Abstract
Large clinical trials demonstrate that control of blood pressure or hyperlipidemia reduces risk for cardiovascular events by ~30%. Factors that may further reduce remaining risk are not definitively established. One potential target is atherosclerosis, a crucial feature in the pathogenesis of cardiovascular diseases whose development is determined by multiple mechanism including complex interactions between endothelial dysfunction and insulin resistance. Reciprocal relationships between endothelial dysfunction and insulin resistance as well as cross-talk between hyperlipidemia and the rennin–angiotensin–aldosterone system may contribute to development of atherosclerosis. Therefore, one appealing strategy for prevention or treatment of atherosclerosis may be to simultaneously address several risk factors with combination therapies that target multiple pathogenic mechanisms. Combination therapy with statins, peroxisome proliferators-activated receptor agonists, and rennin–angiotensin–aldosterone system blockers demonstrate additive beneficial effects on endothelial dysfunction and insulin resistance when compared with monotherapies in patients with cardiovascular risk factors. Additive beneficial effects of combined therapy are mediated by both distinct and interrelated mechanisms, consistent with both pre-clinical and clinical investigations. Thus, combination therapy may be an important concept in developing more effective strategies to treat and prevent atherosclerosis, coronary heart disease, and co-morbid metabolic disorders characterized by endothelial dysfunction and insulin resistance.
Keywords: Combination therapy, Atherosclerosis, Endothelial dysfunction, Insulin resistance, Risk factors
1. Introduction
Development of atherosclerosis is regulated by multiple complex mechanisms that include endothelial dysfunction with impaired nitric oxide (NO) bioavailability, oxidative stress, inflammation, hemostasis, and insulin resistance [1,2]. Hypertension and hypercholesterolemia often occur in conjunction with other metabolic risk factors including glucose intolerance, obesity, diabetes, and metabolic syndrome. This clustering may be due, in part, to reciprocal relationships between endothelial dysfunction and insulin resistance [3,4]. Risk of coronary heart disease increases in stepwise fashion with an increasing number of risk factors. Only 14% of coronary events in hypertensive men and 5% of those in hypertensive women occur in the absence of additional risk factors. Appropriate therapy for hypertensive patients should be guided by risk stratification to improve the multivariate risk profile. An important emerging concept for prevention or treatment of atherosclerosis is that pathophysiological cross-talk between risk factors may be effectively addressed by combination therapy that simultaneously targets several risk factors and addresses multiple molecular mechanisms [2–6]. In this review, we discuss the rationale and importance of combination therapy in treating and preventing cardiovascular events.
2. Differential biological effects among antihypertensive drugs
Systemic hypertension, hypercholesterolemia, and diabetes are all associated with vascular endothelial dysfunction. Drug therapies that simultaneously improve blood pressure (BP), endothelial dysfunction, and insulin resistance may have a larger effect to reduce risk of major cardiovascular events when compared with drugs that target BP alone. Several large-scale randomized clinical studies support the hypothesis that effects of antihypertensive drugs to reduce cardiovascular events are due to actions that go beyond BP reduction per se [7–9]. For example, the angiotensin converting enzyme (ACE) inhibitor ramipril and the angiotensin II (Ang II) type I (AT1) receptor blocker (ARB) losartan may favorably alter endothelial and/or vascular structure/function to mediate reduction of cardiovascular disease beyond effects due to lowering BP per se.
We recently investigated effects of candesartan therapy in hypertensive patients [10,11]. Candesartan treatment inhibits the rennin–angiotensin–aldosterone system (RAAS), and improves markers of oxidative stress, inflammation, and fibrionolysis independent of BP lowering effects. Different classes of anti-hypertensive drugs have differential effects on left ventricular hypertrophy. When effects of losartan or atenolol to alter ultrasound and biochemical markers of myocardial fibrosis were investigated, losartan decreases myocardial collagen content, whereas atenolol does not [12]. In another study, losartan therapy significantly decreases collagen volume fraction [13]. Effects of losartan and atenolol on resistance artery abnormalities in patients with essential hypertension have also been investigated [14]. One year with either treatment reduces blood pressure to a comparable degree. Interestingly, losartan therapy corrects the altered structure and endothelial dysfunction of resistance arteries, whereas atenolol does not. Finally, valsartan has BP-independent effects on left ventricular hypertrophy, reactive oxygen species formation by monocytes, and C-reactive protein in hypertensive patients with left ventricular hypertrophy, when compared with amlodipine [15].
It remains controversial whether reducing blood pressure with thiazide diuretics and beta blockers may provide comparable benefit to ACE inhibitors and ARBs [16,17]. For example, significant benefits of thiazide diuretics and beta blockers were demonstrated in the ALLHAT study [16]. On the other hand, relative inefficacy and multiple adverse metabolic effects of beta blockers and diuretics may make ACE inhibitors and ARBs preferable in some contexts [17]. Nevertheless, in patients with advanced hypertensive cardiovascular disease, benefits of lowering blood pressure may outweigh adverse metabolic events associated with diuretics and, in some specific cases, even with beta blockers. This topic still requires further investigation for definitive conclusions and clinical recommendations.
Many clinical studies show that widely used antihypertensive agents including thiazide diuretics and beta blockers adversely increase blood glucose levels [18]. On the other hand, some intervention trials demonstrate a decrease in incidence of new-onset diabetes in hypertensive subjects treated with ACE inhibitors or ARBs [7,9,19,20]. Although ramipril fails to prevent the new onset of diabetes in one recent clinical study [21], ramipril prevents onset of diabetes in another clinical study [8]. When insulin action and secretion and body fat distribution were investigated after 12-week treatment with candesartan, hydrochlorothiazide, and placebo in hypertensive patients [22], blood pressure was reduced to a comparable extent by both candesartan and hydrochlorothiazide (vs placebo). However, it is important to note that visceral fat redistribution, liver fat accumulation, low-grade inflammation, and aggravated insulin resistance were caused by hydrochlorothiazide but not candesartan treatment.
Endothelial dysfunction associated with diabetes, obesity, metabolic syndrome, and other insulin-resistant states is characterized by impaired NO release from endothelium with a relative decrease in blood flow and delivery of metabolic substrates and hormones to target tissues. Thus, improvement in endothelial function is predicted to enhance insulin sensitivity. The RAAS has multiple effects in the central nervous system, skeletal muscle, liver, and adipose tissue that may interfere with insulin action. Thus, RAAS dysregulation may contribute to the evolution of insulin resistance explaining how RAAS blockades may potentially help prevent new-onset diabetes [23]. Further, cross-talk between Ang II receptor signaling and insulin-signaling pathways may contribute to insulin resistance [24]. RAAS blockade may have direct effects to augment insulin-stimulated glucose uptake, promote adipogenesis [25], and induce peroxisome proliferator-activated receptor-γ activity that promotes differentiation of adipocytes [26]. Recently, it was reported that quinapril increases insulin-stimulated endothelial function and vascular expression of adiponectin in patients with type 2 diabetes [27].
