Abstract
The interaction between gut inflammatory processes and stress is gaining increasing recognition. Corticotropin releasing factor (CRF)-receptor activation in the brain is well established as a key signaling pathway initiating the various components of the stress response including in the viscera. In addition, a local CRF signaling system has been recently established in the gut. This review summarize the present knowledge on mechanisms through which both brain and gut CRF receptors modulate intestinal inflammatory processes and its relevance towards increased inflammatory bowel disease (IBD) activity and post-infectious irritable bowel syndrome (IBS) susceptibility induced by stress.
Introduction
In the past years, the influence of psychosocial and environmental stressors on the pathogenesis of “stress-associated” illnesses such as obesity, metabolic syndrome and type 2 diabetes mellitus, as well as, pain and chronic fatigue syndromes, received increased awareness (Miller et al. 2009; Muscatell et al. 2009; Webster et al. 2008). Early on Hans Selye, who coined the term stress, noticed that the gastrointestinal tract and immune systems are particularly responsive to stressors (Selye 1936). A growing consensus recognized the association of stressful life experiences, and the development and/or exacerbation of functional bowel disorders, such as irritable bowel syndrome (IBS) (Barreau et al. 2008; De Giorgio and Barbara 2008; Santos et al. 2008; Stengel et al. 2009, Taché and Brunnhuber 2008), and inflammatory bowel disease (IBD) activity (Maunder and Levenstein 2008; Mawdsley and Rampton 2005; Santos et al. 2008). IBD, which includes Crohn’s disease and ulcerative colitis (UC), is associated with a measurable inflammatory response leading to gut tissue damage (Shih and Targan 2008). IBS patients, on the other hand, display no or in some cases only low-grade colonic mucosal inflammation (De Giorgio and Barbara 2008). IBS, as defined by Rome III criteria, is characterized by recurrent abdominal pain or discomfort associated with constipation and/or diarrhea, and may show some overlap with microscopic colitis (De Giorgio and Barbara 2008; Limsui et al. 2007). IBS causes long-lasting discomfort and distress in patients, however neither leads to intestinal damage, nor increases the incidence of cancer or chronic inflammatory state. In addition, IBS is a highly prevalent functional bowel disorder, which accounts for up to 40% of gastroenterology outpatients, hence making it an important clinical entity (Taché and Brunnhuber 2008).
The association or impact of psychosocial factors on IBD and IBS prompted investigations to unravel the contributions of stress-related mechanisms in their pathophysiology in order to develop novel therapeutic options targeting these pathways. One principal effector of stress responsive systems is the 41 amino acid corticotropin releasing factor (CRF) that is released in the brain in response to stressors and plays a pivotal role to activate the hypothalamic-pituitary-adrenal (HPA) axis (Lightman, 2008). Brain CRF also acts as a neurotransmitter to modulate behavior and activity of the autonomic nervous system (Taché et al. 2009). In addition, the gut contains peripheral CRF signaling pathways (Fekede and Zorrila, 2007; Stengel and Taché 2009, Taché and Bonaz 2007) that, along with the brain CRF, can contribute to stress-related progression and exacerbation of IBD and IBS manifestations by modulating the autonomic and enteric nervous systems as well as immune function (Fukudo 2007; Gross and Pothoulakis 2007; Mawdsley and Rampton 2005; Paschos et al. 2009; Taché and Brunnhuber 2008).
In this review, we will outline experimental and clinical evidence to show that the central and peripheral CRF systems modulate intestinal inflammation, and show that CRF receptors may be components of the inflammatory processes in IBD and post-infectious IBS, which is relevant to mechanisms underlying the susceptibility of these diseases to stress.
CRF signaling: a brief overview
Corticotropin-releasing factor, a 41-amino-acid peptide, is a major mediator of the endocrine arm of the stress response by centrally stimulating the HPA axis. Neurons of the parvocellular part of the paraventricular nucleus (pPVN) of the hypothalamus produce CRF. The neuropeptide is released into the portal blood vessels of the Zona externa of the median eminence and reaches the pituitary gland where it binds to CRF1 receptors localized on corticotroph cells in the anterior pituitary, thereby inducing the secretion of adrenocorticotropin hormone (ACTH) which stimulates glucocorticoid secretion from the adrenal gland (Lightman, 2008). Other CRF-related peptides include urocortin 1 (Ucn 1), Ucn 2 and Ucn 3 which bear high structural similarities to CRF and high conservation throughout evolution (for review see Fekete and Zorilla 2007; Stengel and Taché 2008). CRF and urocortins are also expressed in peripheral tissues and can be released by regional sensory and sympathetic nerves, immune cells, and gut enteroendocrine and enteric cells to act locally (Fekede and Zorrila, 2007; Karalis et al. 1991; Liu et al. 2006).
Both CRF and urocortins interact with CRF1 and/or CRF2 receptors which are members of the B1 subfamily of seven-transmembrane-domain receptors and show 70% sequence homology (Hillhouse and Grammatopoulos, 2006; Markovic et al. 2008). Eleven alternative splice variants of CRF1 receptors have been identified in humans, in addition to three others in rats, four in mice, and nine in hamsters (Hillhouse and Grammatopoulos 2006; Stengel and Taché 2008). Expression of these variants is tissue specific and can be modified by environmental factors (Fekete and Zorilla 2007; Hillhouse and Grammatopoulos 2006). Recent studies point to functional relevance of these different isoforms by modifying CRF actions in target tissue, either attenuating or amplifying the signaling of functional CRF1a receptors (Markovic et al. 2008). In humans, CRF2 receptor signaling splice variants include the subtypes 2a, 2b, and 2c, whereas most other mammals only express 2a and 2b isoforms with a distinct expression pattern in different tissues (Hauger et al. 2003; Stengel and Taché 2008). Importantly, the binding affinities of CRF and urocortins to CRF1 and CRF2 receptors differ considerably (Hauger et al. 2003). CRF displays its highest affinity to CRF1 receptors (Hauger et al. 2003). Ucn 1 shows an equal affinity to both CRF receptor types whereas Ucn 2 and Ucn 3 are selective agonists for CRF2 receptor (Hauger et al. 2003).
CRF receptors are G-protein coupled receptors and most physiological actions of CRF in the brain and periphery involves the coupling of CRF and urocortins to Gαs proteins that stimulate cAMP mediated signaling cascades (Hauger et al., 2003). Upon agonist activation, both CRF1 and CRF2 receptors can also be coupled to multiple Gα-subunits with an order of potency: Gαs>Gαo>Gαq/11>Gαil/2>Gαz (Hillhouse and Grammatopoulos 2006). Differences in the coupling characteristics of CRF receptors/G protein have been observed within tissues as well as with inbred animal strains (Hauger et al. 2003; Hillhouse and Grammatopoulos 2006; Pioszak et al. 2008). G-protein signaling after ligand receptor interactions can increase intracellular Ca2+ concentrations or result in the activation of different phosphokinases A, B, and C. It can also modify the phosphorylation pattern of intracellular proteins and thereby activate mitogen-activated protein kinases (MAPKs) such as ERK1/2 and p38/MAPK (Hillhouse and Grammatopoulos 2006; Grossini et al. 2009; Gutknecht et al. 2009;). By these multiple signaling pathways, CRF receptor activation influences neuronal, endothelial, endocrine, smooth muscle, epithelial and immune cell activity which are increasingly understood but need further characterization at specific target cells (Black 2002; Hillhouse and Grammatopoulos 2006).
