Recent study has revealed a cephalically directed brain stem system whose stimulation desynchronizes the electrical activity of the cerebral cortex in a manner simulating that observed in awakening from sleep or in the EEG arousal reaction (8). This system was found to be distributed in the reticular formation of the medulla, the tegmentum of the pons and midbrain and the sub- and hypothalamus. The means by which its activating influence became exerted upon the cortex was speculated upon and, because of the generalized distribution of the cortical effects, it seemed likely that the diffuse thalamic projection system was concerned. Some evidence favoring this possibility was obtained but the problem was left for further investigation.
Following preliminary determination of the organization of the diffuse thalamic projection system (12), the present study has explored this matter further: (i) by determining the thalamic regions whose electrical activity is desynchronized by stimulation of the reticular formation of the lower brain stem, (ii) by determining the distribution of thalamic sites from which evoked potentials are recorded upon single shock stimuli to the reticular activating system, (iii) by exploring the thalamic areas whose direct excitation desynchronizes the electrocorticogram, (iv) by ascertaining the distribution of cortical potentials evoked by single shock stimulation of the reticular activating system, and (v) by determining the effect of diencephalic lesions on ascending conduction of the reticular influence. The results indicate that the ventromedial part of the thalamus is most critically involved in transmission of the reticular activating influence, with the rest of the thalamus (including the diffuse thalamic projection system) playing a subsidiary role. Evidence, moreover, is provided that a proportion of the influence of the reticular activating system upon the cortex may be exerted by an extra-thalamic route, paralleling that of the secondary response system described by Morison et al. (1, 10, 11).
Methods
Cats immobilized with B-erythroidine were used, with a Palmer respirator providing artificial respiration. Exposures were made under local procaine. Small doses of chloralosane (5–7 mg./kg.) or nembutal (2–5 mg./kg.) were employed to impart a degree of synchrony to the electrical activity of the brain. Deep pickup or stimulating electrodes were oriented with a Horsley-Clarke instrument. Stimulating current, delivered with bipolar concentric electrodes, consisted of condenser discharges, usually with a falling phase of 0.5 msec., from a Goodwin stimulator. Subcortical activity was recorded with bipolar concentric electrodes consisting of the exposed tips of an insulated stainless steel barrel containing an insulated copper wire, with a polar separation of 0.5–2.0 mm. Cortical tracings were taken with brass screw electrodes resting on dura, or with silver-balled, pial pickups oriented with a Grass multiple electrode carrier. A Grass amplifier with ink-writer was used for recording.
Results
1. Subcortical desynchronization with high frequency bulbar stimulation
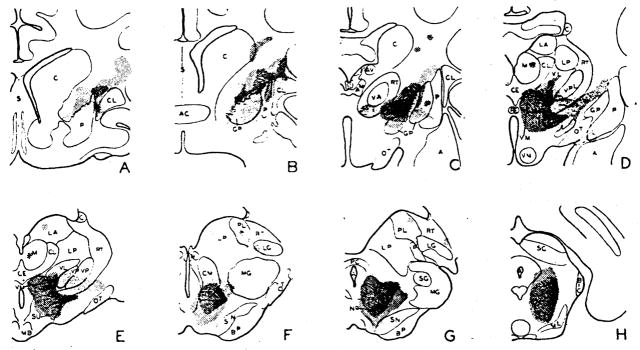
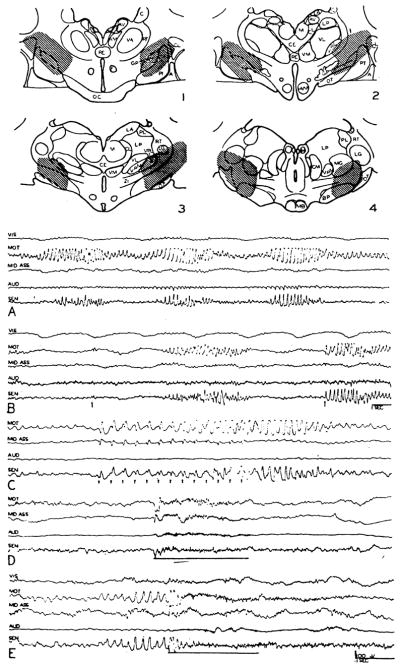
When the bulbar reticular formation was stimulated at 250/sec., usually with 2 volts, and recording electrodes moved systemically through the forebrain, the degree of desynchronization of subcortical electrical activity showed considerable variation. Although there was a tendency for electrical discharge to be activated to some degree in all regions of pickup, immediate strong and repeatable desynchronization was observed in the well-defined areas shown by light shading on transverse sections through the hemisphere in Figure 1. Responding areas included the midbrain tegmentum (Fig. 1G, H), the sub- and hypothalamus (D–F) and the ventromedial portion of the thalamus, in the region of the ventral part of the centromedian (F) and ventromedial and ventrolateral thalamic nuclei (D–E). From the mammillary level forward, the localization of the alteration became more diffuse, with progressive spread lateralward into the internal capsule (A–E). Of the masses of gray matter bordering the anterior limb of the capsule, only the globus pallidus manifested as good activation of electrical activity as did the capsule itself (B, C). The caudate was never affected greatly and responses present in the putamen and claustrum were not as dependable as the capsular ones. Typical examples of subcortical arousal are seen in the right column of Figure 2 from the internal capsule (A), ventromedial thalamic nucleus (B), subthalamus (C, D), centromedian nucleus (E) and reticular formation of the midbrain (F).
