Abstract
Background
In this report, we compare the long-term outcome of pediatric liver transplantation (LTx) patients maintained with tacrolimus-based and with cyclosporine (CsA)-based immunosuppressive therapy. We examine long-term patient and graft survival, the incidence of rejection, and immunosuppression-related complications.
Method
There were 233 consecutive primary LTx in children (ages <18 years) performed between October 1989 and December 1994 with tacrolimus-based immunosuppressive therapy (Group I). These were compared with 120 consecutive primary LTx performed with CsA-based immunosuppressive therapy between January 1988 and October 1989(Group II). Children in both groups were followed until July 1999. Mean follow-up was 91.41±17.7 months (range 55.6–117.8) for Group I, and 128±6.1 months (range 116.7–138.6) for Group II.
Results
At 9 years of follow-up, actuarial patient and graft survival were significantly improved (patient survival 85.4% in Group I vs. 63.8% in Group II, P=0.0001; graft survival Group I 78.9% vs. 60.8% Group II, P=0.0003) and the rate of re -transplantation was significantly lower among patients in Group I (12% in Group I vs. 22.5% in Group II P=0.01). Children in Group I also experienced a significantly reduced incidence of acute rejection (0.97 per patient Group I vs. 1.5 per patient Group II P=0.002) and significantly less steroid resistant acute rejection episodes (3.1% in Group I vs. 8.6% in Group II P=0.0001).
The mean steroid dose was significantly lower in Group I compared with Group II at all time points (P=0.0001) after LTx. Freedom from steroid was also significantly higher in Group I compared with Group II at all time points after LTx (ranging from 78% to 84% in Group I and 9% to 32% in Group II during a 1- to 7-year posttransplant period P=0.0001). The rate of hypertension was significantly lower in Group I than Group II (P=0.0001), and the severity of hypertension (need for more than one anti-hypertensive medication) was also significantly lower in Group I than Group II (P=0.0001).
Although the rate of posttransplant lymphoprolif-erative disorder (PTLD) was not significantly different (13.7% Group I vs.8.3% Group II, P=0.13), the survival after PTLD was significantly better for Group I at 81.2% than for Group II at 50% after 5 years (P=0.034).
Conclusion
The results suggest that tacrolimus-based therapy provides significant long-term benefit to pediatric LTx patients, evidenced by significantly improved patient and graft survival, reduced rate of rejection, and hypertension with lower steroid doses.
Critical developmental time points in clinical transplantation have involved the introduction of immunosuppressive agents that significantly improved patient and graft survival without incurring serious complications or adverse events (1–4). The introduction of cyclosporine A (CsA) to clinical transplantation resulted in a dramatic increase in the survival of various solid organ allografts (5, 6). Expectations were further elevated with the introduction tacrolimus in 1989, initially as rescue therapy for liver allograft recipients for whom CsA-based immunosuppressive therapy failed (7–11). Subsequent success in pilot studies prompted randomized clinical trials of tacrolimus vs. CsA; these were followed by American and European multi-center randomized studies (12–14). On the basis of the results of these trials, tacrolimus was approved for clinical use by the Food and Drug Administration in 1994.
The use of tacrolimus in pediatric patients has paralleled its development in adults. Initial reports by our center dated between 1989 and 1991 (7, 15–17) revealed survival and toxicity comparable to our previous experience with CsA-based regimens. Longer follow-up of an expanded population of children, reported in 1993, substantiated this experience (18). Other pediatric centers have corroborated our findings (19–21).
This is the first report focused on long-term outcome of treatment with tacrolimus in pediatric liver transplantation (LTx) patients. Here, we compare our experience with tacrolimus and CsA, analyzing the effects on patient and graft survival, graft function, causes of death and retransplantation, long-term immunosuppressive management, and major drug-related toxicity.
PATIENTS AND METHODS
The results of LTx in children (ages <18 years) were evaluated in a consecutive series of 233 patients maintained with tacrolimus (Group I) and 120 consecutive patients maintained with CsA-based immunosuppressive therapy (Group II).
Patients in Group I underwent transplantation between October 1989 and December 1994. Patients in Group II received liver allografts between January 1988 and October 1989, just before the adoption of tacrolimus-based immunosuppressive therapy. There were no significant demographic differences between the groups (Table 1). The most frequent indications for LTx among patients in both groups were biliary atresia, cryptogenic cirrhosis, and α1-anti-trypsin deficiency (Table 1). All patients were followed until July 1999. The mean follow-up for Group I was 91.41±17.7 months (range 55.7–117.8) and 128±6.1 (range 116.7–138.5) for Group II.
TABLE 1.
Demographics and indications for liver transplantation
| Demographics: Male/female (%) | Tacrolimus (group I): 144/89 (61.8/38/2) |
Cyclosporin (group II): 59/61 (49.2/50.8) |
|---|---|---|
| Mean age (y) | 5.1 ± 5.3 | 4.6 ± 5.0 |
| Age ≤2 (%) | 107 (45.90) | 59 (49.1) |
| Age ≥2≤6 (%) | 47 (20.2) | 26 (21.7) |
| Age ≥6 (%) | 79 (33.9) | 35 (29.2) |
| Donor age (mean) | 11.1 ± 16.1 | 5.8 ± 13.6 |
| Mean total ischemic time (hr) | 13 ± 5.6 | 12.7 ±7.1 |
| Indications | Tacrolimus (group I) (%) | Cyclosporin (group II) (%) |
| Biliary atresia | 112 (48) | 67 (55.8) |
| Neonatal hepatitis | 5 (2.1) | 7 (5.8) |
| Cryptogenic | 36 (15.4) | 6 (5) |
| A-1 Antitrypsin deficiency | 16 (6.8) | 6 (5) |
| Primary liver malignancy | 6 (2.5) | 5 (4.1) |
| Acute fulminant failure | 5 (2.1) | 4 (3.3) |
| Wilson’s disease | 6 (2.5) | 3 (2.5) |
| Viral hepatitis | 5 (2.1) | 3 (2.5) |
| Drug induced | 3 (1.2) | 3 (2.5) |
| Congenital hepatic fibrosis | 8 (3.4) | 1 (0.8) |
| Familial cholestasis | 7 (3) | 2 (1.6) |
| Cystic fibrosis | 4 (1.7) | 2 (1.6) |
| Secondary cirrhosis | 4 (1.7) | 2 (1.6) |
| Tyrosinemia | 3 (1.2) | 2 (1.6) |
| Primary sclerosing cholangitis | 2 (0.8) | 2 (1.6) |
| Budd chiari syndrome | 3 (1.2) | 1 (0.8) |
| Hamartoma | – | 1 (0.8) |
| Oxalosis | 4 (1.7) | – |
| Other congenital deficiency | 4 (1.7)a | 3 (2.5)b |
| Total | 233 | 120 |
Crigler Najar syndrome, transcarbamylase deficiency, OTC deficiency, carbamyl phosphate synthetase deficiency.
carbamyl phosphate synthetase deficiency, type IV, glycogen storage disease-2.
Protocol
Group I
Until August 1991, tacrolimus was given at a dose of 0.15 mg/kg/day i.v. After that date, the starting dose was reduced to 0.075 mg/kg/day i.v. Oral dosing was commenced at 0.1–0.15 mg/kg twice daily when bowel function returned to normal. Trough concentrations were measured on samples of serum until August 1994 (ELISA) and subsequently on samples of whole blood concentration by TDx assay (Abbott Polyclonal [22–24]) at our institution. Target trough serum whole blood levels at 1 month, 2–3 months, and >3 months were 1.0/15, 0.8/12, and 0.7/8–10 ng/ml respectively.
Group II
CsA at a dose of 4 mg/kg/day was given i.v. until bowel function returned to normal, then at 4–10 mg/kg orally twice a day. Trough concentrations of CsA were measured on samples of whole blood by TDx assay (Abbott [25]). Levels were targeted at 1000–1200 ng/ml in the 1st month, 800–1000 ng/ml in the 2nd month, 600–800 ng/ml in the 3rd month, and 400–600 ng/ml thereafter.
