Abstract
Listening and reading comprehension of paragraph-length material are considered higher-order language skills fundamental to social and academic functioning. Using ecologically relevant language stimuli that were matched for difficulty according to developmental level, we analyze the effects of task, age, neuropsychological skills, and post-task performance on fMRI activation and hemispheric laterality. Areas of supramodal language processing are identified, with the most robust region being left-lateralized activation along the superior temporal sulcus. Functionally, this conjunction has a role in semantic and syntactic processing, leading us to refer to this conjunction as “comprehension cortex.” Different from adults, supramodal areas for children include less extensive inferior frontal gyrus but more extensive right cerebellum and right temporal pole. Broader neuroanatomical pathways are recruited for reading, reflecting the more active processing and larger set of cognitive demands needed for reading compared to listening to stories. ROI analyses reveal that reading is a less lateralized language task than listening in inferior frontal and superior temporal areas, which likely reflects the difficulty of the task as children in this study are still developing their reading skills. For listening to stories, temporal activation is stable by age four with no correlations with age, neuropsychological skills or post-task performance. In contrast, frontal activation during listening to stories occurs more often in older children, and frontal activation is positively correlated with better performance on comprehension questions, suggesting that the activation of frontal networks may reflect greater integration and depth of story processing.
Introduction
The left lateralized Wernicke-Geshwind language network that is connected by the arcuate fasciculus pathway/superior longitudinal fasciculus III and includes posterior superior temporal gyrus (Wernicke's Area), inferior frontal gyrus (Broca's Area), and their homologues (Catani et al., 2005; Frey et al., 2008; Friederici, 2009; Geschwind, 1972; Wernicke, 1874) has been well characterized in adults. Imaging studies reveal a similar network in children across a variety of language tasks (Ahmad et al., 2003; Balsamo et al., 2002; Burman et al., 2008; Cao et al., 2006; Gaillard et al., 2003b; Holland et al., 2001; Schlaggar et al., 2002); however, the influence of age, task performance, and task type on the neural correlates of language remains unclear. Our study provides insight into the influence of these factors by utilizing a novel developmental approach that is ecologically and clinically relevant.
Many pediatric studies use single words or short sentences; however, everyday communication—including discourse and formal academic instruction—entails processing complex language. In the present study we aim to examine the networks involved in processing “whole” language. The language stimuli in our study are paragraph stories that require integration, inference, and derivation of overall meaning based on previous knowledge, as well as phonological, syntactic and semantic information. Thus, our stimuli have greater ecological validity than single words or simple sentences. Although complexity may be operationally defined by various parameters including syntax, semantics, or units (word, sentence, text), extent of activation increases with greater linguistic complexity in left superior temporal cortex, left inferior frontal gyrus and, to a lesser extent, within right homologues (Brauer and Friederici, 2007; Jobard et al., 2007; Just et al., 1996). The greater demands for story comprehension compared to single words require greater neuronal activation and support from secondary areas (Jobard et al., 2007). We examine whether there are developmental differences in how the network is engaged for complex language.
Our task design is novel because we adapt task difficulty to individual skill level. Our study presents stories to children that are designed to match their developmental level with the aim of broadly controlling for performance, which then allows for examination of differences in activation associated with age. This approach is in contrast to previous studies that use a common task typically aimed at the easiest level of comprehension. Each method has advantages and drawbacks; however, from a clinical perspective, the common task method may be unsuitable because it does not optimize the patient's performance. A task that is too difficult or too easy may result in null or misleading activation and we find that success—in the form of a usable dataset—is best achieved when the difficulty level matches one's developmental level (Gaillard, 2004; Gaillard et al., 2004; Yerys et al., 2009).
We also conducted a novel analysis that has not been previously used with children. We make a direct comparison of listening to a story versus silently reading a story. The comparison of reading and listening is informative for identifying the neural correlates unique to each task as well as those involved in supramodal processing of language. There is an evolutionary basis for expecting neural differences associated with listening and reading, particularly when examined during development. Ontogenetically, oral language is acquired earlier with virtually all mammals engaging in a form of oral communication. Recent studies demonstrate that the perinatal brain is selectively primed to process auditory information important and specific to language (Dehaene-Lambertz et al., 2006; Telkemeyer et al., 2009). In contrast, reading is a skill that is unique to humans and is explicitly taught. As such, the neuronal requirements for listening to a story may be more streamlined and automatic than those required for reading a story (Indefrey et al., 2004).
Previous studies with children using single words or with adults using stories show that the overlap between reading and listening activation lies along the superior temporal sulcus into middle temporal gyrus (BA 21,22) (Gaillard et al., 2003a; Jobard et al., 2007; Lindenberg and Scheef, 2007; Turkeltaub et al., 2003). Functionally, temporal lobe activation is critical for lexical and semantic processing. The superior temporal sulcus is also involved in crossmodal integration, a process needed for mapping auditory and visual cues (Calvert, 2001; Naumer et al., 2008). In a study with adults, bilateral inferior frontal gyrus was also an area of overlap for reading and listening (Jobard et al., 2007); however, this may not be found in children who show variable engagement of the frontal lobes during listening tasks (Ahmad et al., 2003; Carpentier et al., 2001; Karunanayaka et al., 2007). Consistent frontal activation in children is found with a large sample size (>300) and by using a different analysis technique (independent component analysis and structural equation modeling) than the standard group t-test comparison (Karunanayaka et al., 2007).
Activation differences for listening over reading are localized mainly in the temporal lobe with the largest differences occurring close to left primary auditory cortex and in right homologues (Carpentier et al., 2001; Jobard et al., 2007; Michael et al., 2001). Activation differences for reading over listening are more extensive. Areas include bilateral occipital lobe and anteriorly along the superior temporal sulcus toward middle and inferior temporal gyrus to include the fusiform gyrus and visual word form areas (Borowsky et al., 2007; Hickok and Poeppel, 2004; Jobard et al., 2007; Saur et al., 2008; Scott and Wise, 2004). Differences in frontal regions include inferior frontal gyrus and supplementary motor area (Carpentier et al., 2001). Activation of inferior temporal areas, including fusiform, is postulated as an association area where there is an incorporation of a visual strategy during language processing (e.g., picturing what is being comprehended) (Balsamo et al., 2006). Frontal activation during reading is associated with several component skills including phonological processing and verbal working memory (Bookheimer, 2002).
We use fMRI to study listening and reading comprehension in healthy children across a wide age range and with a task aimed at each child's developmental level to expand the understanding of neuronal changes associated with language development, particularly whole language, which is the basis of common communication. Our approach is ecologically and clinically relevant. A goal is to investigate the shared and unique components of the language network engaged by different modalities in children as they develop language comprehension skills. We also investigate the extent to which hemispheric laterality strengthens with age for each task as a possible indicator of network maturation. Moreover, task and neuropsychological performance are included in analyses to explore their relationship with activation. We hypothesize that activation from the listening task will overlap with reading primarily in the temporal lobe and to a lesser extent than adults in the frontal lobe. We expect that the reading task will recruit a broader network than the listening task including frontal and occipital regions. We also expect laterality will show a positive correlation with age and performance.
Methods
Participants
Seventy-four healthy, right-handed (Harris, 1947), English-speaking children participated in the protocol. For the listening comprehension task, 59 subjects completed the task (31 boys; mean age=8.72 years, range 4–12). Eleven were excluded due to movement, three due to aborted scans because of technical difficulty, and one due to the child falling asleep in the scanner. The criterion for removal due to movement was if the child moved more than a voxel (3mm3) based on the standard output from the registration preprocessing step that provides six movement parameters (SPM2, University College London, London). For the reading comprehension task, 44 subjects completed the task (24 boys; mean age=10.04 years, range 7–12). Six were excluded due to movement while 24 children did not perform the task because they were not yet reading beyond single words (largely children younger than seven years old). Thirty-six children completed both the reading and listening comprehension tasks (20 boys; mean age=10.2, range 7–12).
Participants underwent a neurological examination by a child neurologist and had no history of developmental, learning, neurological, or psychiatric disorders. The study was approved by Children's National Medical Center Institutional Review Board, with informed consent provided by the parents, and written assent provided by all children prior to any study procedure.