We compared vascular and metabolic effects of antihypertensive drugs in hypertensive patients. Atenolol, amlodipine, and candesartan therapies significantly reduce systolic blood pressure when compared with ramipril. Atenolol and thiazide therapies increase triglycerides levels to a greater extent than either ramipril or candesartan therapy alone. Ramipril and candesartan therapies improve flow-mediated dilation and increase adiponectin levels and insulin sensitivity measured by QUICKI (Quantitative Insulin-Sensitivity Check Index, a surrogate measure of insulin sensitivity) to a greater extent than atenolol or thiazide therapies alone (Fig. 1). Amlodipine therapy increases adiponectin levels more than atenolol therapy. Ramipril, candesartan, and amlodipine therapies significantly decrease leptin levels more than atenolol or thiazide therapies [28]. Leptin may play an important role in atherosclerotic lesion formation and progression and also potentiate pressor effects of hyperinsulinemia in insulin resistant states that may have deleterious cardiovascular effects in obesity [29]. Our observations are consistent with a meta-analysis evaluating differential effects of antihypertensive drugs on incidence of new-onset diabetes [30].
Fig. 1.
Ramipril and candesartan therapies significantly increased adiponectin levels to greater extent than atenolol or thiazide therapies. Amlodipine therapy significantly increased adiponectin levels to a greater extent than atenolol therapy. Ramipril and candesartan therapies significantly increased insulin sensitivity [as assessed by Quantitative Insulin-Sensitivity Check Index (QUICKI)] to a greater extent than atenolol or thiazide therapies. Pl, placebo; At, atenolol; Am, amlodipine, Th, thiazide; Ra, ramipril; Ca, candesartan. Standard error of the mean is identified by the bars. Reproduced with permission from Koh et al. [28].
ACE inhibitors reduce Ang II production and also prevent bradykinin breakdown. However, continued production of Ang II by non-ACE-dependent pathways may occur. ARBs inhibit actions of AT1 receptors resulting in compensatory increases in Ang II that may have biological consequences mediated by other receptors distinct from AT1 receptors [31]. Although ACE inhibitor and ARBs target the RAAS by distinct mechanisms, both give similar clinical advantage except with respect to myocardial infarction (MI) (ARB-MI paradox) [32,33]. As a consequence of AT1 blockade, ARBs increase Ang II levels several-fold above baseline by uncoupling a negative-feedback loop. Increased levels of circulating Ang II result in unopposed stimulation of the AT2 receptors. AT2 receptor stimulation may be harmful under certain circumstances through mediation of growth promotion, fibrosis, and hypertrophy, as well as proatherogenic and proinflammatory effects [34,35]. Ang II may promote plaque rupture by augmenting matrix metalloproteinase-1 in an AT2-dependent fashion and by preventing growth of vascular smooth muscle cells with reduced collagen deposition and additional cellular apoptosis within advanced plaques [36]. These data raise the possibility that ARBs may promote plaque vulnerability and propensity to rupture. Indeed, the reduction in incidence of both MI and cardiovascular death seen with ACE inhibitors is significantly greater than that achieved by ARBs in patients [37–39]. However, new trials ONTARGET/TRANSCEND (ONgoing Telmisartan Alone and in combination with Ramipril Global Endpoint Trial/Telmisartan Randomized AssessmeNt Study in ACEiNtolerant subjects with cardiovascular Disease) report that ARBs give results comparable to ACE inhibitors with respect to MI and cardiovascular death [40,41].
3. Reciprocal relationships between insulin resistance and endothelial dysfunction
Endothelial dysfunction and insulin resistance play crucial roles in the pathogenesis of atherosclerosis and related cardiovascular diseases. Mechanisms contributing independently to both insulin resistance and endothelial dysfunction include glucotoxicity, lipotoxicity, and inflammation [2,3,42,43]. In metabolic and cardiovascular diseases associated with insulin resistance, impairment in the phosphatidylinositol 3-kinase branch of insulin-signaling pathways in both vascular and metabolic tissues contributes to synergistic coupling of insulin resistance and endothelial dysfunction [3]. These reciprocal relationships between insulin resistance and endothelial dysfunction are present in the spontaneously hypertensive rat, a genetic model with characteristics of the human metabolic syndrome [44,45]. In transgenic rats, increased AT1 receptor/NADPH oxidase activation/reactive oxygen species contributes to vascular insulin resistance, endothelial dysfunction, apoptosis, and inflammation [46]. The endogenous NO synthase inhibitor asymmetrical dimethylarginine reduces insulin sensitivity, consistent with previous observations that NO plays a role in insulin sensitivity [47]. In clinical studies, there are positive correlations between insulin resistance with respect to vasodilator actions and metabolic insulin resistance in diabetic and obese subjects [48].
4. Additive beneficial effects on atherosclerosis with combination treatment
4.1. Cross-talk between statin and RAAS: statins combined with ACE inhibitors or ARBs vs monotherapy
4.1.1. Pre-clinical evidence
Statins improve endothelial function via stimulation of NO synthase activity and mediate antioxidant effects that result in enhanced NO bioactivity [1,49]. RAAS blockade also improves endothelial function [10,11]. In addition, low-density lipoprotein (LDL) induces expression of AT1 receptor. Hypercholesterolemic rabbits display enhanced vascular expression of AT1 receptors that mediate increased activity of Ang II [50]. Hypercholesterolemia leads to a significant increase in Ang II-induced BP elevation. Statins that reverses the elevated BP response to Ang II infusion is accompanied by decreased AT1 receptor density [51]. Statins also have indirect NOX inhibitory action through inhibition of Rac isoprenylation [52] and attenuate oxidative stress through inhibition of Rac1 [53]. Atorvastatin protects against cerebral infarction via inhibition of NADPH oxidase-derived superoxide in transient focal ischemia [54]. Cerivastatin may act by inhibiting the prenylation, membrane anchoring, and subsequent activation of Ras proteins [55]. Lovastatin also stimulates protein kinase B/Akt kinase activity, and Akt-dependent phosphorylation forces p21 in the cytoplasm, where it inhibits Rhokinases contributing to the suppression of cardiomyocyte hypertrophy [56] (Fig. 2).
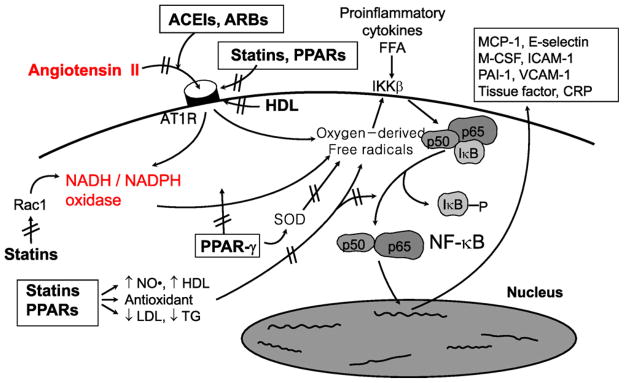
Fig. 2.
Dysregulation of the rennin–angiotensin–aldosterone system (RAAS) may contribute to the pathogenesis of atherosclerosis. Angiotensin II binds to angiotensin II type I receptor (AT1R) resulting in enzymatic production of oxygen-derived free radicals. This leads to dissociation of inhibitory factor, IκB with subsequent activation of NF-κB that stimulates expression of proinflammatory genes, chemokines, and cytokines. Cross-talk between hyperlipidemia and RAAS at multiple steps is illustrated here. This may help to explain why combined therapy with statins, peroxisome proliferators-activated receptors (PPARs), and RAAS blockade have additive beneficial effects on endothelial dysfunction and insulin resistance when compared with monotherapies in patients with cardiovascular risk factors. Modified from Dr. Koh [1,4,5,6].