Both groups of children received 500 mg of hydrocortisone after liver allograft reperfusion if the child’s body weight was ≤20 kg, 1000 mg hydrocortisone if body weight was ≥20 kg, and 1000 mg methylprednisolone if body weight was >50 kg.
Children receiving CsA were subjected to a steroid taper over 5 days starting with 0.75 mg/kg of methylprednisone or the equivalent dose of hydrocortisone every 6 hr on day 1, to 0.6 mg/kg every 6 hr on day 2, 0.45 mg/kg every 6 hr on day 3, 0.3 mg/kg every 6 hr on day 4, and 0.3 mg/kg every 12 hr on day 5. This steroid taper was used only after 1993 in Group I.
Diagnosis and Treatment of Rejection
Rejection was diagnosed by liver biochemical profile in the absence of any biliary or vascular complication, and confirmed by percutaneous liver biopsy. Depending on the severity of rejection and changes in liver biochemistry, minimal rejections were treated with an increase in baseline immunosuppressive therapy (CsA/tacrolimus) and/or prednisone. Mild episodes of rejection were treated with 500 mg of hydrocortisone in children < 20 kg body weight, 1000 mg hydrocortisone in children 20–50 kg, and 1000 mg methylprednisolone in children with body weight > 50 kg. Moderate episodes of rejection were treated with an additional steroid taper, consistent with the induction taper protocol. Steroid-resistant rejection was treated with monoclonal antibody OKT3 at a dose of 5 ml/day (2.5 ml if weight < 20 kg) for 5–7 days in Group I, and for 10–14 days in Group II.
Statistical Analysis
Actuarial survival was calculated using the Kaplan-Meier statistical method. Differences in survival were calculated using log rank analysis. The incidences of various events were compared between the two groups using Pearson chi-square. Differences in mean values between both groups were compared using the equality of variance and independent sample student t test. Statistical Package for Social Sciences 8.0 software (SPSS, Inc., Chicago, IL) was used for generating the statistical data described above. Multiple time points were analyzed to study the statistical significance of differences occurring at various times over the follow-up period. A P value of <0.05 was considered significant.
RESULTS
Patient Survival
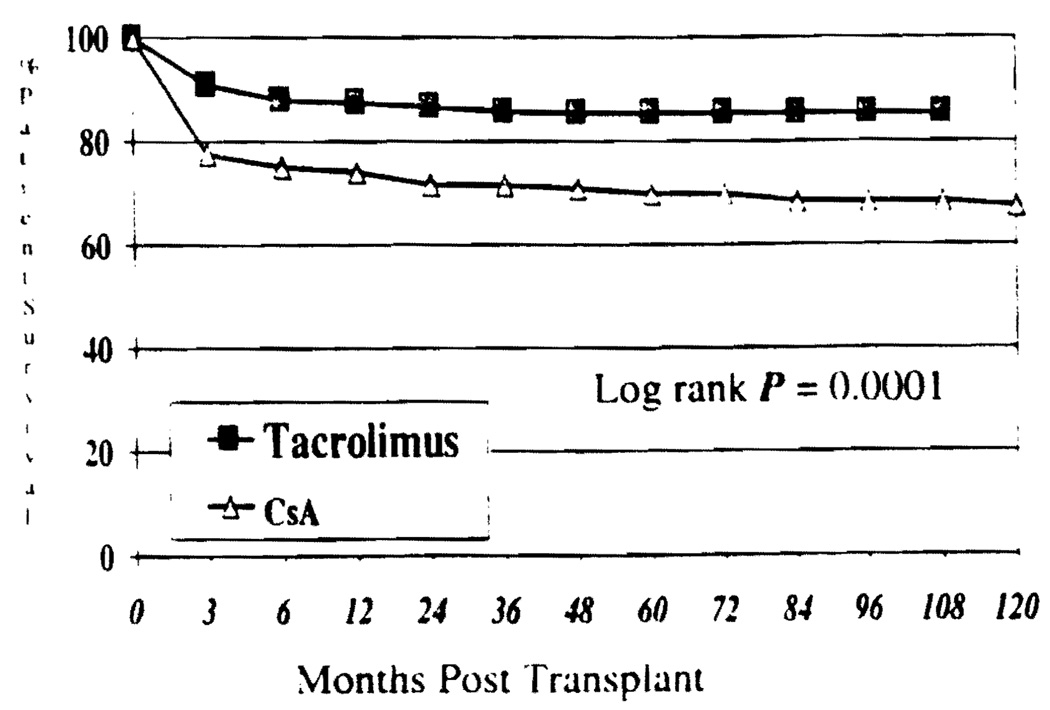
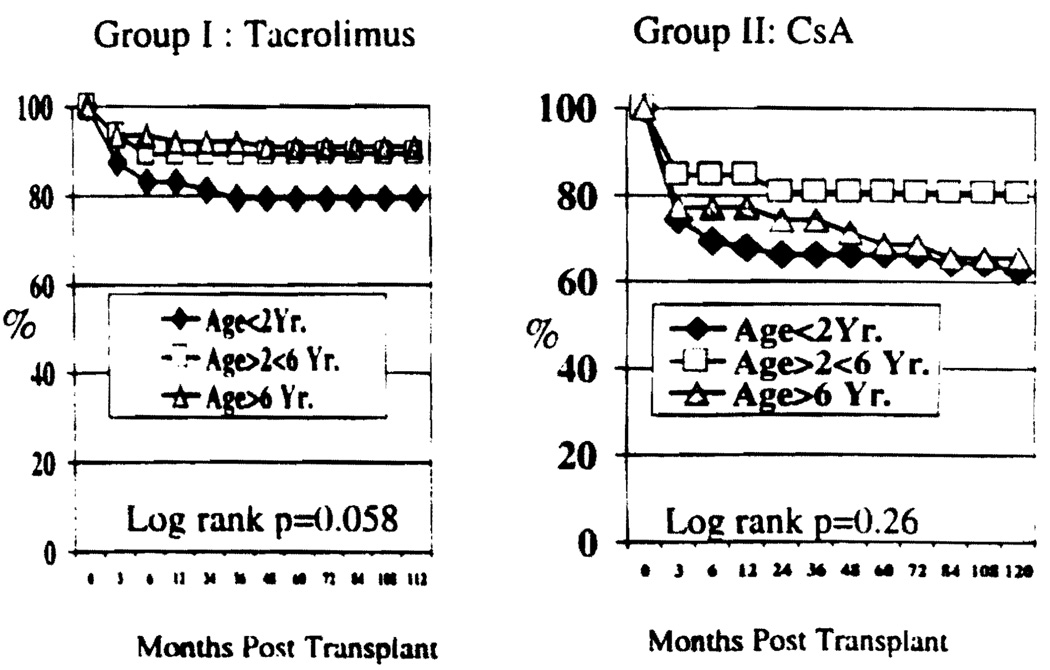
Overall patient survival for both groups is shown in Figure 1. Nine year actuarial patient survival was 85.4% for Group I vs. 68.3% in Group II. This difference was statistically significant (P=0.0001). Survival for children ≤2 years of age was inferior to that of children >2 years of age in either group. However, the difference did not reach statistical significance (P=0.058 Group I; P=0.26 Group II, Figure 2).
FIGURE 1.
Patient survival by Kaplan-Meier.
FIGURE 2.
Patient survival for various age groups under tacrolimus-based or CsA-based therapy.
The majority of deaths occurred within the first 3 months after transplantation (Group I 9%; Group II 24%). The most frequent causes of death in this period were infection, intraoperative and postoperative hemorrhage, and primary nonfunction of the liver allograft (Table 2).
TABLE 2.