The immune system of the gastro-intestinal tract: a brief overview
Due to its position and function, the gastrointestinal tract, along with the other viscera containing mucosal compartments such as the respiratory and reproductive tracts, represents the first line of defense of the body against luminal antigens and pathogens derived from the environment. Intestinal epithelial cells (IECs) form a single layer of polarized cells, tightly sealed by tight junctions that impede paracellular transport of small macromolecules via the paracellular route. IECs produce high amounts of mucus and secrete antimicrobial peptides that limit bacterial colonization (Al-Sadi et al. 2009; Kraehenbuhl and Corbett 2004; Macpherson et al. 2008). Under physiological conditions, the epithelial barrier remains permeable to a few bacteria and antigens in follicle-associated epithelia such as M cells which overlay aggregated mucosal lymphoid tissue, allowing the gut to induce tolerogenic reactions (Al-Sadi et al. 2009; Kraehenbuhl and Corbett 2004). If bacteria and antigens pass at sites of leaky tight junctions, however, there is induction of a transient local low-grade proinflammatory cytokine response that is essential to inhibit invasion of microorganisms into the body (Al-Sadi et al. 2009; Kraehenbuhl and Corbett 2004; Macpherson et al. 2008). This response is rapidly counter-regulated and does not result in a measurable systemic proinflammatory state.
Mechanisms that prevent inflammation in the intestine include the production of neutralizing immunoglobulins (Ig) such as IgA (Kraehenbuhl and Corbett 2004; Macpherson et al. 2008) and a low expression of pattern recognition receptors (PRRs) and co-stimulatory molecules on antigen-presenting cells, which are essential for T cell activation (Akira et al. 2006; Kraehenbuhl and Corbett 2004; van Vliet et al. 2007). There is also local release of the cytokine, thymic stromal lymphopoietin (TSLP), which inhibits the differentiation of dendritic cells (DCs) that drive a proinflammatory T helper (Th) cell 1-mediated immune response (Liu et al. 2007). The anti-inflammatory cytokine bias in the gut is basically maintained by high secretion of interleukin (IL)-10 and tissue growth factor-β (TGF-β) which are released by regulatory T cells (Makita et al. 2007). IL-10 suppresses both Th-1 and Th-2-cell responses as well as the innate immune functions (Kraehenbuhl and Corbett 2004). Thus, under physiological conditions, there is a phenomenon of tolerance where the gut is in a state of active immunosuppression, which prevents a destructive immune response while still eliminating microbes and antigens by neutralization or induction of a local, controlled low-grade and transient inflammation (Gonzalez-Rey et al. 2007; Kraehenbuhl and Corbett 2004). However, when pathogens or increased numbers or bacteria pass the intestinal barrier, a measurable local proinflammatory response is induced to eliminate the threatening agents.
Stress modulate the activity of neuroendocrine, immune and gastrointestinal systems (Black 2002; Lightman 2008; Taché and Brunnhuber 2008; Webster Marketon and Glaser 2008,). Altered release of neuroendocrine factors, such as glucocorticoids, vasoactive intestinal peptides, neurotensin, adrenomedullin, catecholamines or CRF and its related peptides, by stress, may disturb the intestinal cytokine balance and intestinal barrier integrity (Gonzalez-Rey et al. 2007; Gross and Pothoulakis, 2007; Santos et al. 2008). Inflammation is a main component in the pathogenesis of IBD (Shih and Targan 2008; Gross and Pothloulakis, 2007) and there is increasing evidence that low grade proinflammatory processes may also have a role in the development of IBS particularly post-infectious IBS (De Giorgio and Barbara 2008; Spiller and Garsed, 2009; Santos et al. 2008).
CRF receptor signaling and IBD
IBD is associated with a measurable inflammatory response in the gut which includes activation of the innate immune response via recognition of microbial structures by pattern recognition receptors (e.g. Toll-like receptors) as well as activation of the adaptive immune system driving Th-1 and Th-17 responses that trigger the inflammatory processes in the intestine (Arseneau et al. 2007; Shih and Targan, 2008). Th1 responses induce a proinflammatory cytokine bias, including increased release of interferon-gamma (IFNγ) or tumor necrosis factor-alpha (TNFα) to defend microbial and viral infections, but also perpetuate autoimmune responses. Excessive proinflammation may lead to uncontrolled tissue damage. In addition, Th17 cells produce IL-17, which promotes a local inflammatory response including IL-6 and IL-8 release and neutrophils chemotaxis to remove microbes. By triggering an excessive inflammatory response, Th17 cells contribute to the development and exaggeration of autoimmune diseases such as IBD (Shih and Targan, 2008). Under physiological conditions, regulatory T (Treg)- and Th2 cell driven responses, which include the release of IL-10, TGF-β and IL-4, counteract the Th1 mediated microbicidal and autoimmune actions (Arseneau et al. 2007, Sanchez-Munoz et al. 2008).
Considerable evidence show that psychological stress increases the risk of relapse and/or exacerbation of IBD symptoms (Mawdsley and Rampton 2005). Activation of the CRF system may be part of the underlying mechanism of stress-related modulation of IBD activity as reviewed recently (Gross and Pothoulakis 2007; Mawdslely and Rampton 2005; Paschos et al., 2009). Stressors such as hypothermia, hyperosmolarity, and hypoxia as well as inflammatory stimuli induce CRF secretion in human lymphocytes and mouse splenocytes (Baigent and Lowry; 2000; Bellinger et al. 2001; Kravchenco and Furalev 1994). In several tissues, CRF signaling promotes the immune response: CRF stimulates human lymphocyte proliferation by increasing IL-2 receptor expression and enhancing the production of IL-1 and IL-2 (Singh and Leu 990). In addition, CRF and/or urocortins evoke enhances endotoxin-induced cytokine release (TNFα, Il 1 and IL-6) from mice peritoneal macrophages, chemotaxis of mononuclear cells and induces macrophage activation which is associated with local release of oxidative mediators and proinflammatory cytokines (Agelaki et al. 2002; Black 2002; Koshida and Kotake 1994; Moss et al. 2007; Tsatsani et al. 2006, Tsatsani et al. 2007; Wlk et al. 2002). In addition, urocortins are expressed in several immune cells (Gravanis and Margioris 2005; Muramatsu et al. 2000) and can evoke a proinflammatory response. Urocortin 1 induces IL-6 release in a time- and dose-dependent manner which is associated with the activation of ERK and p38 MAP kinases and stimulation of nuclear factor kappa B (NFκB) in cardiomyocytes (Huang et al. 2009).
In the intestine, activation of CRF signaling results in promoting the proinflammatory response. CRF expression is upregulated at the gene and protein levels in inflammatory, mesenchymal and neuronal cells in rat cecum in response to peptidoglycan-induced colitis. Conversely, in experimental models of intestinal inflammation, CRF-deficient mice display an attenuated inflammatory response (Gay et al. 2008; Kokkotou et al. 2006). Clinical studies showed that the colonic mucosa of patients with IBD displays increased numbers of CRF-immunoreactive enterochromaffin and macrophage cells (Gross and Pothoulakis, 2007). Other reports indicate an increased expression of CRF immunoreactive cells in the lamina propria, namely in mononuclear and macrophage in colonic biopsies from patients suffering from active UC (Gross and Pothoulakis, 2007). Likewise, Ucn 1 expression is upregulated in lamina propria macrophages and expressed in enterochromaffin cells and IECs in patients with UC with a positive correlation with the intensity of the disease (Gross and Pothoulakis, 2007; Muramatsu et al. 2000; Saruta et al. 2004).
The contribution of the CRF1 receptor signaling in colonic inflammation induced by trinitrobenzene sulfonic acid combined six weeks later with repeated nociceptive stress of colorectal distensions has been demonstrated by the reduction in the number of colonic neutrophils and eosinophils in rats pretreated with CRF1 antagonist before exposure to repeated colorectal distentions (Saito-Nakaya et al. 2008).