Hypercholesterolemia and hypertension have synergistic deleterious effects on coronary endothelial function [57]. RAAS blockade inhibits binding of Ang II to the AT1 receptor, resulting in decreased production of oxygen-derived free radicals. Statins inhibit upregulation of AT1 receptor expression. Furthermore, statins inhibit production of oxygen-derived free radicals by reducing LDL, increasing NO synthesis, and promoting antioxidant effects. Therefore, combined therapy with statins and RAAS blockade may have additive beneficial effects on endothelial function, insulin resistance, and atherosclerosis [4,5] (Fig. 2). Indeed, in apolipoprotein E null mice fed with a high-cholesterol diet, neither valsartan nor fluvastatin had any effect on BP or cholesterol level. However, combined therapy with both drugs decreases plaque area and lipid deposition after 10 weeks [58].
4.1.2. Clinical evidence
We reported vascular and metabolic responses to therapies with statin and RAAS blockade alone or in combination in hypertensive, hypercholesterolemic patients. Simvastatin combined with losartan improves endothelial function, reduces inflammatory markers, and improves insulin sensitivity to a greater extent than monotherapy with either drug in hypercholesterolemic, hypertensive patients [59,60]. In another study, additive beneficial effects of combined therapy with a statin and the ACE inhibitor ramipril was investigated in hypercholesterolemic patients with type 2 diabetes [61]. Combined therapy with ramipril and simvastatin has beneficial additive effects on tissue factor activity and prothrombin fragment 1 + 2 in patients with type 2 diabetes [62]. In type 2 diabetic patients, short-term atorvastatin combined with irbesartan treatment was more effective than either monotherapy [63]. On-pump coronary artery bypass graft surgery is associated with an intense systemic inflammatory response that is almost completely prevented by early treatment with high doses of ACE inhibitors and statins [64].
4.2. Cross-talk between PPARs and RAAS: PPARs combined with ACE inhibitors or ARBs vs monotherapy
4.2.1. Pre-clinical evidence
Fenofibrate, a synthetic ligand of peroxisome proliferators-activated receptor (PPAR) α, reduces triglycerides and increases high-density lipoprotein cholesterol. Fenofibrate improves endothelial function via stimulation of NO synthase activity and mediates antioxidant effects that result in enhanced NO bioactivity [65,66]. Fenofibrate lowers abdominal and skeletal adiposity and improves insulin sensitivity in OLETF rats [67]. The expression of visfatin and adiponectin mRNA in visceral fat depots is elevated by rosiglitazone or fenofibrate treatment (when compared with untreated OLETF rats). Moreover, tumor necrosis factor-α mRNA is downregulated by these drugs [68].
Thiazolidinediones (PPARγ agonists) improve insulin resistance in patients with type 2 diabetes while simultaneously exerting a broad spectrum of anti-atherogenic effects in vitro and in animal models of atherosclerosis [69–71]. PPARγ ligands increase endothelial NO release without altering endothelial NO synthase expression [72]. Superoxide anion radical decreases NO bioavailability. NADPH oxidase produces superoxide anion radical thereby contributing to NO catabolism in endothelium. PPARγ ligands decrease NADPH-dependent superoxide anion radical production in human umbilical vein endothelial cells and also reduce relative mRNA levels of NADPH oxidase subunits. PPARγ ligands stimulate both activity and expression of Cu/Zn-SOD (superoxide dismutase). Thus, in addition to direct effects on NO production in endothelial cells, PPARγ ligands may enhance NO bioavailability, in part, by altering endothelial superoxide anion radical metabolism through suppression of NADPH oxidase and induction of Cu/Zn-SOD. These findings illuminate additional molecular mechanisms by which PPARγ ligands may directly alter vascular endothelial function [73] (Fig. 2). Also, PPARγ agonists lower BP in diabetic patients and animal models, at least partially independent of their insulin-sensitizing effects [74,75]. This blood pressure-lowering effect is more moderate in human patients than in animal models [76,77].
Cross-talk exists between signaling pathways regulated by PPARγ or PPARγ and Ang II [26,78–80]. Through activation of NF-κB, Ang II stimulates proinflammatory gene expression and downregulation of PPARγ and PPARγ. This promotes vascular inflammation and acceleration of atherosclerosis in apolipoprotein E knockout mice [78]. PPARγ activators attenuate development of hypertension, correct structural abnormalities, and improve endothelial dysfunction induced by Ang II, a potent endogenous vasoconstrictor. The effect of fenofibrate to oppose the elevated BP response to Ang II infusion is accompanied by decreases in oxidative stress and inflammation in the vascular wall [79]. PPARγ ligands reduce AT1 receptor mRNA and protein [80]. High-density lipoprotein also reduces hyperglycemia-induced upregulation of AT1 receptor in human aortic endothelial cells. This is associated with decreases in reactive oxygen species, NAD(P)H oxidase activity and responsiveness to Ang II [81] (Fig. 2). Therefore, combined therapy with PPARγ or PPARγ and RAAS blockade has additive beneficial effects on endothelial function, insulin resistance, and atherosclerosis [4,5] (Fig. 2). Indeed, candesartan or pioglitazone protects against hypertensive cardiovascular damage without lowering BP while combination therapy exerts greater beneficial effects than monotherapy with either drug on hypertensive cardiovascular injury [82].
4.2.2. Clinical evidence
Fenofibrate therapy significantly changes lipoprotein levels, improves endothelial function, reduces markers of inflammation and hemostasis, and raises adiponectin levels in patients with hypertriglyceridemia [83]. We investigated vascular and metabolic responses to either fenofibrate or candesartan either alone or in combination in hypertriglyceridemic, hypertensive patients. Fenofibrate combined with candesartan improves endothelial function and reduces inflammatory markers to a greater extent than monotherapy in hypertriglyceridemic, hypertensive patients [84].
The Fenofibrate Intervention and Event Lowering in Diabetes (FIELD) study assesses the effect of fenofibrate on cardiovascular disease events in 9795 participants aged 50–75 years, with type 2 diabetes mellitus. Fenofibrate does not significantly reduce the risk of the primary outcome of coronary events. It does reduce total cardiovascular events, mainly due to fewer non-fatal myocardial infarctions and revascularizations [85]. There are several important potential confounding factors to consider when interpreting the results of the FIELD study. These include normal to mildly elevated mean basal triglycerides levels in study subjects, a high dropout rate, uneven allocation of patients between placebo (17%) and fenofibrate (8%) treatment, and concurrent treatment with other lipid-lowering agents (predominantly statins) and cardioprotective therapies such as aspirin, beta blockers, and ACE inhibitors [66,86]. Thus, higher rate of starting statin therapy in patients allocated to placebo may mask a moderately larger treatment benefit. Because of these substantial limitations, conclusions drawn from the FIELD study regarding fibrates need to be interpreted cautiously.
In nondiabetic patients with low high-density lipoprotein cholesterol and metabolic syndrome, pioglitazone treatment significantly raises high-density lipoprotein cholesterol and favorably affects lipoprotein particle size, markers of inflammation, and adipokines without changing triglycerides, LDL cholesterol, or weight [87].