Causes of death Group I (n = 233) & Group I (n = 120)
| Causes | Mo after Transplantation |
Total | % |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Group | ≤3 | >3≤6 | >6≤12 | >12≤24 | >24≤36 | >36≤48 | >48≤60 | >60≤72 | >72≤84 | >84≤96 | n | Death | Group population |
|
| Bacterial | Group I | 4 | 2 | 1 | 1 | 8 | 23.53 | 3.43 | ||||||
| Group II | 7 | 7 | 17.95 | 5.83 | ||||||||||
| Fungal | Group I | 1 | 1 | 1 | 3 | 8.82 | 1.29 | |||||||
| Group II | 1 | 1 | 2 | 5.13 | 1.67 | |||||||||
| Viral- Cytomegalovirus |
Group I | – | 1 | 1 | 2.94 | 0.43 | ||||||||
| Group II | 1 | 1 | 2.56 | 0.83 | ||||||||||
| Viral-Adeno | Group I | 1 | 1 | 2.94 | 0.43 | |||||||||
| Group II | 2 | 2 | 5.13 | 1.67 | ||||||||||
| Viral-Epstein-Barr Virus |
Group I | – | 1 | 1 | 2 | 5.88 | 0.86 | |||||||
| Group II | 1 | 2-lymphoma | 1 | 4 | 10.26 | 3.33 | ||||||||
| Multiple Infections | Group I | 3 | 1 | 1 | 2.94 | 0.43 | ||||||||
| Group II | 3 | 7.69 | 2.50 | |||||||||||
| Infection (all) | Group I | (6) | (5) | (1) | (1) | (2) | (1) | (16) | (47.06) | (6.87) | ||||
| Group II | (15) | (1) | (2) | (1) | (19) | (48.72) | (15.83) | |||||||
| Intraoperative | Group I | 6 | 0 | 0.00 | 0.00 | |||||||||
| Group II | 6 | 15.38 | 5.00 | |||||||||||
| Central nervous system |
Group I | 1 | 1 | 2.94 | 0.43 | |||||||||
| Group II | – | 0 | 0.00 | 0.00 | ||||||||||
| Postoperative hemorrhage |
Group I | 3 | 3 | 8.82 | 1.29 | |||||||||
| Group II | – | 1 | 1 | 2.56 | 0.83 | |||||||||
| Cardiac | Group I | 3 | 1 | 4 | 11.76 | 1.72 | ||||||||
| Group II | 3 | 3 | 7.69 | 2.50 | ||||||||||
| Respiratory | Group I | – | 0 | 0.00 | 0.00 | |||||||||
| Group II | 3 | 1 | 1 | 5 | 12.82 | 4.17 | ||||||||
| Recurrence of malignancy |
Group I | – | 1 | 1 | 2.94 | 0.43 | ||||||||
| Group II | 1 | 2 | 3 | 7.69 | 2.50 | |||||||||
| Primary non- function |
Group I | 7 | 7 | 20.59 | 3.00 | |||||||||
| Group II | 1 | 1 | 2.56 | 0.83 | ||||||||||
| Unknown | Group I | 1 | 1 | 2 | 5.88 | 0.86 | ||||||||
| Group II | – | 1 | 1 | 2.56 | 0.83 | |||||||||
| Total n (% death) | Group I | 21 (61.8) | 5 (14.7) | 2 (5.9) | 1 (2.9) | 4 (11.8) | 1 (2.9) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 34 | 100 | 14.59 |
| Group II | 29 (74.5) | 1 (2.6) | 1 (2.6) | 3 (7.7) | 0 (0) | 2 (5.1) | 1 (2.6) | 0 (0) | 2 (5.1) | 0 (0) | 39 | 100 | 32.50 | |
Graft Survival
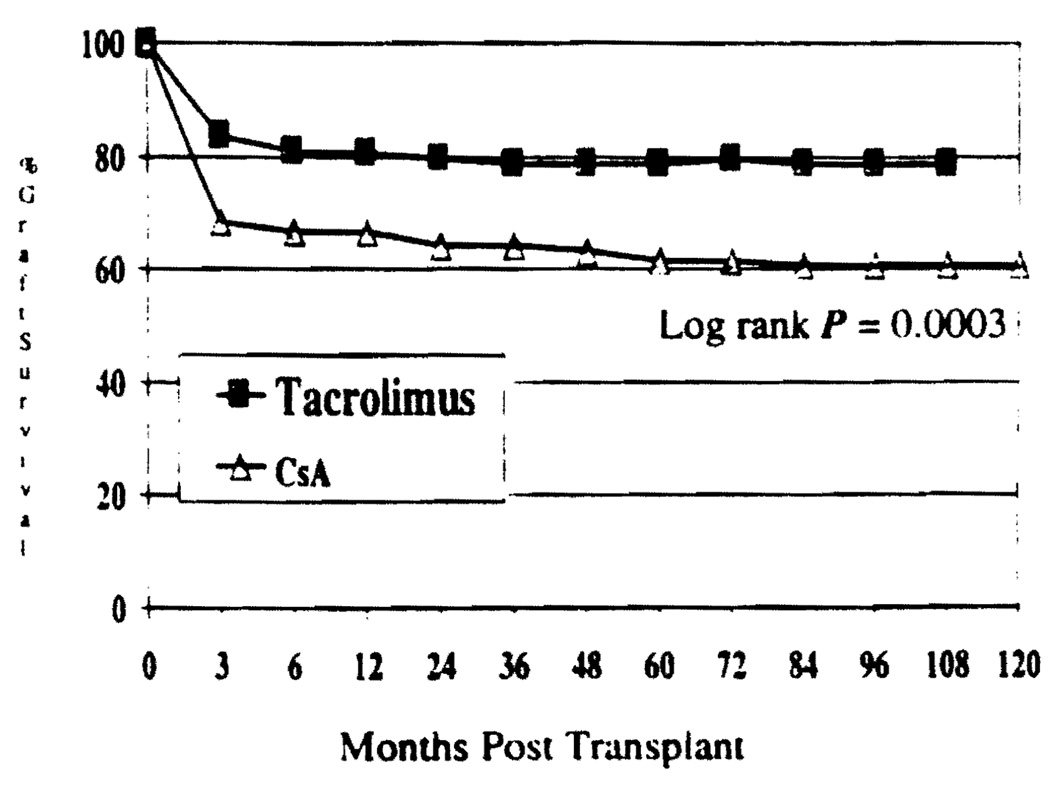
Retransplantation or deaths without retransplantation were considered graft losses. The actuarial graft survival at 9 years was 78.9% in Group I and 60.8% in Group II (Fig. 3). This difference was statistically significant (P=0.0003).
FIGURE 3.
Kaplan-Meier estimates of graft survival, P=0.0003.
Retransplantation
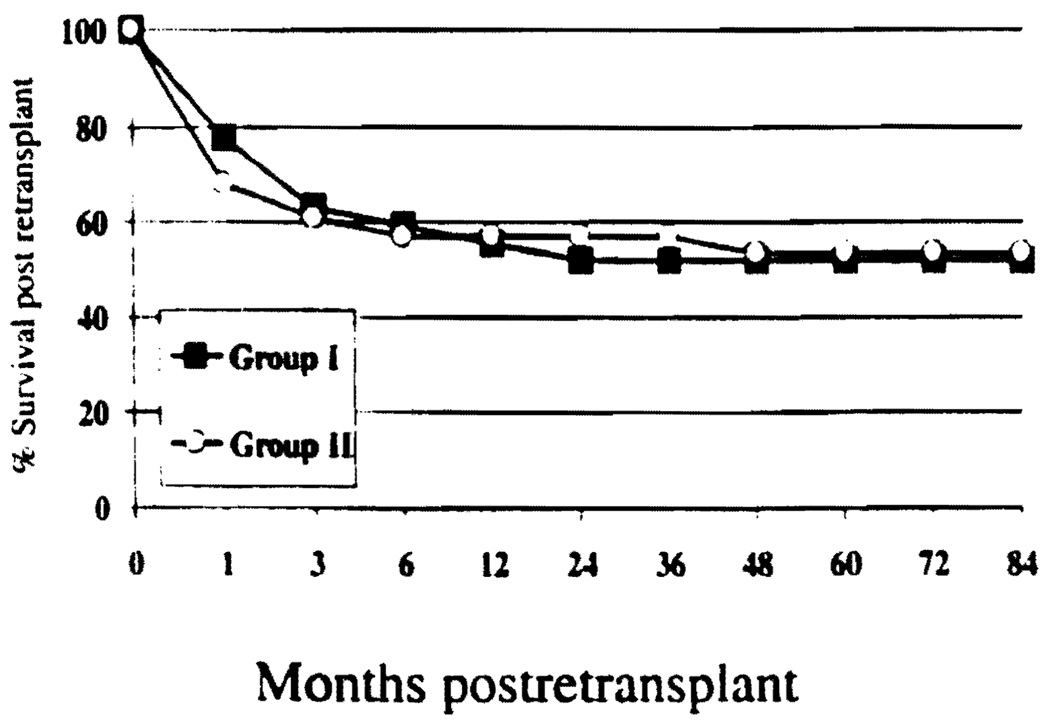
There were 28 children (12.0%) in Group I and 27 in Group II (22.5%) who received a second liver allograft. The difference in rate of retransplantation was statistically significant (P=0.01). Of retransplants >80% occurred within the first 3 months. The most common causes of retransplantation were primary nonfunction of the liver allograft and hepatic artery thrombosis (Table 3). In addition, one child from Group I (0.2%) and six children from Group II (5.0%) received a third liver allograft (P=0.007). The survival at 1 and 7 years after retransplantation was 57.1% and 53.6% in Group I and 55.6% and 51.9% in Group II, respectively. The difference was not statistically significant (Log rank P=0.97). As shown in Figure 4, almost 90% of deaths occurred within the first 3 months after transplantation.