Further studies also established the involvement of the Ucn 2-CRF2 receptor signaling pathway in the modulation of colonic inflammation (Gross and Pothoulakis, 2007; Paschos et al. 2009). Patients who suffer from IBD display high expression of Ucn 2 and CRF2 receptors in the colon (Moss et al. 2007). Likewise, in a rat model of chemically-induced colitis, Ucn 2 expression is increased in a large population of infiltrating immune cells of the distal colon, and associated with compensatory down-regulation of CRF2 receptors in neurons of the myenteric plexus (Chang et al. 2007). Moreover, CRF2 receptor deficient mice show a reduced susceptibility to intestinal inflammatory response to experimental induction of colitis (Kokkotou et al. 2006; Moss et al. 2007). At the cellular level, CRF can modulate macrophage function by augmenting LPS-induced proinflammatory cytokine production in these cells which is CRF2 receptor dependent and enhance Toll-like receptor (TLR)-4 gene expression (Tsatsani et al. 2006). It was also demonstrated that CRF, Ucn 1 and Ucn 2, acting through both CRF1 and CRF2 dependent signaling pathways, enhances TNF-α transcription in macrophages (Tsatsani et al. 2007). Moss et al. demonstrated in human non-transformed NCM460 colonocytes that Ucn 2 induces NFκB signaling via CRF2 receptors which results in an exaggerated release of the chemokine IL-8 (Moss et al. 2007).
Besides the described effects of CRF and its related peptides acting on CRF receptors located on different immune cells, mast cells are also targets of stress and CRF receptor signaling action in inflammation (Farhadi et al. 2007). The plasticity of mast cells allows for a rapid and selective immune response. Mast cells can release a variety of preformed or newly synthesized mediators, among those proteases and inflammatory mediators including histamine, chymase, tryptase, IL-1β and TNF-α (Rao and Brown 2008 Siddiqui and Miner 2004). In particular, mast cell tryptase or chymase (RMCPII) (He 2004) activate protease activated receptor-2 (PAR-2) on intestinal epithelial cells and heighten paracellular permeability (Demaude et al. 2009; He 2004; Santos et al. 2008). Therefore, mast cells are important effectors of the intestinal epithelial response to stress and inflammation, which include ion secretion abnormalities, macromolecules increased permeability and mucin release (Farhadi et al. 2007; Santos et al. 2008). In the human colonic mucosa, CRF receptors are expressed at the gene and protein levels exclusively in resident mast cells, thought not in all (Wallon et al. 2008). In the rat colon, the presence of CRF receptors on mast cells and their modulation by CRF are supported by functional reports (Santos et al. 2008), while immunostaining showed CRF1 receptor in some non-specified lamina propria cells that need to be further characterized (Chatzaki et al. 2004). In addition to expressing CRF receptors, activated mast cells can also release CRF and its related peptides (Theoharides et al. 2004). CRF and urocortins which are locally released by immune and enteroendocrine cells (Baignet and Lowry 2000; Chang et al. 2007; Gross and Pothoulakis 2007) induce a mast-cell dependent increased permeability as shown in human biopsies of sigmoid colon and in the rat proximal colon (Barreau et al. 2007; Santos et al. 2008; Wallon et al. 2008).
Moreover, Barreau et al. demonstrated that neonatal maternal deprivation in rats increases the intestinal permeability by mechanisms involving CRF-induced CRF1 receptor-dependent mast cell activation in adulthood (Barreau et al. 2007). The observation that mast cell-deficient rodents do not exhibit a stress-induced increase in permeability, as well as the reversibility of permeability changes by mast cell reconstitution, highlight the importance of mast cells in stress-related colonic barrier dysfunction (Farhadi et al. 2007; Santos et al. 2008). Of note, however, is that while mast cells are incontestably key players in these events, recent studies in mice support also the involvement of CRF- and mast cell-independent pathways in the early phase of stress-induced alterations of permeability, via proteases most likely originating from the pancreas (Demaude et al. 2009).
Collectively these data support CRF signaling through both CRF1 and CRF2 receptors as an important process in stress-related modulation of immune components in IBD (for overview see Tab. 1, Fig. 1 and Paschos et al., 2009).
Tab 1.
Overview of CRF signaling pathways-induced alterations of inflammatory processes/immune activation in gut associated tissues and/or cells.
| Cell type | Biological actions of CRF signaling | CRF receptor | References |
|---|---|---|---|
| Mast cells | Release of proteases (histamine, heparin, chymase, carboxypeptidase, tryptase) | CRF, Ucn 1/CRF1 |
Barreau et al. 2007 Farhardi et al. 2005 He 2004 |
| Release of arachidonic acid derivates (PGE2) |
Rao and Brown 2008 Santos et al. 2008 |
||
| Release of cytokines (IL-1β, IL-3, IL-4, IL-5, IL-6, IL-9, IL-10, IL-13, IL-16, IL-18, IL-25, TNF-α) |
Shih and Targan 2008 Taché and Brunnhuber 2008 |
||
| Release of chemokines (such as IL-8, MCP-1, 3 and 4, RANTES, eotaxin) | Wallon et al. 2008 | ||
| Macrophages | Increase activation release of oxidative mediators | CRF1 and CRF2 |
Chang et al. 2007 Muramatsu et al. 2000 |
| Dose-dependent inhibition of activation of TNF-α release | CRF1 and CRF2 or CRF2 selectively |
Moss et al. 2007 Tsatsanis et al. 2007 Tsatsani et al. 2006 Tsatsanis et al. 2005 Wallon et al. 2008 |
|
| Induction of COX-2/PGE2 | CRF1 and CRF2 | Wlk et al. 2002 | |
| Time- and dose-dependent enhanced apoptosis | Ucn 1, Ucn2/CRF2 | ||
| Dendritic cells | Decrease the release of IL-18 | not defined | Lee et al. 2009 |
| Mucosal plasma cells | Correlation with inflammation in ulcerative colitis | Ucn 1/CRF1 | Saruta et al. 2004 |
| Lymphocytes | Stimulation of lymphocyte proliferation | not clearly defined |
Agelaki et al. 2002 Koshida and Kotake1994 |
| Increase IL-2 receptor expression | |||
| Enhance IL-1 and IL-2 production | Singh and Leu 1990 | ||
| Increased chemotaxis of mononuclear cells | |||
| Colonocytes | NFκB signaling and IL-8 release | Ucn 2/CRF2 | Moss et al. 2007 |
| Intestinal epithelial cells | Increase permeability of mucosa | CRF1 and CRF2 | Castagliuolo et al. 1998 Santos et al. 2008 Barreau et al. 2007 Wallon et al. 2008 Demaude et al. 2009 |
| Undefined cell type in mucosa | Increase inflammatory response during colitis | CRF/CRF2 or undefined Ucn 2/CRF2 |
Fukudo et al. 2007 Gay et al. 2008 |
| Correlation of Ucn 2 expression and IBD symptoms | Kokkotou et al. 2006 | ||
| Anti-inflammatory effects of CRF | CRF2 | Moss et al. 2007 |
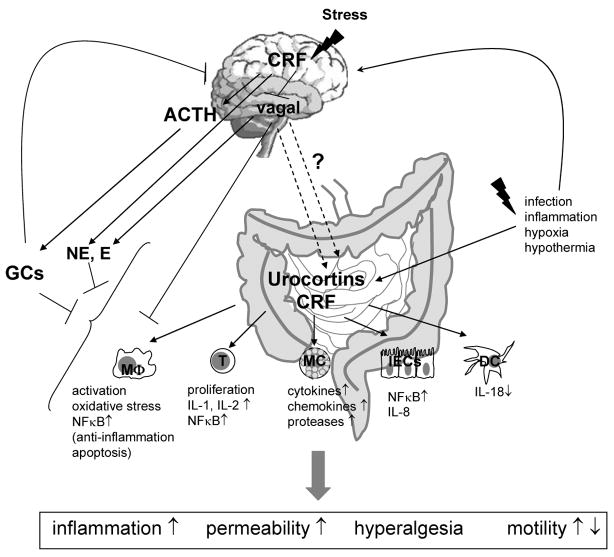
Figure 1. Putative role of central and peripheral CRF signaling pathways to influence immune processes and potential implications in stress-related IBD and IBS symptoms.
