Abstract
Background
Palliative oxygen therapy is widely used for dyspnea in individuals with life-limiting illness ineligible for long-term oxygen therapy.
Methods
This international double-blind randomized controlled trial evaluatedeffectiveness of oxygen vs. medical (room) air for relieving breathlessness in patients with life-limiting illness, refractory dyspnea, and PaO2>55 mm Hg. Participants were recruited from outpatient clinics at 9 sites (Australia, United States, England). Participants received oxygen or medical air via concentrator through nasal cannulae at 2 liters/minute for 7 days. The primary outcome measure was breathlessness (0-10 numerical rating scale [NRS]), measured twice daily.
Findings
Participants (N=239) were: mean age, 73 (standard deviation [SD] 10); 62% male; mean PaO2, 77 mm Hg (SD 12); mean morning dyspnea, 4.5 on NRS (SD 2.2); chronic obstructive pulmonary disease, 64%; cancer, 16%. Oxygen was not significantly superior to medical air for relief of breathlessness. Over the 7-day period, after provision of medical gas, mean morning and evening dyspnea decreased by -0.8 (95% confidence interval [CI]: -1.1, -0.5) and -0.4 (CI: -0.7, 0.1), respectively (p<0.001), regardless of intervention. Baseline dyspnea predicted improvement with medical gas; participants with moderate (4-6 NRS) and severe (7-10 NRS) baseline dyspnea had average decreases in morning dyspnea of -0.7 (CI: -1.1, -0.4) and -2.4 (CI: -3.0, -1.8), respectively.
Interpretation
There is no additional symptomatic benefit of oxygen over room air delivered by nasal cannulae for relieving refractory dyspnea related to life-limiting illness in patients with PaO2>55 mm Hg. Dyspnea intensity decreased in both study arms, temporally related to provision of medical gas.
Keywords: Dyspnea, dyspnoea (MeSH), Palliative care (MeSH), Terminal care (MeSH), Oxygen, Breathlessness
Background
Dyspnea has been defined as “a subjective experience of breathing discomfort that consists of qualitatively distinct sensations varying in intensity. The experience derives from interactions among multiple physiological, psychological, social, and environmental factors.”1 Prevalence of severe dyspnea among terminally ill patients has been reported as 65%, 70%, and 90% for heart failure, lung cancer, and chronic obstructive pulmonary disease (COPD) patients, respectively.2 Dyspnea often presents as a chronic condition that intensifies during the dying process;3 it can erode quality of life (QOL), psychological well-being, and social functioning.4
The exact nature and cause, and therefore appropriate treatment, of dyspnea remain elusive. Objective measures, such as desaturation with exercise, may point toward underlying pathology, but do not reliably indicate subjective experience. Current pharmacologic treatments include opioids, psychotropic drugs, inhaled frusemide, Heliox 28, and oxygen; opioids remain the mainstay of treatment.5, 6 Palliative interventions seek primarily to alleviate the sensation of breathlessness; they are generally applied in palliative care irrespective of underlying pathology and respiratory functioning.7
Long-term oxygen therapy (LTOT) is indicated for COPD patients with severe hypoxemia (PaO2≤55 mm Hg at rest); treatment improves survival, dyspnea, and functional status.8-10 Palliative oxygen is frequently prescribed to manage dyspnea in people with advanced life-limiting illness, irrespective of PaO2, and is generally considered standard of care.11, 12 Over 70% of physicians caring for dyspneic palliative care patients report prescribing palliative oxygen, usually for refractory symptoms (65%) or at patient request (30%).13 There is not, however, clear evidence demonstrating symptomatic benefit of palliative oxygen,14-16 though the intervention entails cost and logistical burden. Across the world, hospices commonly prescribe oxygen based on symptomatic, rather than pulse oximetry, criteria. In Canada, compassionate-use oxygen not meeting LTOT criteria represents 30% of the oxygen therapy budget.9 Lack of evidence to support palliative oxygen use and lack of available clinical practice guidelines have led to inconsistent access and variable utilization.17
This study sought to determine the symptomatic effectiveness of palliative oxygen for patients with life-limiting illness, refractory breathlessness, and PaO2>55 mm Hg. The comparator was medical (room) air provided via a modified concentrator (altered according to a standardized protocol, see below); the null hypothesis was that oxygen therapy is not superior to medical air in this setting.
Methods
This was an international multi-site, double-blind, randomized controlled trial conducted from April 2006 to March 2008. The study protocol was approved by the Duke University Health System Institutional Review Board (IRBs), and local Research & Ethics Committees or IRBs of all participating sites.
Participants and Setting
Participants were recruited from outpatient pulmonary, palliative care, oncology, and primary care clinics at 5 sites in Australia, 2 in United States, and 2 in England. Eligible participants were: >18 years of age, with PaO2>55 mm Hg, experiencing refractory dyspnea related to life-limiting illness (determined by referring physicians), maximally treated for underlying disease, reporting dyspnea at rest or with minimal exertion of ≥3 on the Medical Research Council (MRC) categorical dyspnea scale,18 on stable medications for 1 week prior to participation, and judged by their physicians to have ≥1 month prognosis. Exclusion criteria included: meeting international LTOT eligibility guidelines, history of hypercarbic respiratory failure with oxygen, anemia (hemoglobin <10.0 g/dL), hypercarbia (PaCO2 >50 mm Hg), smoking, cognitive impairment (Folstein Mini-mental Status Exam19 <24/30), and a respiratory or cardiac event in the prior 7 days. All participants provided written informed consent.
Intervention and Procedures
Consenting participants who met screening criteria underwent arterial blood gas assessment either in the outpatient clinic or home using a standardized protocol. If PaO2 >55 mm Hg and all eligibility criteria met participants were randomized 1: 1 to oxygen or medical air, stratified by baseline PaO2 (≤70, 71-80, 81-90, 91-100 mm Hg). A central system available through the pharmacy service at Repatriation General Hospital (Adelaide, Australia) randomized participants in balanced blocks of 4 patients per stratum, based on Fisher and Yates Statistical Tables.20
The intervention lasted 7 days. This duration was selected because, in a preparatory survey, palliative care physicians indicated that a definitive study of palliative oxygen, one that would provide compelling evidence about dyspnea and QOL, would require 3-7 days.13 Although dyspnea caused by hypoxemia or hypoxygenation may be relieved by oxygen within a short period of a few minutes or hours, we chose the conservative estimate of practicing clinicians because these physicians represent the practical audience for the study's results.
A medical gas concentrator was delivered to the participant's home in the afternoon on Day 0 and retrieved in the afternoon on Day 7. Using a standardized protocol, the medical gas company serving each site modified half of the concentrators to dispense room air without setting off the internal alarm that sounds when oxygen levels are low. Concentrators appeared identical. Patients, delivery persons, investigators, and nurses were blinded to assignments. Medical gas was administered continuously at 2 liters/minute through nasal cannulae. Participants were instructed to use the concentrator at least 15 hours daily, during hours of their choosing.
Measurements
The study's primary outcome was “breathlessness right now,” recorded twice daily – within 30 minutes of awakening (“morning”) and bedtime (“evening”) – in a patient diary using a 0-10 numerical rating scale (NRS; anchors, 0=“not breathless at all”, 10=”breathlessness as bad as you can imagine”), a valid instrument for this population.21 A 1-point reduction in self-reported dyspnea is generally considered clinically relevant change;22 therefore, a 1-point reduction was used to define “response” for all NRS measures in the study.
Diaries also captured secondary outcomes: average dyspnea in the past 24 hours (0-10 NRS), worst breathlessness in the past 24 hours (0-10 NRS), relief of dyspnea over the prior 24 hours (0-10 NRS), and ordered categorical scales for functional impact, sleep disturbance, drowsiness, anxiety, nasal irritation, and nose bleeds. QOL was assessed daily using the McGill Quality of Life Questionnaire (MQOLQ);23 comprised of 17 items, the MQOLQ includes a single-item measure of global QOL (0-10 NRS). Functional changes were assessed using the Modified Medical Research Council of Great Britain (MRC) 4-point categorical dyspnea scale 24 and Dyspnea Exertion Scale (DES, categories provided in Table 1).25 Secondary measures were asked once daily, usually in the evening except when more relevant to morning (e.g., sleep).