TABLE 3.
Causes of retransplanta in Group I (n = 233) & II (n = 120)
| Causes | Mo. after transplantation |
Total |
% |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Group | ≤3 | >3≤6 | >6≤12 | >12≤24 | >24≤36 | >36≤48 | >48≤60 | >60≤72 | >72≤84 | >84≤96 | n | Retransplant | Group population |
|
| Primary non-function | Group I | 13 | 13 | 46.43 | 5.58 | |||||||||
| Group II | 7 | 1 | 8 | 29.63 | 6.67 | |||||||||
| Hepatic artery thrombosis | Group I | 10 | 10 | 35.71 | 4.29 | |||||||||
| Group II | 8 | 8 | 29.63 | 6.67 | ||||||||||
| Portal vein thrombosis/stricture | Group I | 2 | 2 | 7.14 | 0.86 | |||||||||
| Group II | 1 | 1 | 3.70 | 0.83 | ||||||||||
| Adeno virus or cytomegalovirus hepatitis |
Group I | 1 | 1 | 3.57 | 0.43 | |||||||||
| Group II | 2 | 1 | 3 | 11.11 | 2.50 | |||||||||
| Biliary complication | Group I | 0 | 0.00 | 0.00 | ||||||||||
| Group II | 1 | 1 | 3.70 | 0.83 | ||||||||||
| Acute rejection | Group I | 0 | 0.00 | 0.00 | ||||||||||
| Group II | 1 | 1 | 3.70 | 0.83 | ||||||||||
| Chronic rejection | Group I | 0 | 0.00 | 0.00 | ||||||||||
| Group II | 2 | 2 | 4 | 14.81 | 3.33 | |||||||||
| PTLD | Group I | 0 | 0.00 | 0.00 | ||||||||||
| Group II | 1 | 1 | 2 | 7.41 | 1.67 | |||||||||
| Unknown | Group I | 0 | 0.00 | 0.00 | ||||||||||
| Group II | 1 | 1 | 3.70 | 0.83 | ||||||||||
| Total n (%) | Group Ib | 26 (92.9) | 1 (4) | 0 (0) | 0 (0) | 1 (3.7) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 28 | 100.00 | 12.02 |
| Group IIb | 22 (81.5) | 0 (0) | 0 (0) | 1 (3.7) | 0 (0) | 0 (0) | 4 (14.8) | 0 (0) | 0 (0) | 0 (0) | 27 | 100.0 | 22.50 | |
Causes of second transplantation only.
Significant difference P, 0.01. In addition, 6 patients (5%) in Group I received third transplant for chronic rejection (4), acute rejection (1), and primary nonfunction (1). In Group I, one patient received third transplant for primary nonfunction (P=0.007).
FIGURE 4.
Incidence of retransplantation (group I=12%, group II=22.5%; P=0.01) and survival after retransplantation (P=0.97).
Incidence of Rejection
The overall mean number of rejection episodes per child was significantly lower in Group I compared with Group II (0.97 vs. 1.5, respectively; P=0.002; Table 4)
TABLE 4.
Incidence of rejection
| Episodes of rejection | Tacrolimus (Group I) |
CsA (Group II) |
||||
|---|---|---|---|---|---|---|
| No. patients | % patients | Total episodes |
No. patients | % patients | Total episodes |
|
| 0 | 100 | 42.9 | 0 | 52 | 43.3 | 0 |
| 1 | 73 | 31.3 | 73 | 25 | 20.8 | 25 |
| 2 | 38 | 16.3 | 76 | 14 | 11.7 | 28 |
| 3 | 15 | 6.4 | 45 | 7 | 5.8 | 21 |
| 4 | 2 | 0.8 | 8 | 9 | 7.5 | 31 |
| 5 | 5 | 2.1 | 25 | 13 | 10.8 | 65 |
| Total | 233 | 227 | 120 | 175 | ||
| Mean rejections per patient | 0.97a | 1.5a | ||||
P value = 0.002 (t test)
Group I
One hundred children (42.9%) remained rejection-free. Seventy-three children (31.3%) experienced one episode of rejection, 38 (16.3%) had 2 episodes, 15 (6.4%) experienced 3 episodes, 2 (0.8%) had 4 episodes, and 5 (2.1%) had 5 episodes
Group II
Fifty-two children (43.3%) remained rejection-free. Twenty-five children (20.8%) experienced one episode of rejection, 14 (11.7%) experienced 2 episodes, 7 (5.8%) had 3 episodes, 9 (7.5%) experienced 4 episodes, and 13 (10.8%) had 5 episodes.
Treatment of Rejection
For children in Group I, 64 episodes of rejection (28.3%) were treated simply by an increase in baseline immunosuppressive therapy either by increasing the baseline dose of tacrolimus (n=19, 8.4%) or increasing the maintenance dose of prednisone (n= 14, 6.2%), or by increasing the maintenance doses of both prednisone and tacrolimus (n=31, 13.7%) without any steroid bolus. There were 156 patients (68.7%) treated with i.v. steroid bolus and 7 (3.1%) treated with OKT3.
For children in Group II, 141 episodes of rejection (80.6%) were treated with i.v. steroids and 15 (8.6%) were treated with OKT3. Nineteen children (10.9%) were converted to tacrolimus-based therapy to control steroid-resistant rejection episodes. The incidence of steroid-resistant rejection requiring OKT3 therapy was significantly lower in tacrolimus-treated children (Group I) compared with CsS-treated children (Group II P=0.0001; Table 5).
TABLE 5.
Treatment of rejection
| Treatment | Group I |
Group II |
||
|---|---|---|---|---|
| n | % | n | % | |
| Corticosteroid | 156 | 68.7 | 141 | 80.6 |
| OKT3 | 7* | 3.1 | 15* | 8.6 |
| Conversion to tacrolimus | 0 | 0 | 19 | 10.9 |
| Increase maintenance CSA/tacrolimus | 19 | 8.4 | 0 | 0 |
| Increase maintenance prednisone dose | 14 | 6.2 | 0 | 0 |
| Increase maintenance prednisone + CSA/tacrolimus |
31 | 13.7 | 0 | 0 |
| Total | 227 | 175 | ||
P = 0.0001 (Pearson chi square).
