Abstract
A series of isogenic methicillin-resistant Staphylococcus aureus isolates recovered from a bacteremic patient were shown to acquire gradually increasing levels of resistance to vancomycin during chemotherapy with the drug (K. Sieradzki, T. Leski, L. Borio, J. Dick, and A. Tomasz, J. Clin. Microbiol. 41:1687-1693, 2003). We compared properties of the earliest (parental) vancomycin-susceptible isolate, JH1 (MIC, 1 μg/ml), to two late (progeny) isolates, JH9 and JH14 (vancomycin MIC, 8 μg/ml). The resistant isolates produced abnormally thick cell walls and poorly separated cells when grown in antibiotic-free medium. Chemical analysis of the resistant isolates showed decreased cross-linkage of the peptidoglycan and drastically reduced levels of PBP4 as determined by the fluorographic assay. Resistant isolates showed reduced rates of cell wall turnover and autolysis. In vitro hydrolysis of resistant cell walls by autolytic extracts prepared from either susceptible or resistant strains was also slow, and this abnormality could be traced to a quantitative (or qualitative) change in the wall teichoic acid component of resistant isolates. Some change in the structure and/or metabolism of teichoic acids appears to be an important component of the mechanism of decreased susceptibility to vancomycin in S. aureus.
Methicillin-resistant Staphylococcus aureus (MRSA) strains with reduced susceptibility to vancomycin (so called VISA strains) have been detected among clinical isolates in several countries (5, 13, 15, 22; M. C. Ploy, C. Grelaud, C. Martin, L. de Lumley, and F. Denis, Letter, Lancet 351:1212, 1998), raising serious concern about the impact of such a resistance mechanism on the chemotherapy of multidrug-resistant staphylococci. A number of studies have described properties of various VISA isolates (4, 7, 9, 18, 22). However, attempts to identify the mechanism of resistance in these VISA strains remain problematic because of conflicting observations concerning the properties of various VISA isolates and also because isogenic vancomycin-susceptible (parental) strains were not available for comparison. This problem is bypassed by the recent identification of a series of isogenic MRSA isolates with gradually increasing vancomycin MICs (23). The isolates, named JH1 through JH15, were recovered in consecutive samples from a single bacteremic patient who underwent extensive chemotherapy with vancomycin during a 2-month period. The JH isolates shared an identical pulsed-field gel electrophoretic pattern, spaA type, and multilocus sequence type and carried the same staphylococcal cassette chromosome mec type III (23). The MIC of vancomycin for the first isolate, JH1, was 1 μg/ml, which increased to 8 μg/ml for the final isolates, JH9 and JH14.
In this communication, we compare the resistant VISA isolates JH9 and JH14 to the parental strain, JH1, in order to identify physiological and biochemical properties that are associated with the mechanism of reduced susceptibility to vancomycin. Examination of these strains should provide unique insights into the mechanism of evolution of vancomycin resistance in vivo.
MATERIALS AND METHODS
Strains and growth conditions.
The S. aureus strains used in the study are listed in Table 1. Bacteria were grown in tryptic soy broth (TSB) (Difco, Detroit, Mich.) at 37°C with aeration, as described previously (19). Growth was followed by monitoring optical density (620 nm, using an LKB spectrophotometer; Pharmacia LKB Biotechnology, Inc., Uppsala, Sweden).
TABLE 1.
Some relevant properties of the JH series of S. aureus isolatesa
| Strain | Clinical source | Vancomycin MIC (μg/ml) | Growth rate (min of doubling time of OD) | PFGEb | MLSTc |
|---|---|---|---|---|---|
| JH1 | Blood | 1.0 | 30 | A0 | 1-4-1-4-12-1-28 |
| JH2 | Blood | 4.0 | 45 | A0 | 1-4-1-4-12-1-28 |
| JH3 | Blood | 4.0 | 45 | A0 | 1-4-1-4-12-1-28 |
| JH9 | Blood | 8.0 | 60 | A0 | 1-4-1-4-12-1-28 |
| JH14 | Heart valve | 8.0 | 60 | A0 | 1-4-1-4-12-1-28 |
| JH15 | Nare of contact | 1.0 | 30 | A0 | 1-4-1-4-12-1-28 |
More details are provided in reference 23.
Pulsed-field gel electrophoretic pattern.
Multilocus sequence type.
Electron microscopy.
Cells suspended in growth medium were fixed with an equal volume of 5% glutaraldehyde and stored for at least 24 h at 4°C until they were further processed for electron microscopy, as described previously (24).
Autolysis assay.
Triton X-100-stimulated autolysis in glycin buffer (pH 8.0) was measured as previously described (19). Cells were grown exponentially to an optical density at 620 nm (OD620) of about 0.3. The cultures were then rapidly chilled, and cells were washed once with ice-cold distilled water and suspended to an OD620 of 1.0 in 50 mM glycine buffer supplemented with 0.01% Triton X-100. Autolysis was measured during incubation at 37°C as the decrease in OD620 using a model 340 spectrophotometer (Sequoia-Turner Corp., Mountain View, Calif.).
Preparation of purified cell walls and peptidoglycan was carried out by methods described previously (8). Briefly, bacteria were extracted with hot sodium dodecyl sulfate (SDS) and processed through mechanical breakage by shaking with glass beads followed by stepwise treatments with proteolytic enzymes, 8 M LiCl, acetone, and several washes with distilled water. The lyophilized pellet containing covalently linked peptidoglycan and wall teichoic acid (TA) was considered “purified cell wall preparations.” Peptidoglycan was prepared from the purified cell walls by extraction with 49% hydrofluoric acid at 0°C for 48 h, a procedure which detaches TA fragments from the peptidoglycan, which can then be recovered by centrifugation and repeated washes with distilled water.