Neuropsychological Testing
Intelligence and language skills were assessed in a separate session within a month prior to scanning. Intelligence was assessed according to standardized administration with an age appropriate measure; either the Differential Scales of Ability (DAS, ages 4–5) (Elliot, 1990) or the Wechsler Abbreviated Scale of Intelligence (WASI, ages 6–12) (Wechsler, 1999). The IQ measures contain several verbal and nonverbal subtests, providing a Full Scale IQ (General Conceptual Ability for the DAS), Verbal IQ (Verbal Cluster), and Performance IQ (Nonverbal Cluster). Language measures were selected to assess a variety of expressive and receptive language skills. Fundamental language skills were assessed with subtests from the Clinical Evaluation of Language Fundamentals (Preschool Version (CELF-P, age 4) (Wiig and Secord, 2004) or Fourth Edition (CELF-4, ages 5–12) (Semel et al., 2003)) that comprise the Core Language composite. Oral reading skills were assessed with the Gray Oral Reading Test: Fourth Edition (GORT-4) (Wiederholt and Bryant, 2001), which measures growth in oral reading based on rate, accuracy, fluency, comprehension, resulting in a composite Oral Reading Quotient (ORQ). Phonological Awareness was assessed with subtests from the Comprehensive Test of Phonological Processing (CTOPP) (Wagner, 1999), and verbal memory was evaluated with the Story Memory subtest of the Wide Range Assessment of Memory and Learning (WRAML) (Sheslow, 1990).
Functional MRI Paradigms
A protocol was followed to ensure that the children felt confident and comfortable about all aspects of the scanning experience, with two mock scanning sessions prior to the actual imaging session. In the scanner, subjects performed a listening to stories task and a reading stories task as part of a larger battery of language tasks that were performed during the same scanning session. Task order was randomized.
Tasks were created by adapting stories from standardized measures that included a narrative (full stories available in the online supplement). These included the Children's Memory Scale (Cohen, 1997), Dynamic Indicators of Basic Early Literacy Skills (DIBELS) (Good, 2002), Gray Silent Reading Test (GSRT) (Wiederholt, 2000), and Gray Oral Reading Test: Fourth Edition (GORT-4), Form B (Wiederholt and Bryant, 2001), which are all measures that were created using large, diverse normative samples. Four ability levels (Level 1-Grade 1 and below, Level 2 – Grade 2, Level 3- Grade 3, Level 4- Grade 4 and above) were created for the listening to stories with grade level being the criterion. Due to the larger differences expected for reading ability, six levels were created with grade being the criterion. The reading levels were: Kindergarten and below, Grade 1, Grade 2, Grade 3, Grades 4–5, and Grades 6 and above. Selections for the fMRI task at were the grade appropriate entry items from the normed measure. To keep the number of levels manageable, the levels were designed for a relatively large developmental window and thus, passages were meant to be well within each ability level. The stories for the listening and reading tasks were adapted from the comparable level of an alternate form of the standardized measure.
For both listening and reading, the task paradigm consisted of alternating 30 second blocks of experimental and baseline conditions. The task consisted of 10 blocks (five of each condition), with total scan time for the entire task being five minutes. Two stories were presented over the 5 experimental blocks, thus one story spanned at least two blocks. For the listening to stories task, the experimental condition consisted of pre-recorded sentences with a tone interspersed at the end of sentences. Subjects were asked to press the button of the MR compatible response box when they heard a tone in order to assure vigilance to the task. The baseline condition consisted of reverse speech, designed to match the experimental condition for primary audition, motor response, length of utterance, and volume of presentation. For the reading stories task, the experimental condition consisted of sentences projected onto a MR compatible screen above the child's head. Subjects were asked to covertly read the sentences on the screen and press the button when they came to a period. If they finished before the screen changed, they were asked to read it again. The baseline condition consisted of viewing black and white dots to control for motor and eye movement. When the subject saw a white square appear on the screen, they were to indicate via a button press. Post-scan questions were posed to measure story comprehension. Ten questions (5 free recall/multiple choice and 5 recognition) assessed story comprehension and indicated performance level.
Image Acquisition
Functional data was acquired using a 3.0 Tesla Siemens Magnetom Trio equipped with a standard CP head coil. Anatomical images of participants were collected using a sagittal T1 MPRAGE sequence, slice thickness of 1.0 mm, TR of 1600ms and TE of 3.37 ms, which served to screen for anatomical abnormalities and used as normative data for structural childhood epilepsy studies. Blood oxygen level-dependent (BOLD) changes were measured using a whole brain EPI sequence with parameters: TR=3000ms, TE =30ms, FoV= 192mm, and effective voxel size=3.0 × 3.0 × 3.0 mm3. Axial images were collected parallel to the anterior commissure-posterior commissure plane, which served as an origin of reference. Whole brain volumes consisted of 50 axial slices of 2.8 mm thickness with a 0.2 mm gap between slices.
Stimuli were presented using Windows compatible E-prime software version 1.1 (Psychology Software tools, Inc., Pittsburgh, PA). All auditory stimuli were presented to subjects through MR compatible headphones, which also facilitated communication between the subject and MR technician and reduced in-scanner noise. Subjects' task held the button box in the left hand.
Functional MRI Data Analysis
Image data preprocessing and group analysis was performed using Statistical Parametric Mapping software (SPM2) (University College London, London) and the Statistical Analysis Toolbox through Matlab (The MathWorks, Inc; Natick, MA). Images were realigned, spatially normalized to the MNI standard anatomical space, spatially smoothed using an 8mm full width at half maximum Gaussian kernel and temporally filtered (high-pass filter: 128 seconds). Individual t-maps were generated by comparing the experimental and baseline conditions on a voxel-wise basis with movement parameters as covariates of noninterest. Movement was included to control for any activation differences related to movement. After rejecting data with excessive motion (> 1 voxel) as described above, no overall differences in motion related to age group were found (p=.28). Specific comparisons revealed that the youngest age group (Mean=0.16 mm, SD=0.2 mm) moved more in the × direction—by less than a millimeter—than the middle age group (Mean=0.05mm, SD=0.4; p=.03). Group maps were generated from individual activation maps using a random-effects model to obtain a whole brain activation maps and determine the network of brain regions activated during each task examining all of the subjects combined. The activation maps were thresholded at p<0.05 corrected for multiple comparisons using Family Wise Error (FWE), and a minimum cluster size of 20 voxels.
Region of Interest Analysis and Laterality
We based our regions of interest (ROI) on anatomical areas that are well established as primary contributors to language processing and validated by invasive means, including the intracarotid amobarbital test (IAT) and electrocorticography (Gaillard et al., 2002b). Three ROIs were defined broadly by anatomic designations using the Wake Forest PickAtlas (Maldjian et al., 2003). They included: 1) Anatomic Label for Inferior frontal gyrus (IFG, including BA 44, 45, 47), 2) Anatomic Label for Middle frontal gyrus (MFG, including BA 9, 46), and 3) Wernicke's Area (WA, including BA 21, 22, 39). Anatomical ROIs were chosen to have an inclusive region that would potentially capture any variability in activation related to development, yet, the selection of specific regions reflect the understanding that it is uncommon to activate much outside these areas for language processing (Mbwana et al., 2009).
In addition to anatomic ROIs, analyses were also conducted using functional ROIs that are based on the activation in the primary language regions—IFG and WA—from the group map and conjunction results. The functional ROIs were smaller than the anatomical ROIs because they are limited to the most common activation, yet the functional ROIs still covered a relatively broad cortical region. In addition, no IFG functional ROI was analyzed for the listening task because the group map rendered only a 29 voxel ROI and individual activation was too variable. We conducted our analyses with functional ROIs to ensure that our results were not influenced by reduced sensitivity based on having an overly inclusive anatomical ROI and to determine if the ROI based on the conjunction rendered different results.
Lateraility index (LI) is computed by comparing the voxels active in each hemisphere: LI = (ΣLvoxels − ΣRvoxels) / (ΣLvoxels + ΣRvoxels). Values range from −1 to 1 with higher positive values indicating greater left lateralization. We utilized a bootstrap method (Wilke and Lidzba, 2007; Wilke and Schmithorst, 2006) to calculate LI and report the “weighted mean” of LI values which is equal to the arithmetic mean of all possible LI values. We categorized language dominance such that left hemisphere dominance is defined by LI values >0.2, right hemisphere dominance is defined by LI values < −0.2, and bilateral representation are LI values between −0.2 and 0.2 (Binder et al., 1995; Gaillard et al., 2002a; Pujol et al., 1999).
Statistical Analyses
Descriptive statistics were used to characterize demographic, neuropsychological, task performance, and LI variables. To examine the relationships among these variables the appropriate parametric and nonparametric two-tailed correlation analysis (depending on the nature and distribution) were conducted. Multivariate analysis of variance (MANOVA) examined age and regional differences in LI. A 3 (WA, IFG, and MFG) by 2-way (reading versus listening) ANOVA was used to assess the effect of region of interest and task type on degree of lateralization. Regression analyses performed were conducted using SPM2 to correlate activation with age, neuropsychological skills, and task performance. Age group comparisons were also conducted by ANOVA and conjunction analysis in SPM2 to determine unique and common activation. Subjects were segregated into three age groups according to the three listening levels: 4–6 years-old, 7–9 years-old, and 10–12 years-old.