Thiazolidinediones also exert beneficial effects on other parameters of the metabolic syndrome. In particular, a considerable number of animal and human studies show that thiazolidinediones treatment results in small but significant reductions of BP [88,89]. A meta-analysis of the effect of thiazolidinediones on BP with 37 clinical trials has been reported. Trials with independent-group design and trials with pre–post design were evaluated separately. When compared with baseline, thiazolidinediones lower systolic BP by 4.70 mmHg (95% confidence interval, −6.13 to −3.27) and diastolic BP by 3.79 mmHg (95% confidence interval, −5.82 to −1.77). When compared with placebo, thiazolidinediones lower systolic BP by 3.47 mmHg (95% confidence interval, −4.91 to −2.02) and diastolic BP by 1.84 mmHg (95% confidence interval, −3.43 to −0.25). Thus, thiazolidinediones lower both systolic and diastolic BP, albeit the BP-lowering effect is small [90].
5. Clinical implication
Dysregulation of RAAS is a critical feature of the pathogenesis of atherosclerosis. This may contribute to reciprocal relationships between insulin resistance and endothelial dysfunction. Cross-talk between hyperlipidemia and RAAS occurs at multiple points. Combined therapy with statins, PPARs, and RAAS blockers demonstrate additive beneficial effects on endothelial dysfunction and insulin resistance when compared with monotherapies in patients with cardiovascular risk factors. This is mediated by both distinct and interrelated mechanisms (Fig. 2). We report the effect of such combined therapy mainly on surrogate parameters of arteriosclerosis such as endothelial function and other biomarkers. However, there is no direct evidence that improvement in endothelial function is associated with improved survival. Nevertheless, several studies have shown that a single measurement of endothelial function in both the coronary and peripheral circulation is of prognostic value in various clinical cohorts including patients with established coronary disease and those with atypical symptoms [91,92]. In a large population of young adults, strong inverse relationships exist between endothelial-dependent dilation and structural arterial disease (by carotid intima media thickness) after multivariable adjustment for traditional risk factors [93]. This is most striking in those with the worst endothelial-dependent dilation. Thus, a protective role for the quiescent endothelial phenotype may exist and complementary use of endothelial function testing and structural measurements for characterization of early disease may be beneficial [94]. With respect to issues such as improved survival, further investigation in larger prospective studies is required [95].
Statins may significantly improve blood pressure control in subjects with both hypercholesterolemia and uncontrolled hypertension. On the other hand, statins may not improve blood pressure control in subjects with both hypercholesterolemia and controlled hypertension [96]. Indeed, pravastatin and atorvastatin did not reduce blood pressure in patients taking antihypertensive drugs in large clinical trials. Nevertheless, statins markedly reduce the burden of cardiovascular disease [97,98]. Additive beneficial effects of combined therapy are consistent with previous pre-clinical and clinical investigations [99–101]. For example, low LDL cholesterol and systolic BP beneficially impact coronary atherosclerosis. However, the association between intensive control of both risk factors and coronary plaque progression remains unclear. Changes in atheroma burden monitored by intravascular ultrasound were studied in 3437 patients with coronary artery disease. Very low LDL cholesterol and normal systolic BP are associated with the slowest progression of coronary atherosclerosis [102]. These findings suggest the need for intensive control of hypercholesterolemia and hypertension in patients with coronary artery disease.
There is a strong scientific rationale for recommending combination therapy to treat or prevent atherosclerosis and coronary heart disease [6,103–106]. Combined therapy may be an important emerging concept in developing optimal treatment and prevention strategies for atherosclerosis, coronary heart disease, and co-morbid metabolic disorders characterized by endothelial dysfunction and insulin resistance.
Acknowledgments
Funding
This study was partly supported by grants from established investigator award (2007–1) (K.K. Koh), Gil Medical Center, Gachon University, Incheon, Korea. This work was supported, in part, by the Intramural Research Program, NCCAM, NIH (M.J. Quon).
Footnotes
We presented part of this work in the Cardiology Grand Round, University of San Diego Hospital, May 7, 2009 and Stanford University Hospital, May 8, 2009.
Disclosure
None.
References
- 1.Koh KK. Effects of statins on vascular wall: vasomotor function, inflammation, and plaque stability. Cardiovasc Res. 2000;47:648–57. doi: 10.1016/s0008-6363(00)00146-2. [DOI] [PubMed] [Google Scholar]
- 2.Kim J, Montagnani M, Koh KK, Quon MJ. Reciprocal relationships between insulin resistance and endothelial dysfunction: molecular and pathophysiological mechanisms. Circulation. 2006;113:1888–904. doi: 10.1161/CIRCULATIONAHA.105.563213. [DOI] [PubMed] [Google Scholar]
- 3.Kim JA, Koh KK, Quon MJ. The union of vascular and metabolic actions of insulin in sickness and in health. Arterioscler Thromb Vasc Biol. 2005;25:889–91. doi: 10.1161/01.ATV.0000164044.42910.6b. [DOI] [PubMed] [Google Scholar]
- 4.Koh KK, Han SH, Quon MJ. Inflammatory markers and the metabolic syndrome: insights from therapeutic interventions. J Am Coll Cardiol. 2005;46:1978–85. doi: 10.1016/j.jacc.2005.06.082. [DOI] [PubMed] [Google Scholar]
- 5.Han SH, Quon MJ, Kim J, Koh KK. Adiponectin and cardiovascular disease: response to therapeutic interventions. J Am Coll Cardiol. 2007;49:531–8. doi: 10.1016/j.jacc.2006.08.061. [DOI] [PubMed] [Google Scholar]
- 6.Koh KK, Quon MJ. Targeting converging therapeutic pathways to overcome hypertension. Int J Cardiol. 2009;132:297–9. doi: 10.1016/j.ijcard.2008.11.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yusuf S, Sleight P, Pogue J, et al. Effects of an angiotensin-converting-enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients. The Heart Outcomes Prevention Evaluation Study Investigators. N Engl J Med. 2000;342:145–53. doi: 10.1056/NEJM200001203420301. [DOI] [PubMed] [Google Scholar]