Enzymatic hydrolysis of purified cell walls or peptidoglycans in vitro.
Purified cell walls or peptidoglycan was suspended in 50 mM Tris-Cl (pH 7.5) at an initial OD620 of 1.0 (cell walls) or OD620 of 0.5 (peptidoglycan). Lysis was measured as a decrease in OD620 during incubation of wall samples with crude autolytic enzyme extracts (10 μg of protein/ml) at 37°C.
Cell wall turnover.
Cells were labeled for six generations in TSB containing 2 μCi and 5 μg of tritium-labeled N-acetylglucosamine (NAGA-3H) per ml (Amersham, Arlington Heights, Ill.), followed by growth in isotope-free medium (supplemented with 5 mM nonradioactive NAGA), during which the rate of release of radioactive cell wall components was measured as described previously (19).
Crude autolytic enzyme extracts were prepared from the vancomycin-susceptible strain JH1 and from the VISA isolates JH9 and JH14 by a method described previously (19). Bacterial cultures were grown to mid-logarithmic phase in 250 ml of TSB at 37°C with aeration, chilled rapidly, harvested by centrifugation, and washed once in ice-cold 50 mM Tris-Cl (pH 7.5), and the bacterial pellet was extracted with 250 μl of 4% SDS or 3 M LiCl at room temperature for 30 min with stirring. Supernatants were used as autolytic extracts.
Preparation of crude cell wall TA.
One-milligram quantities of purified cell walls containing both peptidoglycan and covalently linked wall TA were suspended in 1 ml of 50 mM Tris-CI buffer, pH 7.5, and solubilized by digestion with a combination of the M1 muramidase and lysostaphin. After hydrolysis, the preparations were heated at 80°C for 10 min to inactivate the enzymes, denatured protein was removed by centrifugation, and the clear supernatant solution was used as crude cell wall TA extract.
Bacteriolytic enzyme profiles after SDS-polyacrylamide gel electrophoresis.
Separation of proteins was carried out by the technique of Laemmli (16). Resolving gels (7.5% acrylamide-0.2% bisacrylamide) contained crude cell walls (0.1%). Cell wall substrates were prepared in the following manner. Bacteria were grown to mid-logarithmic phase at 37°C with aeration, chilled rapidly, harvested by centrifugation, and resuspended in boiling 8% SDS. After 30 min of boiling, the samples were washed in distilled water and lyophilized. Visualization of bacteriolytic enzymes was carried out as described previously (19).
Phosphorus content of cell wall samples was determined by the method of Chen et al. (6).
The peptidoglycan synthesis rate was measured by pulse labeling portions of cultures with NAGA-3H (5 uCi and 1 μg per ml; Amersham), as described previously (19).
HPLC analysis of the peptidoglycan.
Enzymatic hydrolysates of peptidoglycan were analyzed with reversed-phase high-pressure liquid chromatography (HPLC) as described previously (8), except that the step involving treatment with phosphatase was omitted.
Membrane purification and analysis of PBPs.
Membranes were prepared from cells grown to the late exponential stage as described previously (21). Proteins (60 μg per sample) were labeled with benzyl[14C]penicillin potassium (155 μCi per mg) (Amersham Pharmacia Biotech, Little Chalfont, United Kingdom) for 10 min at 30°C. The reaction was stopped by the addition of an excess of nonlabeled benzylpenicillin. Labeled penicillin binding proteins (PBPs) were resolved by the technique of Laemmli (16) and visualized by fluorography.
Sequencing of the pbp4 gene.
DNA fragments including the pbp4 gene were amplified by PCR from chromosomal DNA and sequenced as described previously (21).
RESULTS
Abnormal morphology of the vancomycin-resistant strains.
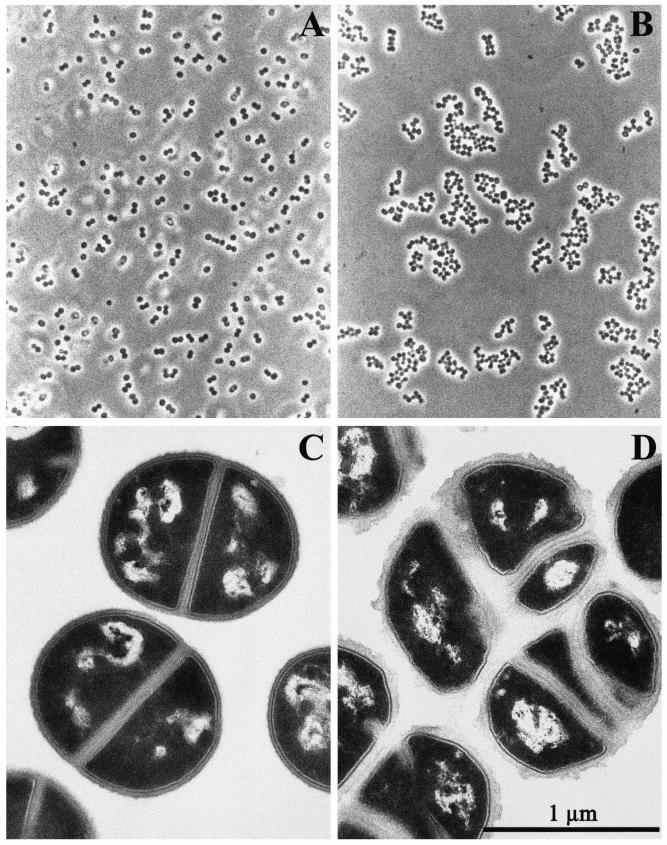
All JH strains were grown from small inocula in the same standard conditions in TSB free of vancomycin. Bacterial cultures were observed by phase-contrast microscopy, and morphology of the cells was also analyzed by transmission electron microscopy. In comparison with the parental strain JH1, the cultures of which were composed of regularly shaped and well separated cocci (Fig. 1A and C), cultures of the VISA strains JH9 and JH14 (vancomycin MIC = 8.0 μg/ml) grew as multicellular aggregates (Fig. 1B). Electromicroscopic thin sections of the VISA strains showed clusters of unseparated cells with abnormally thick cell walls and also surrounded by large amounts of amorphous extracellular material (Fig. 1D). Less-extensive thickening of cell walls was also apparent in isolates JH2 and JH3 (vancomycin MIC = 4 μg/ml) (data not shown).