Fig. 1.
Transverse sections through hemisphere, showing regions exhibiting desynchronization of electrical activity during high frequency stimulation of bulbar or midbrain reticular formation (light shading), and evoked potentials on single shock reticular stimulation (dark shading). Note similarity of distribution. Abbreviations for Figs. 1, 4, 7 and 8 are as follows: A—amygdala, AC—anterior commissure, AM—anteromedial nuc., AV—anteroventral nuc., BIC—brachium of the inferior colliculus, BP—basis pedunculi, C—caudate nuc., CE—nuc. centralis medialis, CL—claustrum, CL—nuc. centralis lateralis, CM—centre median, F—fornix, GP—globus pallidus, H—habenular nuclei, HVM—hypothalamic ventromedial nuc., LA—nuc. lateralis anterior, LG—lateral geniculate nuc., LP—nuc. lateralis posterior, M—medial nuc., MB—mammillary body, MG—medial geniculate nuc., ML—medial lemniscus, NR—red nuc., OC—optic chiasma, OT—optic tract, P—posterior nuc., PC—posterior commissure, PL—pulvinar, PRE—pretectal region, PT—putamen, RE—nuc. reuniens, S—septum, SC—superior colliculus, SG—suprageniculate nuc., SN—substantia nigra, SU—subthalamic nuc., RT—reticular nuc., VA—nuc. ventralis anterior, VL—nuc. ventralis lateralis, VM—ventromedial nuc., VP—nuc. ventralis posterior, VPL—ventroposterolateral nuc., VPM—ventroposteromedial nuc., ZI—zona incerta.
Fig. 2.
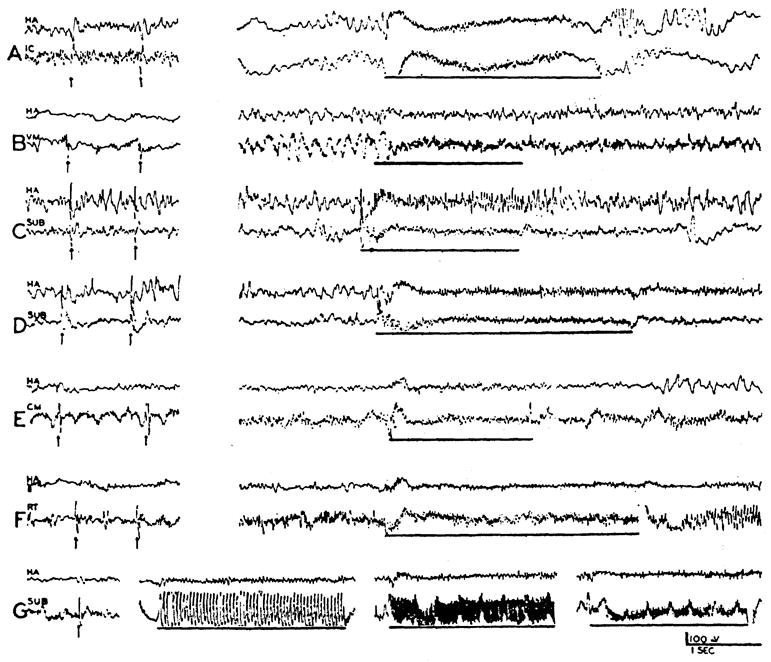
Records of electrical activity of cephalic brain stem structures during single shock (left) and repetitive (right) stimulation of reticular activating system at bulbar (A, C—G) and midbrain (B) levels. Single shock stimuli (arrows) had intensities of 2–5 V., repetitive stimuli (heavy line) had intensities of 2 V. and frequencies of 250/sec. Records were obtained from internal capsule (A), ventromedial thalamic nucleus (B), the same point in subthalamus (C and D) before (C) and after (D) removal of cerebellum, centre median (E), and midbrain tegmentum (F). In (G), records from subthalamus show, from left to right, effect of single shock, 20/sec., 50/sec., and 75/sec. stimuli (5 V.) to bulbar reticular formation. Upper channel, in each strip, is record from homolateral anterior cortex (HA).
When the brain stem reticular formation was stimulated at a more rostral level, in the midbrain tegmentum, the same distribution of subcortical effects was encountered as with excitation at the bulbar plane. Thus it is seen that by stimulating at various points along the activating system all more cephalically located components may be mobilized. Likewise, in one case, the midbrain tegmentum was stimulated, with a pickup electrode placed in the bulbar reticular formation, and a similar descending desynchronization was found in the bulb.
The recruiting nuclei of the thalamus, or the associational thalamic nuclei to which they project (12), were never prominently involved. Only the fringes of the diffusely projecting nuclei (ventral part of the centromedian, lateral wing of the intralaminar, and ventral portion of the ventralis anterior) could be implicated (Fig. 1C–F). The sensory relay nuclei likewise were relatively silent, with only the ventralis posterior, in immediate proximity to the best responsive area, participating regularly in the subcortical arousal (Fig. 1D, E). Although occasionally the associational nuclei showed desynchronization (D–F), they were also usually unaffected.