Table 1. Characteristics of the study population.
| Oxygen (N,%) | Medical Air (N,%) | Overall (N,%) | |||||
|---|---|---|---|---|---|---|---|
| N | 120 | 119 | 239 | ||||
| Male | 76 | 63 | 71 | 60 | 147 | 62 | |
| Etiology | |||||||
| COPD | 71 | 59 | 81 | 68 | 152 | 63 | |
| Restrictive lung disease | 5 | 4 | 9 | 8 | 14 | 6 | |
| Bronchiectasis | 4 | 3 | 3 | 2 | 7 | 3 | |
| Primary pulmonary hypertension | 0 | 0 | 3 | 2 | 3 | 1 | |
| Primary lung cancer | 18 | 15 | 15 | 13 | 33 | 14 | |
| Known secondary lung cancer | 2 | 2 | 3 | 3 | 5 | 2 | |
| Pleural effusion | 2 | 2 | 0 | 0 | 2 | 1 | |
| End stage cardiomyopathy | 2 | 2 | 5 | 4 | 7 | 3 | |
| Other | 16 | 13 | 0 | 0 | 16 | 7 | |
| Age | Mean (SD) | 73 (11) | 74 (10) | 73 (10) | |||
| Baseline morning dyspnea (Day -1; 0-10 NRS) | Mean (SD) | 4.5 (2.2) | 4. 6 (2.4) | 4.5 (2.3) | |||
| Baseline evening dyspnea (Day -1; 0-10 NRS) | Mean (SD) | 4.7 (2.2) | 4.7 (2.3) | 4.7 (2.2) | |||
| Baseline global QOL (Day 0; 0-10 NRS) | Mean (SD) | 6.2 (2.2) | 5.9 (2.0) | 6.0 (2.1) | |||
| MRC dyspnea functional scale | |||||||
| Breathless when walking at own pace* | 0 | 0 | 1 | 1 | 1 | 1 | |
| Breathless when walking 100 yards | 54 | 45 | 59 | 50 | 113 | 47 | |
| Breathless when dressing or undressing | 66 | 55 | 59 | 49 | 125 | 52 | |
| Dyspnea Exertion Scale | |||||||
| 1: Able to walk at own pace on the level without getting out of breath | 11 | 9 | 9 | 8 | 20 | 9 | |
| 2: Becomes breathless when walking around the house or on the hospital ward on the level at own pace | 54 | 46 | 50 | 44 | 104 | 45 | |
| 3: Becomes breathless if moves around in bed or get out of bed | 26 | 22 | 29 | 26 | 55 | 24 | |
| 4: Becomes breathless when talking | 24 | 20 | 23 | 20 | 47 | 20 | |
| 5: Is breathless at rest | 4 | 3 | 2 | 2 | 6 | 2 | |
| ECOG Performance Status | |||||||
| 0: Fully active, able to carry on all pre-disease performance without restriction | 0 | 0 | 1 | 1 | 1 | 0 | |
| 1: Restricted in physically strenuous activity but ambulatory and able to carry out work of a light of sedentary nature, e.g., light house work, office work | 40 | 34 | 29 | 26 | 69 | 30 | |
| 2: Ambulatory and capable of all self-care but unable to carry out any work activities. Up and about more than 50% of waking hours | 48 | 40 | 61 | 54 | 109 | 47 | |
| 3: Capable of only limited self-care, confined to bed or chair more than 50% of waking hours | 31 | 26 | 22 | 19 | 53 | 23 | |
| Regular oxygen previously prescribed | 56 | 48 | 59 | 50 | 115 | 48 | |
| PaO2 | Mean (SD) | 77 (12) | 76 (12) | 77 (12) | |||
| Range | 56-122 | 58-132 | 56-132 | ||||
| PaCO2 | Mean (SD) | 39 (4) | 38 (5) | 39 (5) | |||
| Range | 28-51 | 27-51 | 27-51 | ||||
| Strata for randomization: | |||||||
| PaO2<=70.5 | 40 | 33 | 44 | 37 | 84 | 35 | |
| 70.6<=PaO2<=80.5 | 37 | 31 | 38 | 32 | 75 | 31 | |
| 80.6<=PaO2<=90.5 | 27 | 23 | 22 | 18 | 49 | 21 | |
| PaO2>=90.6 | 16 | 13 | 15 | 13 | 31 | 13 | |
| Total hours of concentrator use** | Mean(SD) | 93 (36) | 98 (44) | 96 (40) | |||
This individual met the MRC eligibility criteria during enrollment.
NOTE: 15 hours/day, per protocol = 105 total hours
Diaries were completed beginning two days prior to intervention (Day -2). Research personnel assessed the full MQOLQ and performance status (Eastern Cooperative Oncology Group [ECOG] Performance Status Scale26) on Days -2, 0, and 6. At the end of the study, respondents were asked to rate their overall experience with the intervention and to state if they wished to continue with oxygen therapy (via concentrator).
Data analysis
Primary analyses were conducted on an intent-to-treat basis using SAS 9.1 (Cary, NC, USA). Descriptive statistics were used to characterize populations. Internal consistency of each subscale of the MQOLQ was confirmed with Cronbach's alpha prior to proceeding with analyses.
Efficacy
Repeated measures models were used to estimate the effect of time and intervention on all endpoints. Mixed Model Repeated Measures analysis (SAS PROC MIXED) with an unstructured covariance matrix was used to estimate the effect of time on mean dyspnea and QOL score by intervention. Separate repeated measures logistic regression models were used to estimate change over time by intervention in (a) participants with high MRC scores, and (b) participants reporting sleep disturbance due to breathlessness. These models were created using generalized estimating equations (GEE, SAS PROC GENMOD), assuming an unstructured covariance matrix and using categorical variables of: time (Days -1 to 6), intervention (oxygen vs. air), and interaction (time × treatment intervention). The interaction term was included to assess the consistency of treatment effect over time. Intention-to-treat principles were followed; all models included participants who completed the baseline assessment (N=239) regardless of whether they received intervention. Missing assessments were minimal and assumed to be missing at random.
Predictors of response
Proportions of responders were calculated, with response defined as a ≥1-point NRS decrease from Day -1 to Day 6 (i.e., participants still indicating improvement at end of intervention). This post hoc analysis included only participants who completed both baseline and Day 6 assessments.
To identify variables that best predicted response, a series of logistic regression models estimated the effect of each predictor on response and the difference in effect between treatment arms. Each model included treatment arm, one predictor, and interaction. Potential predictors were baseline dyspnea (low [0-3], moderate [4-6], severe [7-10]), age, gender, COPD (yes/no), PaO2 at enrollment, rapid decline in breathlessness preceding enrollment (declining MRC scores over 4 weeks), ECOG at Day 0, opioid use, previous oxygen use, and study site. Predictors that indicated a potential effect on response (Type III Wald chi-square test with p≤0.2) were included in a full interaction model. Predictive variables in the interaction model were identified through stepwise selection. Morning and evening changes in breathlessness were modeled separately.
Sample size calculation
The sample size estimate of 240 participants was based on the primary outcome variable, prior experience in a dyspnea trial evaluating morphine vs. placebo,7 and use of a student t test to compare interventions at Day 6. Assumptions were: 20% attrition rate; NRS variance of 6; 1-point NRS change defining clinical relevance. A sample size of 240 participants would provide 80% power to detect a 1-point difference with α=0.05. Actual NRS variance and attrition were less than expected. Repeated measures analyses were used rather than the student t test.
Role of funding source
None of the study sponsors had a role in the conduct of this study or its reporting.
Results
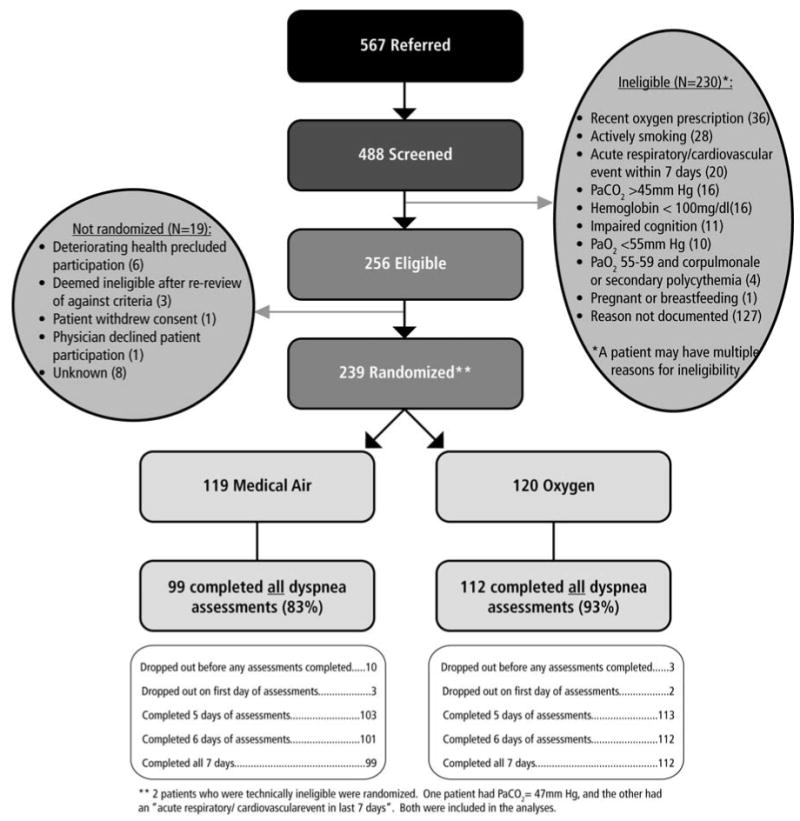
Figure 1 presents participant flow; Table 1 provides participant characteristics (n=239). Thirteen participants (5%; 10 air, 3 oxygen) dropped out before the study commenced and completed no assessments; 15 (6%; 10 air, 5 oxygen) dropped out before completing the final (Day 6) assessment.