Baseline Immunosuppressive therapy
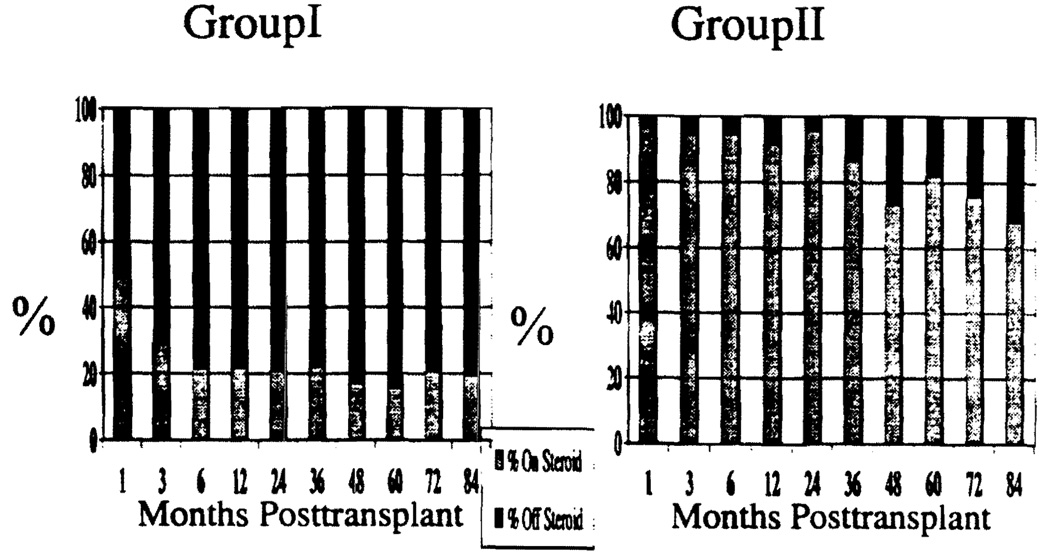
The mean doses of immunosuppressants and mean trough concentrations of tacrolimus and CsA are presented in Table 6. The mean dose of prednisone was 4–8 times lower in Group I compared with Group II at various times after transplantation. This difference reached statistical significance (P=0.0001). Approximately 50% of children in Group I were maintained with a steroid-free immunosuppressive regimen by 1 month. At 6–84 months, 80% of children in Group I were steroid-free. By contrast, only 6% of children in Group II achieved a steroid-free state in the 1st month, and 32% were steroid-free at 84 months. The difference was statistically significant at all time points analyzed (P=0.0001; Figure 5). The reduction in tacrolimus dose in 1991 and addition of steroid in 1993 did not make any significant difference in patient or graft survival. It facilitated the perioperative management by in decreasing the transient oliguria and or neurological disorder.
TABLE 6.
Immunosuppression
| Mo. after transplantation | 1 | 3 | 6 | 12 | 24 | 36 | 48 | 60 | 72 | 84 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Group I (tacro) | Tacroa dose mg/day | 7.1±5.3 | 6.4±4.9 | 4.9±4.0 | 4.3±3.9 | 3.9±3.4 | 3.5±3 | 3.4±2.6 | 3.1±2.5 | 3.2±2.3 | 3.0±2.2 |
| Tacro level (plasma) ng/ml | 1.2±1.7 | 0.8±0.6 | 0.8±0.9 | 0.5±0.4 | 0.4±0.3 | 0.5±0.5 | 0.4±0.4 | - | - | - | |
| Tacro level (whole blood) ng/ml | 14.1±8.2 | 8.8±4.3 | 10.6±8.8 | 7.3±2.6 | 8.2±3.8 | 8.4±6.1 | 6.6±3.0 | 8.0±5.4 | 9.7±8.8 | 5.0±2.5 | |
| Mean Pred dose mg/db | 4±5.4 | 1.8±3.5 | 1.3±3.5 | 1.3±3.2 | 1.3±3.8 | 1.0±3.1 | 1.1±3.2 | 1.1±3.4 | 1.2±3.4 | 1.2±3.7 | |
| Group II (CsA) | CsA dose mg/d | 235±200 | 181±267 | 186±267 | 147±90 | 127±77 | 126±77 | 116±78 | 7.1±5.8 | 6±5.3 | 108±82 |
| CsA level ng/ml | 1017±481 | 821±401 | 530±298 | 473±319 | 332±169 | 236±229 | 175±105 | 155±99 | 155±105 | 169±122 | |
| Mean Pred dose mg/dayb | 8.3±4.6 | 8.2±7.4 | 6.4±4.2 | 6.3±7.3 | 5.1±4.5 | 5.5±12 | 4.7±12 | 4±3.9 | 4.7±4.9 | 4.1±4.5 | |
| Pred dose | Group | ||||||||||
| 0 mg/day | Group Ic | 50.9 | 70.8 | 78.5 | 78.2 | 79.2 | 77.9 | 82.5 | 84.1 | 79.2 | 80.5 |
| Group IIc | 0 | 5.6 | 5.5 | 8.5 | 7.3 | 13.6 | 27.1 | 18.5 | 24.6 | 32.3 | |
| 1–5 mg/day | Group I | 25.1 | 19.8 | 16.2 | 13.6 | 13.6 | 15.5 | 12.5 | 10.9 | 15 | 13.8 |
| Group II | 49.2 | 49.2 | 56.9 | 75.7 | 77.6 | 71.2 | 71.2 | 62.8 | 64.2 | 68.1 | |
| 6–10 mg/day | Group I | 15.2 | 6.2 | 2.5 | 5.7 | 3.2 | 3.2 | 3.3 | 2.4 | 3.7 | 2.7 |
| Group II | 33.8 | 30.9 | 31.9 | 10 | 11.9 | 9.5 | 7.1 | 11.4 | 10.1 | 10.2 | |
| >10 mg/day | Group I | 8.6 | 3.1 | 2.5 | 1.9 | 3.8 | 1.9 | 1.6 | 2.4 | 1.8 | 2.7 |
| Group II | 16.9 | 14 | 5.5 | 5.7 | 5.9 | 5.4 | 2.8 | 5.7 | 11.5 | 8.8 |
Abbreviations: Pred = Prednisone; Tacro = tacrolimus.
Significant difference. P=0.001 (t test).
Significant difference for each time point. P=0.0001 (Pearson chi square).
FIGURE 5.
Freedom from corticosteroid use (P=0.0001).
Liver function
The results of liver function tests at various time intervals after transplantation are shown in Table 7 for both groups. Mean values approach the normal range at all time points, analyzed from 1 month to 8 years after LTx.
TABLE 7.
Liver function and renal functiona
| Liver function | Group | Mo after transplantation |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 3 | 6 | 12 | 24 | 36 | 48 | 60 | 72 | 84 | 96 | ||
| Total bilirubin mg/dl | I | 0.9 | 0.5 | 0.5 | 0.6 | 0.6 | 0.6 | 0.5 | 0.6 | 0.6 | 0.5 | 0.7 |
| II | 1.3 | 1.5 | 0.7 | 1.1 | 1 | 0.8 | 0.9 | 0.7 | 0.9 | 0.8 | 0.8 | |
| ASTb U/L | I | 79 | 124 | 66 | 61 | 56.5 | 45 | 57 | 50 | 58 | 75 | 33 |
| II | 51 | 44 | 46 | 44 | 57 | 56 | 82 | 52 | 58 | 44 | 49 | |
| ALT U/L | I | 66 | 73 | 63 | 60 | 51 | 48 | 49 | 48 | 56 | 39 | 38 |
| II | 46 | 51 | 46 | 41 | 49 | 51 | 59 | 53 | 48 | 43 | 51 | |
| Alk-PO4 U/L | I | 229 | 361 | 372 | 350 | 267 | 259 | 249 | 237 | 235 | 340 | 243 |
| II | 206 | 205 | 244 | 289 | 233 | 236 | 235 | 281 | 212 | 246 | 241 | |
| GGTP U/L | I | 142 | 102 | 65 | 54 | 61 | 50 | 55 | 48 | 72 | 58 | 32 |
| II | 165 | 123 | 113 | 67 | 65 | 61 | 91 | 51 | 73 | 58 | 103 | |
| Renal function | Group | |||||||||||
| BUN mg/dl | I | 18 | 20 | 19 | 18 | 17 | 17 | 16 | 15 | 15 | 14 | 14 |
| II | 24 | 23 | 22 | 19 | 17 | 17 | 17 | 16 | 15 | 17 | 17 | |
| Creatinine mg/l | I | 0.6 | 0.5 | 0.5 | 0.7 | 0.8 | 0.7 | 0.7 | 0.6 | 0.6 | 0.7 | 0.8 |
| II | 0.5 | 0.6 | 0.6 | 0.7 | 0.6 | 0.7 | 0.8 | 0.8 | 0.7 | 0.8 | 0.7 | |
In Group II, 3 children with oxalosis were undergoing dialysis before Ltx received a kidney transplant, and 3 children who went on to end stage renal failure after LTx received a kidney transplant.