The subcortical sites at which activation was observed upon reticular stimulation usually exhibited a spontaneous low fast activity for a time after the recording electrode was placed. In these cases, stimulation was withheld until slow waves appeared to furnish a background of synchrony. While regions of low fast activity occurred in localities outside the activation foci, nonetheless, within the responsive areas the intensity of response usually paralleled the initial degree of spontaneous desynchronization. In a recent cathode-ray oscillograph analysis of cerebellar-like activity (3) the regions outlined in the present study were all involved along other subcortical areas exhibiting fast activity.
In addition to localized pickups with concentric electrodes, records were obtained from broader deep areas by recording between the tips of electrodes placed 3 mm. apart and oriented mediolaterally in the forebrain. With this technique, desynchronization during high frequency bulbar stimulation was found to some extent everywhere in the forebrain (Fig. 3). It was, however, most prominent in the areas defined with the more localized concentric electrodes.
Fig. 3.
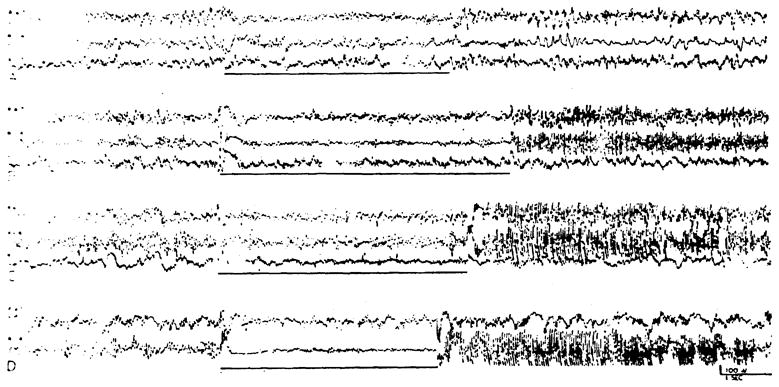
Records of activity between tips of electrodes placed 3 mm. apart and oriented mediolaterally in deep structures, showing subcortical activation and after-discharge of large fast waves induced by 2 V., 250/sec. stimulation of bulbar reticular formation marked by heavy line). In each case electrodes are at 1, 4, and 7 mm. from midline. Areas include: (A) rostral portion of thalamus, with electrodes from medial to lateral in nuc. reuniens, reticular nuc., and internal capsule; (B) mid-thalamus, with electrodes in ventromedial nuc., ventrolateral nuc., and ventroposterolateral nuc.; (C) rostral midbrain, with electrodes in central gray, midbrain tegmentum and substantia nigra; and (D) caudal midbrain, with electrodes in red nucleus, midbrain tegmentum, and substantia nigra. In designating channels, R 1–4 indicates recording to be between tips of electrodes placed at 1 and 4 mm. from midline; R 4–7 between electrodes at 4 and 7 mm. from midline; and R 1–7 between electrodes at 1 and 7 mm. from midline.
The poststimulatory activity of activated deep regions varied greatly. At times, the pre-existing spontaneous activity was immediately restored (Fig. 2A, E). More frequently, desynchronization persisted for some time after cessation of the stimulus (Fig. 2B–D). Irregularly seen was an immediate after-discharge consisting of fast, high amplitude waves. This last effect was noted when recording with concentric bipolar pickups (Fig. 2F), but was seen best with broader pickups (Fig. 3B–D). This exuberant after-discharge was found in the midbrain and diencephalic localities of activation outlined above, but could not be followed into the capsule. It was largest in the midbrain tegmentum and diminished progressively upon moving cephalad. Conversely, with midbrain reticular stimulation, a similar after-discharge was recorded from the bulbar reticular formation.
2. Subcortical potentials evoked by single shock reticular stimulation
It was soon noticed that subcortical regions exhibiting desynchronization upon high frequency reticular stimulation also showed large potentials (either spikes or waves) when a single pulse was delivered to the bulbar or midbrain reticular formation. Distribution of the regions from which these evoked potentials could be recorded is shown by darker shading on the transverse sections through the forebrain in Figure 1. The areas of projection closely parallel those for subcortical activation and include the tegmentum, ventromedial thalamus (centromedian, ventromedial and ventrolateral nuclei), sub- and hypothalamus and internal capsule. Occasional effects were sometimes seen in the dorsal thalamus (D, E), these invariably having a broad wave form and usually being evanescent.
Typical evoked potentials are seen in the left column of Figure 2, from the internal capsule (A), ventromedial thalamic nucleus (B), subthalamus (C, D), centre median (E), and the reticular formation of the midbrain (F). That these electrical alterations are dependent on the integrity of neural channels is shown by their disappearance, either upon death of the animal or by the interposition of a lesion between the stimulus and pickup.
Such rostrally propagated reticular potentials recorded from the subthalamus are shown with different frequencies of stimulation (Fig. 2G): single shock, 20/sec., 50/sec. and 75/sec. Individual evoked potentials follow the stimulus with little diminution of amplitude until a frequency of 40–50/sec. is reached but, with higher stimulus rates, are submerged in the low fast discharge of activation. In the experiment from which this record was taken, as in all, the ventralis posterior and other sensory relay nuclei were also explored, but spikes were not found. It is not possible, therefore, to attribute these potentials to the inadvertent stimulation of lemniscal or other long afferent paths bordering the reticular formation.