FIG. 1.
Phase-contrast and thin-section micrographs of the JH1 (A and C) and JH9 (B and D) cells. Bacteria, grown in TSB, were harvested at the mid-exponential stage of growth. Bar = 1 μm.
Suppressed autolysis in JH isolates with reduced susceptibilities to vancomycin.
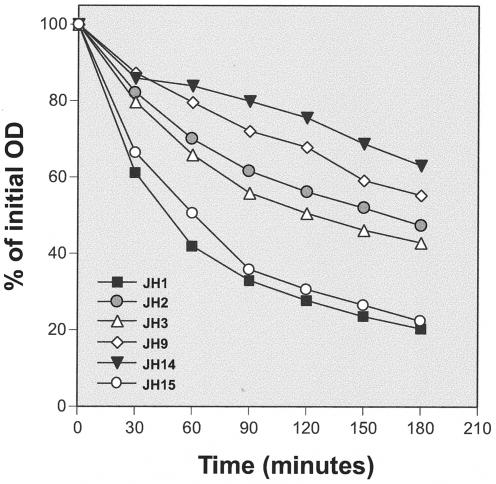
JH isolates were resuspended in glycine buffer containing Triton X-100 to trigger autolysis. Compared with JH1, isolates JH2, JH3, JH9, and JH14 showed gradually decreasing autolytic rates which roughly paralleled the decreasing vancomycin susceptibilities of the isolates (Fig. 2).
FIG. 2.
Triton X-100-stimulated autolysis of the parental strain, JH1, and its progeny, strains JH2 through JH14, as well as strain JH15. Cultures were suspended in autolysis buffer to an initial OD of ≅1.0, and the rates of autolysis were monitored as decrease of OD in time.
Suppressed cell wall turnover in JH9 and JH14 isolates.
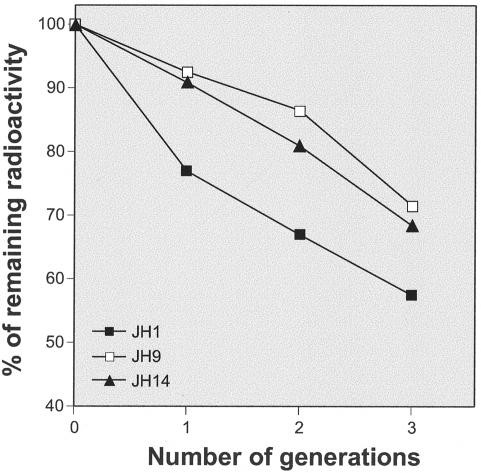
Cultures of the parental strain JH1 and isolates JH9 and JH14 were grown in TSB, which was supplemented with radioactive N-acetylglucosamine. After growth for six generations, the cells were washed and back diluted into isotope-free medium, and the rate of release of radioactivity from the cell walls of bacteria was monitored as a function of cell generation measured as doubling of optical density. Figure 3 shows that the spontaneous cell wall turnover—measured as the initial rate of release of isotope-labeled wall material—was significantly reduced in the vancomycin-resistant strains JH9 and JH14.
FIG. 3.
Suppression of cell wall turnover in strains JH9 and JH14. Bacterial cells were prelabeled with radioactive N-acetylglucosamine and then transferred into isotope-free medium, and the rate of cell wall turnover was measured as described in Materials and Methods.
Zymographic profile of autolytic enzymes.
