Abstract
Cell-type specific regulation of a small number of growth factor signal transduction pathways generates diverse developmental outcomes. The zinc finger protein Churchill (ChCh) is a key effector of Fibroblast Growth Factor (FGF) signaling during gastrulation. ChCh is largely thought to act by inducing expression of the multifunctional Sip1 (Smad Interacting Protein 1). We investigated the function of ChCh and Sip1a during zebrafish somitogenesis. Knockdown of ChCh or Sip1a results in misshapen somites that are short and narrow. As in wild-type embryos, cycling gene expression occurs in the developing somites in ChCh and Sip1a compromised embryos, but expression of her1 and her7 is maintained in formed somites. In addition, tailbud fgf8 expression is expanded anteriorly in these embryos. Finally, we found that blocking FGF8 restores somite morphology in ChCh and Sip1a compromised embryos. These results demonstrate a novel role for ChCh and Sip1a in repression of FGF activity.
Introduction
Fibroblast growth factors (FGFs) play essential roles in cell growth and differentiation in many developmental contexts (Amaya et al., 1991; Sutherland et al., 1996; Borland et al., 2001; Coumoul and Deng, 2003; Furthauer et al., 2004; Bottcher and Niehrs, 2005; Thisse and Thisse, 2005; Huang et al., 2007; Krens et al., 2008). During vertebrate embryogenesis, FGFs are required for induction of both mesoderm and neural ectoderm, patterning of the midbrain, posteriorization of the neural plate and segmentation of the mesoderm. In vertebrates, the FGF family contains over 20 ligands that interact with four receptors. The mechanisms that account for the diverse responses evoked by FGFs have yet to be fully elucidated, but depend on cell-type specific modulators of signaling.
One such FGF effector is the zinc finger protein Churchill (ChCh), which regulates FGF activity during gastrulation. ChCh is slowly induced in response to FGF and acts as a switch between mesoderm and neural inducing activities of FGF in chick, Xenopus and zebrafish (Sheng et al., 2003; Snir et al., 2006; Londin et al., 2007a). ChCh inhibits expression of mesodermal markers including brachyury, Tbx6L, and spt and blocks mesendoderm induction, which requires FGF signaling in cooperation with Activin/Nodal activity (Kimelman and Maas, 1992; LaBonne and Whitman, 1994). ChCh also regulates cell movements during gastrulation. In the chick, Chch blocks ingression of presumptive ectoderm through the primitive streak at the end of gastrulation. Therefore, these cells adopt neural fates instead of ingressing through the streak and becoming paraxial mesoderm (Sheng et al., 2003). Similarly, when zebrafish blastomeres with compromised ChCh activity are transplanted to the animal pole of wild type hosts, they leave the epiblast, migrate to the germ ring and acquire mesodermal fates (Londin et al., 2007b).
ChCh was initially thought to regulate transcription of target genes via a direct DNA interaction. However, subsequent biophysical characterization of ChCh has suggested that it may not be a DNA binding protein (Lee et al., 2007). None-the-less, by direct or indirect mechanisms, ChCh induces sip1 (Smad Interacting Protein 1) transcription (Sheng et al., 2003; Snir et al., 2006; Londin et al., 2007a), which is key to the activity of ChCh. Sip1 is a multifunctional molecule that modulates TGF-β signaling by converting activated forms of both Smad1/5 and Smad2/3 to transcriptional repressors (Remacle et al., 1999; Verschueren et al., 1999; Postigo, 2003; Postigo et al., 2003). In addition, Sip1 regulates cell movements by directly repressing E-cadherin transcription (Remacle et al., 1999; Comijn et al., 2001) and mesoderm induction by directly impeding Xbra transcription (Remacle et al., 1999; Verschueren et al., 1999).