The rostral passage of reticular influences can thus be traced with equivalent results, using for reference either the desynchronization caused by high frequency stimulation or the potential evoked by a single shock. The electrical alteration in either case passes forward through the midbrain, sub- and hypothalamus, ventromedial part of the thalamus, and into the internal capsule. Moving dorsally in the thalamus, effects are increasingly harder to obtain and, similarly, upon pursuing the internal capsule forward, they wane progressively.
3. Activation of electrocorticogram by direct stimulation of brain stem
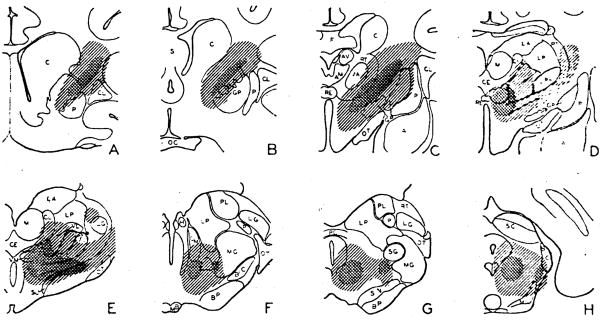
Stimuli were delivered at 250/sec., usually with 2 volts, and electrocorticograms taken from widely distributed cortical areas (Fig. 5). The generalized activation observed during stimulation of the reticular formation at bulbar (Fig. 5A) and midbrain levels (B) is seen also during excitation of the ventromedial thalamus (C) and internal capsule (D), all parts of the cortex exhibiting change. Regions whose stimulation induced such generalized cortical desynchronization are shown with shading on transverse sections in Figure 4, with the most dependable foci more darkly shaded. In the midbrain, the tegmentum was broadly included in the inciting zone. Cortical activation was best upon stimulation of the medial mesencephalon, however, in the same localities outlined for reticular spikes and subcortical activation (Fig. 4G, H). In addition, more lateral structures also yielded a generalized cortical effect. The ubiquitous cortical arousal elicited by excitation of this lateral zone was best when the afferent pathways present there—the brachium of the inferior colliculus and the medial lemniscus—were stimulated. In these instances, however, the cortical projection areas of these pathways were often specifically affected. With stimulation of the medial lemniscus, for example, high amplitude fast waves were evoked in the sensory cortex, in addition to desynchronization of activity in other cortical areas (Fig. 5G), and similar effects in the auditory cortex were observed upon stimulation of the brachium of the inferior colliculus.
Fig. 5.
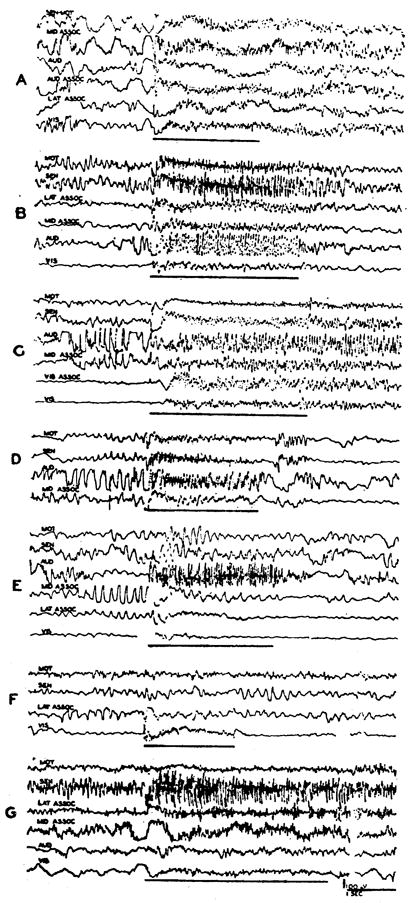
A–D: Records showing generalized cortical activation induced by high frequency stimulation of activating system at various levels: (A) bulbar reticular formation, (B) midbrain, (C) ventromedial thalamic nucleus, and (D) internal capsule. E–G: Also illustrated are the more specific effects observed upon direct stimulation of sensory pathways: (E) medial geniculate, with predominant effect in auditory cortex; (F) lateral geniculate, with effect limited to visual cortex; and (G) medial lemniscus in midbrain, with generalized activation of cortex but with fast high amplitude waves appearing in sensory area. Period of stimulation (2 V., 250/sec.) is marked by dark lines. Abbreviations of channels indicate: AUD—auditory, AUD ASSOC—auditory to middle suprasylvian association area, LAT ASSOC—lateral association area, MID ASSOC—middle suprasylvian association area, MOT—motor, SEN—sensory, SEN MOT—sensory to motor, VIS ASSOC—visual to lateral association area, VIS—visual.
Fig. 4.
Transverse sections through hemisphere with shading indicating regions whose direct stimulation yields generalized cortical activation. More darkly shaded areas are those from which effect was most reliable and had lowest threshold. Note similar distribution to that seen in Fig. 1.
At a thalamic level (Fig. 4C–F), general cortical arousal was best produced by stimulation of the sub- and hypothalamus, and the ventromedial portion of the thalamus, including the ventromedial and most of the ventrolateral nuclei, with a lateral excitable extension into the adjacent reticular nucleus and internal capsule. Stimulation of the diffusely projecting nuclei of the thalamus did not activate the cortex, except in the perimeter of the excitable zone just described and, of the relay nuclei, only the ventralis posterior appeared involved and then only in a marginal sense, along the fringes of the responsive area.