Exposure to central or peripheral stressors activates the HPA axis and/or peripheral CRF/urocortin release via alterations of autonomic nervous system and/or additional unknown pathways. Immune cells such as macrophages (Mφ), T lymphocytes (T), mucosal mast cells (MC), dendritic cells (DC) or intestinal epithelial cells (IECs) respond to CRF receptor signaling mainly by promoting inflammation. Proinflammatory cytokines enhance inflammation, increase permeability and change local neuronal activity which can alter pain reception and intestinal function. Increased circulating glucocorticoids (GC), and catecholamines and decreased parasympathetic activity induced by central CRF exert anti-inflammatory effects which may counter-regulate inflammatory processes in the gut.
Microscopic Inflammation and IBS
The role of the immune system in the pathogenesis of IBS in subsets of patients has gained recognition in the past years (De Giorgio and Barbara 2008; Kindt et al. 2009; Liebregts et al. 2007; Ohman et al. 2009a; Ohman et al 2009b; Spiller and Garsed 2009). In particular, recent clinical studies provide evidence that subsets of IBS patients can display low-grade inflammation in the intestinal mucosa (De Giorgio and Barbara 2008; Kindt et al. 2009). For instance, psychological factors including fatigue and depression are associated with low-grade inflammatory infiltrate and increased mast cell count in biopsies of the colon of IBS patients (Piche et al. 2008). In addition, there is a high correlation between the severity of abdominal pain and mast cell numbers and activation in the colonic mucosa of IBS patients (De Giorgio and Barbara 2008).
Well documented prospective studies show an incidence of 4%–31% of IBS occurrence following bacterial gastroenteritis and stressful life events, increasing the risk to develop such post-infectious IBS (Spiller and Garsed 2009). A significant subset of patients with IBS also has mildly increased counts of bacteria in the upper intestinal tract (Posserud et al. 2007, Scarpellini et al. 2009). However, a correlation between low-grade inflammatory processes and IBS symptoms is still to be evaluated. Systemic monitoring of inflammatory components in IBS patients indicate that peripheral blood mononuclear cells (PBMCs) exhibit heightened baseline levels of TNF-α, IL-1β and IL-6 after 24 hours ex vivo culture and an increased IL-6 inducibility after LPS stimulation (Liebregts et al. 2007). Of interest was the recent report that PBMC supernatants from patients with post-infectious IBS diarrhea had predominantly increased mechanosensory responses of pelvic serosal, muscular or mucosal afferents of the colon. This indicates that secretory factors from PBMCs sensitize colonic afferent nerve endings, leading to a more hyperalgesic response to mechanical stimuli (Hughes et al. 2009). Moreover, an unexpected discovery was that PBMCs under healthy conditions secrete factors that dampen the mechanosensitivity of afferent fibers (Hughes et al. 2009). These findings provide new insight to understand the complex interactions between neural and immune system components in the gut, switching from inhibition to potentiation in IBS patients, which needs to be further characterized.
Animal models are consistent with mild transient low-grade inflammation triggering IBS-like symptoms such as colonic hyperalgesia and long-term sensitization of enteric neurons, leading to prolonged changes of visceral sensory functions (Adam et al. 2006). It is well recognized that proinflammatory cytokines released in the intestinal tract can alter intestinal homeostasis in different neuronal and epithelial effector systems. For instance, IL-1β and IL-6 inhibit acetylcholine release from cholinergic nerve terminals in myenteric ganglia and thereby decrease gastrointestinal motility (Lomax et al. 2005). Other inflammatory mediators such as prostaglandin E2 exert stimulatory effects on intestinal secretion and motility by slowing membrane depolarization which increases the neuronal excitability and therewith a general activation of nearly all enteric neurons (Linden et al. 2004; Manning et al. 2002). There is also evidence that TNF-α, as an important mediator of mucosal mast cells, induces tight junction dysregulation and initiates proapoptotic pathways of intestinal epithelial cells as well as changes in intestinal permeability (Groschwitz and Hogan, 2009).
While the underlying immune component in IBS pathogenesis is gaining recognition, particularly in post-infectious IBS and in association with stress (Dupont 2007; Spiller and Garsed 2009), the implication of brain and peripheral CRF receptor signaling in this inflammatory process has been little investigated so far. This contrasts with the vast literature providing convergent experimental evidence that brain and peripheral CRF1 signaling pathways are involved in experiment models of IBS or stress-induced visceral hypersensitivity, increased colonic permeability and altered secretory and motor functions (Taché and Brunnhuber 2008, Stengel and Taché. 2009). Inflammatory processes are modulated by the autonomous nervous system including levels of sympathetic and parasympathetic activation, which are both altered by activation of brain CRF signaling (Irwin et al. 1990, Wood and Woods 2007). Interestingly, IBS is associated with autonomic dysregulation as confirmed by studies showing that autonomic cardiovascular regulation is impaired in IBS patients due to a reduced parasympathetic but relatively increased sympathetic activity (Tillisch et al. 2006; Waring et al. 2004). In addition, increased activation of the sympathetic nervous system in the etiologic role in IBS is supported by the positive correlation between resting heart rate indicative of sympathetic activity, and visceral sensation during rectal balloon inflation, which was found in IBS patients but not in healthy individuals (Gupta et al. 2002; Tillisch et al. 2006). The known central action of CRF to decrease vagal efferent outflow could be relevant in this context (Taché and Bonaz 2007; Wood and Woods 2007). It is clearly established that vagal cholinergic anti-inflammatory pathways are activated in response to peripheral immune activation (Tracey 2007), a phenomenon similar to the low-grade inflammation observed in post-infectious IBS (Liebregts et al. 2007, Piche et al. 2008). Therefore, central CRF release by reducing the vagal cholinergic anti-inflammatory tone to the intestine may indirectly promote local inflammatory processes. In addition, activation of sympathetic outflow contributes to intestinal inflammation typically by suppressing immune cell functions (Straub et al. 2006, Irwin et al. 1990). Interestingly, the vagus nerve functionally regulates the postganglionic catecholaminergic fibers of the splenic nerve, which induces catecholamine-dependent inhibition of TNF-α production in splenic macrophages (Rosas-Ballina et al. 2008). Whether a similar mechanism of vagal modulation of sympathetic activity in the intestinal tract exists is unknown. However, it is of relevance to note that sympathetic innervation is lost during prolonged inflammatory conditions in the gut, thereby promoting chronic inflammatory processes which has been demonstrated in IBD or rheumatoid arthritis (Straub et al. 2006), but has yet to be investigated under conditions of chronic low-grade inflammation in IBS. Activation of brain CRF receptors by CRF and Ucn 1 increases sympathetic and adrenomedullary activity leading to increased norepinephrine and epinephrine levels (Wood and Woods 2007) and thereby inhibits immune activation as it was shown for rat splenic NK cells (Irwin 1990). Whether central activation of CRF can also indirectly modulate NK cells in the gut and alter intestinal immune homeostasis, at least under physiological or acute inflammatory processes, remains to be elucidated.
Mechanisms of CRF signaling expressed in the gut in post-infectious, low grade inflammation IBS may involve known direct stimulatory actions of CRF on immune cells, in particular mast cells (Santos et al. 2008; Taché and Brunnhuber 2008; Wallon et al. 2008) as detailed in the IBD section. However, further studies are required to assess whether there is an upregulation of the CRF ligands and alterations of CRF variants regulating peptide actions in IBS patients with low grade inflammation.