In zebrafish, chch is expressed after gastrulation (Londin et al., 2007a), but later roles for ChCh have not been studied. We now present data that ChCh and Sip1 are required for somitogenesis. Segmentation is an essential step in formation of the vertebrate body axis. Mesodermal segmentation is established by sequentially dividing the unsegmented presomitic mesoderm into the bilaterally segmented structures called somites. The “clock and wavefront” model describes the mechanisms of regulation of somitogenesis (Cooke, 1978; Palmeirim et al., 1997; Forsberg et al., 1998; McGrew et al., 1998; Jiang et al., 2000; Bessho et al., 2001; Saga and Takeda, 2001). In zebrafish, the “clock and wavefront” model proposes that function of a molecular oscillator in the presomitic mesoderm (PSM) results in controlled expression of a set of genes associated with the Notch pathway (Holley et al., 2000; Jiang et al., 2000; Pourquie, 2001; Pourquie, 2003). Simultaneously, the wavefront modulates the ability of the PSM to respond to the morphogenic signals and produce segments (Cooke and Zeeman, 1976; Dale and Pourquie, 2000; Giudicelli and Lewis, 2004). The pulses generated by the molecular clock are translated into very highly regulated spatial periodicity. Despite the similarities in somitogenesis between species, there are differences between amniotes and mammals in the segmentation program (Giudicelli and Lewis, 2004; Cinquin, 2007). For example, lunatic fringe and delta genes appear to function differently in mouse, chick and zebrafish (Prince et al., 2001; Sato et al., 2002; Dale et al., 2003; Serth et al., 2003; Qiu et al., 2004). In addition, the Wnt pathway is important for regulation of segmentation clock in mice (Takada et al., 1994; Aulehla et al., 2003), but not zebrafish.
Previous studies revealed that FGF signaling at the PSM regulates the wavefront position during somitogenesis. FGF modulates somite size by impeding maturation of PSM (Dubrulle et al., 2001; Sawada et al., 2001; Delfini et al., 2005; Wahl et al., 2007). Blocking FGF signaling increases somite length, while activating signaling has the opposite effect (Sawada et al., 2001). In addition, activation of FGF signaling blocks convergence movements and extends somite width (Furthauer et al., 1997; Krens et al., 2008). It is important to define regulators of FGF signaling in the PSM in order to determine how precise positional cues within the PSM are generated.
Here, we demonstrate that ChCh and Sip1a modulate FGF signaling in the PSM during somitogenesis. Surprisingly, we find that ChCh and Sip1 repress FGF expression. During somitogenesis, knockdown of chch or sip1a results in somites that are less extended thru anterior-posterior (A/P) axis while they are over-extended thru mediolateral axis. We also found that inhibition of ChCh and Sip1a perturbs oscillating gene expression in the forming somites. In ChCh or Sip1 compromised embryos, fgf8 expression in the paraxial mesoderm is expanded rostrally leading to altered somite size. Manipulations that reduced FGF8 signaling in ChCh and Sip1a compromised embryos restored somite size. This demonstrated that ChCh and Sip1a regulate somite morphogenesis by limiting FGF signaling. Together, these findings establish a novel role for ChCh as a repressor of FGF signaling.