At the cephalic pole of the thalamus, the lateral migration of the excitable activating core was completed, and was predominantly capsular, with inclusion of the reticular nucleus and globus pallidus to a lesser extent. More rostrally still (Fig. 4A, B), the region is seen to continue forward in the internal capsule, which structure, rather than the basal ganglia (5), appears to be the excitable focus in this region.
Although high frequency stimulation of the relay nuclei did not cause the generalized cortical desynchronization just described, a localized provocation of fast activity was usually seen in their projection cortex. This effect was particularly clear in the case of the geniculate nuclei, which are far removed from the more medial regions that yield a generalized arousal. Thus, with stimulation of the medial geniculate, fast activity appeared almost exclusively in the auditory cortex, although the lateral association and visual areas also exhibited a mild activation (Fig. 5E) and, with stimulation of the lateral geniculate, the visual cortex alone was desynchronized (Fig. 5F). Similar effects were seen upon excitation of the ventralis posterior, although its proximity to the activating focus made such limited effects difficult to obtain.
It is thus clear that with high frequency stimulation of the brain stem, desynchronization of cortical electrical activity was distributed in two ways: broadly and indiscriminately over the entire cortex and, in a localized manner, in more circumscribed cortical zones. Localized fast activity was evoked by stimulating the relay nuclei of the thalamus, the effect being limited to or best in their projection cortex. Generalized arousal of electrocortical activity was best elicited by stimulating a more medial system running through the tegmentum of the midbrain, sub- and hypothalamus, and ventromedial thalamus, and which could be followed lateralward and forward in the internal capsule. In addition, from the lateral midbrain tegmentum, in the region of the brachium of the inferior colliculus and medial lemniscus, a generalized activation was elicited, but with specific effects, usually in the form of greater amplitude fast waves, in the cortical projection of the afferent path.
4. Cortical potentials evoked by stimulating activating system
Early in the investigation of evoked subcortical potentials, it was noticed that cortical spikes or waves were seen at a time when no potential of any kind could be found in the thalamic relay nuclei. It seemed likely, therefore, that these potentials specifically represented the cortical effect of reticular stimulation, the sub-cortical route being that just traced through the midbrain tegmentum, sub- and hypothalamus and ventromedial thalamus. The catholic nature of the desynchronization of electrocortical activity, upon stimulation of the activation system, had previously been emphasized by Moruzzi and Magoun (8) who further pointed out that it was often more pronounced in the frontal regions. If specificities of reticulocortical projection did exist, however, it seemed impossible to detect them with high frequency reticular stimulation, due to the generalized nature of the effect. Therefore, a survey of the distribution of evoked potentials on the dorsolateral cortex was conducted with single shock stimuli delivered to the bulbar or midbrain reticular formation. Cortical pickups were taken with bipolar concentric electrodes, with the tip buried in the deeper layers of cortex and the barrel on the surface.
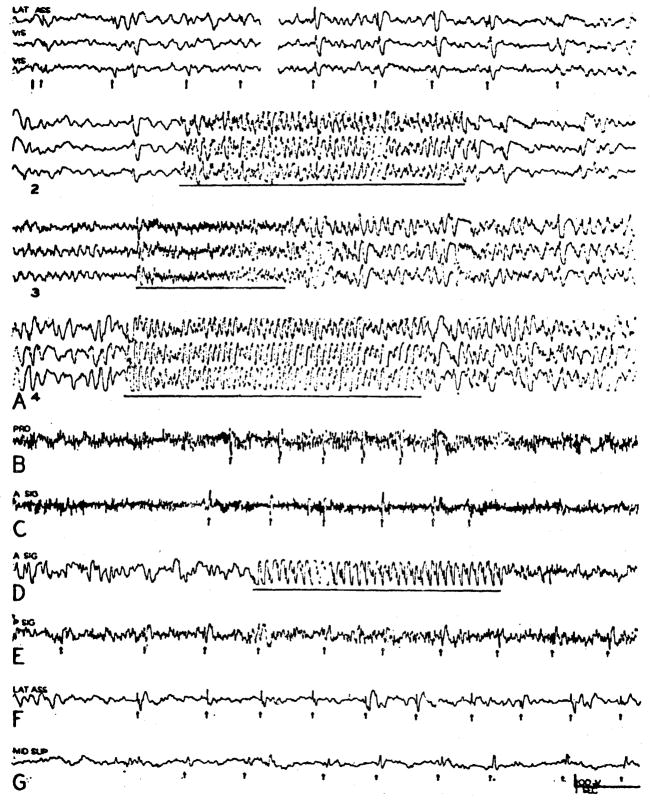
The distribution of the cortical potentials evoked by single shock stimulation of the reticular activating system, either in the midbrain or medulla, was extremely widespread, all of the dorsolateral cortex being implicated to a greater or less degree. However, the most intense and reliable effects were grouped toward the frontal pole of the hemisphere in the proreal and anterior and posterior sigmoid gyri (Fig. 6B–E). In these regions the potentials could follow frequencies up to 8–10/sec. as seen in the anterior sigmoid (Fig. 6D). Moreover, the evoked potentials were routinely sharper and less wave-like than those recorded from the more caudal cortical areas. In Fig. 6B, D, and E the midbrain tegmentum was excited and in C the bulbar reticular formation. The most characteristic feature of more caudal cortical effects was their great variability and their ability to be augmented by serially repeated stimuli (Fig. 6A, G).