Results
Neuropsychological Data
Subjects' cognitive skills fell in the average to above average range for all measures, with no significant differences among age groups (p>.05, Table 1). Moreover, no significant differences in skills were evident for the subsets of children who completed particular tasks; 59 children for listening to stories (Mean FSIQ=115, SD=14, range 80−156), 44 children for reading stories (Mean FSIQ=117, SD =15); and 36 children who completed both (Mean FSIQ=118, SD=15).
Table 1.
Neuropsychological performance, LI, and Post-Task Question performance across regions for the sample and by age group. Cognitive performance was not different across age. SS=Standard Score (Normative Sample Mean = 100, SD=15); ss= scaled score (Normative Sample Mean = 10, SD=3).
| Total Sample Mean (SD) n=67 | 4–6 y.o. Youngest n=17 | 7–9 y.o. Middle n=23 | 10–12 y.o. Oldest n=27 | |
|---|---|---|---|---|
| GCA or Full Scale IQ (SS) | 115 (14) | 111 (11) | 121 (15) | 113 (14) |
| CELF Core Language (SS) | 112 (13) | 110 (16) | 112 (8) | 114 (14) |
| GORT4 Oral Reading Quotient (SS) | 117 (18) | - | 118 (17) | 117 (18) |
| CTOPP Phonological Awareness (SS) | 102 (12) | 104 (9) | 103 (10) | 100 (15) |
| WRAML Immediate Story Memory (ss) | 12 (3) | 12 (4) | 12 (2) | 11 (3) |
| Listening WA LI (n=59) | 0.52 (0.28) | 0.46 (0.32) | 0.52 (0.31) | 0.57 (0.22) |
| Listening IFG LI (n=38) | 0.50 (0.46) | 0.68 (0.17) | 0.58 (0.43) | 0.36 (0.54) |
| Listening MFG LI (n=38) | 0.38 (0.49) | 0.46 (0.38) | 0.36 (0.48) | 0.36 (0.55) |
| Reading WA LI (n=44) | 0.51 (0.36) | - | 0.53 (0.39) | 0.50 (0.33) |
| Reading IFG LI (n=44) | 0.41 (0.42) | - | 0.40 (0.34) | 0.41 (0.48) |
| Reading MFG LI (n=44) | 0.32 (0.38) | - | 0.30 (0.38) | 0.33 (0.38) |
| Listening Recognition Correct (%) | 77 (19) | 71 (27) | 77 (16) | 79 (18) |
| Listening Recall Correct (%) | 79 (21) | 69 (20) | 76 (24) | 85 (18) |
| Listening All Questions Correct (%) | 78 (16) | 71 (20) | 76 (17) | 82 (16) |
| Reading Recognition Correct (%) | 87 (15) | - | 87 (14) | 88 (15) |
| Reading Recall Correct (%) | 86 (17) | - | 89 (11) | 82 (20) |
| Reading All Questions Correct (%) | 87 (13) | - | 88 (11) | 85 (15) |
Listening to Stories Task
Task Performance and Neuropsychological Skills
Subjects answered post-task questions with 78% accuracy indicating good task compliance and comprehension of material (Table 1). Performance on post-task questions was positively correlated with immediate story memory (p<.05) and age (p<.05), but age and story memory were not significantly correlated (Table included in supplementary material). There was a trend for performance on post-task questions to be positively correlated with Verbal IQ (p=.07), CTOPP Phonological Awareness (p=.07) and CELF Core Language (p=.09).
Functional Activation
Group Map
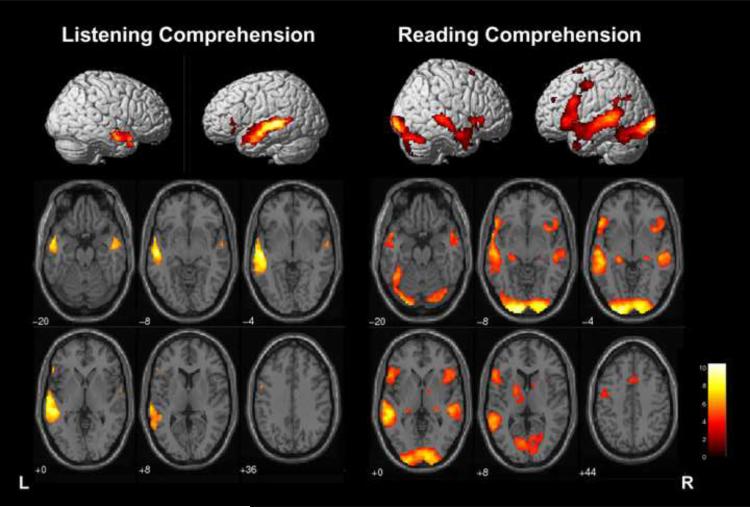
Activation was robust along the left superior temporal sulcus and to a lesser extent, within right homologues (FWE p<.05, >20 voxels in cluster; Table 2, Figure 1). Small clusters of activation in the left inferior frontal gyrus (BA 45, 47; 31 voxels) and left orbitofrontal cortex (BA 11; 26 voxels) are also activated. On an individual basis, 38 children had frontal cortex activation in IFG and/or MFG (BA 44, 45, 46 or 47).
Table 2.
Listening and Reading Comprehension Tasks and the conjunction of both tasks. MNI coordinates and Brodmann Areas for regions displayed in Figures 1 and 3 (Group Maps FWE Corrected, p=.05, >20 voxels).
| Listening Task | ||||||
|---|---|---|---|---|---|---|
| Location | Hemisphere, gyrus | Brodmann Area (BA) | Cluster size | T value | ||
| x | y | z | ||||
| −54 | −18 | −6 | Left MTG | 2073 | 10.42 | |
| −58 | −16 | 0 | Left STG | 8.98 | ||
| 54 | −4 | −22 | Right MTG | 21 | 415 | 7.26 |
| −54 | 28 | 0 | Left IFG | 45/47 | 31 | 6.61 |
| −2 | 42 | −16 | Left orbitofrontal | 11 | 26 | 6.33 |
| −54 | 4 | 36 | Left precentral gyrus | 6 | 22 | 5.93 |
| Reading Task | ||||||
|---|---|---|---|---|---|---|
| Location | Hemisphere, gyrus | Brodmann Area (BA) | Cluster size | T value | ||
| x | y | z | ||||
| −16 | −98 | −6 | Left occipital lobe | 17/18 | 10536 | 14.05 |
| 28 | −94 | −10 | Right occipital lobe | 17/18 | 13.15 | |
| −60 | −40 | 0 | Left MTG | 21 | 11.71 | |
| −56 | −42 | 6 | Left STG | 22 | 10.44 | |
| −52 | 24 | −4 | Left IFG | 47 | 9.99 | |
| −32 | −86 | −22 | Left cerebellum | 9.46 | ||
| 44 | −68 | −28 | Right cerebellum | 9.29 | ||
| 12 | 22 | 34 | Right cingulate gyrus | 778 | 7.38 | |
| 50 | −26 | −2 | Right STG | 21 | 785 | 10.23 |
| 32 | 22 | −10 | Right IFG | 47 | 680 | 7.97 |
| 54 | 4 | −30 | Right MTG | 21 | 292 | 7.18 |
| −40 | −6 | 46 | Left precentral gyrus | 6 | 122 | 6.36 |
| −22 | 44 | 26 | Left SFG | 54 | 6.54 | |
| 12 | 16 | 62 | Right SFG | 6 | 21 | 6.54 |
| −6 | 48 | 42 | Left MFG | 8/9 | 20 | 6.04 |
| Conjunction of Listening and Reading | ||||||
|---|---|---|---|---|---|---|
| Location | Hemisphere, gyrus | Brodmann Area (BA) | Cluster size | T value | ||
| x | y | z | ||||
| −56 | 2 | −12 | Left STG/MTG | 21 | 1625 | 9.59 |
| 18 | −82 | −38 | Right cerebellum | 119 | 7.23 | |
| 56 | 10 | −14 | Right STG | 38 | 72 | 6.87 |
| −52 | 28 | −10 | Left IFG | 47 | 29 | 5.57 |
Figure 1.
3-D Rendering of whole brain activation for Listening Comprehension and Reading Comprehension tasks for children ages 4–12 years old (FWE Corrected, p<.05, >20 voxels in cluster). Right sagittal view (on left), Left sagittal view (on right); and axial slices in neurological convention (left is left hemisphere).