Results
Role of ChCh and Sip1 in somitogenesis
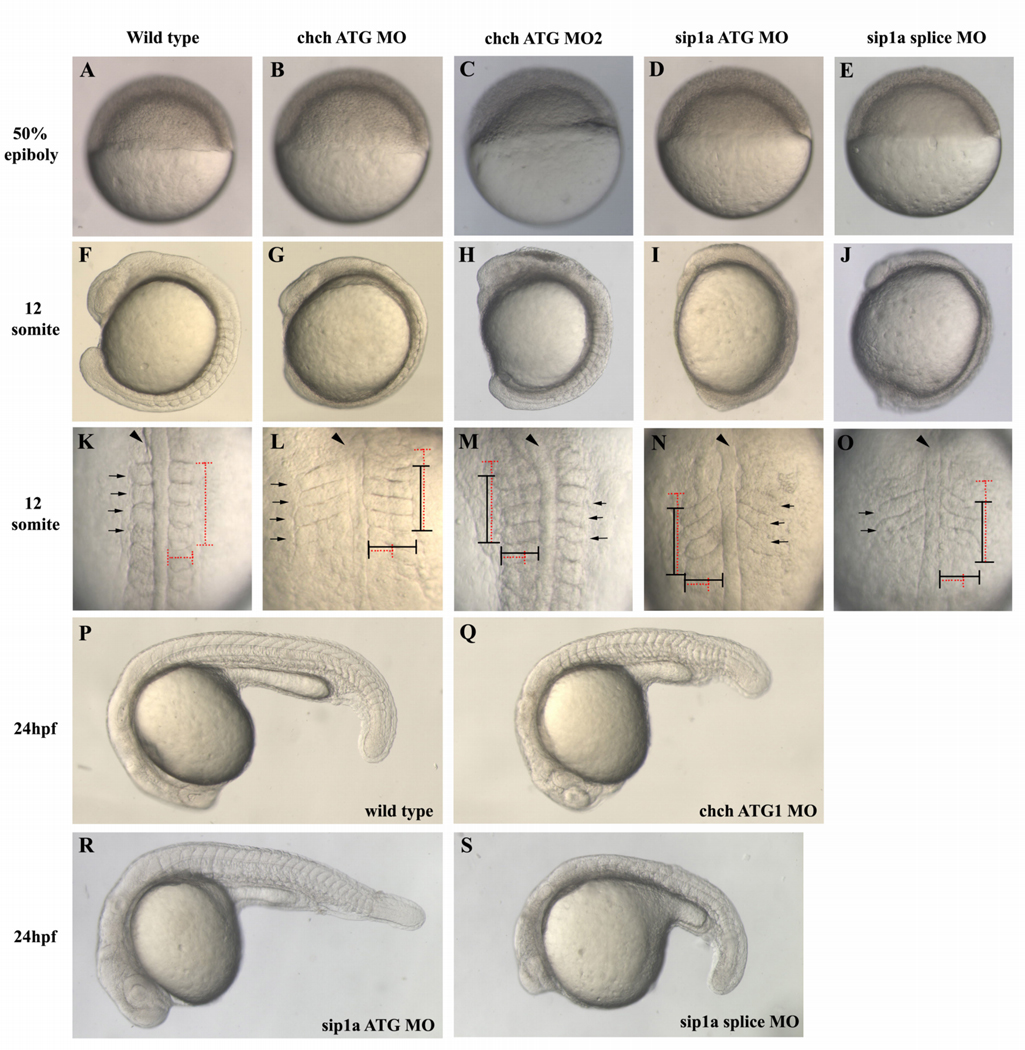
In a previous study, we observed that ChCh is required for proper somite formation (Londin et al., 2007b). However, the mechanism of action of ChCh in somitogenesis is unknown. To investigate the function of ChCh in zebrafish somitogenesis, we inhibited ChCh activity using two translation blocking morpholinos (chch ATG MO and MO2) (Londin et al., 2007b). Microinjection of chch ATG MO presents a similar, but stronger somite phenotype than that produced by chch ATG MO2 microinjection. chch morpholino injected embryos are morphologically indistinguishable from control morpholino injected siblings until 75–95% epiboly stage (Fig 1B, C). Dorsal views of the 12-somite stage chch morphants reveals that somites are less extended thru anterior-posterior axis while they are over-extended thru mediolateral axis (Fig. 1K–M, 52%, n= 128). Moreover, these embryos have a shorter and wider body axis (Fig. 1 G, L; H, M). At 24 hours post fertilization, somites in ChCh compromised embryos are enlarged and lack their characteristic chevron shape (Fig. 1Q). Furthermore, although roughly 30 pairs of somites are formed in wild type siblings, chch morphants only form 22–26 somites (Fig. 1Q and data not shown). Taken together, these results demonstrate that ChCh is required during somitogenesis.
Figure 1. ChCh and Sip1a are required for somitogenesis.