Figure 1. CONSORT diagram showing flow of participants through the study.
Primary analysis: Relief of dyspnea
The primary outcome was the sensation of breathlessness, measured twice daily (0-10 NRS). No significant difference was found in the effect of the two gases on this measure (Figure 2). Dyspnea scores were not significantly lower for oxygen at any time over the study period. For morning dyspnea, 62 (52%) and 48 (40%) of patients responded to the oxygen and medical air interventions, respectively. For evening dyspnea, response rates were 42% for both interventions.
Figure 2. Impact of medical gas intervention on dyspnea.
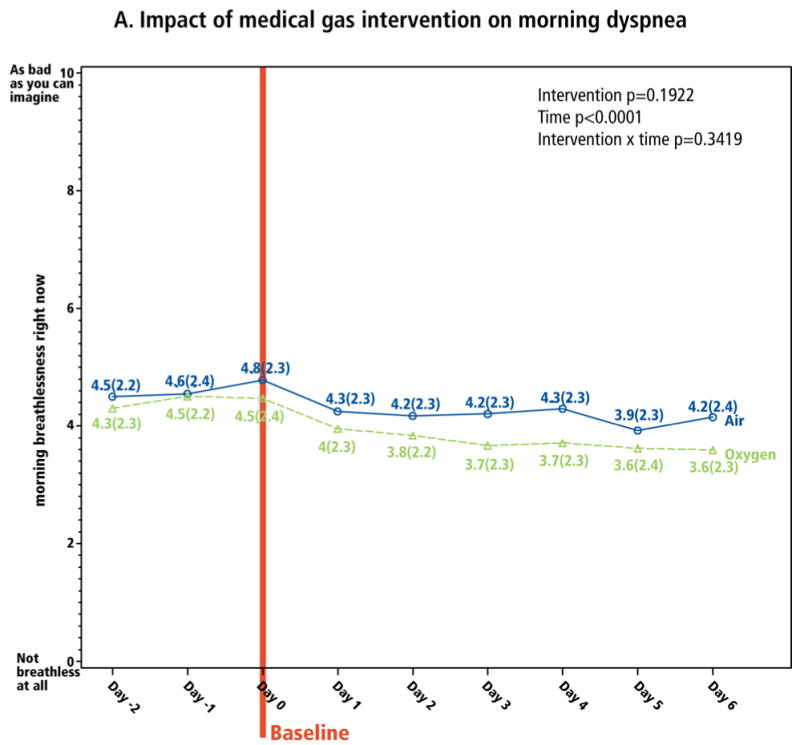
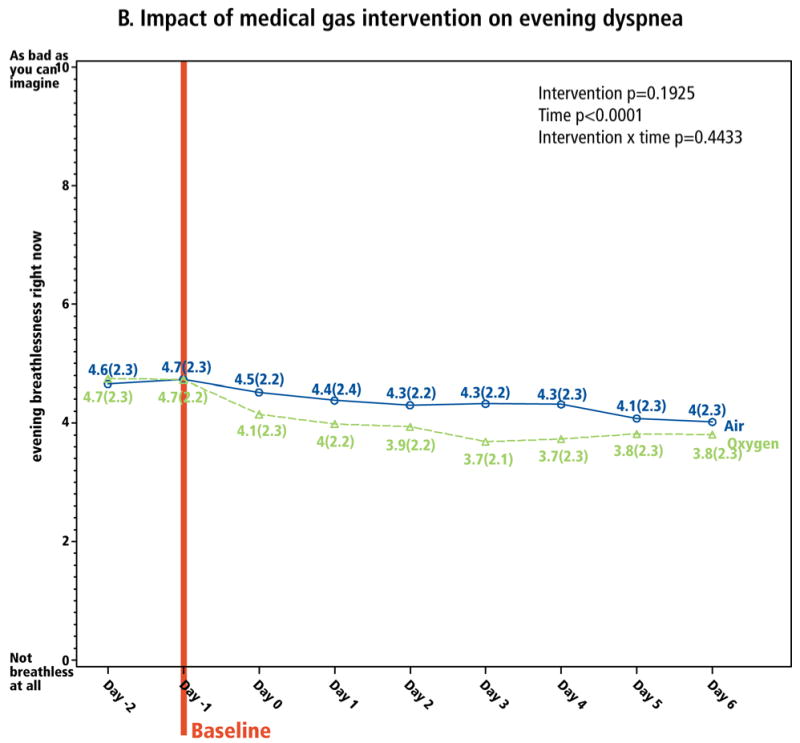
Dyspnea was measured on a 0-10 NRS, with which the patient reported “breathlessness right now.” Panel A is morning dyspnea, and panel B is evening dyspnea. The baseline assessment was the last assessment completed before initiation of the intervention on Day 0.
| Day -2 | Day -1 | Day 0 | Day 1 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| ARM | Total per arm | N | Mean (95% CI) |
N | Mean (95% CI) |
N | Mean (95% CI) |
N | Mean (95% CI) |
| Oxygen | 117 | 117 | 4.3 (3.9,4.7) |
117 | 4.5 (4.1,4.9) |
117 | 4.5 (4,4.9) |
115 | 4 (3.5,4.4) |
| Air | 110 | 108 | 4.5 (4.1,4.9) |
108 | 4.6 (4.1,5) |
109 | 4.8 (4.4,5.2) |
105 | 4.3 (3.8,4.7) |
| Day 2 | Day 3 | Day 4 | Day 5 | Day 6 | |||||
|---|---|---|---|---|---|---|---|---|---|
| N | Mean (95% CI) |
N | Mean (95% CI) |
N | Mean (95% CI) |
N | Mean (95% CI) |
N | Mean (95% CI) |
| 114 | 3.8 (3.4,4.3) |
113 | 3.7 (3.2,4.1) |
113 | 3.7 (3.3,4.1) |
112 | 3.6 (3.2,4.1) |
112 | 3.6 (3.2,4) |
| 106 | 4.2 (3.7,4.6) |
103 | 4.2 (3.8,4.7) |
103 | 4.3 (3.8,4.8) |
101 | 3.9 (3.5,4.4) |
101 | 4.2 (3.7,4.6) |
| Day -2 | Day -1 | Day 0 | Day 1 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| ARM | Total per Arm | N | Mean (95% CI) |
N | Mean (95% CI) |
N | Mean (95% CI) |
N | Mean (95% CI) |
| Oxygen | 117 | 117 | 4.7 (4.3,5.1) |
117 | 4.7 (4.3,5.1) |
116 | 4.1 (3.7,4.5) |
113 | 4.0 (3.5,4.4) |
| Air | 110 | 108 | 4.6 (4.2,5.1) |
108 | 4.7 (4.3,5.1) |
106 | 4.5 (4.1,4.9) |
105 | 4.4 (3.9,4.8) |
| Day 2 | Day 3 | Day 4 | Day 5 | Day 6 | |||||
|---|---|---|---|---|---|---|---|---|---|
| N | Mean (95% CI) |
N | Mean (95% CI) |
N | Mean (95% CI) |
N | Mean (95% CI) |
N | Mean (95% CI) |
| 113 | 3.9 (3.5,4.3) |
111 | 3.7 (3.3,4.1) |
111 | 3.7 (3.3,4.1) |
110 | 3.8 (3.4,4.2) |
112 | 3.8 (3.4,4.2) |
| 103 | 4.3 (3.8,4.7) |
101 | 4.3 (3.9,4.7) |
101 | 4.3 (3.8,4.8) |
101 | 4.1 (3.6,4.5) |
99 | 4 (3.5,4.5) |
Secondary analyses
Longitudinal analyses explored the interventions' clinical impact. Over the intervention period, there was significant improvement from both gases in both morning and evening dyspnea (time p<0.0001, both models; Figure 2). From baseline to Day 6, mean overall morning and evening dyspnea decreased by -0.8 (CI: -1.1, -0.5) and -0.4 (CI: -0.7,-0.1), respectively (p<0.001), reflecting 18% and 9% relative improvement (calculated as mean decrease in dyspnea ÷ mean baseline dyspnea, i.e., 0.8/4.5 and 0.4/4.7, respectively; Table 2). Oxygen appeared to have greater impact on relative change in morning dyspnea, whereas medical air had greater impact on relative change in evening dyspnea (Table 2). Morning dyspnea dropped most substantially between Day 0 and Day 1 (Figure 2A) and evening dyspnea dropped between Day -1 and Day 0 (Figure 2B), both less than a day after the concentrator arrived. Of the 177 (74%) of patients whose evening breathlessness decreased by ≥1 point, 97 (55%) improved within the first 24 hours, and 156 (88%) within the first 72 hours, of the intervention. Relief of dyspnea in the prior 24 hours, measured on a 0-10 NRS based on the Brief Pain Inventory27, reflected similar results (Figure 3).