- 8.HOPE/HOPE-TOO Study Investigators. Long-term effects of ramipril on cardiovascular events and on diabetes results of the HOPE Study Extension. Circulation. 2005;112:1339–46. doi: 10.1161/CIRCULATIONAHA.105.548461. [DOI] [PubMed] [Google Scholar]
- 9.Dahlöf B, Devereux RB, Kjeldsen SE, et al. Cardiovascular morbidity and mortality in the Losartan Intervention For Endpoint reduction in hypertension study (LIFE): a randomised trial against atenolol. Lancet. 2002;359:995–1003. doi: 10.1016/S0140-6736(02)08089-3. [DOI] [PubMed] [Google Scholar]
- 10.Koh KK, Ahn JY, Han SH, et al. Pleiotropic effects of angiotensin II receptor blocker in hypertensive patients. J Am Coll Cardiol. 2003;42:905–10. doi: 10.1016/s0735-1097(03)00846-5. [DOI] [PubMed] [Google Scholar]
- 11.Koh KK, Quon MJ, Han SH, et al. Anti-inflammatory and metabolic effects of candesartan in hypertensive patients. Int J Cardiol. 2006;108:96–100. doi: 10.1016/j.ijcard.2005.07.040. [DOI] [PubMed] [Google Scholar]
- 12.Ciulla MM, Paliotti R, Esposito A, et al. Different effects of antihypertensive therapies based on losartan or atenolol on ultrasound and biochemical markers of myocardial fibrosis: results of a randomized trial. Circulation. 2004;110:552–7. doi: 10.1161/01.CIR.0000137118.47943.5C. [DOI] [PubMed] [Google Scholar]
- 13.Diez J, Querejeta R, Lopez B, et al. Losartan-dependent regression of myocardial fibrosis is associated with reduction of left ventricular chamber stiffness in hypertensive patients. Circulation. 2002;105:2512–7. doi: 10.1161/01.cir.0000017264.66561.3d. [DOI] [PubMed] [Google Scholar]
- 14.Schiffrin EL, Park JB, Intengan HD, Touyz RM. Correction of arterial structure and endothelial dysfunction in human essential hypertension by the angiotensin receptor antagonist losartan. Circulation. 2000;101:1653–9. doi: 10.1161/01.cir.101.14.1653. [DOI] [PubMed] [Google Scholar]
- 15.Yasunari K, Maeda K, Watanabe T, et al. Comparative effects of valsartan versus amlodipine on left ventricular mass and reactive oxygen species formation by monocytes in hypertensive patients with left ventricular hypertrophy. J Am Coll Cardiol. 2004;43:2116–23. doi: 10.1016/j.jacc.2003.12.051. [DOI] [PubMed] [Google Scholar]
- 16.Cutler JA, Davis BR. Thiazide-type diuretics and beta-adrenergic blockers as first-line drug treatments for hypertension. Circulation. 2008;117:2691–704. doi: 10.1161/CIRCULATIONAHA.107.709931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Messerli FH, Bangalore S, Julius S. Risk/benefit assessment of beta-blockers and diuretics precludes their use for first-line therapy in hypertension. Circulation. 2008;117:2706–15. doi: 10.1161/CIRCULATIONAHA.107.695007. [DOI] [PubMed] [Google Scholar]
- 18.Gurwitz JH, Bohn RL, Glynn RJ, et al. Antihypertensive drug therapy and the initiation of treatment for diabetes mellitus. Ann Intern Med. 1993;118:273–8. doi: 10.7326/0003-4819-118-4-199302150-00005. [DOI] [PubMed] [Google Scholar]
- 19.Yusuf S, Ostergren JB, Gerstein HC, et al. Effects of candesartan on the development of a new diagnosis of diabetes mellitus in patients with heart failure. Circulation. 2005;112:48–53. doi: 10.1161/CIRCULATIONAHA.104.528166. [DOI] [PubMed] [Google Scholar]
- 20.Julius S, Kjeldsen SE, Weber M, et al. Outcomes in hypertensive patients at high cardiovascular risk treated with regimens based on valsartan or amlodipine: the VALUE randomised trial. Lancet. 2004;363:2022–31. doi: 10.1016/S0140-6736(04)16451-9. [DOI] [PubMed] [Google Scholar]
- 21.DREAM Trial Investigators. Bosch J, Yusuf S, et al. Effect of ramipril on the incidence of diabetes. N Engl J Med. 2006;355:1551–62. doi: 10.1056/NEJMoa065061. [DOI] [PubMed] [Google Scholar]
- 22.Eriksson JW, Jansson PA, Carlberg B, et al. Hydrochlorothiazide, but not Candesartan, aggravates insulin resistance and causes visceral and hepatic fat accumulation: the mechanisms for the diabetes preventing effect of Candesartan (MEDICA) Study. Hypertension. 2008;52:1030–7. doi: 10.1161/HYPERTENSIONAHA.108.119404. [DOI] [PubMed] [Google Scholar]
- 23.Sjöstrand M, Eriksson JW. Neuroendocrine mechanisms in insulin resistance. Mol Cell Endocrinol. 2009;297:104–11. doi: 10.1016/j.mce.2008.05.010. [DOI] [PubMed] [Google Scholar]
- 24.Folli F, Kahn CR, Hansen H, Bouchie JL, Feener EP. Angiotensin II inhibits insulin signaling in aortic smooth muscle cells at multiple levels. A potential role for serine phosphorylation in insulin/angiotensin II crosstalk. J Clin Invest. 1997;100:2158–69. doi: 10.1172/JCI119752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sharma AM, Janke J, Gorzelniak K, Engeli S, Luft FC. Angiotensin blockade prevents type 2 diabetes by formation of fat cells. Hypertension. 2002;40:609–11. doi: 10.1161/01.hyp.0000036448.44066.53. [DOI] [PubMed] [Google Scholar]
- 26.Schupp M, Janke J, Clasen R, Unger T, Kintscher U. Angiotensin type 1 receptor blockers induce peroxisome proliferator-activated receptor-γ activity. Circulation. 2004;109:2054–7. doi: 10.1161/01.CIR.0000127955.36250.65. [DOI] [PubMed] [Google Scholar]
- 27.Hermann TS, Li W, Dominguez H, et al. Quinapril treatment increases insulin-stimulated endothelial function and adiponectin gene expression in patients with type 2 diabetes. J Clin Endocrinol Metab. 2006;91:1001–8. doi: 10.1210/jc.2005-1231. [DOI] [PubMed] [Google Scholar]
- 28.Koh KK, Quon MJ, Han SH, et al. Distinct vascular and metabolic effects of different classes of anti-hypertensive drugs. Int J Cardiol. 2008 doi: 10.1016/j.ijcard.2008.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Koh KK, Park SM, Quon MJ. Leptin and cardiovascular disease: response to therapeutic interventions. Circulation. 2008;117:3238–49. doi: 10.1161/CIRCULATIONAHA.107.741645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Elliott WJ, Meyer PM. Incident diabetes in clinical trials of antihypertensive drugs: a network meta-analysis. Lancet. 2007;369:201–7. doi: 10.1016/S0140-6736(07)60108-1. [DOI] [PubMed] [Google Scholar]
- 31.Azizi M, Menard J. Combined blockade of the rennin–angiotensin system with angiotensin-converting enzyme inhibitors and angiotensin II type 1 receptor antagonists. Circulation. 2004;109:2492–9. doi: 10.1161/01.CIR.0000131449.94713.AD. [DOI] [PubMed] [Google Scholar]