CRF receptor signaling and anti-inflammatory processes
It is well established that proinflammatory mediators such as IL-1β, TNF-α and IL-6, which are released as a consequence of peripheral immune activation, stimulate the hypothalamic secretion of CRF which evokes adrenal glucocorticoid release and activation of the sympathetic nervous system (Lightman 2009; Silberstein et al. 2009; Stengel and Taché 2009). Both, glucocorticoids and catecholamines secondarily display immunosuppressive actions by promoting anti-inflammatory processes and inhibiting proinflammatory responses of the innate and adaptive immune system (also see above) (Goetzl et al. 2008; Webster Marketon and Glaser 2008, Straub et al. 2006, Irwin et al. 1990). Additionally, a few reports also indicate indirect local anti-inflammatory actions of CRF receptor signaling mediated by CRF2 receptors (Lee et al. 2009; Tsatsani et al. 2005, Tstsani 2007), which contrasts with reports of local proinflammatory action as described earlier in this review (Kokkotou et al. 2006; Moss et al. 2007; Paschos et al. 2009). In particular, Lee et al. showed that human monocyte-derived DCs express CRF1 and CRF2 receptors. CRF stimulation of these DCs decreased the release of IL-18 which is a proinflammatory mediator that promotes a Th-1 shift of the T cell response, thus showing that it is anti-inflammatory (Lee et al. 2009). Other authors showed that CRF, Ucn 1 and Ucn 2 can transiently inhibit an LPS-induced TNFα-response in the murine macrophage cell line RAW264.7 and freshly-isolated primary murine peritoneal macrophages through interaction with both CRF receptor types via the induction of a COX-2/PGE2 pathway. This immune inhibitory phase is followed by a second phase of heightened TNF-α production due to enhanced transcription of the TNF-gene indicating that the anti-inflammatory effects of CRF-receptor signaling in macrophages is confined at the early stage of inflammation (Tsatsanis et al. 2007). The same authors previously demonstrated that low doses of Ucn 1 and Ucn 2 (10−10–10−8 M) activated CRF2 receptor signaling in macrophages which enhances apoptosis and thereby promotes an anti-inflammatory response whereas higher doses of peptides did not (Tsatsanis et al. 2005). This indicates that differential modulation of inflammatory processes by CRF peptides is not only time- but also dose-dependent.
Conclusions
Activation of peripheral CRF receptor signaling in the gut is mostly considered to be proinflammatory because it is in general associated with intestinal immune cell activation (for overview: Tab. 1 and Fig. 1). During inflammatory processes in the intestine, CRF and its related peptides activate mast cells and possibly other immune cells such as T cells, dendritic cells and macrophages. Increased colonic permeability after local immune cell activation may increase the antigenic challenge in lamina propria and submucosa which can induce and/or exaggerate inflammatory processes in IBD. CRF signaling may also contribute to the development of post-infectious IBS through similar local action of the CRF system in the gut which needs further investigation so that it may lead to novel therapeutic strategies for this subgroup of patients. In addition, in the brain CRF alters the autonomic nervous system activity by decreasing vagal output and increasing sympathetic outflow that is known to have an impact on the inflammatory process by withdrawing the cholinergic anti-inflammatory action of the vagal efferent tone and promoting sympathetic immunomodulation. The detailed cellular events and possible treatment options of inflammatory intestinal disorders regarding interference with CRF signaling pathways remain to be elucidated.
Acknowledgments
The authors thank Miss Eugenia Hu for reviewing the manuscript. Dr. C. Kiank is supported by the German Research Foundation Grant KI 1389/2–1 and Dr. Y. Taché is in receipt of Veteran Administration Research Career Scientist Award and NIHDDK R01grants DK 33061 and DK57238.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Adam B, Gschossmann JM, Krippner C, Scholl F, Ruwe M, Holtmann G. Severity of mucosal inflammation as a predictor for alterations of visceral sensory function in a rat model. Pain. 2006;123:179–86. doi: 10.1016/j.pain.2006.02.029. [DOI] [PubMed] [Google Scholar]
- 2.Agelaki S, Tsatsanis C, Gravanis A, Margioris AN. Corticotropin-releasing hormone augments proinflammatory cytokine production from macrophages in vitro and in lipopolysaccharide-induced endotoxin shock in mice. Infect Immun. 2002;70:6068–6074. doi: 10.1128/IAI.70.11.6068-6074.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 4.Al-Sadi R, Boivin M, Ma T. Mechanism of cytokine modulation of epithelial tight junction barrier, Front. Biosc. 2009;14:2765–2778. doi: 10.2741/3413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arseneau KO, Tamagawa H, Pizarro TT, Cominelli F. Innate and adaptive immune responses related to IBD pathogenesis. Curr Gastroenterol Rep. 2007;9:508–512. doi: 10.1007/s11894-007-0067-3. [DOI] [PubMed] [Google Scholar]
- 6.Baignet SM, Lowry PJ. mRNA expression profiles for corticotrophin-releasing factor (CRF), urocortin, CRF receptors and CRF-binding protein in peripheral rat tissues. J Mol Endocrinol. 2000;25:43–52. doi: 10.1677/jme.0.0250043. [DOI] [PubMed] [Google Scholar]
- 7.Barreau F, Cartier C, Leveque M, Ferrier L, Moriez R, Laroute V, Rosztoczy A, Fioramonti J, Bueno L. Pathways involved in gut mucosal barrier dysfunction induced in adult rats by maternal deprivation: corticotrophin-releasing factor and nerve growth factor interplay. J Physiol. 2007;580:347–356. doi: 10.1113/jphysiol.2006.120907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bellinger DL, Felten DL, Lorton D, Brouxhon S. Effects of interleukin-2 on the expression of corticotropin-releasing hormone in nerves and lymphoid cells in secondary lymphoid organs from the Fisher 344 rat. J Neuroimmunol. 2001;119:37–50. doi: 10.1016/s0165-5728(01)00362-9. [DOI] [PubMed] [Google Scholar]
- 9.Black RH. Stress and the inflammatory response: a review of neurogenic inflammation. Brain Behav Immun. 2002;16:622–653. doi: 10.1016/s0889-1591(02)00021-1. [DOI] [PubMed] [Google Scholar]
- 10.Chang J, Hoy JJ, Idumalla PS, Clifton MS, Bhargava A. Urocortin 2 expression in the rat gastrointestinal tract under basal conditions and in chemical colitis. Peptides. 2007;28:1453–1460. doi: 10.1016/j.peptides.2007.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chatzaki E, Crowe PD, Wang L, Million M, Taché Y, Grigoriadis DE. CRF receptor type 1 and 2 expression and anatomical distribution in the rat colon. J Neurochem. 2004;90:309–316. doi: 10.1111/j.1471-4159.2004.02490.x. [DOI] [PubMed] [Google Scholar]