Laterality
Subjects were predominantly left lateralized for the three regions of interest (IFG, MFG, and WA) (Table 1, Figure 1). Fifty-four children (92%) were left lateralized in WA (average LI=0.58). Of the subset of subjects who had frontal activation, 81% were left lateralized for IFG and 73% were left lateralized for MFG (average LI=0.70 and 0.64, respectively). To examine the strength of laterality independent of hemisphere dominance, we used the absolute value of LI and found that the degree of lateralization was comparable among regions, IFG Mean Absolute LI=0.64; MFG Mean absolute LI=0.58; and WA Mean Absolute LI=0.55 (p>.10).
Age
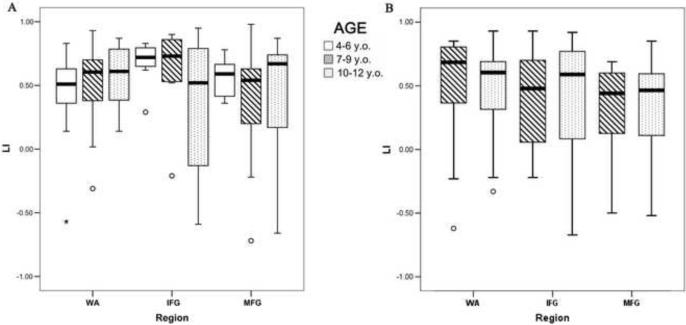
Using a threshold of p<.001 and minimum of 20 voxels in a cluster, no significant clusters of activation were correlated with age. Moreover, there were no age group differences in laterality based on either categorical dominance or Mean Absolute LI (p>.10, Figure 2). However, with age as a continuous variable, there is a trend for age to be positively correlated with lateralization of WA LI (p=.098, supplemental figure). The effect size of age was small (Cohen's f2 =.03). The variance in LI was affected by region and sample size: IFG was the most variable, but does not appear so in younger groups where the number of subjects with activation was less. There was a trend for fewer younger children to activate frontal regions (p=.09): 41% of 4–6 year olds, 72% of 7–9 year olds, and 71% of 10–12 year olds. In Wernicke's, where there were equal samples per age group, variance was similar across age (p>.05).
Figure 2.
Boxplots of Absolute Mean LI value for Age Group for Wernicke's Area (WA), Inferior Frontal Gyrus (IFG), and Middle Frontal Gyrus (MFG). (A) Listening Comprehension Task. (B) Reading Comprehension Task.
Task Performance, Neuropsychological Skills, and Activation
No significant clusters of activation were correlated with post-scan task performance. There was a trend for core language skills to be positively correlated with LI in MFG (p=.08), but no other associations between any other neuropsychological skill and region of interest were evident (p>.05). Post-scan test performance was not correlated with LI of any region (p>.05).
Based on observed patterns of frontal activation, we examined whether children who activated frontal regions showed performance differences. Children who engaged frontal areas to perform the listening task had better post-task recall comprehension (83% vs 70%, p<.05), but groups performed similarly on recognition questions (76% vs 78%, p>.05). However, these two groups of children (frontal vs. non-frontal engagers) did not differ across cognitive skills (WASI, CELF, CTOPP, or WRAML, p>.05).
Reading Comprehension
Task Performance and Neuropsychological Skills
Subjects answered post-task questions with 87% accuracy indicating good task compliance and comprehension of material (Table 1). Stronger performance on post-task reading questions was positively correlated with FSIQ, Performance IQ, CTOPP Phonological Awareness, WRAML Immediate Story Memory (p<.05) and reading ability (p=.05), with a trend for Verbal IQ (p=.07) and CELF Core Language (p=0.10). Post-task performance was not significantly correlated with age (p>.05).
Functional Activation
Group Map
Activation during the reading task was robust along the left superior temporal sulcus extending inferiorly into fusiform area (BA 20) (Table 2, Figure 1). Right homologous regions for superior and middle temporal gyrus were also active, but to a lesser extent. In addition, bilateral (left greater than right) activation was evident in the cerebellum, occipital lobe, and inferior and middle frontal gyri (BA 6, 44, 45).
Laterality
As a group, subjects were left lateralized for the three regions of interest (IFG, MFG, and WA) (Table 1, Figure 1). Thirty-one children (71%) were left lateralized for IFG, 29 (66%) were left lateralized for MFG, and 38 (87%) were left lateralized in WA. Strength of laterality independent of hemisphere dominance was comparable among regions: IFG Mean Absolute LI=0.51; MFG Mean absolute LI=0.44; and WA Mean Absolute LI=0.58 (p>.05).
Age
Age was negatively correlated with activation in IFG bilaterally and basal ganglia (p<.001, uncorrected, >20 voxels in cluster). There were no age differences in degree or categorical dominance of laterality (p>.05, Figure 2). The variance in LI was also similar across age (p>.05).
Task Performance, Neuropsychological Skills, and Activation
Across several analyses, the relationship between activation, lateralization of activation, and cognitive performance was examined. Reading skills were negatively correlated with activation in right middle frontal gyrus (BA6/9) (p=.001, uncorrected, >20 voxels in a cluster). No neuropsychological skills were associated with laterality across any region (p>.05). Post-scan test performance on recall questions was positively correlated with IFG LI (p<.05).
Comparison of Listening and Reading
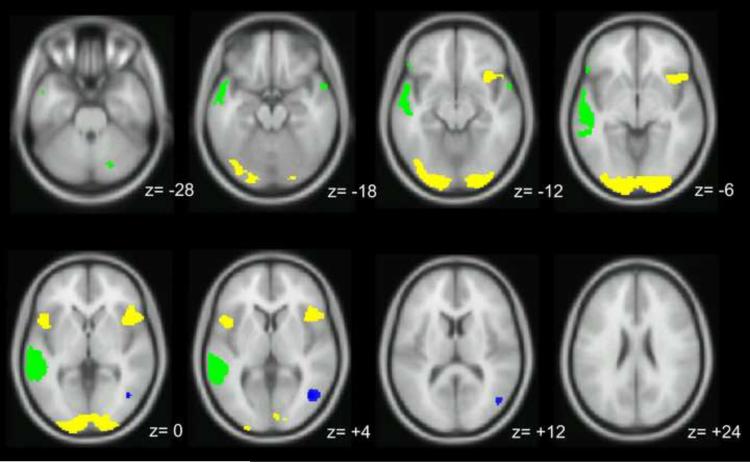
For the 36 subjects ages 7–12 who completed both the reading and listening comprehension tasks, direct task comparisons revealed both overlapping and distinct areas for each task (Figure 3). A conjunction analysis revealed that both tasks strongly activated the left superior temporal sulcus (p<.05, FWE, 1625 voxels, (green in Figure 3, Table 2)). In addition, the conjunction analysis showed overlapping activation in the right cerebellum (119 voxels), right STG (72 voxels), and left IFG (BA 47; 29 voxels). Activation differences showed that the reading task invoked a broader network than the listening task including bilateral occipital, right greater than left IFG (BA 47), as well as superior frontal gyrus (BA 6) (p<.05, FWE). The listening task activated the junction of the right angular/fusiform gyrus (BA 39/37 (p<.05, FWE, 174 voxels) to a greater degree than the reading task. The overall distribution of patterns of language dominance was similar to those described above; however, discordance for language dominance across the two tasks was evident. Seven (27%) children were discordant for laterality within IFG, while five (14%) children were discordant within WA.
Figure 3.
Task-Dependent Activation for subjects who completed both Listening and Reading Comprehension tasks; yellow activation shows areas recruited by the Reading Comprehension task; blue activation shows areas recruited by the Listening Comprehension task; green areas represent activation overlap between the two tasks (FWE Corrected, p<.05, >20 voxels in cluster).
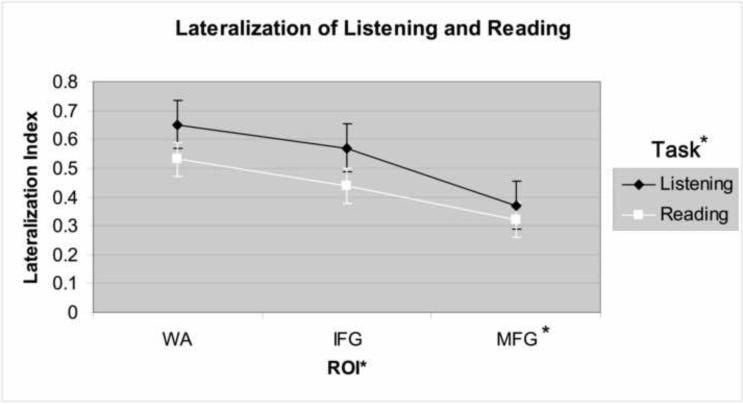
The 3 × 2 ANOVA revealed a main effect of region such that MFG was less lateralized than IFG and WA (p<.05), while IFG and WA had comparable degrees of lateralization (p>.10; Figure 4). For task type, there was a trend for reading to be less lateralized than listening (p=.08). There was no significant interaction between ROI and task type.