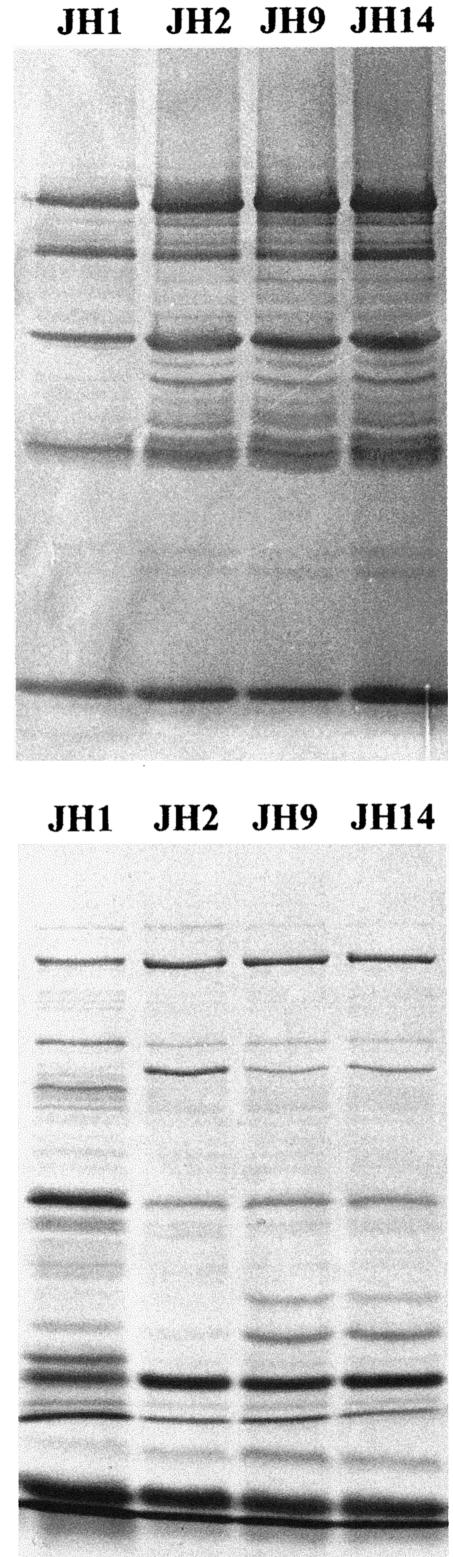
Alterations in the rate of autolysis and cell wall turnover suggested possible changes in the production and/or activity of the various autolytic enzymes present in S. aureus. In order to test the lytic activity of peptidoglycan hydrolases, SDS extracts prepared from the JH strains were analyzed by zymography using crude cell walls of strain NCTC 8325 as the substrate. The zymogen assay revealed that the patterns of autolytic enzymes were similar in parental strain JH1 and its progeny (Fig. 4). However, in the case of strains JH2, JH9, and JH14, one could observe stronger intensity in several bands, suggesting overexpression of some peptidoglycan hydrolases (Fig. 4, top). The same protein extracts, when run in a clear gel and stained with Coomassie brilliant blue, showed a number of quantitative and qualitative changes in the protein band patterns (Fig. 4, bottom).
FIG. 4.
Bacteriolytic enzyme profiles on an SDS-7.5% polyacrylamide gel containing NCTC 8325 cell walls (0.1%) as a substrate (top panel). Autolytic enzyme extracts were prepared and subjected to electrophoresis (40 μg of proteins per line). After electrophoresis, the gels were renatured and, after overnight incubation at 37°C, bands with lytic activity were observed as clear zones in the opaque gel. The clear zones appeared as dark bands against a dark background. The same protein samples, resolved on gels that did not contain cell walls, were subsequently stained with Coomassie brilliant blue (bottom).
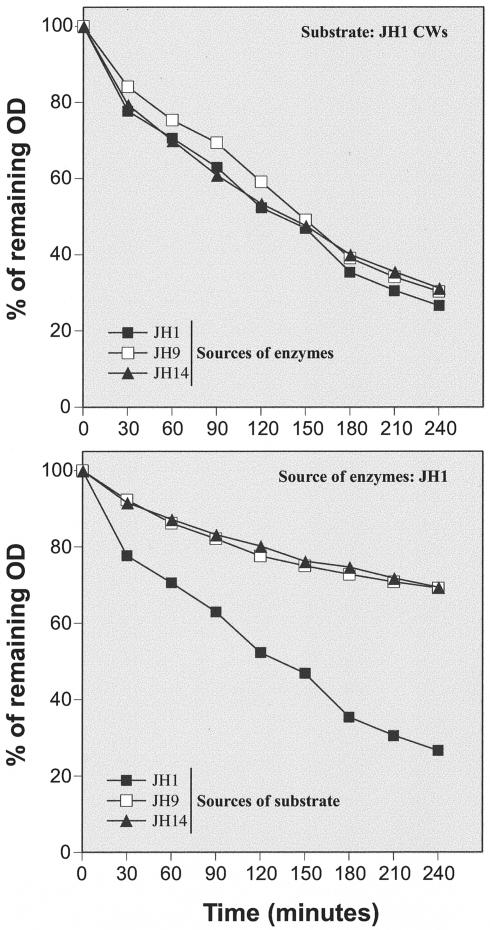
Reduced susceptibilities of cell walls of strains JH9 and JH14 to enzymatic degradation in vitro.
Crude autolytic enzyme extracts prepared from strains JH1, JH9, and JH14 degraded JH1 walls at identical rates (Fig. 5, top). On the other hand, walls purified from strains JH9 and JH14 were degraded at significantly lower rates than walls of strain JH1 when treated with the same autolytic enzyme extracts (Fig. 5, bottom).
FIG. 5.
Solubilization of cell walls (CWs) by LiCl cell extracts. Crude autolytic extracts prepared from strains JH1, JH9, and JH14 were used to test the susceptibility of JH1 cell walls for autolytic degradation in vitro (upper panel). In a parallel set of experiments, isolated cell walls of JH1, JH9, and JH14 were subjected to degradation in vitro by autolytic extract prepared from strain JH1 (bottom).
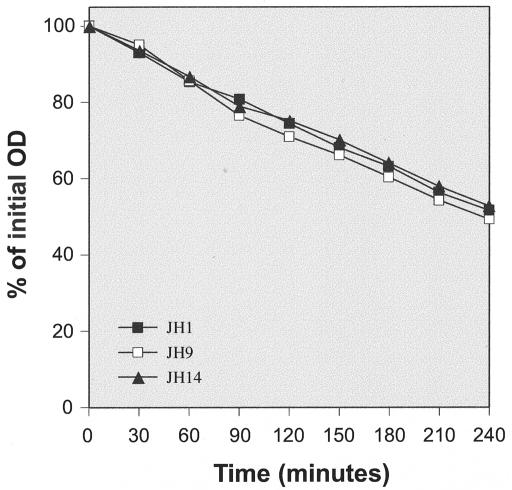
Uniform susceptibilities of peptidoglycan purified from strains JH1, JH9, and JH14 to enzymatic degradation in vitro.