- 12.De Giorgio R, Barbara G. Is irritable bowel syndrome an inflammatory disorder? Curr Gastroenterol Rep. 2008;10:385–390. doi: 10.1007/s11894-008-0073-0. [DOI] [PubMed] [Google Scholar]
- 13.Demaude J, Levêque M, Chaumaz G, Eutamène H, Fioramonti J, Buéno L, Ferrier L. Acute stress increases colonic paracellular permeability in mice through a mast cell-independent mechanism: involvement of pancreatic trypsin. Life Sci. 2009;84:847–852. doi: 10.1016/j.lfs.2009.03.016. [DOI] [PubMed] [Google Scholar]
- 14.Dupont AW. Post-infectious irritable bowel syndrome. Curr Gastroenterol Rep. 2007;9:378–384. doi: 10.1007/s11894-007-0046-8. [DOI] [PubMed] [Google Scholar]
- 15.Farhadi A, Fields JZ, Keshavarzian A. Mucosal mast cells are pivotal elements in inflammatory bowel disease that connect the dots L stress, intestinal permeability and inflammation. World J Gastroenterol. 2007;13:3027–3030. doi: 10.3748/wjg.v13.i22.3027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fekete EM, Zorrilla EP. Physiology, pharmacology, and therapeutic relevance of urocortins in mammals: Ancient CRF paralogs. Front Neuroendocrinol. 2007;28:1–27. doi: 10.1016/j.yfrne.2006.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fukudo S. Role of corticotropin-releasing hormone in irritable bowel syndrome and intestinal inflammation. J Gastroenterol. 2007;42(Suppl 17):48–51. doi: 10.1007/s00535-006-1942-7. [DOI] [PubMed] [Google Scholar]
- 18.Gay J, Kokkotou E, O’Brien M, Pothoulakis C, Karalis KP. Corticotropin-releasing hormone deficiency is associated with reduced local inflammation in a mouse model of experimental colitis. Endocrinology. 2008;149:3403–3409. doi: 10.1210/en.2007-1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goetzl EJ, Chan RC, Yadav M. Diverse mechanisms and consequences of immunoadoption of neuromediator systems. Ann N Y Acad Sci. 2008;1144:56–60. doi: 10.1196/annals.1418.008. [DOI] [PubMed] [Google Scholar]
- 20.Gonzalez-Rey E, Chorny A, Delgado M. Regulation of immune tolerance by anti-inflammatory neuropeptides. Nat Rev Immunol. 2007;7:52–63. doi: 10.1038/nri1984. [DOI] [PubMed] [Google Scholar]
- 21.Gravanis A, Margioris AN. The corticotropin-releasing factor (CRF) family of neuropeptides in inflammation: potential therapeutic applications. Curr Med Chem. 2005;12:1503–1152. doi: 10.2174/0929867054039008. [DOI] [PubMed] [Google Scholar]
- 22.Groschwitz KR, And Hogan SP. Intestinal barrier function: molecular regulation and disease pathogenesis. J All Clin Immuno. 2009;124:3–20. doi: 10.1016/j.jaci.2009.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gross KJ, Pothoulakis C. Role of neuropeptides in inflammatory bowel disease. Inflamm Bowel Dis. 2007;13:918–32. doi: 10.1002/ibd.20129. [DOI] [PubMed] [Google Scholar]
- 24.Grossini E, Molinari C, Mary DA, Uberti F, Ribichini F, Caimmi PP, Vacca G. Urocortin II induces nitric oxide production through cAMP and Ca2+ related pathways in endothelial cells. Cell Physiol Biochem. 2009;23:87–96. doi: 10.1159/000204097. [DOI] [PubMed] [Google Scholar]
- 25.Gupta V, Sheffield D, Verne GN. Evidence for autonomic dysregulation in the irritable bowel syndrome, Dig. Dis Sci. 2002;47:1716–22. doi: 10.1023/a:1016424007454. [DOI] [PubMed] [Google Scholar]
- 26.Gutknecht E, Van der Linden I, Van Kolen K, Verhoeven KF, Vauquelin G, Dautzenberg FM. Molecular mechanisms of corticotropin-releasing factor receptor-induced calcium signaling, Mol. Pharmacol. 2009;75:648–657. doi: 10.1124/mol.108.050427. [DOI] [PubMed] [Google Scholar]
- 27.Hauger RL, Grigoriadis DE, Dallman MF, Plotsky PM, Vale WW, Dautzenberg FM. International Union of Pharmacology. XXXVI Current status of the nomenclature for receptors for corticotropin-releasing factor and their ligands. Pharmacol Rev. 2003;55:21–26. doi: 10.1124/pr.55.1.3. [DOI] [PubMed] [Google Scholar]
- 28.Hillhouse EW, Grammatopoulos DK. The molecular mechanisms underlying the regulation of the biological activity of corticotropin-releasing hormone receptors: implications for physiology and pathophysiology. Endocr Rev. 2006;27:260–286. doi: 10.1210/er.2005-0034. [DOI] [PubMed] [Google Scholar]
- 29.Huang M, Kempuraj D, Papadopoulou N, Kourelis T, Donelan J, Manola A, Theoharides TC. Urocortin induces interleukin-6 release from rat cardiomyocytes through p38 MAP kinase, ERK and NF-{kappa}B activation. J Mol Endocrinol. 2009;42:397–405. doi: 10.1677/JME-08-0120. [DOI] [PubMed] [Google Scholar]
- 30.Hughes PA, Brierley SM, Martin CM, Brookes SJ, Linden DR, Blackshaw LA. Post-inflammatory colonic afferent sensitization: different subtypes, different pathways, and different time-courses. Gut Online. 2009 doi: 10.1136/gut.2008.170811. [DOI] [PubMed] [Google Scholar]
- 31.Irwin M, Vale W, Rivier C. Central corticotropin-releasing factor mediates the suppressive effects of stress on natural killer cell cytotoxicity. Endocrinology. 1990;126:2837–2844. doi: 10.1210/endo-126-6-2837. [DOI] [PubMed] [Google Scholar]
- 32.Karalis K, Sano H, Redwine J, Listwak S, Wilder RL, Chrousos GP. Autocrine or paracrine inflammatory actions of corticotropin-releasing hormone in vivo. Science. 1991;254:421–423. doi: 10.1126/science.1925600. [DOI] [PubMed] [Google Scholar]
- 33.Kawahito Y, Sano H, Mukai S, Asai K, Kimura S, Yamamura Y, Kato H, Chrousos GP, Wilder RL, Kondo M. Corticotropin releasing hormone in colonic mucosa in patients with ulcerative colitis. Gut. 1995;37:544–51. doi: 10.1136/gut.37.4.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kindt S, Van Oudenhove L, Broekaert D, Kasran A, Ceuppens JL, Bossuyt X, Fischler B, Tack J. Immune dysfunction in patients with functional gastrointestinal disorders. Neurogastroenterol Motil. 2009;21:389–398. doi: 10.1111/j.1365-2982.2008.01220.x. [DOI] [PubMed] [Google Scholar]
- 35.Kokkotou E, Torres D, Moss AC, O’Brien M, Grigoriadis DE, Karalis K, Pothoulakis C. Corticotropin-releasing hormone receptor 2-deficient mice have reduced intestinal inflammatory responses. J Immunol. 2006;177:3355–3361. doi: 10.4049/jimmunol.177.5.3355. [DOI] [PubMed] [Google Scholar]
- 36.Koshida H, Kotake Y. Corticotropin-releasing hormone enhances the superoxide anion production of rabbit peritoneal macrophages stimulated with N-formyl-methionyl-leucyl-phenylalanine. Life Sci. 1994;54:539–543. doi: 10.1016/0024-3205(94)90004-3. [DOI] [PubMed] [Google Scholar]
- 37.Kraehenbuhl JP, Corbett M. Keeping the gut at bay. Science. 2004;303:1624–1625. doi: 10.1126/science.1096222. [DOI] [PubMed] [Google Scholar]
- 38.Kravchenco IV, Furalev VA. Secretion of immunoreactive corticotropin releasing factor and adrenocorticotropic hormone by T- and B-lymphocytes in response to cellular stress factors. Biochem Biophys Res Commun. 1994;204:828–834. doi: 10.1006/bbrc.1994.2534. [DOI] [PubMed] [Google Scholar]