AST, aspartate amino transferase; ALT, alanine amino transferase; AIK Po4, alkaline phosphatase; GGTP, Gamma glutamyl transferase.
Renal Function
At 1 month after transplantation, mean serum creatinine was 0.6 mg/dl and 0.5 mg/dl among children in Group I and Group II, respectively. There was little change over 9 years of follow up in both groups (Table 7). In Group I, 4 children received LTx for oxaloses; 3 of these were undergoing dialysis at the time of LTx and received a kidney allograft. Three additional children developed end stage renal failure and underwent kidney transplantation at 3, 5, and 9 years after LTx, respectively. All three children are alive at 7, 4, and 0.5 years after kidney transplantation, with normal renal and liver function. None of the children in Group II developed end stage renal failure or required dialysis or kidney transplantation.
Posttransplant Lymphoproliferative Disorder (PTLD)
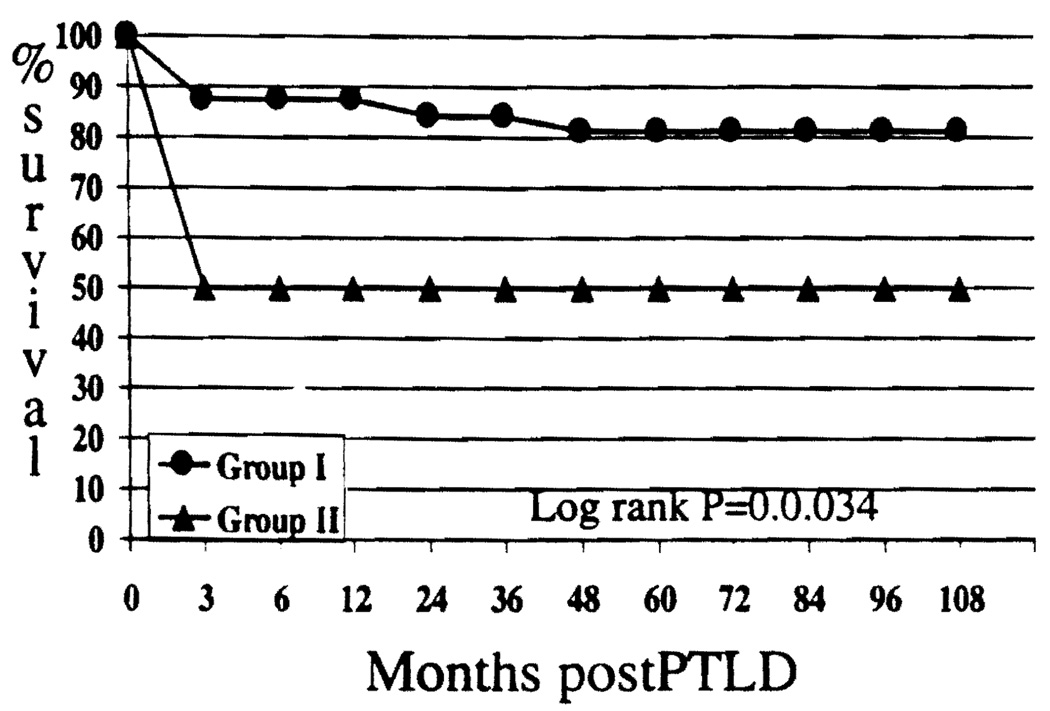
The incidence of PTLD was higher in Group I (n=32,13.7%) than in Group II (n=10, 8.3%); but the difference was not significant (chi-square P=0.13). However, the long-term survival after the diagnosis of PTLD was significantly higher among children in Group I compared with those in Group II (81.3% vs. 50% at 9 years; P=0.034; Fig 6).
FIGURE 6.
Incidence of PTLD (group I=13.7%, group II=8.3%; P=ns)/and survival after PTLD significantly better in Group I (P=0.034).
Group I
Thirty-two children (13.7%) developed PTLD after a mean interval of 11.0 +12.4 months (range 1.6 – 62.1 months). The gastrointestinal tract was the most common site (n=14, 43.7%; five of these also had lymph node involvement), followed by lymph nodes (n=8, 25%). Three children developed PTLD in the spleen, two in the liver allograft, and one each in the tonsil, larynx, and brain. One child had Burkitt’s lymphoma involving the cervical lymph nodes, and another child developed PTLD in multiple sites. Six children (18.7%) have died after the diagnosis of PTLD. Twenty-six children (81.3%) are alive and well, 79.5±15.7 months (range 44.9 – 107.4) after developing PTLD.
Group II
Ten children (8.3%) developed PTLD after a mean interval of 35.15±26.5 months after transplantation (range 1.1 – 69.1 months). The most common sites for the development of PTLD were lymph nodes (n=3, 30%) and the liver allograft (n=2, 30%; one child also had lymph node involvement). The gastrointestinal tract (n=2, 20%), tonsil (n=1,10%), and multiple sites (n=1, 10%) were also reported. Five children (50.0%) died after the diagnosis of PTLD, and 5 children (50.0%) are currently alive 95.4+16.9 months (range 73–108 months) after PTLD.
Hypertension
Hypertension was defined by the requirement of antihypertensive medications, excluding diuretics, to control hypertension. Overall incidence of hypertension was lower in Group I compared with Group II. The percentage of children requiring multiple hypertensive medications were also lower in Group I compared with Group II.
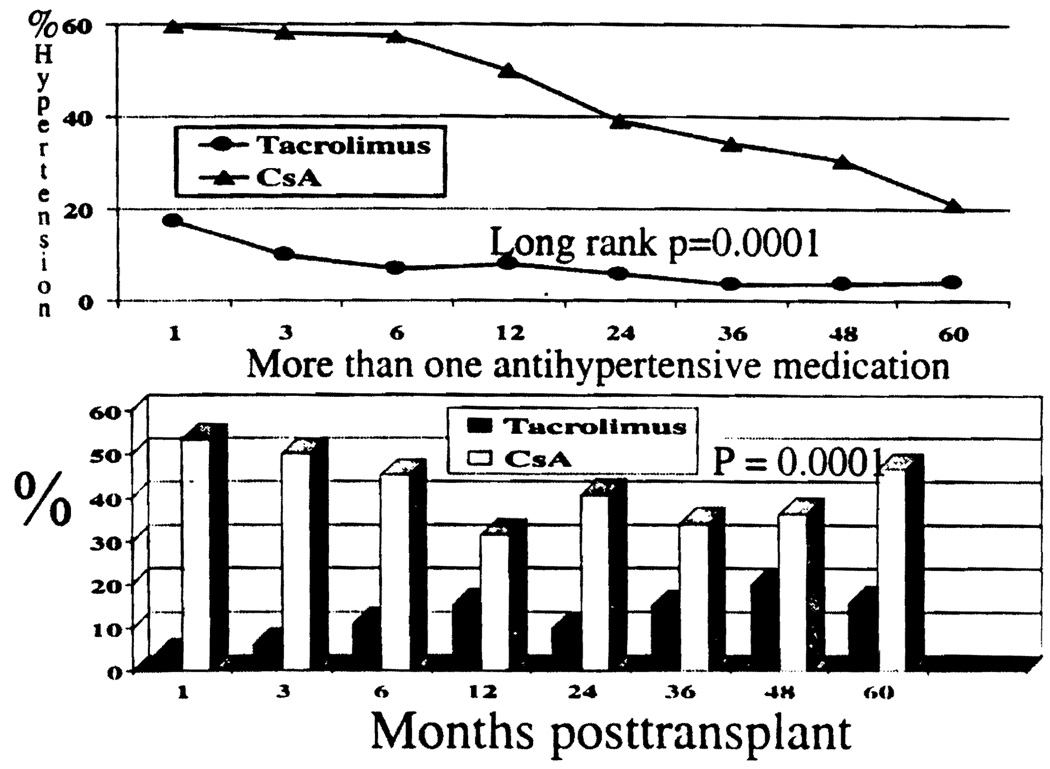
In Group I, the incidence of hypertension requiring antihypertensive medications, was 17% at 1 month, declining to 4% at 6 years. The incidence of hypertension in Group II was significantly higher. At 1 month, and 6 years, 60% and 21% of children were hypertensive, respectively (P=0.0001 at all time points analyzed; Figure 7).