The same enzyme extracts that were used with whole cell walls were also used to test the susceptibilities of purified peptidoglycans. Figure 6 shows that peptidoglycan samples obtained from strains JH1, JH9, and JH14 were degraded by JH1 extract at equal rates.
FIG. 6.
Solubilization of peptidoglycan by LiCl JH1 cell extract. Purified peptidoglycan samples prepared from strains JH1, JH9, and JH14 were incubated with autolytic extract prepared from strain JH1, and the turbidity (OD620) of the samples was monitored at intervals.
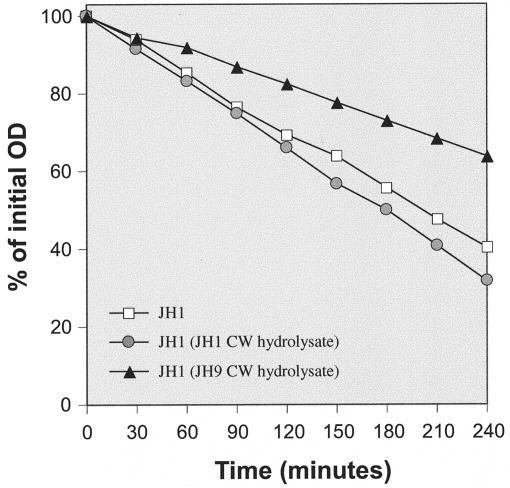
Cell walls of JH9 and JH14 contain increased amounts of TA that interfere with peptidoglycan hydrolysis.
The sharply different results of experiments with cell wall hydrolysis and hydrolysis of peptidoglycan suggested that the reduced rates of wall degradation observed with the vancomycin-resistant JH strains were related to a wall component removable by extraction with hydrofluoric acid, presumably a TA. S. aureus cell wall TA are composed of ribitol residues linked by phosphodiester bridges (2), and phosphorus content has been used to estimate relative amounts of TA in the cell walls. Indeed, cell walls purified from strains JH9 and JH14 contained an excess of 15 and 19 percent phosphorous compared to strain JH1: the phosphorous content of cell walls was 890 and 925 mM/mg of cell wall in strains JH9 and JH14, respectively, compared to 775 mM/mg in the susceptible strain JH1.
In order to further test the involvement of TA in the reduced susceptibilities of JH9 and JH14 cell walls to autolytic degradation, crude autolytic enzyme extracts prepared from strain JH1 were used to hydrolyze peptidoglycan purified from strain JH1 in three different experimental settings: JH1 peptidoglycan alone; JH1 peptidoglycan mixed with wall TA extract equivalent to the amount present in 1 mg of cell wall material per ml (see Materials and Methods) prepared from JH1 cell walls by enzymatic hydrolysis; and JH1 peptidoglycan mixed with wall TA extract prepared from JH9 cell walls. The JH9 wall TA extract (but not the extract from JH1) suppressed the rate of peptidoglycan degradation (Fig. 7).
FIG. 7.
Solubilization by LiCl JH1 cell extract of JH1 peptidoglycan suspended in clear cell wall hydrolysates. Cell walls (CW) of JH9 and JH14 were digested overnight with muramidase and lysostaphin, incubated for 10 min at 100°C, and centrifuged, and clear hydrolysates were used as a medium for JH1 peptidoglycan hydrolysis.
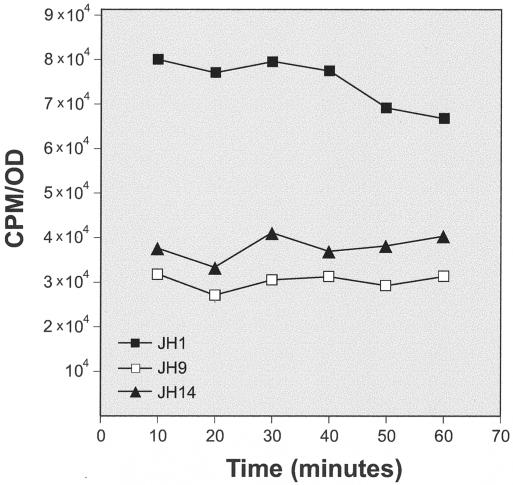
Reduced rates of cell wall synthesis for strains JH9 and JH14.
Incorporation of radioactive N-acetylglucosamine into exponentially growing JH1, JH9, and JH14 was tested at several time intervals during culture growth. Figure 8 shows that the rates of cell wall synthesis in strains JH9 and JH14 were approximately half of that of the parental strain JH1. The slower cell wall synthesis rates were consistent with the lower growth rates of strains JH9 and JH14 (23).
FIG. 8.
Reduced rate of peptidoglycan synthesis for the strain JH9. Cultures of the parental strain, JH1, and its vancomycin-resistant derivative JH9 were grown in TSB. Optical densities were recorded, and the rate of incorporation of radioactive N-acetylglucosamine into the cell wall material during 5-min pulses was determined. The specific rates of incorporation (the counts per minute [CPM] of radioactive label associated with the cell wall divided by the OD) are plotted as a function of time.
Alteration in the peptidoglycan composition of strains with reduced susceptibility to vancomycin.