Figure 4.
Main effect of task and ROI on mean laterality for 36 subjects who completed both Listening and Reading tasks. Regions of Interest (ROI): Wernickes' Area (WA), Inferior Frontal Gyrus (IFG), and Middle Frontal Gyrus (MFG). Post-hoc, MFG is less lateralized than IFG and WA (*p<.05).
Comparison of Anatomical and Functional ROIs
LI values using functional ROIs including the conjunction ROI were higher than LI values using the anatomical ROIs for WA (p<.01, Supplemental Table); no differences in LI values based on functional ROIs were evident for IFG. Despite these differences in magnitude of WA LI, results remained unchanged related to laterality differences associated with task, performance, or age.
Discussion
We find that children engage the same fundamental cortical regions of the language network as adults; however, there are influences of age, task, and methodology on neural activation.
Supramodal activation: Developmental implications
In children as young as four years old, we find a supramodal area of robust activation along the superior temporal sulcus as well as less extensive activation in the left inferior frontal gyrus and right cerebellum. This conjunction represents cortex that is common to listening and reading independent of modality, complexity, or performance and reflects a stable area of the language network. Functionally, these regions have a role in semantic and syntactic processing (Bookheimer, 2002; Pugh et al., 1996), leading us to refer to this conjunction as “comprehension cortex.”
The principal portion of the comprehension cortex is comprised of the temporal lobe including Wernicke's Area. Specifically, activation is in the left posterior aspect of the superior temporal lobe and extends down the superior temporal sulcus into middle temporal gyrus. This temporal region is smaller than the region activated in the listening task alone, but nevertheless is a broad region of interest and comparable to the temporal supramodal areas in studies with adults (Jobard et al., 2007; Lindenberg and Scheef, 2007). Our finding of activation in the right cerebellum is also similar to the studies with adults, albeit our cluster size is larger. In contrast, in the frontal lobe, studies with adults demonstrate a more extensive area of supramodal activation than children in our study. Adult activation includes several regions of the inferior frontal gyrus (BA 44, 45, 47) and superior precentral gyrus/supplementary motor area while our study with children shows a modest area of activation within BA 47. Children also demonstrate a supramodal area of right temporal activation in BA 38 at the temporal pole that is not evident in adult studies.
The overlap between previous adult studies and our study with children further supports the comprehension cortex as a stable and reliably active region of the language network. Stability is also supported by the absence of significant relationships among magnitude or laterality of temporal activation with age, neuropsychological functioning, or task performance. In comparison, a series of studies with a large cohort of children find age-related changes in language lateralization; however, their results vary according to ROI and task (Holland et al., 2007). Findings on the task most similar to our study—story listening—are comparable to our results where no age related differences for the ROI in the temporal lobe are found. Although age-related changes for an anterior ROI are detected, the effect size is small (Cohen's f2 =.03)—which is comparable to our study—and reaches significance with a large sample size of over 300 children but not in our study of 67 children. Therefore, age-related changes in lateralization are modest and more likely to be reflected in frontal regions rather than temporal regions.
The supramodal regions that are unique to children—left IFG, right temporal pole, and right cerebellum—may reflect the developmental timing of cognitive skills and the neural structures that underlie them. Previous anatomical studies show that frontal cortex and other association areas have protracted development compared to other areas of the brain (Chung et al., 2003; Giedd et al., 1999; Gogtay et al., 2004; Sowell et al., 1999; Yakovlev and Lecours, 1967) which parallels the developmental progression of executive functioning skills that are also on a protracted developmental trajectory (Denckla, 1994; Diamond, 2006; Huizinga et al., 2006).
Frontal activation is less lateralized and more variable on an individual basis, across age, and by task than temporal activation. Children over age six engage frontal regions more often than children between the ages of four to six while listening to stories. Other studies with children detect frontal activation to a greater degree for listening to stories than our study, which may be related to methodological differences. One possible issue is that other studies use tones as a baseline task while we use reverse speech (Holland et al., 2007; Karunanayaka et al., 2007). Reverse speech may be processed by children—especially at younger ages—as unknown language and attempts to process an unknown language activate prefrontal regions (Lindenberg and Scheef, 2007; Wang et al., 2003). Thus, the contrast of the baseline and experimental conditions might render decreased frontal activation. Also, detection of consistent frontal activation in children is not demonstrated by traditional group comparison analysis—which is used in our study—but is identified with a different statistical approach using structural equation modeling and independent components analysis (Karunanayaka et al., 2007). Nonetheless, it is evident that frontal areas are more vulnerable to individual and developmental differences.
We find that frontal activation is associated with differences in neuropsychological functioning. Children of any age who engage frontal areas during listening are more accurate on post-scan task recall, suggesting that using frontal regions of the language network may facilitate better comprehension. For reading, greater left lateralization of IFG is associated with post-scan task performance. Frontal activation may reflect active engagement in the form of expressive not just passive listening, allowing for deeper semantic processing. While left posterior temporal areas help with accessing lexical and syntactic information, ventrolateral prefrontal cortex is involved in semantic processing and unification (Bookheimer, 2002; Snijders et al., 2008; Vigneau et al., 2006). Greater activation of left prefrontal cortex correlates with better performance including strength of verbal encoding (Wagner et al., 2001; Wagner et al., 1998) and better reading comprehension (Shankweiler et al., 2008). Connectivity analyses further support and characterize the synchrony between frontal and temporal regions of the language network (Allen et al., 2008; Chow et al., 2008; Frey et al., 2008; Karunanayaka et al., 2007; Powell et al., 2006). Bitan and colleagues (2007) suggest that changes in activation may represent developmental shifts in strategy, specifically from reliance on sensory auditory representations to phonological segmentation and covert articulation. As processes relevant to these types of tasks mature, there is a decreased reliance on primary sensory processing, allowing children to become more fluent readers with better comprehension skills. In summary, comprehension is better for individuals who engage IFG and other prefrontal cortical regions during the listening task, which may be due to better integration of information. Integration represents how one's thoughts become organized, which is one aspect of executive functioning.
A supramodal area in our study with children, but not reported in adult studies, is right hemisphere activation in the temporal pole. This region is an association area that integrates social, emotional, and lexical information (Sabsevitz et al., 2005; Zahn et al., 2007). Similar to the IFG findings, this age-related difference in supramodal activation occurs within a hetromodal area important for higher-order cognitive processing.
Age-related differences found in the cerebellum and reduced laterality of MFG implicate working memory as a possible marker of developmental and performance differences. Working memory is a component skill of executive functioning that moderates language development (Alloway and Gathercole, 2006; Gathercole et al., 2006). The right cerebellum appears to be a more robust area of supramodal processing for children than adults. The cerebellum has a role in verbal working memory and phonological processing (Ackermann et al., 2007; Booth et al., 2007) and is also a region that reaches maturity late in childhood (Diamond, 2000). Thus, the larger area of supramodal activation for the cerebellum when processing language may be because the cerebellum is burdened to a greater extent in children than in adults. In addition, middle frontal gyrus is the least lateralized region of interest across tasks for this group of children. More bilateral engagement of MFG likely reflects high working memory demands when processing language (Gabrieli et al., 1998).
Although by age four the language network is left lateralized and engages the same broad network as older children and adults, differences in our study are in association areas and related to higher-order skills that are known to continue to be refined later in development.
Task-related differences
Activation differences between reading and listening reflect varying task demands. Reading invokes anterior regions, right homologues, and visual processing areas to a greater extent than listening. In addition to engaging a broader network, laterality is lower on average and more bilateral activation is evident than for the listening comprehension task. However, laterality does not differ by age. The reduced leftward asymmetry for reading compared to listening may reflect the engagement of homologous regions to aid performance during paragraph reading, which is necessary as reading is a developing skill in this age range of children. Other developmental studies show that with better skills, there is less activation. The magnitude of activation in posterior brain regions decreases as phonological processing becomes more automatic with age (Church et al., 2008) and increased reading ability correlates with decreased activity in the right extrastriate cortex (Turkeltaub et al., 2003). Other studies find recruitment of homologous regions when language tasks are more difficult (Brauer and Friederici, 2007; Just et al., 1996); an observation supported by our findings that greater right activation and right lateralization are associated with worse reading skills and post-scan test performance, respectively.