FIGURE 7.
Incidence of hypertension (P=0.0001) and requirement for > 1 antihypertensive agent (P=0.0001).
The number of children requiring more than one antihypertensive medication varied over time. Of children in Group I, 4–20% required multiple anti-hypertensive medications, whereas 32–53% of children in Group II required similar therapy (P=0. 0001; Figure 7).
Hyperkalemia
In Group I, the incidence of hyperkalemia requiring fludrocortisone therapy was 54% at 1 year and 7% at 7 years. Hyperkalemia that developed among children in Group II was milder and more readily controlled with ion exchange resins.
Diabetes
Currently, all children in both groups are normoglycemic and free of insulin therapy. However, 23.6% of children maintained with tacrolimus-based therapy required insulin for a short time while receiving total parental nutrition during periods of relatively high corticosteroid dosing.
DISCUSSION
There are several reports summarizing the advantages of tacrolimus over CsA in Ltx (12–14). Overall, these advantages are more pronounced for the pediatric population. Specifically, both the reduced incidence of steroid-resistant rejection and bile-independent absorption properties of tacrolimus have been cited as particular short-term benefits in the treatment of pediatric patients (18–21, 26–28). However, with introduction of the microemulsion formulation of CsA, these kinetics advantages may be of less value (29, 30). There are few reports that compare the long-term outcome of pediatric liver transplantation with tacrolimus-based and CsA-based therapies (31).
The present study reveals significantly improved patient and graft survival with tacrolimus-based therapy after 9 years of follow-up. The data are supported by a significantly reduced rate of retransplantation among tacrolimus-treated patients. These beneficial effects of tacrolimus may in part be related to the advantage of significantly reduced need of steroid. The majority of graft loss and death has occurred early on in the first 3 months after transplantation. In the past, we have reported that reduction in mortality and graft loss with tacrolimus, in 4-years of follow-up, was found to be related to uncontrolled rejection, sepsis, and technical failure by regression analysis (32).
Despite improvement in the surgical technique and better postoperative management, early mortality and graft failure in the first 3 months has remained troubling, particularly for children <2 years of age, regardless of treatment regimen. The incidence of late death or late graft loss reported here is low when compared with findings in adult populations in which age-related disease, de novo cancer, and recurrence of viral hepatitis have been major concerns after successful liver transplantation (33).
Although Cao et al. reported a reduced incidence and severity of rejection episodes in patients maintained with tacrolimus therapy (31), Andrew et al. did not observe any difference in severity or rate of rejection between tacrolimus-based or CsA-based therapies in pediatric populations (3). The present results suggest that the improved survival seen among tacrolimus-treated patients may be related to a reduced requirement to treat severe acute rejection episodes with OKT3. Almost 30% of acute episodes were controlled by increasing the baseline maintenance doses of either tacrolimus or prednisone (or both). In contrast, with CsA-based therapy, the addition of an additional steroid bolus was significantly higher. As reported for adults (12–14) the rate of steroid-resistant rejection requiring OKT3 in patients undergoing CsA-based therapy is significantly higher in children.
The present results also corroborate several reports of reduced requirement for baseline maintenance of corticosteroid therapy among tacrolimus-treated patients. Of tacrolimus-treated children, 70–80% were maintained with monotherapy without corticosteroids beyond 6 months after transplantation. This served as the litmus test for the development of immunosuppressive weaning trials, which eventually resulted in the withdrawal of drug therapy from some patient’s (34).
The principal advantage of monotherapy for the pediatric population has been normal growth and development. This, together with the absence of hirsutism and cushingoid faces, has promoted improvements in quality of life (1, 35, 36)
An additional corollary benefit of the reduced corticosteroid requirement may be seen in the reduced incidence of hypertension among tacrolimus-treated patients. The long-term incidence and severity of hypertension was significantly lower with tacrolimus-based than with CsA-based therapy; others have observed this (4). More important, the requirement for more than one anti-hypertensive medication was significantly lower with tacrolimus at all time points analyzed.
The rate of development of PTLD with tacrolimus-based therapy in this study population was numerically higher than that after treatment with CsA. However, the difference did not reach statistical significance when compared with the number of children at risk 1 month after LTx. At the same time, a higher rate of PTLD in the pediatric population has been reported by others (31). Survival after PTLD was significantly improved with tacrolimus-based compared with CsA-based therapy. Mortality related to PTLD was <20% after 9 years of follow-up with tacrolimus-based versus 50% with CsA-based therapy. PTLD in Group II occurring with relatively high doses of steroid may in part have affected the adverse outcome. Since reducing the induction dosage of tacrolimus after 1991and in subsequent years, the rate of PTLD has been lower, as previously reported (37). Currently, the ability to identify children at high risk for PTLD has allowed for appropriate dosing of immunosuppressants in the peritransplant period. Recommendations have been made to identify Epstein-Barr virus seronegative pediatric liver allograft candidates and the use of polymerase chain reaction to monitor viral load in guiding long-term adjustments of immunosuppressive and antiviral therapies (4). These practices are expected to further reduce the incidence of PTLD as well as the mortality resulting from PTLD.
The incidence of hyperkalemia requiring fludrocortisone was higher in tacrolimus patients, but diminished over the course of follow-up. Progressive nephrotoxicity leading to end stage renal failure has been low in both groups of children, with mean serum creatinine remaining stable into adulthood. Tacrolimus-associated diabetes has been acceptable among both pediatric and adult populations (32, 34, 35).
In conclusion, our experience has revealed that tacrolimus-based therapy offers better long-term graft and patient survival, a higher rate of freedom from steroids, fewer steroid-resistant rejections, and less hypertension. There were fewer deaths attributed to the development of PTLD among tacrolimus-treated pediatric liver allograft recipients. Furthermore, tacrolimus-treated patients experienced no gum hyperplasia, hirsutism, or cushingoid faces. On the basis of this report, tacrolimus should constitute the first line of immunosuppressive therapy for primary liver transplantation in children.