- 39.Lee HJ, Kwon YS, Park CO, Oh SH, Lee JH, Wu WH, Chang NS, Lee MG, Lee KH. Corticotropin-releasing factor decreases IL-18 in the monocyte-derived dendritic cell. Exp Dermatol. 2009;18:199–204. doi: 10.1111/j.1600-0625.2008.00781.x. [DOI] [PubMed] [Google Scholar]
- 40.Liebregts T, Adam B, Bredack C, Roth A, Heinzel S, Lester S, Downie-Doyle S, Smith E, Drew P, Talley NJ, Holtmann G. Immune activation in patients with irritable bowel syndrome. Gastroenterology. 2007;132:913–920. doi: 10.1053/j.gastro.2007.01.046. [DOI] [PubMed] [Google Scholar]
- 41.Lightman SL. The neuroendocrinology of stress: a never ending story. J Neuroendocrinol. 2008;20:880–884. doi: 10.1111/j.1365-2826.2008.01711.x. [DOI] [PubMed] [Google Scholar]
- 42.Limsui D, Pardi DS, Camilleri M, Loftus EV, Jr, Kammer PP, Tremaine WJ, Sandborn WJ. Symptomatic overlap between irritable bowel syndrome and microscopic colitis. Inflamm Bowel Dis. 2007;13:175–181. doi: 10.1002/ibd.20059. [DOI] [PubMed] [Google Scholar]
- 43.Linden DR, Sharkey KA, Ho W, Mawe GM. Cyclooxygenase-2 contributes to dysmotility and enhanced excitability of myenteric AH neurones in the inflamed guinea pig distal colon. J Physiol. 2004;557:191–205. doi: 10.1113/jphysiol.2004.062174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu S, Gao N, Hu HZ, Wang X, Wang GD, Fang X, Gao X, Xia Y, Wood JD. Distribution and chemical coding of corticotropin-releasing factor-immunoreactive neurons in the guinea pig enteric nervous system. J Comp Neurol. 2006;494:63–74. doi: 10.1002/cne.20781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu YJ, Soumelis V, Watanabe N, Ito T, Wang YH, Malefyt Rde W, Omori M, Zhou B, Ziegler SF. TSLP: an epithelial cell cytokine that regulates T cell differentiation by conditioning dendritic cell maturation. Annu Rev Immunol. 2007;25:193–219. doi: 10.1146/annurev.immunol.25.022106.141718. [DOI] [PubMed] [Google Scholar]
- 46.Lomax AE, Fernández E, Sharkey KA. Plasticity of the enteric nervous system during intestinal inflammation. Neurogastroenterol Motil. 2005;17:4–15. doi: 10.1111/j.1365-2982.2004.00607.x. [DOI] [PubMed] [Google Scholar]
- 47.Macpherson A, McCoy KD, Johansen FE, Brandtzaeg P. The immune geography of IgA induction and function. Mucosal Immunol. 2008;1:11–22. doi: 10.1038/mi.2007.6. [DOI] [PubMed] [Google Scholar]
- 48.Makita S, Kanai T, Nemoto Y, Totsuka T, Okamoto R, Tsuchiya K, Yamamoto M, Kiyono H, Watanabe M. Intestinal lamina propria retaining CD4+CD25+ regulatory T cells is a suppressive site of intestinal inflammation. J Immunol. 2007;178:4937–4946. doi: 10.4049/jimmunol.178.8.4937. [DOI] [PubMed] [Google Scholar]
- 49.Manning BP, Sharkey KA, Mawe GM. Effects of PGE2 in guinea pig colonic myenteric ganglia. Am J Physiol Gastrointest Liver Physiol. 2002;283:G1388–1397. doi: 10.1152/ajpgi.00141.2002. [DOI] [PubMed] [Google Scholar]
- 50.Markovic D, Lehnert H, Levine MA, Grammatopoulos DK. Structural determinants critical for localization and signaling within the seventh transmembrane domain of the type 1 corticotropin releasing hormone receptor: lessons from the receptor variant R1d. Mol Endocrinol. 22:2505–2519. doi: 10.1210/me.2008-0177. [DOI] [PubMed] [Google Scholar]
- 51.Maunder RG, Levenstein S. The role of stress in the development and clinical course of inflammatory bowel disease: epidemiological evidence. Curr Mol Med. 2008;8:247–252. doi: 10.2174/156652408784533832. [DOI] [PubMed] [Google Scholar]
- 52.Miller G, Chen E, Cole SW. Health psychology: developing biologically plausible models linking the social world and physical health, Annu. Rev Psychol. 2009;60:501–524. doi: 10.1146/annurev.psych.60.110707.163551. [DOI] [PubMed] [Google Scholar]
- 53.Moss AC, Anton P, Savidge T, Newman P, Cheifetz AS, Gay J, Paraschos S, Winter MW, Moyer MP, Karalis K, Kokkotou E, Pothoulakis C. Urocortin II mediates pro-inflammatory effects in human colonocytes via corticotropin-releasing hormone receptor 2α. Gut. 2007;56:1210–1217. doi: 10.1136/gut.2006.110668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Muramatsu Y, Fukushima K, Iino K, Totsune K, Takahashi K, Suzuki T, Hirasawa G, Takeyama J, Ito M, Nose M, Tashiro A, Hongo M, Oki Y, Nagura H, Sasano H. Urocortin and corticotropin-releasing factor receptor expression in the human colonic mucosa. Peptides. 2000;21:1799–1809. doi: 10.1016/s0196-9781(00)00335-1. [DOI] [PubMed] [Google Scholar]
- 55.Muscatell KA, Slavich GM, Monroe SM, Gotlib IH. Stressful life events, chronic difficulties, and the symptoms of clinical depression. J Nerv Ment Dis. 2009;197:154–160. doi: 10.1097/NMD.0b013e318199f77b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ohman L, Isaksson S, Lindmark AC, Posserud I, Stotzer PO, Strid H, Sjövall H, Simrén M. T-cell activation in patients with irritable bowel syndrome. Am J Gastroenterol. 2009;104:1205–1212. doi: 10.1038/ajg.2009.116. [DOI] [PubMed] [Google Scholar]
- 57.Ohman L, Lindmark AC, Isaksson S, Posserud I, Strid H, Sjövall H, Simrén M. B-cell activation in patients with irritable bowel syndrome (IBS) Neurogastroenterol Motil. 2009;21:644–e27. doi: 10.1111/j.1365-2982.2009.01272.x. [DOI] [PubMed] [Google Scholar]
- 58.Paschos KA, Kolios G, Chatzaki E. The corticotropin-releasing factor system in inflammatory bowel disease: prospects for new therapeutic approaches. Drug Discovery Today. 2009;14:713–720. doi: 10.1016/j.drudis.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 59.Piche T, Saint-Paul MC, Dainese R, Marine-Barjoan E, Iannelli A, Montoya ML, Peyron JF, Czerucka D, Cherikh F, Fillipi J, Tran A, Hbuterne X. Mast cells and cellularity of the colonic mucosa correlated with fatigue and depression in irritable bowel syndrome. Gut. 2008;57:468–473. doi: 10.1136/gut.2007.127068. [DOI] [PubMed] [Google Scholar]
- 60.Pioszak AA, Parker NR, Suino-Powell K, Xu HE. Molecular recognition of corticotropin-releasing factor by its G-protein-coupled receptor CRFR1. J Biol Chem. 2008;283:32900–32912. doi: 10.1074/jbc.M805749200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Posserud I, Stolzer A, Lindström L, Tack J, Abrahamsson H, Simrén M. Small intestinal bacterial overgrowth in patients with irritable bowel syndrome. Gut. 2007;56:802–808. doi: 10.1136/gut.2006.108712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rao KN, Brown MA. Mast cells: multifaceted immune cells with diverse roles in health and disease. Ann N Y Acad Sci. 2008;1143:83–104. doi: 10.1196/annals.1443.023. [DOI] [PubMed] [Google Scholar]
- 63.Rosas-Ballina M, Ochani M, Parrish WR, Ochani K, Harris YT, Huston JM, Chavan S, Tracey KJ. Splenic nerve is required for cholinergic antiinflammatory pathway control of TNF endotoxemia. Proc Natl Acad Sci U S A. 2008;105:11008–11013. doi: 10.1073/pnas.0803237105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Saito-Nakaya K, Hasegawa R, Nagura Y, Ito H, Fukudo S. Corticotropin-releasing hormone receptor 1 antagonist blocks colonic hypersensitivity induced by a combination of inflammation and repetitive colorectal distension. Neurogastroenterol Motil. 2008;20:1147–1156. doi: 10.1111/j.1365-2982.2008.01151.x. [DOI] [PubMed] [Google Scholar]