The broader network for reading than for listening also likely reflects the different cognitive demands and strategies inherent to reading and are maturing in children. The most obvious difference is the engagement of inferior temporo-occipital areas, including fusiform gyrus (BA 37), that process words and utilize visual strategies in analyzing language. Hypoactivity of this region is a sensitive marker of dyslexia (Maisog et al., 2008). Within the extensive imaging literature with reading tasks, engagement of these posterior areas as well as frontal areas varies with the numerous lexical and word form variables such as word frequency, word length, orthographic complexity, and morphology (Berninger et al., 2008). The broader network for reading generally reflects a fundamental difference in active versus passive processing of language as well as incorporating the additional layer of orthographic representations.
In addition to differences in network activation, comparing consistency in laterality across regions between the two tasks informs how language dominance is determined. Up to 25% of children have inconsistent laterality for the two tasks despite similar stimuli and methods for calculating laterality. There is no discernable pattern for which region—IFG or WA—or task is likely to be categorized as atypical. In addition, the LI values fall across a wide range and were not just values close to the boundary (0.2) used for categorizing dominance. This suggests that a percentage of people exhibit mixed dominance across different tasks and argues for use of a panel of tasks to increase reliability of determining language dominance using fMRI (Gaillard et al., 2004).
Methodological issues: Limitations and clinical implications
Two methodological issues of our study also have important clinical implications when using fMRI to determine language dominance for surgical planning. First, anatomical ROIs cover a large cortical area. This is potentially a drawback because a large region of interest may collapse distinct areas of activation that represent different functional demands of the task into one calculation for laterality. However, we deliberately chose the anatomical approach because we expect variability in location of activation related to development and aim to apply these methods to patient populations whom we know have variability in location of activation (Mbwana et al., 2009; Rosenberger et al., 2009). A possible disadvantage of functional ROIs is that they often encompass a smaller area and may fail to capture this variability in activation that may lie outside the group map—thus impacting LI calculations. The functional ROIs render a higher—more left lateralized—average LI value than the anatomical ROI method, which is expected given that the functional ROI is predicated on activation that is common to the group. However, no other analyses are affected by the different methods for ROI selection; the associations between laterality and other variables (i.e., age, performance, neuropsychological skill) are unchanged. Therefore, given our concern of failing to capture variability with development, there may be reason to use the anatomical ROI in studies with patients that may have greater variability in location of activation.
The second issue is our decision to utilize a developmentally appropriate task by giving children of different ages different stimuli. Our study is the first developmental neuroimaging study to use this approach. By targeting our stimuli to the developmental level of the child we mirror their everyday functioning, which increases our success in obtaining a good study (Yerys et al., 2009), allowing us to image children as young as four years old. A concern with our approach is that differential content may account for activation differences. However, if content drives activation differences, we should find an age effect for listening and reading which we do not observe. A study with the specific aim of examining the impact of linguistic complexity during reading and listening also indicates that our results are not simply a reflection of using different stimuli (Jobard et al., 2007). Their study finds differences in unimodal areas such as visual and auditory cortex, which are not the areas where we report differences. Alternatively, studies that use a task aimed at the youngest age level may not be burdening the language system of older children to the same degree and thus, it is possible that their age-related differences may be related to performance differences for the task. Our aim to control for performance was successful as indicated by having no age group differences on post-scan task performance. Thus, using a developmentally appropriate task does not appear to confound our results, but rather provides a clinically useful tool that optimizes performance. Similar to other neuroimaging studies with children (Holland et al., 2007; Schlaggar et al., 2002), a limitation of our study is that our population had high average IQ. No single approach is ideal and conducting developmental studies presents challenges (Berl et al., 2006); however, flexibility with developmental level of task stimuli has great relevance for conducting neuroimaging studies with young and cognitively impaired populations.
In our two tasks that have similar levels of linguistic complexity presented orally and in written form, the common and unique areas of activation provide insights into language development with tasks that are clinically and ecologically valid. Developmental functional imaging studies that use tasks that are reflective of everyday communication provide an opportunity to further understand the complexities of language functioning and their neural correlates.
Supplementary Material
Acknowledgments
This publication was made possible by NINDS R01 NS44280 (WDG), Partnership for Pediatric Research Epilepsy Foundation (MMB); Children's Research Institute Avery Award (MMB), the NINDS Clinical Epilepsy Section Division of Intramural Research, Grant HD040677-07 from the IDDRC, and Grant M01RR020359 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of NCRR or NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errorsmaybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ackermann H, Mathiak K, Riecker A. The contribution of the cerebellum to speech production and speech perception: clinical and functional imaging data. Cerebellum. 2007;6:202–13. doi: 10.1080/14734220701266742. [DOI] [PubMed] [Google Scholar]
- Ahmad Z, Balsamo LM, Sachs BC, Xu B, Gaillard WD. Auditory comprehension of language in young children: Neural networks identified with fMRI. Neurology. 2003;60:1598–605. doi: 10.1212/01.wnl.0000059865.32155.86. [DOI] [PubMed] [Google Scholar]
- Allen P, Mechelli A, Stephan KE, Day F, Dalton J, Williams S, et al. Fronto-temporal interactions during overt verbal initiation and suppression. J Cogn Neurosci. 2008;20:1656–69. doi: 10.1162/jocn.2008.20107. [DOI] [PubMed] [Google Scholar]
- Alloway TP, Gathercole SE, editors. Working Memory and Neurodevelopmental Disorders. Psychology Press; New York, NY: 2006. [DOI] [PubMed] [Google Scholar]
- Balsamo LM, Xu B, Gaillard WD. Language lateralization and the role of the fusiform gyrus in semantic processing in young children. Neuroimage. 2006;31:1306–14. doi: 10.1016/j.neuroimage.2006.01.027. [DOI] [PubMed] [Google Scholar]
- Balsamo LM, Xu B, Grandin CB, Petrella JR, Braniecki SH, Elliott TK, et al. A functional magnetic resonance imaging study of left hemisphere language dominance in children. Arch Neurol. 2002;59:1168–74. doi: 10.1001/archneur.59.7.1168. [DOI] [PubMed] [Google Scholar]
- Berl MM, Vaidya CJ, Gaillard WD. Functional imaging of developmental and adaptive changes in neurocognition. Neuroimage. 2006;30:679–91. doi: 10.1016/j.neuroimage.2005.10.007. [DOI] [PubMed] [Google Scholar]
- Berninger VW, Raskind W, Richards T, Abbott R, Stock P. A multidisciplinary approach to understanding developmental dyslexia within working-memory architecture: genotypes, phenotypes, brain, and instruction. Dev Neuropsychol. 2008;33:707–44. doi: 10.1080/87565640802418662. [DOI] [PubMed] [Google Scholar]
- Binder J, Rao S, Hammeke T, Frost JA, Bandettini P, Jesmanowicz A, et al. Lateralized human brain language systems demonstrated by task subtraction functional magnetic resonance imaging. Arch Neurology. 1995;52:593–601. doi: 10.1001/archneur.1995.00540300067015. [DOI] [PubMed] [Google Scholar]