Fig. 6.
Records showing alterations in cortical electrical activity evoked by 2–5 V. stimulation of activating system in midbrain or bulb (C). Pickups with bipolar concentric electrodes, with point in deeper layers of cortex or subjacent white matter and barrel on surface. Designation of channels indicate: A SIG—anterior sigmoid or motor, LAT ASSOC—lateral association, MID SUP—middle suprasylvian association, PRO—proreus or frontal association, P SIG—posterior sigmoid or sensory, VIS—visual. In (A) are shown the variable responses recorded from visual and lateral association areas initially (1—left) and after 90 sec. of intermittent stimulation (1—right). Potential changes induced by 25/sec. (2) and 250/sec. (3, 4) stimulation are also shown. Evoked potentials are shown in gyrus proreus (B); anterior sigmoid (C, D) with frequency of 7.5 sec. in D; posterior sigmoid (E); lateral association area (F); and middle suprasylvian association area (G) in which gradual increment of response occurred with continued stimulation.
Thus the salient features of reticulocortical connections, as studied with single pulse stimuli either to the bulbar reticular formation or midbrain tegmentum, were the generality of effect upon the proper conditions, the predominance of effect in the rostral part of the cortex, and the ability of the cortex to be “conditioned” in the caudal regions where the potentials were more capricious.
5. Effect of diencephalic lesions on activation elicited from lower brain stem
From the data presented, it appeared that reticular influences might follow alternative ascending routes to the cortex (see Figs. 1, 4): (a) extrathalamically, directly into the internal capsule from the sub- and hypothalamus, and (b) thalamically, with the principal influx through its ventromedial portion. In order to determine the relative importance of these respective pathways, electrolytic lesions were made eliminating one or the other.
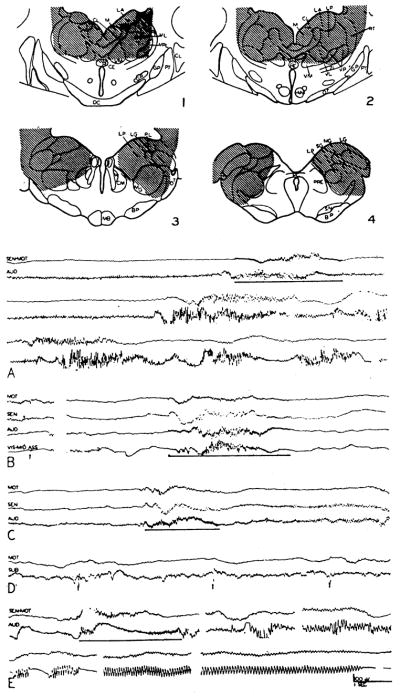
First, the thalamus was destroyed, leaving the basal diencephalon and internal capsule intact (Fig. 7, 1–4). In the lesion shown, the thalamus was destroyed almost in its entirety, with only nuclear borders being spared. Although a portion of the centre median and the caudal part of the medial nucleus were not directly injured, all possible radiations lateralward and forward from them were severed (Fig. 7, 3), indicating a complete functional thalamic destruction. With such a lesion, the spontaneous electrocorticogram showed almost no activity in the sensory-motor region and small fast discharge in the auditory area (Fig. 7A, top record). Stimulation either in the midbrain (A, top record, and B) or in the bulbar reticular formation (C) could still activate the cortex. The after-effect of stimulation differed, however, from that observed in the intact animal. After high frequency stimulation of the activation system, large amplitude, fast frequency, recurring bursts frequently followed after some seconds’ delay (Fig. 7A). Moreover, with the thalmus destroyed, single pulse stimuli to the tegmental or bulbar activating areas still evoked cortical potentials (Fig. 7B).
Fig. 7.
Transverse sections through diencephalon (1–4), showing complete functional destruction of thalamus in preparation from which records A–E were obtained. Channels are designated as follows: AUD—auditory cortex, MOT—motor cortex, SEN—sensory cortex, SEN-MOT—sensory to motor cortex, SUB—subthalamus, VIS-MID ASS— visual to lateral association cortex. Records obtained after a lesion show: A: (upper strip), activation of EEG during 250/sec. stimulation of reticular system, with subsequent sporadic high frequency bursts (middle strip), still continuing 25 sec. later (lower strip); B: wave in motor and other cortical areas evoked by single shock stimulation to midbrain’s tegmentum (left) and generalized arousal upon high frequency stimulation (right); C: similar effect induced by bulbar stimulation; D: potential changes in subthalamus induced by single shock bulbar stimuli; E: seizure induced by midbrain’s stimulation (upper left) to succeeding strips showing its course at 15, 25, 35, 50 and 110 sec. after stimulation.
An incidental finding in this case was the ease with which seizures could be provoked by brain stem stimulation. Although ordinarily 3 or 5 volt high frequency stimulation never initiated a cortical seizure, in this animal seizures occurred twice. In Figure 7E is seen the development and course of such a cortical fit, apparently provoked by midbrain tegmental stimulation at 5 volts, 250/sec.