Table 2. Absolute and relative changes in dyspnea and quality of life (QOL) over the 7 day study period.
Relative change is the absolute change in dyspnea/QOL during the study period divided by the baseline mean dyspnea/QOL score.
| Oxygen | Medical Air | Overall | |
|---|---|---|---|
| Change in morning dyspnea (Baseline to Day 6) | |||
| Absolute change (95% CI) | -0.9 (-1.3,0.5) | -0.7 (-1.2, 0.2) | -0.8 (-1.1, -0.5) |
| Relative change | -20% | -15% | -18% |
| Change in evening dyspnea (Baseline to Day 6) | |||
| Absolute change (95% CI) | -0.3 (-0.7, 0.1) | -0.5 (-0.9, -0.1) | -0.4 (-0.7, -0.1) |
| Relative change | -7% | -11% | -9% |
| Change in global QOL (Baseline to Day 6) | |||
| Absolute change (95% CI) | 0.7 (0.4, 1.0) | 0.7 (0.4, 1.0) | 0.7 (0.5, 0.9) |
| Relative change | 11% | 12% | 12% |
Figure 3. Relief of dyspnea over the prior 24 hours.
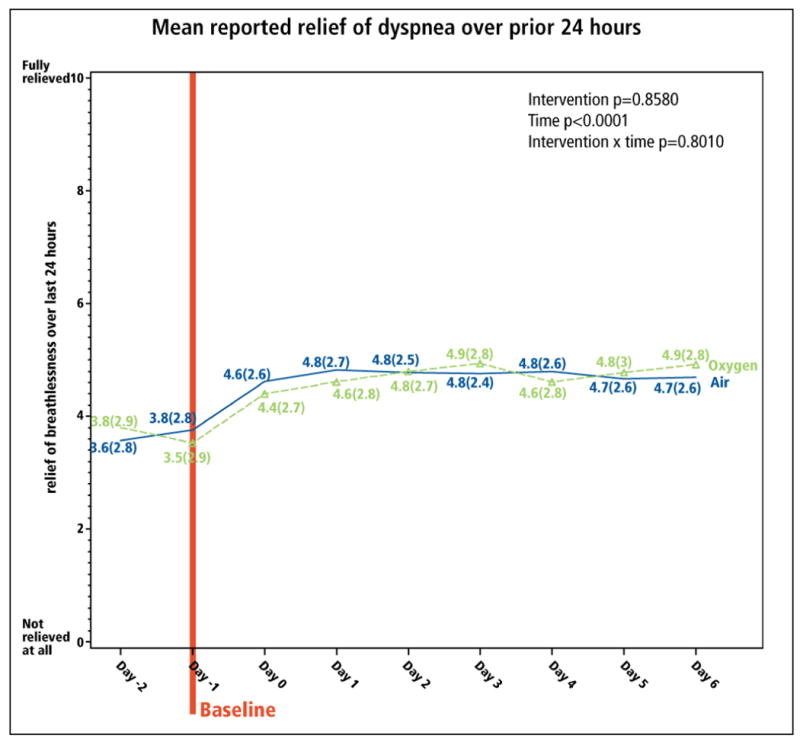
Patients reported their “relief of breathlessness over the prior 24 hours” using a 0-10 NRS. Baseline is Day -1 since the assessment reflected the experience of dyspnea over the previous day.
| Day -2 | Day -1 | Day 0 | Day 1 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| ARM | Total per Arm | N | Mean (95% CI) |
N | Mean (95% CI) |
N | Mean (95% CI) |
N | Mean (95% CI) |
| Oxygen | 117 | 115 | 3.8 (3.3,4.3) |
116 | 3.5 (3,4.1) |
115 | 4.4 (3.9,4.9) |
111 | 4.6 (4.1,5.1) |
| Air | 110 | 108 | 3.6 (3,4.1) |
107 | 3.8 (3.2,4.3) |
106 | 4.6 (4.1,5.1) |
104 | 4.8 (4.3,5.4) |
| Day 2 | Day 3 | Day 4 | Day 5 | Day 6 | |||||
|---|---|---|---|---|---|---|---|---|---|
| N | Mean (95% CI) |
N | Mean (95% CI) |
N | Mean (95% CI) |
N | Mean (95% CI) |
N | Mean (95% CI) |
| 113 | 4.8 (4.3,5.3) |
111 | 4.9 (4.4,5.5) |
110 | 4.6 (4.1,5.1) |
108 | 4.8 (4.2,5.4) |
112 | 4.9 (4.4,5.4) |
| 102 | 4.8 (4.3,5.3) |
103 | 4.8 (4.3,5.2) |
102 | 4.8 (4.3,5.3) |
101 | 4.7 (4.1,5.2) |
101 | 4.7 (4.2,5.2) |
Paralleling dyspnea change, QOL change did not differ between groups (Figure 4). Results from MQOLQ individual items and sub-scales were similar. Overall, the absolute increase in global QOL scores was 0.7 (CI: 0.5, 0.9) (Table 2); 87% of QOL improvement occurred within the first 3 days.
Figure 4. Impact of the medical gas intervention on quality of life (QOL).
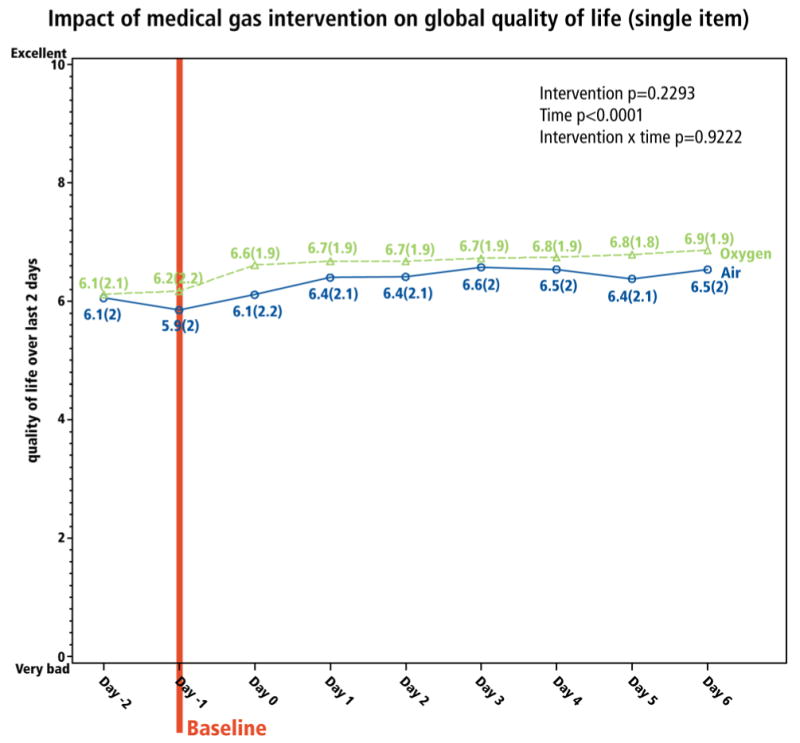
Global QOL was reported daily on a single-item 0-10 NRS patterned after the McGill QOL Questionnaire. The baseline reflects the timing of the survey in relation to initiation of medical gas.
| Day -2 | Day -1 | Day 0 | Day 1 | Day 2 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| ARM | Total per Arm | N | Mean (95% CI) |
N | Mean (95% CI) |
N | Mean (95% CI) |
N | Mean (95% CI) |
N | Mean (95% CI) |
| Oxygen | 117 | 117 | 6.1 (5.7,6.5) |
116 | 6.2 (5.8,6.6) |
106 | 6.6 (6.3,7) |
112 | 6.7 (6.3,7) |
111 | 6.7 (6.3,7) |
| Air | 110 | 108 | 6.1 (5.7,6.4) |
108 | 5.9 (5.5,6.3) |
100 | 6.1 (5.7,6.5) |
105 | 6.4 (6,6.8) |
102 | 6.4 (6,6.8) |
| Day 3 | Day 4 | Day 5 | Day 6 | ||||
|---|---|---|---|---|---|---|---|
| N | Mean (95% CI) |
N | Mean (95% CI) |
N | Mean (95% CI) |
N | Mean (95% CI) |
| 110 | 6.7 (6.4,7.1) |
110 | 6.8 (6.4,7.1) |
109 | 6.8 (6.4,7.1) |
112 | 6.9 (6.5,7.2) |
| 101 | 6.6 (6.2,7) |
102 | 6.5 (6.2,6.9) |
100 | 6.4 (6,6.8) |
101 | 6.5 (6.2,6.9) |
All other patient-reported outcomes reflected the dyspnea (and QOL) trends. The proportion of patients reporting the worst level of functioning on the MRC dyspnea scale (MRC=4; “breathless when undressing”), and sleep disturbed by breathlessness, reduced over the 7-day study, without differential impact by intervention (Figures 5 and 6).