- 65.Santos J, Alonso C, Vicario M, Ramos L, Lobo B, Malagelada JR. Neuropharmacology of stress-induced mucosal inflammation: implications for inflammatory bowel disease and irritable bowel syndrome. Curr Mol Med. 2008;8:258–273. doi: 10.2174/156652408784533788. [DOI] [PubMed] [Google Scholar]
- 66.Saruta M, Takahashi K, Suzuki T, Torii A, Kawakami M, Sasano H. Urocortin 1 in colonic mucosa in patients with ulcerative colitis. J Clin Endocrinol Metab. 2004;89:5352–5361. doi: 10.1210/jc.2004-0195. [DOI] [PubMed] [Google Scholar]
- 67.Scarpellini E, Giorgio V, Gabrielli M, Lauritano EC, Patanella A, Fundaor C, Gasbarrini A. Prevalence of small intestinal bacterial overgrowth in children with irritable bowel syndrome: A case-control study. J Pediatr. 2009 doi: 10.1016/jpeds.2009.03.033. [DOI] [PubMed] [Google Scholar]
- 68.Shih DQ, Targan SR. Immunopathogenesis of inflammatory bowel disease, World. J Gastroenterol. 2008;14:390–400. doi: 10.3748/wjg.14.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Siddiqui AA, Miner PB., Jr The role of mast cells in common gastrointestinal diseases. Curr Allergy Asthm Rep. 2004;4:47–54. doi: 10.1007/s11882-004-0043-z. [DOI] [PubMed] [Google Scholar]
- 70.Silberstein S, Vogl AM, Bonfiglio JJ, Wurst W, Holsboer F, Arzt E, Deussing JM, Refojo D. Immunology, signal transduction, and behavior in hypothalamic-pituitary-adrenal axis-related genetic mouse models. Ann N Y Acad Sci. 2009;153:120–130. doi: 10.1111/j.1749-6632.2008.03967.x. [DOI] [PubMed] [Google Scholar]
- 71.Spiller RC, Garsed K. Postinfectious irritable bowel syndrome. Gastroenterology. 2009;136:1979–1988. doi: 10.1053/j.gastro.2009.02.074. [DOI] [PubMed] [Google Scholar]
- 72.Singh VK, Leu SJ. Enhancing effect of corticotropin-releasing neurohormone on the production of interleukin-1 and interleukin-2. Neurosci Lett. 1990;120:151–154. doi: 10.1016/0304-3940(90)90025-5. [DOI] [PubMed] [Google Scholar]
- 73.Stengel A, Taché Y. Neuroendocrine Control of the Gut During Stress: Corticotropin-Releasing Factor Signaling Pathways in the Spotlight. Annu Rev Physiol. 2009;71:219–239. doi: 10.1146/annurev.physiol.010908.163221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Selye H. A Syndrome Produced by Diverse Nocuous Agents. Nature. 1936;138:p32. doi: 10.1176/jnp.10.2.230a. [DOI] [PubMed] [Google Scholar]
- 75.Straub RH, Wiest R, Strauch UG, Härle P, Schölmerich J. The role of the sympathetic nervous system in intestinal inflammation. Gut. 2006;55:1640–1649. doi: 10.1136/gut.2006.091322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Taché Y, Brunnhuber S. From Hans Selye’s discovery of biological stress to the identification of corticotropin-releasing factor signaling pathways: implication in stress-related functional bowel diseases, Ann. NY Acad Sci. 2008;1148:29–41. doi: 10.1196/annals.1410.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Taché Y, Bonaz B. Corticotropin-releasing factor receptors and stress-related alterations of gut motor function. J Clin Invest. 2007;117:33–40. doi: 10.1172/JCI30085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Taché Y, Kiank C, Stengel A. A Role for Corticotropin-releasing Factor in Functional Gastrointestinal Disorders. Curr Gastroenterol Rep. 2009;11:270–277. doi: 10.1007/s11894-009-0040-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tillisch K, Mayer EA, Labus JS, Stains J, Chang L, Naliboff BD. Sex specific alterations in autonomic function among patients with irritable bowel syndrome. Gut. 2005;54:1396–1401. doi: 10.1136/gut.2004.058685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Theoharides TC, Donelan JM, Papadopoulou N, Cao J, Kempuraj D, Conti P. Mast cells as targets of corticotropin-releasing factor and related peptides. Trends Pharmacol Sci. 2004;25:563–568. doi: 10.1016/j.tips.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 81.Tracey KJ. Physiology and immunology of the cholinergic antiinflammatory pathway. J Clin Invest. 2007;117:289–296. doi: 10.1172/JCI30555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tsatsanis C, Androulidaki A, Dermitzaki E, Gravanis A, Margioris AN. Corticotropin releasing factor receptor 1 (CRF1) and CRF2 agonists exert an anti-inflammatory effect during the early phase of inflammation suppressing LPS-induced TNF-alpha release from macrophages via induction of COX-2 and PGE2. J Cell Physiol. 2007;210:774–83. doi: 10.1002/jcp.20900. [DOI] [PubMed] [Google Scholar]
- 83.Tsatsani C, Androulidaki A, Alissafi T, Charalampopoilos I, Dermitzaki E, Roger T, Gravanis A, Margioris AN. Corticotropin-releasing factor and the urocortins induce the expression of TLR4 in macrophages via activation of the transcription factors PU.1 and AP-1. J Immunol. 2006;176:1869–1877. doi: 10.4049/jimmunol.176.3.1869. [DOI] [PubMed] [Google Scholar]
- 84.Tsatsanis C, Androulidaki A, Dermitzaki E, Charalampopoulos I, Spiess J, Gravanis A, Margioris AN. Urocortin 1 and Urocortin 2 induce macrophage apoptosis via CRFR2, FEBS. Lett. 2005;579:4259–4264. doi: 10.1016/j.febslet.2005.06.057. [DOI] [PubMed] [Google Scholar]
- 85.van Vliet SJ, den Dunnen J, Gringhuis SI, Geijtenbeek TB, van Kooyk Y. Innate signaling and regulation of dendritic cell immunity. Curr Opin Immunol. 2007;19:435–440. doi: 10.1016/j.coi.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 86.Wallon C, Yang PC, Keita AV, Ericson AC, McKay DM, Sherman PM, Perdue MH, Soderholm JD. Corticotropin-releasing hormone (CRH) regulates macromolecular permeability via mast cells in normal human colonic biopsies in vitro. Gut. 2008;57:50–58. doi: 10.1136/gut.2006.117549. [DOI] [PubMed] [Google Scholar]
- 87.Waring WS, Chui M, Japp A, Nicol EF, Ford MJ. Autonomic cardiovascular responses are impaired in women with irritable bowel syndrome. J Clin Gastroenterol. 2004;38:658–663. doi: 10.1097/01.mcg.0000135362.35665.49. [DOI] [PubMed] [Google Scholar]
- 88.Webster Marketon JI, Glaser R. Stress hormones and immune function. Cell Immunol. 2008;252:16–26. doi: 10.1016/j.cellimm.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 89.Wlk M, Wang CC, Venihaki M, Liu J, Zhao D, Anton PM, Mykoniatis A, Pan A, Zack J, Karalis K, Pothoulakis C. Corticotropin-releasing hormone antagonists possess anti-inflammatory effects in the mouse ileum. Gastroenterology. 2002;123:505–515. doi: 10.1053/gast.2002.34783. [DOI] [PubMed] [Google Scholar]
- 90.Wood SK, Woods JH. Corticotropin-releasing factor receptor-1: a therapeutic target for cardiac autonomic disturbances. Expert Opin Ther Targets. 2007;11:1401–1413. doi: 10.1517/14728222.11.11.1401. [DOI] [PubMed] [Google Scholar]