REFERENCES
- 1.Reyes J, Mazariegos GV. Pediatric transplantation. Surg Clin North Am. 1999;79(1):163. doi: 10.1016/s0039-6109(05)70013-x. [DOI] [PubMed] [Google Scholar]
- 2.Alonso EM, Gonzalez-Vallina R, Whitington PF. Update of pediatric liver transplantation. Eur J Pediatr. 1992;151 suppl 1:S23. doi: 10.1007/BF02125799. [DOI] [PubMed] [Google Scholar]
- 3.Andrews W, Sommerauer J, Roden J, Andersen J, Conlin C, Moore P. 10 years of pediatric liver transplantation. J Pediatr Surg. 1996;31(5):619. doi: 10.1016/s0022-3468(96)90660-0. [DOI] [PubMed] [Google Scholar]
- 4.Andrews WS, Sommerauer J, Conlin C, Moore P. Comparison of cyclosporine- vs tacrolimus-based immunosuppression in pediatric liver transplantation. Transplant Proc. 1996;28(2):897. [PubMed] [Google Scholar]
- 5.Dhawan A, Muiesan P. Pediatric liver transplantation. Acta Paediatr Jpn. 1998;40(6):525. doi: 10.1111/j.1442-200x.1998.tb01985.x. [DOI] [PubMed] [Google Scholar]
- 6.Starzl TE, Esquivel C, Gordon R, Todo S. Pediatric liver transplantation. Transplant Proc. 1987;19(4):3230. [PMC free article] [PubMed] [Google Scholar]
- 7.Starzl TE, Todo S, Fung JJ, Demetris AJ, Venkataramman R, Jain A. FK 506 for liver, kidney, pancreas transplantation. Lancet. 1989;2(8670):1000. doi: 10.1016/s0140-6736(89)91014-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fung JJ, Todo S, Jain A, et al. Conversion from cyclosporine to FK 506 in liver allograft recipients with cyclosporine-related complications. Transplant Proc. 1990;22(1):6. [PMC free article] [PubMed] [Google Scholar]
- 9.Fung JJ, Todo S, Tzakis A, et al. Conversion of liver allograft recipients from cyclosporine to FK 506-based immunosuppression: benefits and pitfalls. Transplant Proc. 1991;23(1 Pt. 1):14. [PMC free article] [PubMed] [Google Scholar]
- 10.Demetris AJ, Fung JJ, Todo S, et al. Conversion of liver allograft recipients from cyclosporine to FK506 immunosuppressive therapy: a clinicopathologic study of 96 patients. Transplantation. 1992;53(5):1056. doi: 10.1097/00007890-199205000-00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sher LS, Cosenza CA, Michel J, et al. Efficacy of tacrolimus as rescue therapy for chronic rejection in orthotopic liver transplantation: a report of the U.S Multicenter Liver Study Group. Transplantation. 1997;64(2):258. doi: 10.1097/00007890-199707270-00014. [DOI] [PubMed] [Google Scholar]
- 12.Fung JJ, Eliasziw M, Todo S, et al. The Pittsburgh randomized trial of tacrolimus compared to cyclosporine for hepatic transplantation. J Am Coll Surg. 1996;183(2):117. [PMC free article] [PubMed] [Google Scholar]
- 13.Anonymous. Randomised trial comparing tacrolimus (FK506) and cyclosporin in prevention of liver allograft rejection. European FK506 Multicentre Liver Study Group [see comments] Lancet. 1994;344(8920):423. [PubMed] [Google Scholar]
- 14.Anonymous. A comparison of tacrolimus (FK 506) and cyclosporine for immunosuppression in liver transplantation. The U.S. Multicenter FK506 Liver Study Group [see comments] N Engl J Med. 1994;331(17):1110. doi: 10.1056/NEJM199410273311702. [DOI] [PubMed] [Google Scholar]
- 15.Tzakis AG, Fung JJ, Todo S, Reyes J, Green M, Starzl TE. Use of FK 506 in pediatric patients. Transplant Proc. 1991;23(1 Pt 2):924. [PMC free article] [PubMed] [Google Scholar]
- 16.Todo S, Fung JJ, Demetris AJ, Jain A, Venkataramanan R, Starzl TE. Early trials with FK 506 as primary treatment in liver transplantation. Transplant Proc. 1990;22(1):13. [PMC free article] [PubMed] [Google Scholar]
- 17.Jain AB, Fung JJ, Todo S, et al. Incidence and treatment of rejection episodes in primary orthotopic liver transplantation under FK 506. Transplant Proc. 1991;23(1 Pt 2):928. [PMC free article] [PubMed] [Google Scholar]
- 18.Tzakis AG, Reyes J, Todo S, et al. Two-year experience with FK 506 in pediatric patients. Transplant Proc. 1993;25(1 Pt 1):619. [PMC free article] [PubMed] [Google Scholar]
- 19.Cox KL, Freese DK. Tacrolimus (FK506): the pros and cons of its use as an immunosuppressant in pediatric liver transplantation. Clin Invest Med. 1996;19(5):389. [PubMed] [Google Scholar]
- 20.McDiarmid SV, Busuttil RW, Ascher NL, et al. FK506 (tacrolimus) compared with cyclosporine for primary immunosuppression after pediatric liver transplantation. Results from the U.S. Multicenter Trial. Transplantation. 1995;59(4):530. [PubMed] [Google Scholar]
- 21.McDiarmid SV. The use of tacrolimus in pediatric liver trans-plantation. J Pediatr Gastroenterol Nutr. 1998;26(1):90. doi: 10.1097/00005176-199801000-00016. [DOI] [PubMed] [Google Scholar]
- 22.Cadoff EM, Venkataramanan R, Krajack A, et al. Assay of FK 506 in plasma. Transplant Proc. 1990;22(1):50. [PMC free article] [PubMed] [Google Scholar]
- 23.Grenier FC, Luczkiw J, Bergmann M, et al. A whole blood FK 506 assay for the IMx analyzer. Transplant Proc. 1991;23(6):2748. [PubMed] [Google Scholar]
- 24.Warty VS, Venkataramanan R, Zendehrouh P, et al. Practical aspects of FK 506 analysis (Pittsburgh experience) Transplant Proc. 1991;23(6):2730. [PMC free article] [PubMed] [Google Scholar]
- 25.Burckart GJ, Jain A, Diven W, Venkataramanan R, Starzl TE. Cyclosporine measurement by FPIA, PC-RIA, and HPLC following liver transplantation. Transplant Proc. 1990;22(3):1319. [PMC free article] [PubMed] [Google Scholar]
- 26.Tzakis AG, Reyes J, Todo S, et al. FK 506 versus cyclosporine in pediatric liver transplantation. Transplant Proc. 1991;23(6):3010. [PMC free article] [PubMed] [Google Scholar]
- 27.Jain AB, Venkataramanan R, Cadoff E, et al. Effect of hepatic dysfunction and T tube clamping on FK 506 pharmacokinetics and trough concentrations. Transplant Proc. 1990;22(1):57. [PMC free article] [PubMed] [Google Scholar]
- 28.Jain AB, Fung JJ, Tzakis AG, et al. Comparative study of cyclosporine and FK 506 dosage requirements in adult and pediatric orthotopic liver transplant patients. Transplant Proc. 1991;23(6):2763. [PMC free article] [PubMed] [Google Scholar]
- 29.Kahan BD, Dunn J, Fitts C, et al. The Neoral formulation: improved correlation between cyclosporine trough levels and exposure in stable renal transplant recipients. Transplant Proc. 1994;26(5):2940. [PubMed] [Google Scholar]
- 30.Winkler M, Haller G, Oldhafer K, et al. A new oral formulation of cyclosporine for early oral immunosuppressive therapy in liver transplant recipients. Transplantation. 1996;62(8):1063. doi: 10.1097/00007890-199610270-00006. [DOI] [PubMed] [Google Scholar]
- 31.Cao S, Cox KL, Berquist W, et al. Long-term outcomes in pediatric liver recipients: comparison between cyclosporin A and tacrolimus. Pediatr Transplant. 1999;3(1):22. doi: 10.1034/j.1399-3046.1999.00002.x. [DOI] [PubMed] [Google Scholar]
- 32.Todo S, Fung JJ, Starzl TE, et al. Single-center experience with primary orthotopic liver transplantation with FK 506 immunosuppression. Ann Surg. 1994;220(3):297. doi: 10.1097/00000658-199409000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jain AR, Kashyap R, Rohal S, Abu-Elmagd K, Starzl T, Fung J. What have we learned about primary liver transplantation under tacrolimus immunosuppression ? Long-term follow-up of the first 1000 patients. Ann Surg. 1999;230(3):441. doi: 10.1097/00000658-199909000-00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mazariegos GV, Reyes J, Marino IR, et al. Weaning of immunosuppression in liver transplant recipients. Transplantation. 1997;63(2):243. doi: 10.1097/00007890-199701270-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Senninger N, Golling M, Datsis K, Sido B, Herfarth C, Otto G. Glucose metabolism following liver transplantation and immunosuppression with cyclosporine A or FK 506. Transplant Proc. 1995;27(1):1127. [PubMed] [Google Scholar]
- 36.Krentz AJ, Dmitrewski J, Mayer D, et al. Postoperative glucose metabolism in liver transplant recipients. A two-year prospective randomized study of cyclosporine versus FK506. Transplantation. 1994;57(11):1666. [PubMed] [Google Scholar]
- 37.Cacciarelli TV, Green M, Jaffe R, et al. Management of post-transplant lymphoproliferative disease in pediatric liver transplant recipients receiving primary tacrolimus (FK506) therapy. Transplantation. 1998;66(8):1047. doi: 10.1097/00007890-199810270-00014. [DOI] [PubMed] [Google Scholar]