Figure 5. Impact of interventions on functional performance.
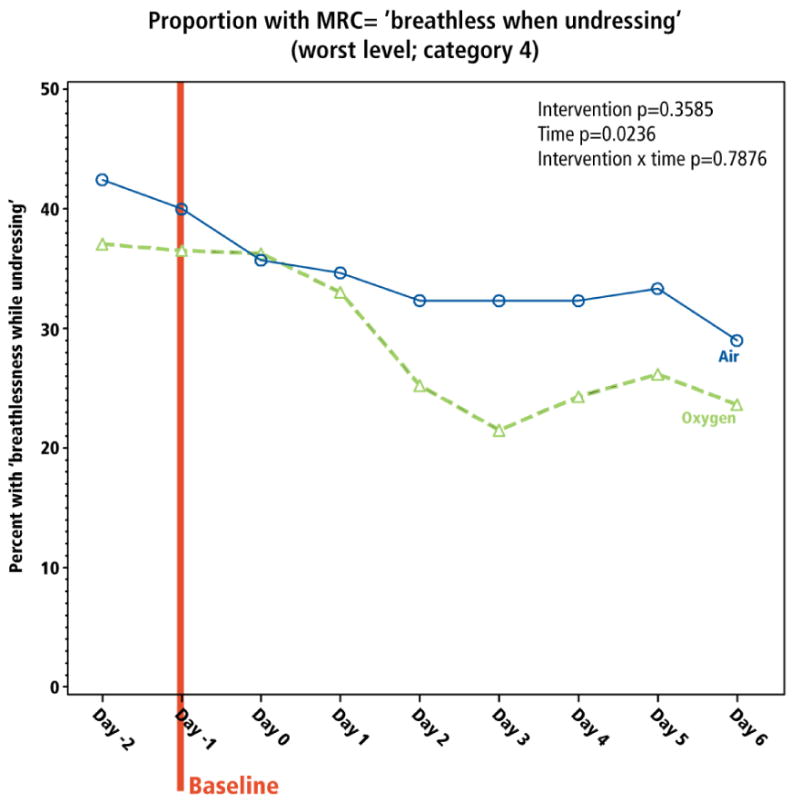
The proportion of participants reporting the worse level of function on the MRC scale (level 4; “breathless while undressing”) is presented. The baseline is Day -1 since the measure is reported in the evening.
| Day -2 | Day -1 | Day 0 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ARM | Total per Arm | N | # ‘yes’ | % ‘yes’ (95% CI) |
N | # ‘yes’ | % ‘yes’ (95% CI) |
N | # ‘yes’ | % ‘yes (95% CI) |
| Oxygen | 117 | 116 | 43 | 37.1% (28.3%,45.9%) |
115 | 42 | 36.5% (27.7%,45.3%) |
102 | 37 | 36.3% (26.9%,45.6%) |
| Air | 110 | 106 | 45 | 42.5% (33%,51.9%) |
105 | 42 | 40% (30.6%,49.4%) |
98 | 35 | 35.7% (26.2%,45.2%) |
| Day 1 | Day 2 | Day 3 | ||||||
|---|---|---|---|---|---|---|---|---|
| N | # ‘yes’ | % ‘yes’ (95% CI) |
N | # ‘yes’ | % ‘yes’ (95% CI) |
N | # ‘yes’ | % ‘yes’ (95% CI) |
| 112 | 37 | 33% (24.3%,41.7%) |
107 | 27 | 25.2% (17%,33.5%) |
107 | 23 | 21.5% (13.7%,29.3%) |
| 101 | 35 | 34.7% (25.4%,43.9%) |
102 | 33 | 32.4% (23.3%,41.4%) |
99 | 32 | 32.3% (23.1%,41.5%) |
| Day 4 | Day 5 | Day 6 | ||||||
|---|---|---|---|---|---|---|---|---|
| N | # ‘yes’ | % ‘yes’ (95% CI) |
N | # ‘yes’ | % ‘yes’ (95% CI) |
N | # ‘yes’ | % ‘yes’ (95% CI) |
| 107 | 26 | 24.3% (16.2%,32.4%) |
107 | 28 | 26.2% (17.8%,34.5%) |
110 | 26 | 23.6% (15.7%,31.6%) |
| 99 | 32 | 32.3% (23.1%,41.5%) |
99 | 33 | 33.3% (24%,42.6%) |
100 | 29 | 29% (20.1%,37.9%) |
Figure 6. Impact of interventions on sleep.
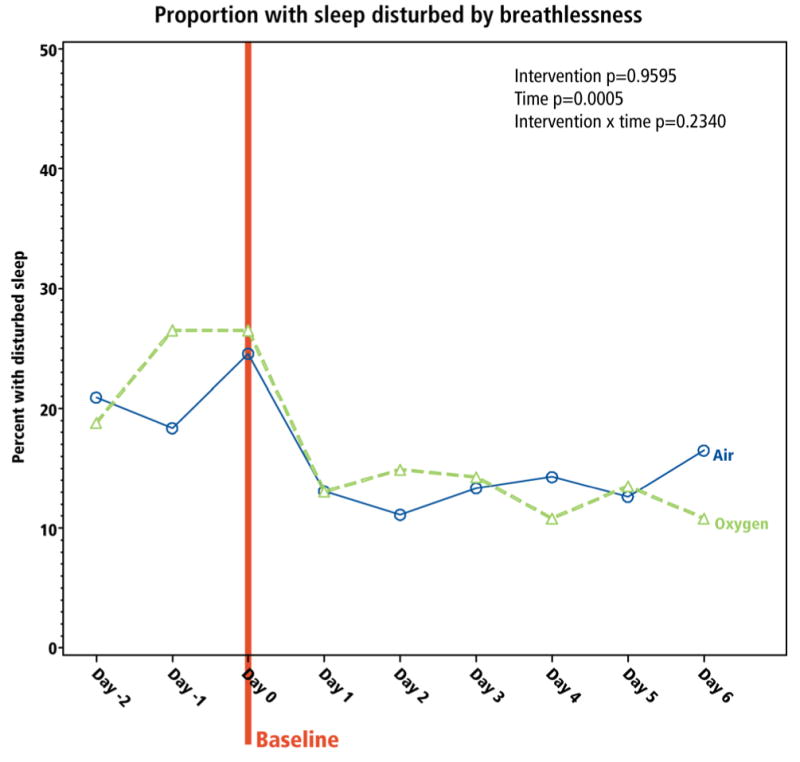
Participants were asked the dichotomous question “was your sleep disturbed by breathlessness?” The proportion responding “yes” is presented. The baseline is Day 0 since the measure is reported in the morning.
| Day -2 | Day -1 | Day 0 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ARM | Total per Arm | N | # ‘yes’ | % ‘yes’ (95% CI) |
N | # ‘yes’ | % ‘yes’ (95% CI) |
N | # ‘yes’ | % ‘yes’ (95% CI) |
| Oxygen | 117 | 117 | 22 | 18.8% (11.7%,25.9%) |
117 | 31 | 26.5% (18.5%,34.5%) |
117 | 31 | 26.5% (18.5%,34.5%) |
| Air | 110 | 110 | 23 | 20.9% (13.3%,28.5%) |
109 | 20 | 18.3% (11.1%,25.6%) |
110 | 27 | 24.5% (16.5%,32.6%) |
| Day 1 | Day 2 | Day 3 | ||||||
|---|---|---|---|---|---|---|---|---|
| N | # ‘yes’ | % ‘yes’ (95% CI) |
N | # ‘yes’ | % ‘yes’ (95% CI) |
N | # ‘yes’ | % ‘yes’ (95% CI) |
| 115 | 15 | 13% (6.9%,19.2%) |
114 | 17 | 14.9% (8.4%,21.5%) |
112 | 16 | 14.3% (7.8%,20.8%) |
| 107 | 14 | 13.1% (6.7%,19.5%) |
108 | 12 | 11.1% (5.2%,17%) |
105 | 14 | 13.3% (6.8%,19.8%) |
| Day 4 | Day 5 | Day 6 | ||||||
|---|---|---|---|---|---|---|---|---|
| N | # ‘yes’ | % ‘yes’ (95% CI) |
N | # ‘yes’ | % ‘yes’ (95% CI) |
N | # ‘yes’ | % ‘yes’ (95% CI) |
| 111 | 12 | 10.8% (5%,16.6%) |
111 | 15 | 13.5% (7.2%,19.9%) |
111 | 12 | 10.8% (5%,16.6%) |
| 105 | 15 | 14.3% (7.6%,21%) |
103 | 13 | 12.6% (6.2%,19%) |
103 | 17 | 16.5% (9.3%,23.7%) |
Predictors of response
Statistically significant predictors of morning response were intervention (oxygen vs. air) and baseline dyspnea (severe vs. moderate vs. low; Table 3). Compared to those receiving air, participants receiving oxygen were twice as likely to have an improvement in morning dyspnea (OR 2.0; CI: 1.1, 3.5); participants with severe baseline dyspnea were 5 times more likely to have a response than were participants with low baseline dyspnea (OR 5.3; CI: 2.2, 12.8); participants with severe baseline dyspnea were 3 times more likely to have a response than were those with moderate baseline breathlessness (OR 3.4; CI: 0.8, 3.0). Baseline dyspnea, but not intervention, similarly predicted evening response. No other participant characteristic predicted response. The impact of the gases was similar regardless of dyspnea etiology, performance status, opioid use, and baseline oxygenation.
