scholar]
- 20.Lacy, M. K., D. P. Nicolau, M. A. Banevicius, C. H. Nightingale, and R. Quintiliani. 1999. Protective effect of trovafloxacin, ciprofloxacin and ampicillin against Streptococcus pneumoniae in a murine sepsis model. J. Antimicrob. Chemother. 44:477-481. [DOI] [PubMed] [Google Scholar]
- 21.Lyman, F. L., and T. Furia. 1969. Toxicology of 2,4,4′-trichloro-2′-hydroxy-diphenyl ether. Ind. Med. 38:45-52. [PubMed] [Google Scholar]
- 22.Milano, S., F. Arcoleo, P. D'Agostino, and E. Cillari. 1997. Intraperitoneal injection of tetracyclines protects mice from lethal endotoxemia downregulating inducible nitric oxide synthase in various organs and cytokine and nitrate secretion in blood. Antimicrob. Agents Chemother. 41:117-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mitruka, M., and H. M. Rawnsley. 1977. Clinical, biochemical and hematological reference values in normal experimental animals. Mason Publishing, New York, N.Y.
- 24.Mitten, M. J., J. Meulbroek, M. Nukkala, L. Paige, K. Jarvis, A. Oleksijew, A. Tovcimak, L. Hernandez, J. D. Alder, P. Ewing, Y. S. Or, Z. Ma, A. M. Nilius, K. Mollison, and R. K. Flamm. 2001. Efficacies of ABT-773, a new ketolide, against experimental bacterial infections. Antimicrob. Agents Chemother. 45:2585-2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morita, Y. S., K. S. Paul, and P. T. Englund. 2000. Specialized fatty acid synthesis in African trypanosomes: myristate for GPI anchors. Science 288:140-143. [DOI] [PubMed] [Google Scholar]
- 26.Reitman, S., and S. Frankel. 1957. A colorimetric method for the determination of serum glutamic oxalacetic and glutamic pyruvic transaminases. Am. J. Clin. Pathol. 28:56. [DOI] [PubMed] [Google Scholar]
- 27.Rock, C. O., and J. E. Cronan. 1996. Escherichia coli as a model for the regulation of dissociable (type II) fatty acid biosynthesis. Biochim. Biophys. Acta 1302:1-16. [DOI] [PubMed] [Google Scholar]
- 28.Saugar, J. M., T. Alarcón, S. López-Hernandez, M. López-Brea, D. Andreu, and L. Rivas. 2002. Activities of polymyxin B and cecropin A-melittin peptide CA(1-8)M(1-18) against a multiresistant strain of Acinetobacter baumannii. Antimicrob. Agents Chemother. 46:875-878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shapira, L., W. A. Soskolne, Y. Houri, V. Barak, A. Halabi, and A. Stabholz. 1996. Protection against endotoxic shock and lipopolysaccharide-induced local inflammation by tetracycline: correlation with inhibition of cytokine secretion. Infect. Immun. 64:825-828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Slater-Radosti, C., G. Van Aller, R. Greenwood, R. Nicholas, P. M. Keller, W. E. DeWolf, Jr., F. Fan, D. J. Payne, and D. D. Jaworski. 2001. Biochemical and genetic characterization of the action of triclosan on Staphylococcus aureus. J. Antimicrob. Chemother. 48(1):1-6. [DOI] [PubMed] [Google Scholar]
- 31.Stewart, M. J., S. Parikh, X. Guoping, P. J. Tonge, and C. Kisker. 1999. Structural basis and mechanism of enoyl reductase inhibition by triclosan. J. Med. Biol. 290:859-865. [DOI] [PubMed] [Google Scholar]
- 32.Suguna, K., A. Surolia, and N. Surolia. 2001. Structural basis for triclosan and NAD binding to enoyl-ACP reductase of Plasmodium falciparum. Biochem. Biophys. Res. Commun. 283:224-228. [DOI] [PubMed] [Google Scholar]
- 33.Surolia, N., and A. Surolia. 2001. Triclosan offers protection against blood stages of malaria by inhibiting enoyl-ACP reductase of Plasmodium falciparum. Nat. Med. 7:167-173. (Erratum, 7:636.) [DOI] [PubMed] [Google Scholar]
- 34.Szkaradkiewicz, A., and T. Tulecka. 1998. Tumor necrosis factor alpha (TNF-alpha) production in experimental infection with Staphylococcus epidermidis. Med. Sci. Monit. 4:53-56. [Google Scholar]
- 35.Thomas, C. J., N. Surolia, and A. Surolia. 2001. Kinetic and thermodynamic analysis of the interactions of 23-residue peptides with endotoxin. J. Biol. Chem. 276:35701-35706. [DOI] [PubMed] [Google Scholar]
- 36.Trinder, P. 1969. Determination of blood glucose using an oxidase-peroxidase system with a non-carcinogenic chromogen. J. Clin. Pathol. 22:158-161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tulp, M. T., G. Sundstrom, L. B. Martron, and O. Hutzinger. 1979. Metabolism of chlorodiphenyl ethers and Irgasan DP 300. Xenobiotica 9:65-77. [DOI] [PubMed] [Google Scholar]
- 38.Turnowsky, F., K. Fuchs, C. Jeschek, and G. Högenauer. 1989. envM genes of Salmonella typhimurium and Escherichia coli. J. Bacteriol. 171:6555-6565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Villalain, J., C. R. Mateo, F. J. Aranda, S. Shapiro, and V. Micol. 2001. Membranotropic effects of the antibacterial agent triclosan. Arch. Biochem. Biophys. 390:128-136. [DOI] [PubMed] [Google Scholar]
- 40.Walker, C. B., D. Nitzan, and T. D. Wilkins. 1977. Chemotherapy of an experimental Bacteriodes fragilis infection in mice. Antimicrob. Agents Chemother. 11:435-440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Webster, D. 1977. The immediate reaction between bromocresol green and serum as a measure of albumin content. Clin. Chem. 23:663-665. [PubMed] [Google Scholar]
- 42.World Health Organization. 2002. WHO fact sheet no. 194, revised January 2002. [Online.] World Health Organization, Geneva, Switzerland. http://www.who.int/inf-fs/en/fact194.html.
- 43.Young, D. S., L. C. Pestaner, and V. Gibberman. 1975. Effects of drugs on clinical laboratory tests. Clin. Chem. 21(5):1D-432D. [PubMed] [Google Scholar]