- Bitan T, Cheon J, Lu D, Burman DD, Gitelman DR, Mesulam MM, et al. Developmental changes in activation and effective connectivity in phonological processing. Neuroimage. 2007;38:564–75. doi: 10.1016/j.neuroimage.2007.07.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bookheimer S. Functional MRI of language: new approaches to understanding the cortical organization of semantic processing. Annu Rev Neurosci. 2002;25:151–88. doi: 10.1146/annurev.neuro.25.112701.142946. [DOI] [PubMed] [Google Scholar]
- Booth JR, Wood L, Lu D, Houk JC, Bitan T. The role of the basal ganglia and cerebellum in language processing. Brain Res. 2007;1133:136–44. doi: 10.1016/j.brainres.2006.11.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borowsky R, Esopenko C, Cummine J, Sarty GE. Neural representations of visual words and objects: a functional MRI study on the modularity of reading and object processing. Brain Topogr. 2007;20:89–96. doi: 10.1007/s10548-007-0034-1. [DOI] [PubMed] [Google Scholar]
- Brauer J, Friederici AD. Functional neural networks of semantic and syntactic processes in the developing brain. J Cogn Neurosci. 2007;19:1609–23. doi: 10.1162/jocn.2007.19.10.1609. [DOI] [PubMed] [Google Scholar]
- Burman DD, Bitan T, Booth JR. Sex differences in neural processing of language among children. Neuropsychologia. 2008;46:1349–62. doi: 10.1016/j.neuropsychologia.2007.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvert GA. Crossmodal processing in the human brain: insights from functional neuroimaging studies. Cereb Cortex. 2001;11:1110–23. doi: 10.1093/cercor/11.12.1110. [DOI] [PubMed] [Google Scholar]
- Cao F, Bitan T, Chou TL, Burman DD, Booth JR. Deficient orthographic and phonological representations in children with dyslexia revealed by brain activation patterns. J Child Psychol Psychiatry. 2006;47:1041–50. doi: 10.1111/j.1469-7610.2006.01684.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpentier A, Pugh KR, Westerveld M, Studholme C, Skrinjar O, Thompson JL, et al. Functional MRI of language processing: dependence on input modality and temporal lobe epilepsy. Epilepsia. 2001;42:1241–54. doi: 10.1046/j.1528-1157.2001.35500.x. [DOI] [PubMed] [Google Scholar]
- Catani M, Jones DK, ffytche DH. Perisylvian language networks of the human brain. Ann Neurol. 2005;57:8–16. doi: 10.1002/ana.20319. [DOI] [PubMed] [Google Scholar]
- Chow HM, Kaup B, Raabe M, Greenlee MW. Evidence of fronto-temporal interactions for strategic inference processes during language comprehension. Neuroimage. 2008;40:940–54. doi: 10.1016/j.neuroimage.2007.11.044. [DOI] [PubMed] [Google Scholar]
- Chung MK, Worsley KJ, Robbins S, Paus T, Taylor J, Giedd JN, et al. Deformation-based surface morphometry applied to gray matter deformation. Neuroimage. 2003;18:198–213. doi: 10.1016/s1053-8119(02)00017-4. [DOI] [PubMed] [Google Scholar]
- Church JA, Coalson RS, Lugar HM, Petersen SE, Schlaggar BL. A developmental fMRI study of reading and repetition reveals changes in phonological and visual mechanisms over age. Cereb Cortex. 2008;18:2054–65. doi: 10.1093/cercor/bhm228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen MJ. CMS: Children's Memory Scale. Psychological Corporations: Harcourt Brace and Company; San Antonio, TX: 1997. [Google Scholar]
- Dehaene-Lambertz G, Hertz-Pannier L, Dubois J, Meriaux S, Roche A, Sigman M, et al. Functional organization of perisylvian activation during presentation of sentences in preverbal infants. Proc Natl Acad Sci U S A. 2006;103:14240–5. doi: 10.1073/pnas.0606302103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denckla MB. A theory and model of executive functioning: A neuropsychological perspective. Paul H. Brooks; Baltimore, MD: 1994. [Google Scholar]
- Diamond A. Close interrelation of motor development and cognitive development and of the cerebellum and prefrontal cortex. Child Dev. 2000;71:44–56. doi: 10.1111/1467-8624.00117. [DOI] [PubMed] [Google Scholar]
- Diamond A. The early development of executive functions. In: Bialystok E, Craik F, editors. Lifespan Cognition: Mechanisms of Change. Oxford University; New York: 2006. pp. 70–95. [Google Scholar]
- Elliot C. Differential Abilities Scale. Psychological Corporations: Harcourt Brace and Company; San Antonio, TX: 1990. [Google Scholar]
- Frey S, Campbell JS, Pike GB, Petrides M. Dissociating the human language pathways with high angular resolution diffusion fiber tractography. J Neurosci. 2008;28:11435–44. doi: 10.1523/JNEUROSCI.2388-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friederici AD. Pathways to language: fiber tracts in the human brain. Trends Cogn Sci. 2009;13:175–81. doi: 10.1016/j.tics.2009.01.001. [DOI] [PubMed] [Google Scholar]
- Gabrieli JD, Poldrack RA, Desmond JE. The role of left prefrontal cortex in language and memory. Proceedings of the National Academy of Science. 1998;95:906–913. doi: 10.1073/pnas.95.3.906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaillard WD. Functional MR imaging of language, memory, and sensorimotor cortex. Neuroimaging Clin N Am. 2004;14:471–85. doi: 10.1016/j.nic.2004.04.005. [DOI] [PubMed] [Google Scholar]
- Gaillard WD, Balsamo L, Xu B, Grandin CB, Braniecki SH, Papero PH, et al. Language dominance in partial epilepsy patients identified with an fMRI reading task. Neurology. 2002a;59:256–65. doi: 10.1212/wnl.59.2.256. [DOI] [PubMed] [Google Scholar]
- Gaillard WD, Balsamo L, Xu B, McKinney C, Papero PH, Weinstein S, et al. fMRI language task panel improves determination of language dominance. Neurology. 2004;63:1403–8. doi: 10.1212/01.wnl.0000141852.65175.a7. [DOI] [PubMed] [Google Scholar]
- Gaillard WD, Balsamo LM, Ibrahim Z, Sachs BC, Xu B. fMRI identifies regional specialization of neural networks for reading in young children. Neurology. 2003a;60:94–100. doi: 10.1212/wnl.60.1.94. [DOI] [PubMed] [Google Scholar]
- Gaillard WD, McKinney CM, Balsamo L, Xu B, Sachs B, Pearl PL, et al. fMRI panel of verbal fluency, auditory, and reading comprehension identifies language dominance compared to the intracarotid amytal test. American Epilepsy Society Epilepsia. 2002b;43(suppl 7):89. [Google Scholar]
- Gaillard WD, Sachs BC, Whitnah JR, Ahmad Z, Balsamo LM, Petrella JR, et al. Developmental aspects of language processing: fMRI of verbal fluency in children and adults. Hum Brain Mapp. 2003b;18:176–85. doi: 10.1002/hbm.10091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gathercole SE, Alloway TP, Willis C, Adams AM. Working memory in children with reading disabilities. J Exp Child Psychol. 2006;93:265–81. doi: 10.1016/j.jecp.2005.08.003. [DOI] [PubMed] [Google Scholar]
- Geschwind N. Language and the brain. Scientific American. 1972;226:76–83. doi: 10.1038/scientificamerican0472-76. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, et al. Brain development during childhood and adolescence: a longitudinal MRI study. Nat Neurosci. 1999;2:861–3. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, et al. Dynamic mapping of human cortical development during childhood through early adulthood. Proc Natl Acad Sci U S A. 2004;101:8174–9. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good RH. In: Dynamic Indicators of Basic Early Literacy Skills. 6th ed. Kaminski RA, editor. Institute for the Development of Education Achievement; Eugene, OR: 2002. [Google Scholar]
- Harris A. Harris test of lateral dominance: Manual of directions for administration and interpretation. Psychological Corporation; New York: 1947. [Google Scholar]
- Hickok G, Poeppel D. Dorsal and ventral streams: a framework for understanding aspects of the functional anatomy of language. Cognition. 2004;92:67–99. doi: 10.1016/j.cognition.2003.10.011. [DOI] [PubMed] [Google Scholar]
- Holland SK, Plante E, Byars A, Strawsburg RH, Schmithorst VJ, Ball WS., Jr. Normal fMRI brain activation patterns in children performing a verb generation task. Neuroimage. 2001;14:837–43. doi: 10.1006/nimg.2001.0875. [DOI] [PubMed] [Google Scholar]
- Holland SK, Vannest J, Mecoli M, Jacola LM, Tillema JM, Karunanayaka PR, et al. Functional MRI of language lateralization during development in children. Int J Audiol. 2007;46:533–51. doi: 10.1080/14992020701448994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huizinga M, Dolan CV, van der Molen MW. Age-related change in executive function: developmental trends and a latent variable analysis. Neuropsychologia. 2006;44:2017–36. doi: 10.1016/j.neuropsychologia.2006.01.010. [DOI] [PubMed] [Google Scholar]
- Indefrey P, Hellwig F, Herzog H, Seitz RJ, Hagoort P. Neural responses to the production and comprehension of syntax in identical utterances. Brain Lang. 2004;89:312–9. doi: 10.1016/S0093-934X(03)00352-3. [DOI] [PubMed] [Google Scholar]