Table 3. Predictors of response to medical gas.
Response was defined as a ≥1-point decrease in the NRS from baseline. Logistic regression was used to identify predictors of response.
| Parameter (# responders) | Reference group (# responders) | OR (95%CI) | Wald Chi-square p-value |
|---|---|---|---|
| Morning dyspnea (N=111 responders, 102 non-responders) | |||
| Intercept | 0.008 | ||
| Oxygen (66) | Medical Air (45) | 2.0 (1.1, 3.5) | 0.02 |
| Severe (32) | Low baseline dyspnea (27) | 5.3 (2.2, 12.8) | 0.0002 |
| Moderate (52) | Low baseline dyspnea (27) | 1.6 (0.8,3.0) | 0.16 |
| Severe (32) | Moderate (52) | 3.4 (1.5, 7.7) | 0.004 |
| Evening dyspnea (N=112 responders, 99 non-responders) | |||
| Intercept | 0.01 | ||
| Oxygen (64) | Medical Air (48) | 1.5(0.8, 2.6) | 0.20 |
| Severe (38) | Low baseline dyspnea (22) | 8.7(3.4, 22.0) | <0.0001 |
| Moderate (52) | Low baseline dyspnea (22) | 1.8(1.0, 3.5) | 0.07 |
| Severe (38) | Moderate (52) | 4.8(2.0, 11.3) | 0.0004 |
Preference for intervention
Among the 239 participants, 43 (18%) did not want to receive oxygen after the study; 63 (26%) indicated that they derived no benefit; 41 (17%) requested and received unblinded oxygen after the study; 74 (31%) requested oxygen but did not receive it; 18 (8%) did not respond. Distributions were similar between treatment arms.
Side effects
There was no clinically meaningful difference between interventions in side effects, and few adverse effects (Table 4).
Table 4. Patient-reported rating of side effects.
| Oxygen | Medical Air | Overall | ||||
|---|---|---|---|---|---|---|
| N | % | N | % | N | % | |
| How drowsy have you felt today? | ||||||
| Not drowsy at all | 14 | 12 | 14 | 13 | 28 | 13 |
| Mildly drowsy | 47 | 41 | 39 | 36 | 86 | 38 |
| Moderately drowsy | 43 | 37 | 41 | 38 | 84 | 37 |
| Extremely drowsy | 12 | 10 | 14 | 13 | 26 | 12 |
| Total answering question | 116 | 100 | 108 | 100 | 224 | 100 |
| How much nasal irritation have you experienced today? | ||||||
| None at all | 21 | 18 | 26 | 24 | 47 | 21 |
| Mild symptoms | 62 | 53 | 44 | 41 | 106 | 47 |
| Moderate symptoms | 31 | 27 | 31 | 29 | 62 | 28 |
| Extreme symptoms | 2 | 2 | 7 | 6 | 9 | 4 |
| Total answering question | 116 | 100 | 108 | 100 | 224 | 100 |
| Have you experienced any nose bleeds today? | ||||||
| No | 89 | 76 | 69 | 64 | 158 | 71 |
| Yes but not troublesome | 21 | 18 | 27 | 25 | 48 | 22 |
| Yes and mildly troublesome | 3 | 3 | 9 | 8 | 12 | 5 |
| Yes moderately troublesome | 2 | 2 | 3 | 3 | 5 | 2 |
| yes and extremely troublesome | 1 | 1 | 1 | 0 | ||
| Total answering question | 116 | 100 | 108 | 100 | 224 | 100 |
| How anxious have you felt today? | ||||||
| Not anxious at all | 31 | 27 | 17 | 16 | 48 | 21 |
| Mildly anxious | 54 | 47 | 48 | 44 | 102 | 45 |
| Moderately anxious | 27 | 23 | 37 | 34 | 64 | 29 |
| Extremely anxious | 4 | 3 | 6 | 6 | 10 | 5 |
| Total answering question | 116 | 100 | 108 | 100 | 224 | 100 |
Discussion
This adequately powered study demonstrated no additional symptomatic benefit of oxygen over room air delivered by nasal cannulae for relieving refractory breathlessness in patients with PaO2 >55mm Hg. Dyspnea intensity decreased across the study period in both arms, temporally related to the provision of the gas; improvement in QOL scores and exertional capacity mirrored changes in breathlessness. Breathlessness scores of patients with moderate to severe dyspnea improved most, irrespective of medical gas administered.
Historically, a compassion-based rationale has underpinned clinical decisions regarding the use of palliative oxygen. Physicians often prescribe palliative oxygen for patients with refractory dyspnea and PaO2>55 mm Hg despite a lack of definitive evidence to support efficacy in this setting. Prior studies of palliative oxygen and medical air have been difficult to interpret because they were small, inadequately controlled, or had unclear outcomes. This effectiveness study ensured: (1) masked identical standardized interventions; (2) adequate sample size; (3) sufficient study duration to evaluate outcomes; and, (4) patient-centered outcomes meaningful for the target population.
The temporal relationship between gas delivery and breathlessness reduction suggests that medical air is an intervention, not a placebo. Prior small studies of palliative oxygen vs. medical air have also demonstrated improvements with both gases.28, 29 Possible reasons are that: the movement of any gas across the nasal passages influences the sensation of dyspnea; the obvious presence of an intervention alleviates the patient's anxiety and related breathlessness; the concentrator itself may function as a placebo, inducing expectation of benefit; or, the extra attention that the patient receives during study participation improves psychological status, thereby reducing breathlessness. In a similar longitudinal study, dyspnea gradually worsened over an 8-day period, suggesting that study participation does not, in itself, lessen dyspnea.7
In both study arms, a temporal relationship between dyspnea, QOL, exertional capacity, and sleep improvement after introducing medical gas is apparent (Figures 2-6). Because patients with intractable symptoms achieved significant benefit from both interventions, these results warrant further exploration to determine the gases' relative impact and feasibility, whether this was placebo effect from study participation or a meaningful medical intervention, and to guide clinicians in best use of medical gases to relieve patients' breathlessness.
First, are results clinically significant? The absolute mean reduction in dyspnea [-0.8 (CI: -1.1, -0.5) in the morning; -0.4 (CI: -0.7, -0.1) in the evening] reflects an 18% and 9% relative reduction, respectively. In patients with refractory symptoms, a 9% reduction in intensity may be clinically meaningful and most individuals would find 18% improvement important. Overall, 46% and 42% of individuals responded in the morning and evening, respectively. These proportions are similar to opioid response proportions.7, 30 Sub-group analyses demonstrated that the impact of the gases was similar regardless of current opioid use. The “morning effect”, given anecdotal evidence that most people used oxygen at night, may warrant further exploration, in conjunction with study of breathlessness on exertion, to hone in on potential windows of time when a medical gas intervention is most likely to benefit the patient.
Second, how do results compare to other intervention studies of air movement to treat breathlessness? Animal studies dating back to the 1960s have demonstrated the role of upper airway receptors associated with the trigeminal nerve in reducing ventilation requirements.31 Among people with COPD, blowing air on the face (e.g., open window, fan) significantly diminished the sensation of dyspnea induced by a resistive load and hypercapnia without causing significant reduction in ventilation.31 Cold temperature appears to improve efficacy,32 though the relative roles of mechanics and temperature remain unclear. Exploratory studies found that a handheld fan improved dyspnea when blowing air towards the face, but not towards the leg;33,34 a randomized controlled crossover trial has recently confirmed that a handheld fan directed at the face is effective in reducing the symptom of breathlessness, as compared to the same fan directed at the leg.33 Our study adds to the evidence by demonstrating (1) change over time in dyspnea after medical gas delivery by nasal cannulae, and (2) corollary impact on QOL and physical functioning.
Third, how do we interpret the conflicting findings that there were no differences in the effects of the two gases, and yet the oxygen intervention predicted morning dyspnea improvement? The graphs provide insight; there was a non-significant trend for oxygen to confer more benefit (Figures 2, 4, 5, 6); predictor analysis upheld this trend. The interventions were equivalent in proportional improvement (Figure 3).