Living wild-type, ChCh and Sip1a-compromised embryos. Early epiboly movements appear normal in chch or sip1a morphants (A–E). By 12s misshapen somites are apparent in both chch and sip1 morpholino treated embryos (F–J). In these embryos, somites are less extended thru anterior-posterior axis and over-extended thru mediolateral axis (K–O). Horizontal and vertical red dotted lines span the width and length of the first four wild type somites for comparison to the first four somites of morphant embryos, which are marked with a black line (K–O). At 24hpf, somites are enlarged and misshapen in both ChCh and Sip1a compromised embryos (P–S). Arrowheads denote notochord and black arrows denote somites. (A–J, P–S) are lateral views; (K–O), dorsal views.
Because ChCh activity is mediated by Sip1 during gastrulation (Sheng et al., 2003) and homozygous Sip1 knockout mice have a somite phenotype similar to chch morphants (Maruhashi et al., 2005), we asked whether Sip1 inhibition produced similar somite defects in zebrafish. Zebrafish have two sip1 genes (sip1a and sip1b) (Delalande et al., 2008). To investigate the function of Sip1 in zebrafish somitogenesis, we inhibited Sip1 activity using previously characterized sip1a and sip1b morpholinos (Delalande et al., 2008). The sip1a ATG MO injected embryos have a somite phenotype similar to chch MO injected embryos, but the overall phenotype is more severe (Fig. 1I, N (89%, n=112) and data not shown). As in chch morphants, the somites are shorter thru anterior-posterior axis and over-extended thru mediolateral axis (Fig. 1N). Microinjection of sip1a splice morpholino also produced embryos were also short in anterior-posterior axis and elongated in the mediolateral axis (Fig. 1 J, O). Similar to chch morphants, somites are enlarged and lost their characteristic chevron shape at 24 hpf (Fig. 1R, S). Because this morpholino alters mRNA structure, we were able to monitor the effectiveness of the knockdown on eliminating wild-type mRNA (Supp. Fig. 1). Until dome stage, only wild-type mRNA is detected, which is presumably maternal message that is unaltered by the splice morpholino. Beginning at dome stage (4.3 hpf), the smaller misspliced product is detected. By 75% epiboly (8 hpf), no wild-type mRNA is apparent.
An alternatively spliced form of sip1a that lacks a portion of exon 8 has been described (Delalande et al., 2008). The alternative splice form lacks one zinc finger that is present in the longer form (Supp Fig. 2, blue bar, Supp Fig. 3A, dark blue box). We identified a similar alternatively spliced form of Sip1b that contains a deletion of 66 bp of exon 8 (Supp. Fig. 2). Since both sip1a and sip1b contained forms that lack the same region, we sought to determine whether either form of sip1a was required for somite formation. Both sip1a forms were detected by RT-PCR during a series of stages spanning the first 24 hpf (data not shown). We designed morpholinos to target the exon 8/intron 8 boundary (sip1a splice MO2, Supp Fig. 3A, pink bar) to drive production of the shorter form and a morpholino (sip1a splice MO3, Supp Fig. 3A, orange bar) that targets the alternative splice site in exon 8 and blocks production of the shorter form (leaving only the long form). Analysis of cDNA from embryo injected with each morpholino revealed that sip1a splice MO2 efficiently altered splicing so that only the shorter form was produced, while sip1a splice MO3 eliminated the shorter form (Supp. Fig. 3B, C). Somitogenesis was not altered by microinjection of either morpholino (data not shown). Due to toxic effects of co-injecting high doses of the two morpholinos (10 ng each), we were unable to analyze the effects of blocking production of both the long and short Sip1a forms in the same embryos. However, these experiments suggest that each Sip1a form is likely sufficient for normal somite development.
In a previous report, sip1b morphants were reported to have severe defects and produce only a few somites (Delalande et al., 2008). We observed a more severe phenotype than Delalande et. al. when we microinjected low doses of sip1b MO (data not shown). Therefore, we were unable to address the role of sip1b in somite formation using morpholino approaches.