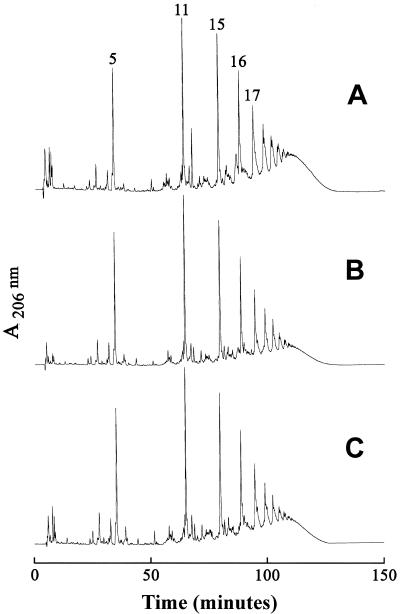
Peptidoglycan samples were prepared from the susceptible strain JH1 and strains JH2, JH3, JH9, and JH14 and were digested with M1 muramidase and analyzed by reversed-phase HPLC. Strains JH9 and JH14 produced a peptidoglycan which showed approximately twofold reduction in the proportion of oligomeric muropeptides that eluted from the HPLC column with retention times longer than 100 min. This was paralleled by a relative increase in the representation of monomeric (muropeptide 5) and dimeric (muropeptide 11) components (Fig. 9).
FIG. 9.
Muropeptide profiles of the parental strain JH1 (A) and its vancomycin-resistant progeny, JH9 (B) and JH14 (C). Peptidoglycan was purified and digested with muramidase, and the muropeptides were separated by HPLC.
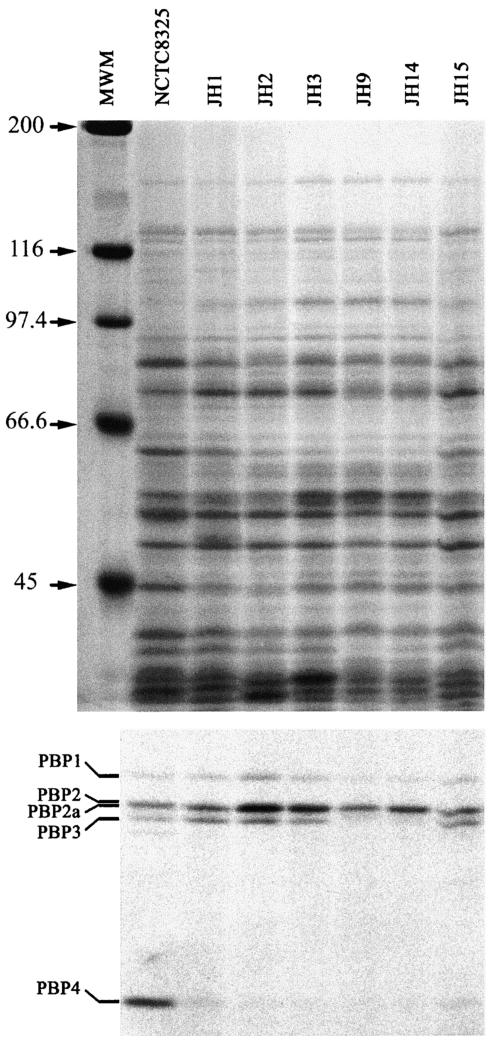
Suppressed expression of PBP4.
Plasma membrane preparations isolated from the parental strain JH1 and its resistant progeny isolates were tested for the presence of staphylococcal PBPs by fluorography with [14C]penicillin. Figure 10 shows the greatly decreased intensity in PBP4 label in strain JH1 compared to the control strain, NCTC 8325. Further decrease in PBP4 label was apparent in strains JH2, JH3, JH9, and JH14. Sequencing of the pbp4 gene and its promoter region from those strains did not show any differences (data not shown).
FIG. 10.
Membrane proteins (upper panel) and the PBP patterns (bottom) of the JH strains. The purified plasma membranes were incubated with (14C)benzylpenicillin and subjected to SDS-polyacrylamide gel electrophoresis, gels were stained with Coomassie brilliant blue, and the PBPs were finally detected by fluorography. MWM, molecular weight markers.
DISCUSSION
In this communication we compared physiological, biochemical, and genetic properties of a series of bacteremic MRSA isolates that were recovered from a patient at different times during extensive vancomycin chemotherapy (23).
The isogenic nature of these isolates (23) should allow one to interpret with more confidence the altered properties of strains JH9 and JH14 as changes related to the mechanism of vancomycin resistance rather than accidental variations in the phenotypes of unrelated clinical isolates. Similar isogenic, vancomycin-susceptible parental isolates have been lacking for other VISA strains described in the literature, making the interpretation of unusual properties of VISA strains as part of the mechanisms of resistance less convincing.
The two most striking alterations identified in the VISA strains JH9 and JH14 were the abnormality of chemical composition (reduced cross-linkage) of peptidoglycan and the abnormally thick cell walls of the bacteria.
The proportion of highly cross-linked muropeptides (i.e., muropeptides with retention times higher than 100 min on the HPLC column) dropped from 50% for strain JH1 to 30 and 33% in JH9 and JH14, respectively (Fig. 9). Figure 10 shows that the decreased cross-linking of peptidoglycan was paralleled by a decrease in PBP4 of the VISA strains JH2, JH3, JH9, and JH14 compared to the susceptible parental strain JH1 (and strain JH15, which is a vancomycin-susceptible colonizing isolate [23]).This finding is consistent with the documented role of S. aureus PBP4 in peptidoglycan cross-linking (21). Decreased PBP4 was also demonstrated for other VISA strains (9); however, the molecular basis for altered PBP4 expression remains unknown. Sequencing of pbp4 from JH1 and from JH9 and JH14 detected no differences.