Implications for clinical practice
Palliative oxygen is widely prescribed in palliative care. These results should therefore be placed in clinical context, providing practical guidance to inform care of patients with refractory breathlessness and advanced life-limiting illness. Interpreted cautiously, these results suggest that moving gas near the nasal passages, and specifically delivered via nasal cannulae, may lead to improved symptoms. The gas, however, need not be oxygen. Effect can achieved in the setting of other palliative interventions, such as opioids (the option best supported by evidence). Currently, it is difficult to prescribe medical air; prescription of oxygen may be substituted but with important caveats. Oxygen is flammable; smoking patients, and those with smoking caregivers, should not be prescribed oxygen.35 Oxygen is expensive and may be difficult to obtain. Potentially hypercarbic patients, and especially people with central hypoventilation syndromes, should have close supervision when prescribed oxygen. Given that air motion seems to be an operative factor in relieving breathlessness, a simple hand-held or table-top fan may be a helpful, inexpensive, first step. Treatment of breathlessness with a medical gas – whether oxygen or moving air – may be advisable to alleviate other related symptoms in addition to dyspnea, such as fatigue. Additionally, and especially for patients with less severe dyspnea, nonpharmacological options such as pulmonary rehabilitation should be considered.
If medical gas is prescribed, treatment should focus on patients with dyspnea scores (NRS) of ≥4, and especially those with scores ≥7. Recurrent assessment with standardized scales is prudent, especially when using an N-of-1 approach, as it is difficult to predict which patients will benefit.16 This study demonstrates that most benefit occurred in the first 24 hours, and nearly all symptomatic and functional improvements happened in the first 3 days. Assessment in an N-of-1 study should therefore happen at 72 hours. Discontinuing the intervention after 3 days, if ineffective in that time, will require substantial re-education of clinicians and caregivers who often perceive palliative oxygen as a critical element for relief of suffering. The logistical burden of this intervention, as well as its burden in terms of social stigma and interpersonal barrier,36 should be considered. Clinical practice guidelines should be updated to avoid offering a burdensome treatment, or continuing it, if patients are unlikely to benefit through symptom relief.
Limitations
First, we did not collect the exact time of morning and evening assessments, nor did we know the exact times during which participants used the gases; we omitted these details to reduce participant burden. Second, our inclusion/exclusion criteria prevent extrapolation of study results to terminally ill, dyspneic patients with less than 1-month expected survival, and to patients eligible for LTOT. Third, we considered palliative oxygen within the context of general clinical practice, regardless of dyspnea etiology, and therefore we deliberately enrolled a heterogeneous population. This approach reflects standard practice in palliative medicine, in which the symptom is treated similarly for patients with different underlying diseases. It is possible that palliative oxygen is more beneficial than medical air for some sub-groups (e.g., COPD patients vs. cancer patients), and that our study was not adequately powered to identify these patients. We plan to combine results from this trial with the main systematic reviews for palliative oxygen in cancer and COPD to explore this question.14,37 Fourth, more randomized participants dropped out of the medical air arm than out of the oxygen arm, thereby introducing potential for skewing; however, most drop-outs occurred before the intervention began. Fifth, because most participants had ECOG performance status of 2 or 3, and did not indicate breathlessness at rest, this population may not be representative of the sickest palliative care patients who frequently receive palliative oxygen. Sixth, the relatively small definition of response (1-point change on NRS) calls into question the clinical significance of demonstrated benefit. Each patient should be the final arbiter; patients can and do exercise this role discerningly.38 Seventh, secondary analyses may be underpowered, and, given multiple comparisons, some findings may occur by chance. Eighth, since our focus was on subjective experiences of breathlessness, we did not track objective measures of oxygen saturation, hemodynamics and sleep, which might have provided insight into the gases' benefits. Finally, although participants were instructed to use the gas 15 hours per day, total hours of use recorded by concentrator meters suggest a slightly lower daily usage (14 hours per day; Table 1). Since the majority of response occurred in the first 24 hours, when participants were presumably most likely to use the intervention, it is unlikely that stricter adherence would change outcomes. In predictor analyses, we did not see a dose-response between level of PaO2 and dyspnea relief by intervention, although it is possible that underuse of concentrators contributed to this lack of effect.
Conclusion
Quality care for people with life-limiting illness and refractory symptoms requires the judicious use of interventions that provide greatest patient-defined benefit with least harm. Palliative oxygen does not provide incremental benefit over room air, when provided at 2L/min by nasal cannulae, for patients with PaO2 >55mm Hg. There was a temporal relationship between provision of medical gas, symptomatic benefit, and improved QOL, especially for people with moderate to severe dyspnea. Results can be efficiently defined through careful monitoring of symptoms using basic standardized scales (e.g., 0-10 NRS), with patient preference being a guiding factor in decisions to continue or discontinue therapy. A future research agenda should explore these findings in the context of health service utilization, caregiver confidence, exertional breathlessness, and additional interventions for refractory dyspnea in the setting of life-limiting illness.
Supplementary Material
Acknowledgments
The authors wish to thank the many patients with advanced life-limiting illness who generously participated in this study, and their caregivers. We also thank Ms. Bernadette Kenny and Ms. Belinda Fazekas (Southern Adelaide Palliative Services, Adelaide Australia) for their important contributions in study and data management, and Dr. David Woods (Tasmania Statewide Palliative Care Service, Australia) for his assistance with participant recruitment in Tasmania, Australia.
Funding: This study was funded through grants from: the United States National Institutes of Health (NIH; R01 AG026469), Australian National Health and Medical Research Council (324770 and 375127), Duke Institute for Care at the End of Life, and Doris Duke Charitable Foundation. None of the study sponsors had a role in the conduct of this study or its reporting, except through the input of the Data Safety Monitoring Board as mandated by the NIH.
Complete Funding Declaration: This study was funded through grants from: the United States National Institutes of Health (R01 AG026469), Australian National Health and Medical Research Council (324770 and 375127), Duke Institute for Care at the End of Life, and Doris Duke Charitable Foundation.
Footnotes
Conflicts of interest and disclosures: The authors have no relevant conflicts of interest that threaten integrity of data or results. Christine MacDonald has received honoraria for presenting at scientific meetings sponsored by Astra Zeneca, GlaxoSmithKline, and Boehringer Ingelheim, and has served as an Advisory Board member for these same corporations. Janet Bull, MD is on the Speaker's Bureau for Wyeth Pharmaceuticals.
Guarantor: Amy Abernethy, MD, Principal Investigator, had full access to all study data and takes responsibility for the integrity of the data and the accuracy of data analyses.
Author contributions
APA: conception and design; acquisition of data; analysis and interpretation of data; drafting of manuscript; critical revision of manuscript; obtaining funding; administrative, technical or material support; supervision
CFM: conception and design; acquisition of data; analysis and interpretation of data; critical revision of manuscript; obtaining funding; supervision
PF: conception and design; acquisition of data; analysis and interpretation of data; critical revision of manuscript; obtaining funding; supervision
KC: conception and design; acquisition of data; analysis and interpretation of data; critical revision of manuscript; obtaining funding; supervision
JEH: conception and design; statistical analyses; analysis and interpretation of data; drafting of manuscript; critical revision of manuscript; supervision
JM: conception and design; statistical analyses; analysis and interpretation of data; drafting of manuscript; critical revision of manuscript
IY: conception and design; acquisition of data; analysis and interpretation of data; critical revision of manuscript; obtaining funding; supervision
JB: acquisition of data; analysis and interpretation of data; critical revision of manuscript; supervision
AW: conception and design; analysis and interpretation of data; critical revision of manuscript; obtaining funding