- Jobard G, Vigneau M, Mazoyer B, Tzourio-Mazoyer N. Impact of modality and linguistic complexity during reading and listening tasks. Neuroimage. 2007;34:784–800. doi: 10.1016/j.neuroimage.2006.06.067. [DOI] [PubMed] [Google Scholar]
- Just MA, Carpenter PA, Keller TA, Eddy WF, Thulborn KR. Brain activation modulated by sentence comprehension. Science. 1996;274:114–6. doi: 10.1126/science.274.5284.114. [DOI] [PubMed] [Google Scholar]
- Karunanayaka PR, Holland SK, Schmithorst VJ, Solodkin A, Chen EE, Szaflarski JP, et al. Age-related connectivity changes in fMRI data from children listening to stories. Neuroimage. 2007;34:349–60. doi: 10.1016/j.neuroimage.2006.08.028. [DOI] [PubMed] [Google Scholar]
- Lindenberg R, Scheef L. Supramodal language comprehension: role of the left temporal lobe for listening and reading. Neuropsychologia. 2007;45:2407–15. doi: 10.1016/j.neuropsychologia.2007.02.008. [DOI] [PubMed] [Google Scholar]
- Maisog JM, Einbinder ER, Flowers DL, Turkeltaub PE, Eden GF. A meta-analysis of functional neuroimaging studies of dyslexia. Ann N Y Acad Sci. 2008;1145:237–59. doi: 10.1196/annals.1416.024. [DOI] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage. 2003;19:1233–9. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- Mbwana J, Berl MM, Ritzl EK, Rosenberger L, Mayo J, Weinstein S, et al. Limitations to plasticity of language network reorganization in localization related epilepsy. Brain. 2009;132:347–56. doi: 10.1093/brain/awn329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michael EB, Keller TA, Carpenter PA, Just MA. fMRI investigation of sentence comprehension by eye and by ear: modality fingerprints on cognitive processes. Hum Brain Mapp. 2001;13:239–52. doi: 10.1002/hbm.1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naumer MJ, Doehrmann O, Muller NG, Muckli L, Kaiser J, Hein G. Cortical Plasticity of Audio-Visual Object Representations. Cereb Cortex. 2008 doi: 10.1093/cercor/bhn200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell HW, Parker GJ, Alexander DC, Symms MR, Boulby PA, Wheeler-Kingshott CA, et al. Hemispheric asymmetries in language-related pathways: a combined functional MRI and tractography study. Neuroimage. 2006;32:388–99. doi: 10.1016/j.neuroimage.2006.03.011. [DOI] [PubMed] [Google Scholar]
- Pugh KR, Shaywitz BA, Shaywitz SE, Constable RT, Skudlarski P, Fulbright RK, et al. Cerebral organization of component processes in reading. Brain. 1996;119(Pt 4):1221–38. doi: 10.1093/brain/119.4.1221. [DOI] [PubMed] [Google Scholar]
- Pujol J, Deus J, Losilla JM, Capdevila A. Cerebral lateralization of language in normal left-handed people studies by functional fMRI. Neurology. 1999;52:1038–1043. doi: 10.1212/wnl.52.5.1038. [DOI] [PubMed] [Google Scholar]
- Rosenberger LR, Zeck J, Berl MM, Moore EN, Ritzl EK, Shamim S, et al. Interhemispheric and intrahemispheric language reorganization in complex partial epilepsy. Neurology. 2009;72:1830–6. doi: 10.1212/WNL.0b013e3181a7114b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabsevitz DS, Medler DA, Seidenberg M, Binder JR. Modulation of the semantic system by word imageability. Neuroimage. 2005;27:188–200. doi: 10.1016/j.neuroimage.2005.04.012. [DOI] [PubMed] [Google Scholar]
- Saur D, Kreher BW, Schnell S, Kummerer D, Kellmeyer P, Vry MS, et al. Ventral and dorsal pathways for language. Proc Natl Acad Sci U S A. 2008;105:18035–40. doi: 10.1073/pnas.0805234105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlaggar BL, Brown TT, Lugar HM, Visscher KM, Miezin FM, Petersen SE. Functional neuroanatomical differences between adults and school-age children in the processing of single words. Science. 2002;296:1476–9. doi: 10.1126/science.1069464. [DOI] [PubMed] [Google Scholar]
- Scott SK, Wise RJ. The functional neuroanatomy of prelexical processing in speech perception. Cognition. 2004;92:13–45. doi: 10.1016/j.cognition.2002.12.002. [DOI] [PubMed] [Google Scholar]
- Semel E, Wiig E, Secord W. CELF 4: Clinical Evaluation of Language Fundamentals. Fourth Edition Psychological Corporations Harcourt Assessment, Inc; San Antonio, TX: 2003. [Google Scholar]
- Shankweiler D, Mencl WE, Braze D, Tabor W, Pugh KR, Fulbright RK. Reading differences and brain: cortical integration of speech and print in sentence processing varies with reader skill. Dev Neuropsychol. 2008;33:745–75. doi: 10.1080/87565640802418688. [DOI] [PubMed] [Google Scholar]
- Sheslow D, Adams W. Wide range assessment of memory and learning. Jastak Associates, Inc; Wilmington, DE: 1990. [Google Scholar]
- Snijders TM, Vosse T, Kempen G, Van Berkum JJ, Petersson KM, Hagoort P. Retrieval and Unification of Syntactic Structure in Sentence Comprehension: an fMRI Study Using Word-Category Ambiguity. Cereb Cortex. 2008 doi: 10.1093/cercor/bhn187. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Holmes CJ, Jernigan TL, Toga AW. In vivo evidence for post-adolescent brain maturation in frontal and striatal regions. Nat Neurosci. 1999;2:859–61. doi: 10.1038/13154. [DOI] [PubMed] [Google Scholar]
- Telkemeyer S, Rossi S, Koch SP, Nierhaus T, Steinbrink J, Poeppel D, et al. Sensitivity of newborn auditory cortex to the temporal structure of sounds. J Neurosci. 2009;29:14726–33. doi: 10.1523/JNEUROSCI.1246-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turkeltaub PE, Gareau L, Flowers DL, Zeffiro TA, Eden GF. Development of neural mechanisms for reading. Nat Neurosci. 2003;6:767–73. doi: 10.1038/nn1065. [DOI] [PubMed] [Google Scholar]
- Vigneau M, Beaucousin V, Herve PY, Duffau H, Crivello F, Houde O, et al. Meta-analyzing left hemisphere language areas: phonology, semantics, and sentence processing. Neuroimage. 2006;30:1414–32. doi: 10.1016/j.neuroimage.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Wagner AD, Pare-Blagoev EJ, Clark J, Poldrack RA. Recovering meaning: Left prefrontal cortex guides controlled semantic retrieval. Neuron. 2001;31:329–338. doi: 10.1016/s0896-6273(01)00359-2. [DOI] [PubMed] [Google Scholar]
- Wagner AD, Schacter DL, Rotte M, Koutstaal W, Maril A, Dale AM, et al. Building memories: remembering and forgetting of verbal experiences as predicted by brain activity. Science. 1998;281:1188–91. doi: 10.1126/science.281.5380.1188. [DOI] [PubMed] [Google Scholar]
- Wagner RK, Torgensen JK, Rashotte CA. CTOPP: Comprehensive Test of Phonological Processing. Pro-Ed, Inc; Austin, TX: 1999. [Google Scholar]
- Wang Y, Sereno JA, Jongman A, Hirsch J. fMRI evidence for cortical modification during learning of Mandarin lexical tone. J Cogn Neurosci. 2003;15:1019–27. doi: 10.1162/089892903770007407. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Manual for the Wechsler Abbreviated Scale of Intelligence. The Psychological Corporation; San Antonio, TX: 1999. [Google Scholar]
- Wernicke C. The aphasic symptom-complex: A psychological study on an anatomical basis (Translated from German) Cohn and Weigert; Breslau, Germany: 1874. [Google Scholar]
- Wiederholt J, Bryant B. GORT4: Gray Oral Reading Test. Fourth Edition Pro-Ed, Inc; Austin, TX: 2001. [Google Scholar]
- Wiederholt JL, Blalock G. Gray silent reading tests. Pro-Ed, Inc; Austin, TX: 2000. [Google Scholar]
- Wiig E, Secord W. CELF P2: Clinical Evaluation of Language Fundamentals, Preschool. Second Edition Psychological Corporations Harcourt Assessment, Inc; San Antonio, TX: 2004. [Google Scholar]
- Wilke M, Lidzba K. LI-tool: a new toolbox to assess lateralization in functional MR-data. J Neurosci Methods. 2007;163:128–36. doi: 10.1016/j.jneumeth.2007.01.026. [DOI] [PubMed] [Google Scholar]
- Wilke M, Schmithorst VJ. A combined bootstrap/histogram analysis approach for computing a lateralization index from neuroimaging data. Neuroimage. 2006;33:522–30. doi: 10.1016/j.neuroimage.2006.07.010. [DOI] [PubMed] [Google Scholar]
- Yakovlev PI, Lecours AR. The myelogenic cycles of regional maturation of the brain. In: Minkowski, editor. Regional development in early life. Blackwell; Oxford: 1967. pp. 3–23. [Google Scholar]
- Yerys BE, Jankowski KF, Shook D, Rosenberger LR, Barnes KA, Berl MM, et al. The fMRI success rate of children and adolescents: Typical development, epilepsy, attention deficit/hyperactivity disorder, and autism spectrum disorders. Hum Brain Mapp. 2009 doi: 10.1002/hbm.20767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahn R, Moll J, Krueger F, Huey ED, Garrido G, Grafman J. Social concepts are represented in the superior anterior temporal cortex. Proc Natl Acad Sci U S A. 2007;104:6430–5. doi: 10.1073/pnas.0607061104. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.