- 32.Verma S, Strauss M. Angiotensin receptor blockers and myocardial infarction. BMJ. 2004;329:1248–9. doi: 10.1136/bmj.329.7477.1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Strauss MH, Hall AS. Angiotensin receptor blockers may increase risk of myocardial infarction: unraveling the ARB-MI paradox. Circulation. 2006;114:838–54. doi: 10.1161/CIRCULATIONAHA.105.594986. [DOI] [PubMed] [Google Scholar]
- 34.Reudelhuber TL. The continuing saga of the AT2 receptor: a case of the good, the bad, and the innocuous. Hypertension. 2005;46:1261–2. doi: 10.1161/01.HYP.0000193498.07087.83. [DOI] [PubMed] [Google Scholar]
- 35.Widdop RE, Jones ES, Hannan RE, Gaspari TA. Angiotensin AT2 receptors: cardiovascular hope or hype? Br J Pharmacol. 2003;140:809–24. doi: 10.1038/sj.bjp.0705448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim MP, Zhou M, Wahl LM. Angiotensin II increases human monocyte matrix metalloproteinase-1 through the AT2 receptor and prostaglandin E2: implications for atherosclerotic plaque rupture. J Leukoc Biol. 2005;78:195–201. doi: 10.1189/jlb.1204715. [DOI] [PubMed] [Google Scholar]
- 37.Strauss MH, Lonn EM, Verma S. Is the jury out? Class specific differences on coronary outcomes with ACE-inhibitors and ARBs: insight from meta-analysis and The Blood Pressure Lowering Treatment Trialists’ Collaboration. Eur Heart J. 2005;26:2351–3. doi: 10.1093/eurheartj/ehi574. [DOI] [PubMed] [Google Scholar]
- 38.Verdecchia P, Angeli F, Gattobigio R, Reboldi GP. Do angiotensin II receptor blockers increase the risk of myocardial infarction? Eur Heart J. 2005;26:2381–6. doi: 10.1093/eurheartj/ehi445. [DOI] [PubMed] [Google Scholar]
- 39.McDonald MA, Simpson SH, Ezekowitz JA, Gyenes G, Tsuyuki RT. Angiotensin receptor blockers and risk of myocardial infarction: systematic review. BMJ. 2005;331:873. doi: 10.1136/bmj.38595.518542.3A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.ONTARGET Investigators. Yusuf S, Teo KK, et al. Telmisartan, ramipril, or both in patients at high risk for vascular events. N Engl J Med. 2008;358:1547–59. doi: 10.1056/NEJMoa0801317. [DOI] [PubMed] [Google Scholar]
- 41.Telmisartan Randomised AssessmeNt Study in ACE iNtolerant subjects with Cardiovascular Disease (TRANSCEND) Investigators. Yusuf S, Teo K, et al. Effects of the angiotensin-receptor blocker telmisartan on cardiovascular events in high-risk patients intolerant to angiotensin-converting enzyme inhibitors: a randomised controlled trial. Lancet. 2008;372:1174–83. doi: 10.1016/S0140-6736(08)61242-8. [DOI] [PubMed] [Google Scholar]
- 42.Han SH, Quon MJ, Koh KK. Reciprocal relationships between abnormal metabolic parameters and endothelial dysfunction. Curr Opin Lipidol. 2007;18:58–65. doi: 10.1097/MOL.0b013e328012b627. [DOI] [PubMed] [Google Scholar]
- 43.Muniyappa R, Montagnani M, Koh KK, Quon MJ. Cardiovascular actions of insulin. Endocr Rev. 2007;28:463–91. doi: 10.1210/er.2007-0006. [DOI] [PubMed] [Google Scholar]
- 44.Potenza MA, Marasciulo FL, Chieppa DM, et al. Insulin resistance in spontaneously hypertensive rats (SHR) is associated with endothelial dysfunction characterized by imbalance between NO and ET-1 production. Am J Physiol Heart Circ Physiol. 2005;289:H813–22. doi: 10.1152/ajpheart.00092.2005. [DOI] [PubMed] [Google Scholar]
- 45.Potenza MA, Marasciulo FL, Tarquinio M, Quon MJ, Montagnani M. Treatment of spontaneously hypertensive rats (SHR) with rosiglitazone and/or enalapril simultaneously improves hypertension and insulin resistance by restoring balance between vasodilator and vasoconstrictor actions of insulin. Diabetes. 2006;55:3594–603. doi: 10.2337/db06-0667. [DOI] [PubMed] [Google Scholar]
- 46.Wei Y, Whaley-Connell AT, Chen K, et al. NADPH oxidase contributes to vascular inflammation, insulin resistance, and remodeling in the transgenic (mRen2) rat. Hypertension. 2007;50:384–91. doi: 10.1161/HYPERTENSIONAHA.107.089284. [DOI] [PubMed] [Google Scholar]
- 47.Sydow K, Mondon CE, Schrader J, Konishi H, Cooke JP. Dimethylarginine dimethylaminohydrolase overexpression enhances insulin sensitivity. Arterioscler Thromb Vasc Biol. 2008;28:692–7. doi: 10.1161/ATVBAHA.108.162073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Laakso M, Edelman SV, Brechtel G, Baron AD. Decreased effect of insulin to stimulate skeletal muscle blood flow in obese man: a novel mechanisms for insulin resistance. J Clin Invest. 1990;85:1844–52. doi: 10.1172/JCI114644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Koh KK, Son JW, Ahn JY, et al. Vascular effects of diet and statin in hypercholesterolemic patients. Int J Cardiol. 2004;95:185–91. doi: 10.1016/j.ijcard.2003.05.018. [DOI] [PubMed] [Google Scholar]
- 50.Nickenig G, Sachinidis A, Michaelsen F, et al. Upregulation of vascular angiotensin II receptor gene expression by low-density lipoprotein in vascular smooth muscle cells. Circulation. 1997;95:473–8. doi: 10.1161/01.cir.95.2.473. [DOI] [PubMed] [Google Scholar]
- 51.Nickenig G, Bäumer AT, Temur Y, et al. Statin-sensitive dysregulated AT1 receptor function and density in hypercholesterolemic men. Circulation. 1999;100:2131–4. doi: 10.1161/01.cir.100.21.2131. [DOI] [PubMed] [Google Scholar]
- 52.Bokoch GM, Prossnitz V. Isoprenoid metabolism is required for stimulation of the respiratory burst oxidase of HL-60 cells. J Clin Invest. 1992;89:402–8. doi: 10.1172/JCI115599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nakagami H, Jensen KS, Liao JK. A novel pleiotropic effect of statins: prevention of cardiac hypertrophy by cholesterol-independent mechanisms. Ann Med. 2003;35:398–403. doi: 10.1080/07853890310001294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hong H, Zeng JS, Kreulen DL, Kaufman DI, Chen AF. Atorvastatin protects against cerebral infarction via inhibiting NADPH oxidase-derived superoxide in ischemic stroke. Am J Physiol Heart Circ Physiol. 2006;291:H2210–5. doi: 10.1152/ajpheart.01270.2005. [DOI] [PubMed] [Google Scholar]
- 55.Dechend R, Fiebler A, Lindschau C, et al. Modulating angiotensin II-induced inflammation by HMG Co-A reductase inhibition. Am J Hypertens. 2001;14(6 Pt 2):55S–61S. doi: 10.1016/s0895-7061(01)02070-2. [DOI] [PubMed] [Google Scholar]
- 56.Hauck L, Harms C, Grothe D, et al. Critical role for FoxO3a-dependent regulation of p21CIP1/WAF1 in response to statin signaling in cardiac myocytes. Circ Res. 2007;100:50–60. doi: 10.1161/01.RES.0000254704.92532.b9. [DOI] [PubMed] [Google Scholar]