ChCh and Sip1a are required for pattering of presomitic mesoderm
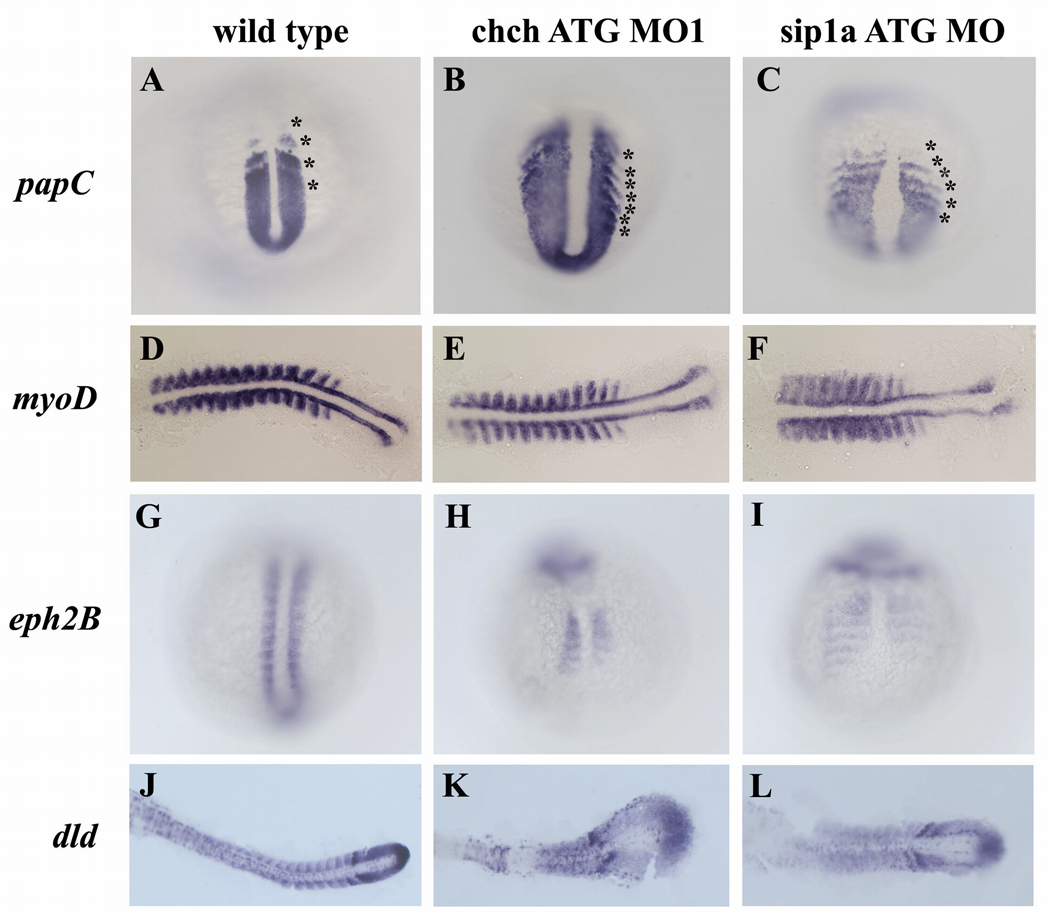
During somitogenesis, a series of highly regulated morphogenetic processes produce periodic and symmetrically formed somite boundaries. To characterize the origin of the severe somite defects in ChCh and Sip1a-compromised embryos, we performed a series of RNA in situ hybridizations with 14 somite stage chch and sip1a MO injected embryos. Since somites are derived from paraxial mesoderm cells, we analyzed the state of the presomitic mesoderm cells in ChCh and Sip1a compromised embryos. papC encodes a structural transmembrane protein and regulates the mesodermal segmentation during zebrafish development. In wild-type control embryos, four papC expression stripes corresponds to the (prospective) somites at the segmentation plate (Yamamoto et al., 1998), Fig. 2A). However, in chch and sip1a morphants, the number of stripes ranges from 5–8 (Fig. 2B, C). Moreover, tail bud expression domain of papC in ChCh and Sip1a compromised embryos is much broader mediolaterally than in wild type siblings (Fig. 2A–C).