Our observations suggest that the abnormal wall thicknesses of strains JH9 and JH14 are related to lower rates of cell wall degradation rather than increased rates of cell wall biosynthesis. Both the growth rates (23) and the rates of cell wall synthesis were reduced for strains JH9 and JH14 from that of the parental strain, JH1 (Fig. 8). Isolates JH2, JH3, JH9, and JH14, for which MICs of vancomycin MICs increased, showed gradually decreasing rates of autolysis, and for JH9 and JH14, the turnover of cell wall was clearly reduced from that of the parental strain, JH1. The results of in vitro cell wall degradation experiments indicate that at least one of the mechanisms responsible for the reduced cell wall turnover and autolysis involves decreased susceptibility of the cell walls of VISA strains to in vitro degradation by autolytic extracts. Furthermore, the experiments documented in Fig. 6 and 7 clearly show that the decreased susceptibility of VISA cell walls to autolytic degradation was associated with the TA component. Involvement of TA in vancomycin susceptibility of S. aureus was recently demonstrated (17). Whether this effect is a reflection of the larger amounts of TA or some structural changes in the wall TA of the VISA strains remains to be determined. Regulation of hydrolytic activity of bacterial cell wall hydrolases by TA has been established (12, 14, 25).
VISA isolates described previously showed considerable variation in properties. Reduced susceptibility to vancomycin was reported to be unstable for some (1, 3) but not for other (18, 22) VISA isolates. Reduced rates of autolysis were observed for several VISA isolates (18). However, one of the first VISA isolates, strain Mu50, was reported to exhibit a greatly increased rate of autolysis compared to arbitrarily selected vancomycin-susceptible S. aureus strains (10). Increased thicknesses of cell walls have been demonstrated for many VISA isolates (7, 18) but not nearly to the same extent as seen in strains JH9 and JH14. Reduction in the proportion of highly cross-linked muropeptides apparently in parallel with the increase in the MIC of vancomycin was demonstrated in some but not all VISA isolates (4, 22). The presence of nonamidated glutamic acid residues in the peptidoglycan of some but not all VISA strains was also described (11).
It is conceivable that S. aureus can achieve reduction in susceptibility to vancomycin by more than one mechanism or may develop an essentially similar “antibiotic-trapping” resistance mechanism by alternative routes (see below). In any case, the lack of isogenic vancomycin-susceptible parental strains makes the interpretation of the numerous altered properties in VISA strains described earlier problematic. The availability of strain JH1 as a comparison eliminates this problem: it allows one to interpret with more confidence the physiological and structural changes in JH9 and JH14 as correlates of the vancomycin resistance mechanism.
The properties of JH9 and JH14 described in this communication show several striking similarities to the properties of isogenic vancomycin-resistant laboratory mutants which were selected for gradually increasing levels of vancomycin resistance in vitro (19, 20). Both the JH strains and the laboratory mutants had reduced rates in growth and cell wall synthesis; reduced levels of β-lactam resistance (20); a decrease in the proportion of highly cross-linked muropeptides in the peptidoglycan; and decrease in PBP4 as detected by the fluorographic assay (21). However, the most striking morphological alteration observed in the clinical VISA strains JH9 and JH14, namely, the increased thickness of cell walls, and the inhibition of cell separation were only observed in the vancomycin-resistant laboratory mutants when they were grown in the presence of a subinhibitory concentration of vancomycin which was shown to inhibit wall turnover and autolysis by blocking the access of murein hydrolyses to their cell wall substrate (19). A similar substrate-blocking mechanism related to some qualitative or quantitative alteration in the wall TA appears to be the biochemical basis of inhibited autolysis and thickened cell walls in isolates JH9 and JH14. These observations point to some alteration in TA structure or biosynthesis as one of the key components of the vancomycin resistance mechanism in S. aureus.
Based on the properties of vancomycin-resistant laboratory mutants, we have proposed that the mechanism of staphylococcal vancomycin resistance may involve entrapment of the antibiotic molecules in the cell wall, preventing or hindering the access of the antibiotic molecules to sites of cell wall biosynthesis located at the plasma membrane (19, 21). The key observations on which this model was based included the increased proportion of muropeptides with free d-alanyl-d-alanine termini, the sequestration of vancomycin from the medium by the cell walls of the mutant bacteria, and the massive deposition of cell wall material on the outer surfaces of resistant cells grown in the presence of vancomycin (19, 21). The observations described in this communication indicate that such an antibiotic-trapping mechanism may also operate in VISA isolates that emerge in the clinical environment in vivo. Experiments are in progress to identify the exact nature and order of appearance of genetic changes that form the basis of reduced susceptibilities to vancomycin for the JH isolates.
Acknowledgments
Partial support for these investigations was provided by a grant from the U.S. Public Health Service, RO1 AI37275.
REFERENCES
- 1.Aeschlimann, J. R., E. Hershberger, and M. J. Rybak. 1999. Analysis of vancomycin population susceptibility profiles, killing activity, and postantibiotic effect against vancomycin-intermediate Staphylococcus aureus. Antimicrob. Agents Chemother. 43:1914-1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Archibald A. R. 1972. The chemistry of staphylococcal cell walls, p. 75. In J. O. Cohen (ed.), The staphylococci. Wiley Interscience, New York, N.Y.