SB: conception and design; acquisition of data; analysis and interpretation of data; critical revision of manuscript; obtaining funding; supervision
JLW: analysis and interpretation of data; drafting of manuscript; critical revision of manuscript
JAT: conception and design; analysis and interpretation of data; critical revision of manuscript; obtaining funding
AJC: conception and design; analysis and interpretation of data; critical revision of manuscript; obtaining funding
DCC: conception and design; acquisition of data; analysis and interpretation of data; drafting of manuscript; critical revision of manuscript; obtaining funding; administrative, technical or material support; supervision
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.American Thoracic Society. Dyspnea. Mechanisms, assessment, and management: a consensus statement. Am J Respir Crit Care Med. 1999;159:321–340. doi: 10.1164/ajrccm.159.1.ats898. [DOI] [PubMed] [Google Scholar]
- 2.Lynn J, Teno JM, Phillips RS, et al. Perceptions by family members of the dying experience of older and seriously ill patients. SUPPORT Investigators. Ann Intern Med. 1997;126(2):97–106. doi: 10.7326/0003-4819-126-2-199701150-00001. [DOI] [PubMed] [Google Scholar]
- 3.Mercadante S, Casuccio A, Fulfaro F. The course of symptom frequency and intensity in advanced cancer patients followed at home. J Pain Symptom Manage. 2000;20(2):104–112. doi: 10.1016/s0885-3924(00)00160-3. [DOI] [PubMed] [Google Scholar]
- 4.Tanaka K, Akechi T, Okuyama T, Nishiwaki Y, Uchitomi Y. Prevalence and screening of dyspnea interfering with daily life activities in ambulatory patients with advanced lung cancer. J Pain Symptom Manage. 2002;23(6):484–489. doi: 10.1016/s0885-3924(02)00394-9. [DOI] [PubMed] [Google Scholar]
- 5.Abernethy AP, Uronis HE, Wheeler JL, Currow DC. Pharmacological management of breathlessness in advanced disease. Prog Pailliat Care. 2008;16(1):15–20. [Google Scholar]
- 6.Uronis HE, Currow DC, Abernethy AP. Palliative management of refractory dyspnea in COPD. Int J COPD. 2006;1(3):289–304. doi: 10.2147/copd.2006.1.3.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abernethy AP, Currow DC, Frith P, Fazekas BS, McHugh A, Bui C. Randomised, double blind, placebo controlled crossover trial of sustained release morphine for the management of refractory dyspnoea. BMJ. 2003;327(7414):523–528. doi: 10.1136/bmj.327.7414.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.O'Donohue WJ, Jr, Plummer AL. Magnitude of usage and cost of home oxygen therapy in the United States. Chest. 1995;107(2):301–302. doi: 10.1378/chest.107.2.301. [DOI] [PubMed] [Google Scholar]
- 9.Guyatt GH, McKim DA, Austin P, et al. Appropriateness of domiciliary oxygen delivery. Chest. 2000;118(5):1303–1308. doi: 10.1378/chest.118.5.1303. [DOI] [PubMed] [Google Scholar]
- 10.Cranston JM, Crockett A, Currow D. Oxygen therapy for dyspnoea in adults. Cochrane Database Syst Rev. 2008;3 doi: 10.1002/14651858.CD004769.pub2. CD004769. [DOI] [PubMed] [Google Scholar]
- 11.Booth S, Anderson H, Swannick M, et al. The use of oxygen in the palliation of breathlessness. A report of the expert working group of the Scientific Committee of the Association of Palliative Medicine Respir Med. 2004;98(1):66–77. doi: 10.1016/j.rmed.2003.08.008. [DOI] [PubMed] [Google Scholar]
- 12.Luce JM, Luce JA. Perspectives on care at the close of life. Management of dyspnea in patients with far-advanced lung disease: “once I lose it, it's kind of hard to catch it… ”. JAMA. 2001;285(10):1331–1337. doi: 10.1001/jama.285.10.1331. [DOI] [PubMed] [Google Scholar]
- 13.Abernethy AP, Currow DC, Frith P, Fazekas B. Prescribing palliative oxygen: a clinician survey of expected benefit and patterns of use. Palliat Med. 2005;19(2):168–170. doi: 10.1177/026921630501900219. [DOI] [PubMed] [Google Scholar]
- 14.Uronis HE, Currow DC, McCrory DC, et al. Oxygen for relief of dyspnoea in mildly- or non-hypoxaemic patients with cancer: a systematic review and meta-analysis. Brit J Cancer. 2008;98(2):294–299. doi: 10.1038/sj.bjc.6604161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Uronis HE, Abernethy AP. Oxygen for relief of dyspnea: What is the evidence? Cur Opinion Palliat Support Care. 2008;2(2):89–94. doi: 10.1097/SPC.0b013e3282ff0f5d. [DOI] [PubMed] [Google Scholar]
- 16.Nonoyama ML, Brooks D, Guyatt GH, Goldstein RS. Effect of oxygen on health quality of life in patients with chronic obstructive pulmonary disease with transient exertional hypoxemia. Am J Respir Crit Care Med. 2007;176:343–349. doi: 10.1164/rccm.200702-308OC. [DOI] [PubMed] [Google Scholar]
- 17.Young IH, Crockett AJ, McDonald CF. Adult domiciliary oxygen therapy. Position statement of the Thoracic Society of Australia and New Zealand. Med J Australia. 1998;168(1):21–25. doi: 10.5694/j.1326-5377.1998.tb123340.x. [DOI] [PubMed] [Google Scholar]
- 18.Tierney RM, Horton SM, Hannan TJ, Tierney WM. Relationships between symptom relief, quality of life, and satisfaction with hospice care. Palliat Med. 1998;12(5):333–344. doi: 10.1191/026921698670933919. [DOI] [PubMed] [Google Scholar]
- 19.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psych Res. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 20.Fisher RA, Yates F. Statistical tables for biological, agricultural and medical research. [1974 printing] London: Longman; 1963. [Google Scholar]
- 21.Fierro-Carrion G, Mahler DA, Ward J, et al. Comparison of continuous and discrete measurements of dyspnea during exercise in patients with COPD and normal subjects. Chest. 2004;125(1):77–84. doi: 10.1378/chest.125.1.77. [DOI] [PubMed] [Google Scholar]
- 22.Norman GR, Sloan JA, Wyrwich KW. Interpretation of changes in health-related quality of life: the remarkable universality of half a standard deviation. Med Care. 2003;41(5):582–592. doi: 10.1097/01.MLR.0000062554.74615.4C. [DOI] [PubMed] [Google Scholar]
- 23.Cohen SR, Mount BM, Bruera E, Provost M, Rowe J, Tong K. Validity of the McGill Quality of Life Questionnaire in the palliative care setting: a multi-centre Canadian study demonstrating the importance of the existential domain. Palliat Med. 1997;11(1):3–20. doi: 10.1177/026921639701100102. [DOI] [PubMed] [Google Scholar]
- 24.Mahler DA, Wells CK. Evaluation of clinical methods for rating dypnea. Chest. 1988;93(3):580–585. doi: 10.1378/chest.93.3.580. [DOI] [PubMed] [Google Scholar]
- 25.Heyse-Moore LH. On dyspnoea in advanced cancer. Southampton, UK: Southampton University; 1993. [Google Scholar]
- 26.Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5(6):649–655. [PubMed] [Google Scholar]
- 27.Daut RL, Cleeland CS, Flanery RC. Development of the Wisconsin Brief Pain Questionnaire to assess pain in cancer and other diseases. Pain. 1983;17(2):197–210. doi: 10.1016/0304-3959(83)90143-4. [DOI] [PubMed] [Google Scholar]
- 28.Bruera E, Sweeney C, Willey J, et al. A randomized controlled trial of supplemental oxygen versus air in cancer patients with dyspnea. Palliat Med. 2003;17(8):659–663. doi: 10.1191/0269216303pm826oa. [DOI] [PubMed] [Google Scholar]
- 29.Booth S, Kelly MJ, Cox NP, Adams L, Guz A. Does oxygen help dyspnea in patients with cancer? Am J Respir Crit Care Med. 1996;153(5):1515–1518. doi: 10.1164/ajrccm.153.5.8630595. [DOI] [PubMed] [Google Scholar]
- 30.Jennings AL, Davies AN, Higgins JP, Gibbs JS, Broadley KE. A systematic review of the use of opioids in the management of dyspnoea. Thorax. 2002;57(11):939–944. doi: 10.1136/thorax.57.11.939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schwartzstein RM, Lahive K, Pope A, Weinberger SE, Weiss JW. Cold facial stimulation reduces breathlessness induced in normal subjects. Am Rev Respir Dis. 1987;136(1):58–61. doi: 10.1164/ajrccm/136.1.58. [DOI] [PubMed] [Google Scholar]
- 32.Burgess KR, Whitelaw WA. Effects of nasal cold receptors on pattern of breathing. J Applied Phys. 1988;64(1):371–376. doi: 10.1152/jappl.1988.64.1.371. [DOI] [PubMed] [Google Scholar]
- 33.Galbraith S, Perkins P, Lynch A, Booth S. Does a handheld fan improve intractable breathlessness?. Paper presented at: European Association for Palliative Care Research Forum; May, 2008; Trondheim, Norway. [Google Scholar]
- 34.Bausewein C, Booth S, Gysels M, et al. Non-pharmacological interventions for breathlessness in advanced stages of malignant and non-malignant diseases. Cochrane Database Syst Rev. 2008;2 doi: 10.1002/14651858.CD005623.pub2. CD005623. [DOI] [PubMed] [Google Scholar]
- 35.Robb BW, Hungness ES, Hershko DD, Warden GD, Kagan RJ. Home oxygen therapy: adjunct or risk factor? J Burn Care Rehab. 2003;24(6):403–406. doi: 10.1097/01.BCR.0000096275.27946.68. [DOI] [PubMed] [Google Scholar]
- 36.Gysels M, Higginson IJ. Access to services for patients with chronic obstructive pulmonary disease: the invisibility of breathlessness. J Pain Symptom Manage. 2008;36:451–460. doi: 10.1016/j.jpainsymman.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 37.Uronis HE, McCrory DC, Samsa GP, Currow DC, Abernethy AP. Palliative oxygen for non-hypoxaemic chronic obstructive pulmonary disease. Cochrane Airways Group. Cochrane Database Sys Rev. 2010 doi: 10.1002/14651858.CD006429.pub2. In Press. [DOI] [PubMed] [Google Scholar]
- 38.Currow DC, Fazekas B, Abernethy AP. Oxygen use--patients define symptomatic benefit discerningly. J Pain Symptom Manage. 2007;34(2):113–114. doi: 10.1016/j.jpainsymman.2007.03.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