- 57.Rodriguez-Porcel M, Lerman LO, Herrmann J, et al. Hypercholesterolemia and hypertension have synergistic deleterious effects on coronary endothelial function. Arterioscler Thromb Vasc Biol. 2003;23:885–91. doi: 10.1161/01.ATV.0000069209.26507.BF. [DOI] [PubMed] [Google Scholar]
- 58.Li Z, Iwai M, Wu L, et al. Fluvastatin enhances the inhibitory effects of a selective AT1 receptor blocker, valsartan, on atherosclerosis. Hypertension. 2004;44:758–63. doi: 10.1161/01.HYP.0000145179.44166.0f. [DOI] [PubMed] [Google Scholar]
- 59.Koh KK, Quon MJ, Han SH, et al. Additive beneficial effects of losartan combined with simvastatin in the treatment of hypercholesterolemic, hypertensive patients. Circulation. 2004;110:3687–92. doi: 10.1161/01.CIR.0000143085.86697.13. [DOI] [PubMed] [Google Scholar]
- 60.Han SH, Koh KK, Quon MJ, Lee Y, Shin EK. The effects of simvastatin, losartan, and combined therapy on soluble CD40 ligand in hypercholesterolemic, hypertensive patients. Atherosclerosis. 2007;190:205–11. doi: 10.1016/j.atherosclerosis.2006.01.021. [DOI] [PubMed] [Google Scholar]
- 61.Koh KK, Quon MJ, Han SH, et al. Vascular and metabolic effects of combined therapy with ramipril and simvastatin in patients with type 2 diabetes. Hypertension. 2005;45:1088–93. doi: 10.1161/01.HYP.0000166722.91714.ba. [DOI] [PubMed] [Google Scholar]
- 62.Koh KK, Quon MJ, Han SH, et al. Combined therapy with ramipril and simvastatin has beneficial additive effects on tissue factor activity and prothrombin fragment 1 + 2 in patients with type 2 diabetes. Atherosclerosis. 2007;194:230–7. doi: 10.1016/j.atherosclerosis.2006.07.031. [DOI] [PubMed] [Google Scholar]
- 63.Ceriello A, Assaloni R, Da Ros R, et al. Effect of atorvastatin and irbesartan, alone and in combination, on postprandial endothelial dysfunction, oxidative stress, and inflammation in type 2 diabetic patients. Circulation. 2005;111:2518–24. doi: 10.1161/01.CIR.0000165070.46111.9F. [DOI] [PubMed] [Google Scholar]
- 64.Radaelli A, Loardi C, Cazzaniga M, et al. Inflammatory activation during coronary artery surgery and its dose-dependent modulation by statin/ACE-inhibitor combination. Arterioscler Thromb Vasc Biol. 2007;27:2750–5. doi: 10.1161/ATVBAHA.107.149039. [DOI] [PubMed] [Google Scholar]
- 65.Han SH, Quon MJ, Koh KK. Beneficial vascular and metabolic effects of peroxisome proliferator-activated receptor (PPAR)α Activators. Hypertension. 2005;46:1086–92. doi: 10.1161/01.HYP.0000187900.36455.4c. [DOI] [PubMed] [Google Scholar]
- 66.Koh KK, Quon MJ, Rosenson RS, Chung W-J, Han SH. Vascular and metabolic effects of treatment of combined hyperlipidemia: focus on statins and fibrates. Int J Cardiol. 2008;124:149–59. doi: 10.1016/j.ijcard.2007.04.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lee HJ, Choi SS, Park MK, et al. Fenofibrate lowers abdominal and skeletal adiposity and improves insulin sensitivity in OLETF rats. Biochem Biophys Res Commun. 2002;296:293–9. doi: 10.1016/s0006-291x(02)00822-7. [DOI] [PubMed] [Google Scholar]
- 68.Choi KC, Ryu OH, Lee KW, et al. Effect of PPAR-alpha and -gamma agonist on the expression of visfatin, adiponectin, and TNF-alpha in visceral fat of OLETF rats. Biochem Biophys Res Commun. 2005;336:747–53. doi: 10.1016/j.bbrc.2005.08.203. [DOI] [PubMed] [Google Scholar]
- 69.Ishibashi M, Egashira K, Hiasa K, et al. Antiinflammatory and antiarteriosclerotic effects of pioglitazone. Hypertension. 2002;40:687–93. doi: 10.1161/01.hyp.0000036396.64769.c2. [DOI] [PubMed] [Google Scholar]
- 70.Tao L, Liu HR, Gao E, et al. Antioxidative, antinitrative, and vasculoprotective effects of a peroxisome proliferator-activated receptor-gamma agonist in hypercholesterolemia. Circulation. 2003;108:2805–11. doi: 10.1161/01.CIR.0000097003.49585.5E. [DOI] [PubMed] [Google Scholar]
- 71.Hsueh WA, Bruemmer D. Peroxisome proliferator-activated receptor gamma: implications for cardiovascular disease. Hypertension. 2004;43:297–305. doi: 10.1161/01.HYP.0000113626.76571.5b. [DOI] [PubMed] [Google Scholar]
- 72.Calnek DS, Mazzella L, Roser S, Roman J, Hart CM. Peroxisome proliferator-activated receptor γ ligands increase release of nitric oxide from endothelial cells. Arterioscler Thromb Vasc Biol. 2003;23:52–7. doi: 10.1161/01.atv.0000044461.01844.c9. [DOI] [PubMed] [Google Scholar]
- 73.Hwang J, Kleinhenz DJ, Lassègue B, et al. Peroxisome proliferator-activated receptor-gamma ligands regulate endothelial membrane superoxide production. Am J Physiol Cell Physiol. 2005;288:C899–905. doi: 10.1152/ajpcell.00474.2004. [DOI] [PubMed] [Google Scholar]
- 74.Berger JP, Akiyama TE, Meinke PT. PPARs: therapeutic targets for metabolic disease. Trends Pharmacol Sci. 2005;26:244–51. doi: 10.1016/j.tips.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 75.Chetty VT, Sharma AM. Can PPARgamma agonists have a role in the management of obesity-related hypertension? Vasc Pharmacol. 2006;45:46–53. doi: 10.1016/j.vph.2005.11.010. [DOI] [PubMed] [Google Scholar]
- 76.Duan SZ, Usher MG, Mortensen RM. Peroxisome proliferator-activated receptor-gamma-mediated effects in the vasculature. Circ Res. 2008;102:283–94. doi: 10.1161/CIRCRESAHA.107.164384. [DOI] [PubMed] [Google Scholar]
- 77.Nicol CJ, Adachi M, Akiyama TE, Gonzalez FJ. PPARgamma in endothelial cells influences high fat diet-induced hypertension. Am J Hypertens. 2005;18:549–56. doi: 10.1016/j.amjhyper.2004.10.032. [DOI] [PubMed] [Google Scholar]
- 78.Tham DM, Martin-McNulty B, Wang YX, et al. Angiotensin II is associated with activation of NF-kappaB-mediated genes and downregulation of PPARs. Physiol Genom. 2002;11:21–30. doi: 10.1152/physiolgenomics.00062.2002. [DOI] [PubMed] [Google Scholar]
- 79.Diep QN, Amiri F, Touyz RM, et al. PPARalpha activator effects on Ang II-induced vascular oxidative stress and inflammation. Hypertension. 2002;40:866–71. doi: 10.1161/01.hyp.0000037969.41360.cc. [DOI] [PubMed] [Google Scholar]
- 80.Sugawara A, Takeuchi K, Uruno A, et al. Transcriptional suppression of type 1 angiotensin II receptor gene expression by peroxisome proliferator-activated receptor-gamma in vascular smooth muscle cells. Endocrinology. 2001;142:3125–34. doi: 10.1210/endo.142.7.8272. [DOI] [PubMed] [Google Scholar]
- 81.Van Linthout S, Spillmann F, Lorenz M, et al. Vascular-protective effects of high-density lipoprotein include the downregulation of the angiotensin II type 1 receptor. Hypertension. 2009;53:682–7. doi: 10.1161/HYPERTENSIONAHA.108.118919. [DOI] [PubMed] [Google Scholar]