Figure 2. Patterning of the presomitic and somitic mesoderm is disrupted in ChCh and Sip1a compromised embryos.
Whole mount (A–C, G–I) and flat mount (D–F, J–L) RNA in situ hybridization of somite markers in wild type, ChCh and Sip1a compromised embryos. All views are dorsal; anterior to the top (A–C, G–I) and anterior to the left (D–F, J–L)). The expression domains of the PSM marker, papC is broader mediolaterally in ChCh and Sip1a compromised embryos than the wild type siblings (A–C). The number of the papC expression stripes corresponding to the prospective and formed somites at the segmentation plate in chch and sip1a MO is higher than in wild type siblings (A–C, compare asterisks number). The myogenic regulatory factor, myoD, is expressed in the posterior somite compartment in chch and sip1a MO injected embryos as in wild-type embryos (D–F). ephB2 and dld expression at the anterior half of the somites (G–L) is reduced and diffuse. Asterisks denote (prospective) somites.
Rostrocaudal polarity of the somites is also disrupted in ChCh and Sip1a compromised embryos. The segmental expression of myogenic regulatory factor myoD in the posterior half of the somites (Weinberg et al., 1996) is extended in the mediolateral axis (Fig. 2D–F). On the other hand, expression of ephB2 (Durbin et al., 1998) (Fig. 2G–I), dld (Jiang et al., 2000; Holley et al., 2002) (Fig. 2J–L) and fgf8 (Fig.3 G–I) in the anterior half of the somites is reduced and expression is no longer restricted to the anterior region of the somites in both chch and sip1a morphants. Therefore, we conclude that rostrocaudal somite polarity requires ChCh and Sip1.
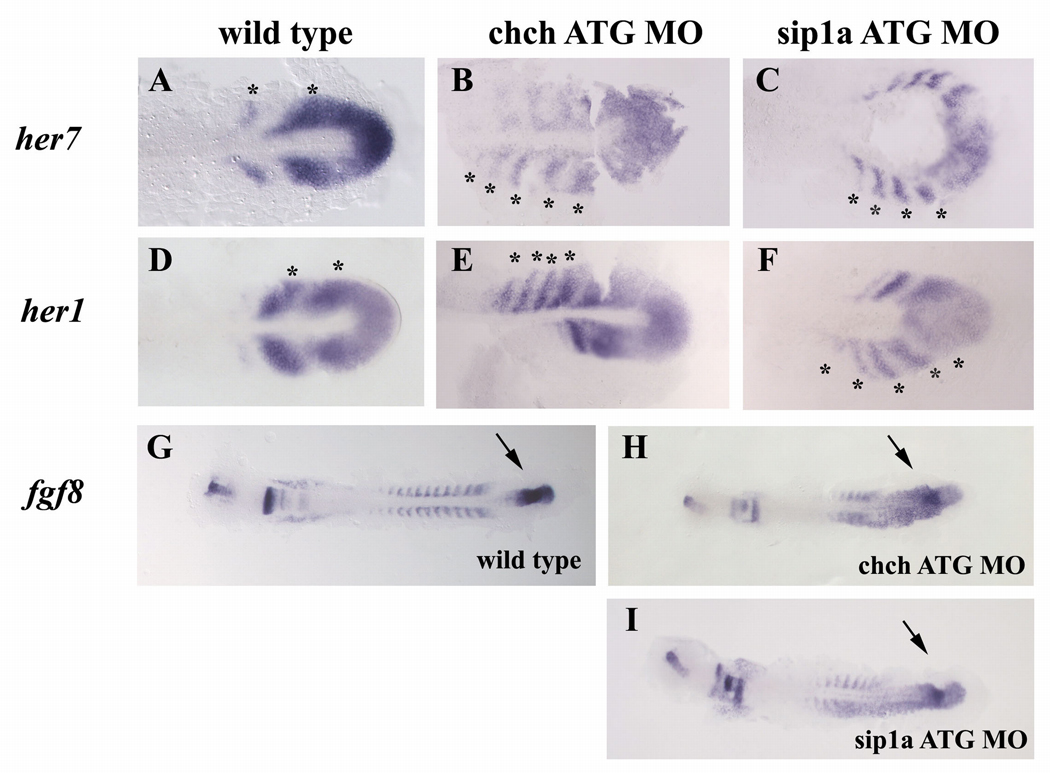
Figure 3. Inhibition of ChCh and Sip1a affects the components of the “clock and wavefront model”.