Next, since it appeared that activation pathways partially followed an extrathalamic route, electrolytic lesions were produced in the lower part of the internal capsule, throughout the length of the diencephalon, by inserting the electrode from a lateral approach through the auditory cortex (Fig. 8, 1–4). This lesion destroyed extrathalamic pathways from the midbrain tegmentum and sub- and hypothalamus to the internal capsule while sparing connections of these structures with the thalamus as well as leaving intact a good proportion of thalamocortical connections. Therefore, any desynchronization of electrocortical activity, upon stimulation of the reticular system in the lower brain stem of this preparation, might be said to be mediated through the thalamus.
Fig. 8.
Transverse sections through diencephalon (1–4) from animal in which lesions destroyed that part of internal capsule transmitting extrathalamic corticipetal influences of reticular activating system. Records obtained after the lesion show: (A) spontaneous activity of cortex; (B) potentials evoked in cortical areas by click stimuli, although geniculate bodies were inadvertently injured and no primary auditory potentials could be evoked; (C) cortical potentials induced by 3/sec. stimulation of midbrain reticular substance; (D, E) generalized cortical arousal produced by 2–5 V. stimulation at 250/sec. in bulbar or midbrain reticular formation. Channels are as designated: AUD—auditory, MID ASS—middle suprasylvian association, MOT—motor, SEN—sensory, VIS—visual.
With such a lesion, spontaneous synchrony was a prominent feature of the electrocorticogram, with large recurrent 7–10/sec. spindle bursts dominating the picture, especially in the motor area (Fig. 8A). This finding resembled that observed earlier upon elimination of the activation system at more caudal levels, and has been attributed to the release of synchronizing thalamic elements (particularly the recruiting system) from activating influences (6).
In the instance shown in Figure 8, the medial geniculate bodies were inadvertently almost entirely destroyed. However, click stimuli were still capable of evoking cortical potentials. Although no evoked discharge occurred in the auditory cortex, waves appeared in the motor and sensory areas (Fig. 8B). The amplitude of these evoked potentials varied with their relation to spontaneous spindle bursts for during an interspindle period the effect was extremely small, while just before the occurrence of a burst, the potential was much more prominent (Fig. 8B) and, indeed, at times appeared to initiate the spindle (see also 9).
Single shock or low frequency stimulation of the activation system was likewise capable of evoking waves in the cortex. The strongest effects, as illustrated by stimulation of the midbrain tegmentum at 3/sec. (Fig. 8C), were in the same cortical areas exhibiting responses to click stimuli. Following the lesion shown in Figure 8, 1–4, high frequency stimulation of the bulbar or midbrain reticular formation was still fully capable of desynchronizing the electrocorticogram (Fig. 8D, E). There was, moreover, no regional specificity or effect, all areas recorded being desynchronized.
It thus appears that the ascending reticular activating influence may follow alternative routes to the cortex and course both by an extrathalamic path from basal diencephalic structures directly into the internal capsule and, as is usually the case with corticipetal discharge, by relay through the thalamus. In the latter route, the core of the system is concentrated in the ventromedial portion of the thalamus and probably fans in a diffuse manner dorsally to other thalamic regions, becoming progressively less well-defined in its ascent.
Since the cephalic course of the activating influence overlaps cerebellothalamic projections, the cerebellum was removed to see whether its presence was essential for mediation of any of the effects described above. A control was first obtained with pickups in the subthalamus and frontal cortex (Fig. 2C), in both of which spike potentials were evoked with single shock stimuli and desynchronization with 250/sec. stimulation of the bulbar reticular formation. After complete ablation of the cerebellum, the picture was not significantly changed either with single pulse or high frequency stimulation (Fig. 2D).
Discussion
In this attempt to determine the manner in which the reticular formation of the brain stem activates or desynchronizes the EEG, the mediation of reticulocortical influences has been studied both with single shock and with high frequency stimulation. Although critical interaction data are not available, it seems likely that the neural pathways responsible for rostral passage of single evoked potentials also mediate the activating influences produced with high frequency stimulation. Localization of these alterations, on recording through the forebrain, is essentially the same with either parameter of stimulation to the caudal reticular formation. Moreover, those areas which on direct high frequency stimulation yield generalized cortical arousal also evoke generalized cortical potentials when excited with single pulse stimuli. The change from the discrete effect of a single stimulus to the desynchronization produced with high frequencies can furthermore be followed with equal ease when recording either deeply or from the cortex.
Both reticular activation and single potentials have been seen to reach the cortex by two routes: over an extrathalamic path into the internal capsule, and by action upon the thalamus. In the thalamus, the effects are prominent only in its ventromedial portion, immediately above the sub- and hypothalamus, with a progressive diminution upon passing dorsally, the diffusely projecting nuclei (centre median, intralaminar, and ventralis anterior) lying on the fringes of the best responsive areas. However, when broader pickups were used through the thalamus, desynchronization was found everywhere.
It appears, then, that in thalamic mediation of the arousal reaction reticular influences enter the ventromedial part of the thalamus and are communicated to dorsal and lateral thalamic structures in an increasingly diffuse brush. Ultimate effect on the cortex might occur simply through the radiations of the individual nuclei. Whatever the channel involved, desynchronization of the spontaneous activity of its ventromedial region would appear to be the primary event in electrocortical activation exerted through the thalamus.