- 82.Nakamura T, Yamamoto E, Kataoka K, et al. Beneficial effects of pioglitazone on hypertensive cardiovascular injury are enhanced by combination with candesartan. Hypertension. 2008;51:296–301. doi: 10.1161/HYPERTENSIONAHA.107.099044. [DOI] [PubMed] [Google Scholar]
- 83.Koh KK, Han SH, Quon MJ, Ahn JY, Shin EK. Beneficial effects of fenofibrate to improve endothelial dysfunction and raise adiponectin levels in patients with primary hypertriglyceridemia. Diabetes Care. 2005;28:1419–24. doi: 10.2337/diacare.28.6.1419. [DOI] [PubMed] [Google Scholar]
- 84.Koh KK, Quon MJ, Han SH, et al. Additive beneficial effects of fenofibrate combined with candesartan in the treatment of hypertriglyceridemic hypertensive patients. Diabetes Care. 2006;29:195–201. doi: 10.2337/diacare.29.02.06.dc05-1418. [DOI] [PubMed] [Google Scholar]
- 85.Keech A, Simes RJ, Barter P, et al. Effects of long-term fenofibrate therapy on cardiovascular events in 9795 people with type 2 diabetes mellitus (the FIELD study): randomised controlled trial. Lancet. 2005;366:1849–61. doi: 10.1016/S0140-6736(05)67667-2. [DOI] [PubMed] [Google Scholar]
- 86.Rosenson RS. Field of confusion: future prospects for fibrate therapy in cardiovascular disease. Curr Atheroscler Rep. 2006;8:219–22. doi: 10.1007/s11883-006-0076-y. [DOI] [PubMed] [Google Scholar]
- 87.Szapary PO, Bloedon LT, Samaha FF, et al. Effects of pioglitazone on lipoproteins, inflammatory markers, and adipokines in nondiabetic patients with metabolic syndrome. Arterioscler Thromb Vasc Biol. 2006;26:182–8. doi: 10.1161/01.ATV.0000195790.24531.4f. [DOI] [PubMed] [Google Scholar]
- 88.Sarafidis PA, Nilsson PM. The effect of thiazolidinediones on blood pressure levels—a systematic review. Blood Press. 2006;15:135–50. doi: 10.1080/08037050600853720. [DOI] [PubMed] [Google Scholar]
- 89.Qayyum R, Schulman P. Cardiovascular effects of the thiazolidinediones. Diabetes Metab Res Rev. 2006;22:88–97. doi: 10.1002/dmrr.596. [DOI] [PubMed] [Google Scholar]
- 90.Qayyum R, Adomaityte J. A meta-analysis of the effect of thiazolidinediones on blood pressure. J Clin Hypertens (Greenwich) 2006;8:19–28. doi: 10.1111/j.1524-6175.2005.04784.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Suwaidi JA, Hamasaki S, Higano ST, et al. Long-term follow-up of patients with mild coronary artery disease and endothelial dysfunction. Circulation. 2000;101:948–54. doi: 10.1161/01.cir.101.9.948. [DOI] [PubMed] [Google Scholar]
- 92.Halcox JPJ, Schenke WH, Zalos G, et al. Prognostic value of coronary vascular endothelial dysfunction. Circulation. 2002;106:653–8. doi: 10.1161/01.cir.0000025404.78001.d8. [DOI] [PubMed] [Google Scholar]
- 93.Juonala M, Viikari JS, Laitinen T, et al. Interrelations between brachial endothelial function and carotid intimamedia thickness in young adults: the Cardiovascular Risk in Young Finns study. Circulation. 2004;110:2918–29. doi: 10.1161/01.CIR.0000147540.88559.00. [DOI] [PubMed] [Google Scholar]
- 94.Halcox JP, Donald AE, Ellins E, et al. Endothelial function predicts progression of carotid intimamedia thickness. Circulation. 2009;119:1005–12. doi: 10.1161/CIRCULATIONAHA.108.765701. [DOI] [PubMed] [Google Scholar]
- 95.Deanfield JE, Halcox JP, Rabelink TJ. Endothelial function and dysfunction: testing and clinical relevance. Circulation. 2007;115:1285–95. doi: 10.1161/CIRCULATIONAHA.106.652859. [DOI] [PubMed] [Google Scholar]
- 96.Koh KK, Quon MJ, Waclawiw MA. Are statins effective for simultaneously treating dyslipidemias and hypertension? Atherosclerois. 2008;196:1–8. doi: 10.1016/j.atherosclerosis.2007.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kushiro T, Mizuno K, Nakaya N, et al. Pravastatin for cardiovascular event primary prevention in patients with mild-to-moderate hypertension in the Management of Elevated Cholesterol in the Primary Prevention Group of Adult Japanese (MEGA) Study. Hypertension. 2009;53:135–41. doi: 10.1161/HYPERTENSIONAHA.108.120584. [DOI] [PubMed] [Google Scholar]
- 98.Williams B, Lacy PS, Cruickshank JK, et al. Impact of statin therapy on central aortic pressures and hemodynamics: principal results of the Conduit Artery Function Evaluation-Lipid-Lowering Arm (CAFE-LLA) Study. Circulation. 2009;119:53–61. doi: 10.1161/CIRCULATIONAHA.108.785915. [DOI] [PubMed] [Google Scholar]
- 99.Sever PS, Dahlöf B, Poulter NR, et al. Prevention of coronary and stroke events with atorvastatin in hypertensive patients who have average or lower-than-average cholesterol concentrations, in the Anglo-Scandinavian Cardiac Outcomes Trial–Lipid Lowering Arm (ASCOT-LLA): a multicentre randomised controlled trial. Lancet. 2003;361:1149–58. doi: 10.1016/S0140-6736(03)12948-0. [DOI] [PubMed] [Google Scholar]
- 100.Demers C, McMurray JJ, Swedberg K, et al. Impact of candesartan on nonfatal myocardial infarction and cardiovascular death in patients with heart failure. JAMA. 2005;294:1794–8. doi: 10.1001/jama.294.14.1794. [DOI] [PubMed] [Google Scholar]
- 101.Dahlof B, Sever PS, Poulter NR, et al. Prevention of cardiovascular events with an antihypertensive regimen of amlodipine adding perindopril as required versus atenolol adding bendroflumethiazide as required, in the Anglo-Scandinavian Cardiac Outcomes Trial-Blood Pressure Lowering Arm (ASCOT-BPLA): a multicentre randomised controlled trial. Lancet. 2005;366:895–906. doi: 10.1016/S0140-6736(05)67185-1. [DOI] [PubMed] [Google Scholar]
- 102.Chhatriwalla AK, Nicholls SJ, Wang TH, et al. Low levels of low-density lipoprotein cholesterol and blood pressure and progression of coronary atherosclerosis. J Am Coll Cardiol. 2009;53:1110–5. doi: 10.1016/j.jacc.2008.09.065. [DOI] [PubMed] [Google Scholar]
- 103.Koh KK. Combination treatment to prevent atherosclerosis. Hypertension. 2007;50:e67. doi: 10.1161/HYPERTENSIONAHA.107.092064. [DOI] [PubMed] [Google Scholar]
- 104.Koh KK, Quon MJ. Combination therapy for treatment or prevention of atherosclerosis. Hypertension. 2008;52:e18. doi: 10.1161/HYPERTENSIONAHA.108.115840. [DOI] [PubMed] [Google Scholar]
- 105.Koh KK, Oh PC, Quon MJ. Does reversal of oxidative stress and inflammation provide vascular protection? Cardiovasc Res. 2009;81:649–59. doi: 10.1093/cvr/cvn354. [DOI] [PubMed] [Google Scholar]
- 106.Koh KK, Quon MJ. The importance of considering alternative or combination strategies for lowering LDL-C. Int J Cardiol. 2009;136:115–9. doi: 10.1016/j.ijcard.2009.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]