- 3.Boyle-Vavra, S., S. K. Berke, J. C. Lee, and R. S Daum. 2000. Reversion of the glycopeptide resistance phenotype in Staphylococcus aureus clinical isolates. Antimicrob. Agents Chemother. 44:272-277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boyle-Vavra, S., H. Labischinski, C. C. Ebert, K. Ehlert, and R. S. Daum. 2001. A spectrum of changes occurs in peptidoglycan composition of glycopeptide-intermediate clinical Staphylococcus aureus isolates. Antimicrob. Agents Chemother. 45:280-287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Centers for Disease Control. 1997. Update: Staphylococcus aureus with reduced susceptibility to vancomycin—United States, 1998. Morb. Mortal. Wkly. Rep. 46:813-815. [PubMed] [Google Scholar]
- 6.Chen, P. S., T. Y. Toribara, and H. Warner. 1956. Micro determination of phosphorus. Anal. Chem. 28:1756-1758. [Google Scholar]
- 7.Cui, L., X. Ma, K. Sato, K. Okuma, F. C. Tenover, E. M. Mamizuka, C. G. Gemmell, M.-N. Kim, M.-C. Ploy, N. El Solh, V. Ferraz, and K. Hiramatsu. 2003. Cell wall thickening is a common feature of vancomycin resistance in Staphylococcus aureus. J. Clin. Microbiol. 41:5-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.De Jonge, B. L. M., Y.-S. Chang, D. Gage, and A. Tomasz. 1992. Peptidoglycan composition of a highly methicillin-resistant Staphylococcus aureus strain: the role of penicillin binding protein 2A. J. Biol. Chem. 267:11248-11254. [PubMed] [Google Scholar]
- 9.Finan, J. E., G. L. Archer, M. J. Pucci, and M. W. Climo. 2001. Role of penicillin-binding protein 4 in expression of vancomycin resistance among clinical isolates of oxacillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 45:3070-3075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hanaki, H., K. Kuwahara-Arai, S. Boyle-Vavra, R. S. Daum, H. Labischinski, and K. Hiramatsu. 1998. Activated cell-wall synthesis is associated with vancomycin resistance in methicillin-resistant Staphylococcus aureus clinical strains Mu3 and Mu50. J. Antimicrob. Chemother. 42:199-209. [DOI] [PubMed] [Google Scholar]
- 11.Hanaki, H., H. Labischinski, Y. Inaba, N. Kondo, H. Murakami, and K. Hiramatsu. 1998. Increase in glutamine-non-amidated muropeptides in the peptidoglycan of vancomycin-resistant Staphylococcus aureus strain Mu50. J. Antimicrob. Chemother. 42:315-320. [DOI] [PubMed] [Google Scholar]
- 12.Herbold, D. R., and L. Glaser. 1975. Interaction of N-acetylmuramic acid L-alanine amidase with cell wall polymers. J. Biol. Chem. 250:7231-7238. [PubMed] [Google Scholar]
- 13.Hiramatsu, K., H. Hanaki, T. Ino, K. Yabuta, T. Oguri, and F. C. Tenover. 1997. Methicillin resistant Staphylococcus aureus clinical strain with reduced vancomycin susceptibility. J. Antimicrob. Chemother. 40:135-136. [DOI] [PubMed] [Google Scholar]
- 14.Höltje, J.-V., and A. Tomasz. 1975. Specific recognition of choline residues in the cell wall teichoic acid by the N-acetylmuramyl-L-alanine amidase of Pneumococcus. J. Biol. Chem. 250:6072-6076. [PubMed] [Google Scholar]
- 15.Kim, M. N., C. H. Pai, J. H. Woo, J. S. Ryu, and K. Hiramatsu. 2000. Vancomycin-intermediate Staphylococcus aureus in Korea. J. Clin. Microbiol. 38:3879-3881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature (London) 277:680-685. [DOI] [PubMed] [Google Scholar]
- 17.Peschel, A., C. Vuong, M. Otto, and F. Götz. 2000. The D-alanine residues of Staphylococcus aureus teichoic acids alter the susceptibility to vancomycin and the activity of autolytic enzymes. Antimicrob. Agents Chemother. 44:2845-2847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pfeltz, R. F., V. K. Singh, J. L. Schmidt, M. A. Batten, C. S. Baranyk, M. J. Nadakavukaren, R. K. Jayaswal, and B. J. Wilkinson. 2000. Characterization of passage-selected vancomycin-resistant Staphylococcus aureus strains of diverse parental backgrounds. Antimicrob. Agents Chemother. 44:294-303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sieradzki, K., and A. Tomasz. 1997. Inhibition of cell wall turnover and autolysis by vancomycin in a highly vancomycin-resistant mutant of Staphylococcus aureus. J. Bacteriol. 179:2557-2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sieradzki, K., and A. Tomasz. 1999. Gradual alterations in cell wall structure and metabolism in vancomycin-resistant mutants of Staphylococcus aureus. J. Bacteriol. 181:7566-7570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sieradzki, K., M. G. Pinho, and A. Tomasz. 1999. Inactivated pbp4 in highly glycopeptide-resistant laboratory mutants of Staphylococcus aureus. J. Biol. Chem. 274:18942-18946. [DOI] [PubMed] [Google Scholar]
- 22.Sieradzki, K., R. B. Roberts, S. W. Haber, and A. Tomasz. 1999. The development of vancomycin resistance in a patient with methicillin-resistant Staphylococcus aureus. N. Engl. J. Med. 340:517-523. [DOI] [PubMed] [Google Scholar]
- 23.Sieradzki, K., T. Leski, L. Borio, J. Dick, and A. Tomasz. 2003. Evolution of a VISA strain in vivo: multiple changes in the antibiotic resistance phenotypes of a single lineage of MRSA under the impact of antibiotics administered for chemotherapy. J. Clin. Microbiol. 41:1687-1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tomasz, A., J. D. Jamieson, and E. Ottolenghi. 1964. The fine structure of Diplococcus pneumoniae. J. Cell Biol. 22:453-467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wecke, J., K. Madela, and W. Fischer. 1997. The absence of D-alanine from lipoteichoic acid and wall teichoic acid alters surface charge, enhances autolysis and increases susceptibility to methicillin in Bacillus subtilis. Microbiology 143:2953-2960. [DOI] [PubMed] [Google Scholar]