The extrathalamic component of the ascending reticular system has been seen to migrate laterally from the sub- and hypothalamus into the internal capsule, with the principal departure at and above the mammillary level. Unequivocal evidence for this route is provided by persistence of generalized cortical activation upon high frequency stimulation of the caudal portion of the reticular system after destruction of the thalamus. From the data presented, it is clear that the extrathalamic ascending pathway of the activation system closely parallels that for the “secondary response,” described in 1936 by Derbyshire et al (2) as a late cortical wave induced by sciatic stimulation under deep anesthesia. This secondary response was distributed widely and bilaterally in the cortex, and could only follow stimuli up to 3/sec. under deep barbiturate anesthesia (4). By means of lesions it was shown to be transmitted through the medial midbrain tegmentum and subthalamus to reach the cortex by an ill-defined extrathalamic route (1). It persisted after section of the medial lemniscus and could be reproduced by direct stimulation of the midbrain and basal diencephalon (11). While the path of the secondary response has been regarded as purely extrathalamic, the present study has shown that after low capsular lesions click stimuli or single shocks to the medial brain stem still evoked widely distributed cortical waves which were evidently mediated through the thalamus.
It may be emphasized that, as with the reticular activating system, high frequency stimulation of sensory pathways in the brain stem also causes a generalized cortical arousal, but with the distribution of effects diminishing progressively as the brain stem is ascended until, with direct excitation of the thalamic relay nuclei, desynchronization of electrocortical activity is limited to the respective projection area. An explanation for this is offered by the results of recent study of collaterals from afferent somatic and auditory paths, in which an extensive brush of collateral connections has been found moving into the medial brain stem as far forward as the thalamus (13). Thus with stimulation of peripheral receptors, collateral afferent impulses feed into the activating system, functionally mobilizing it (8). That this may occur even at a thalamic level is seen by the slight over-all cortical desynchronization sometimes encountered during direct stimulation of the relay nuclei of the thalamus.
Summary
The ascending course of the reticular activating system has been investigated in the brain stem of the cat, with special reference to conduction through the diencephalon.
With repetitive stimulation of this system in the bulb or mid-brain, desynchronization of electrical activity has been observed in the sub- and hypothalamus, ventromedial thalamus and internal capsule. Potentials evoked by single shock reticular stimuli are recorded from the same areas. Excitation of these diencephalic regions, in turn, induces generalized desynchronization of electrocortical activity, and single shock stimuli delivered to them evoke widely distributed cortical potentials.
These results suggest that alternative routes are available for corticipetal conduction of the reticular activating influence over: (i) a thalamic path involving transmission to the ventromedial part of the thalamus, with relays to the cortex from the remainder of this structure; and (ii) an extrathalamic path involving direct passage into the internal capsule from the sub- and hypothalamus. In agreement, after selective destruction of either one of these routes, leaving the other intact, generalized desynchronization of electrocortical activity could still be elicited by lower brain stem stimulation.
References
- 1.Dempsey EW, Morison RS, Morison BR. Some afferent diencephalic pathways related to cortical potentials in the cat. Amer J Physiol. 1941;131:718–731. [Google Scholar]
- 2.Derbyshire AS, Rempel B, Forbes A, Lambert EF. Effects of anesthetics on action potentials in the cerebral cortex of the cat. Amer J Physiol. 1936;116:577–596. [Google Scholar]
- 3.Eldred E, Snider RS. Anatomical distribution of fast cerebellar-like activity within the brain stem. Anat Rec. 1950;106:190–191. [Google Scholar]
- 4.Forbes A, Morison BR. Cortical response to sensory stimulation under deep barbiturate narcosis. J Neurophysiol. 1939;2:112–128. [Google Scholar]
- 5.Gerebtzoff MA. Contribution a la physiologie du corps strié. Arch int Physiol. 1941;51:333–351. [Google Scholar]
- 6.Lindsley DB, Bowden JW, Magoun HW. Effect upon the EEG of acute injury to the brain stem activating system. EEG clin Neurophysiol. 1949;1:475–486. [PubMed] [Google Scholar]
- 7.Lindsley DB, Schreiner LH, Knowles WB, Magoun HW. Behavioral and EEG changes following chronic brain stem lesions in the cat. EEG clin Neurophysiol. 1950;2:483–498. doi: 10.1016/0013-4694(50)90086-1. [DOI] [PubMed] [Google Scholar]
- 8.Moruzzi G, Magoun HW. Brain stem reticular formation and activation of the EEG. EEG clin Neurophysiol. 1949;1:455–473. [PubMed] [Google Scholar]
- 9.Moruzzi G, Brookhart JM, Niemer WT, Magoun HW. Augmentation of evolved electro-cortical activity during spindle bursts. EEG clin Neurophysiol. 1950;2:29–31. [Google Scholar]
- 10.Morison RS, Dempsey EW, Morison BR. On the propagation of certain cortical potentials. Amer J Physiol. 1941;131:744–751. [Google Scholar]
- 11.Morison RS, Dempsey EW, Morison BR. Cortical responses from electrical stimulation of the brain stem. Amer J Physiol. 1941;131:732–743. [Google Scholar]
- 12.Starzl TE, Magoun HW. Organization of the diffuse thalamic projection system. J Neurophysiol. 1951;14:133–146. doi: 10.1152/jn.1951.14.2.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Starzl TE, Taylor CW, Magoun HW. Collateral afferent excitation of reticular formation of brain stem. J Neurophysiol. 1951;14:479–496. doi: 10.1152/jn.1951.14.6.479. [DOI] [PMC free article] [PubMed] [Google Scholar]