Flat mount RNA in situ hybridization of 10 somite stage wild type, ChCh and Sip1a compromised embryos(A–I). All views are dorsal; anterior to the left. In wildtype embryos, periodic activation of Notch signaling provides cycling gene expression of Notch pathway genes such as her1 and her7 (A–F). The number of the her7 expressing stripes in chch and sip1a ATG MO injected embryos ranges from 4 to 5 (B,C; E,F). fgf8 expression domain at tailbud is expanded anteriorly in ChCh and Sip1a compromised embryos (G–I). Asterisks denote each her1 or her7 expressing premesoderm stripe (A–F). Arrows denotes fgf8 tailbud expression domain (G–I).
Inhibition of ChCh and Sip1a alters periodic gene expression
The “clock and wavefront” model describes the timing and positioning of somite boundaries during segmentation of the mesoderm (Cooke, 1978; Palmeirim et al., 1997; Forsberg et al., 1998; McGrew et al., 1998; Jiang et al., 2000; Bessho et al., 2001). In this model, somite size is the synchronous function of frequency of “molecular clock” oscillations and of the pace of “wavefront” progression. In chch and sip1a morphants, formed somites are smaller thru anterior-posterior axis. Either a faster ticking “molecular clock” or slowed down “wavefront” progression during somitogenesis can trigger reduction in somite size.
To test whether cyclic gene expression is altered in ChCh and Sip1a compromised embryos, we assayed her1 and her7 expression by RNA in-situ hybridization. her1 and her7 are both the output of the “molecular clock” and have characteristic 1 to 2 stripe expression domain in the PSM at 10 somite stage (Fig. 3A,D) (Oates and Ho, 2002). However, the number of stripes observed in the ChCh and Sip1a compromised embryos ranged from 3 to 5 (Fig. 3A–C; D–F, marked with asterisks) indicating that impeding ChCh or Sip1a averts the proper termination of cyclic her1 and her7 expression in the anterior PSM. However, the performance and pace of the “molecular clock” is not substantially altered because despite the size difference, duration of somite formation in ChCh and Sip1a compromised embryos is comparable to control morpholino injected siblings (Supp Fig 4 and data not shown).
Failure to properly terminate her1 and her7 cyclic expression in the anterior PSM can be the result of slowed wavefront progression because the “wavefront” facilitates the transition of the PSM cells from the immature state to mature state by arresting the oscillating her1 and her7 wave. If the pace of the wavefront progression is slower, the overall rate of maturation of the PSM and therefore arresting the expression of cyclic her1 and her7 expression would also be slowed.
FGF signaling at the PSM is required for the regulation of the position of the wavefront during somitogenesis (Dubrulle et al., 2001; Sawada et al., 2001; Delfini et al., 2005; Wahl et al., 2007). A threshold level of FGF signaling facilitates the transition of the PSM cells from the immature state to mature state (Dubrulle et al., 2001; Sawada et al., 2001). Ectopic activation of the FGF signaling in zebrafish by surgically inserting FGF8 soaked beads in one side of the PSM gives rise to narrower somites in the region anterior to the FGF bead, while blocking FGF signaling has the opposite effect. On the FGF8 soaked bead implanted side, her1 expression domain also extends more anteriorly than the control side (Sawada et al., 2001).
To determine whether the somite alterations in chch or sip1 morphants are associated with altered fgf8 gene expression, we assayed fgf8 expression in these embryos by RNA in-situ hybridization. In both chch and sip1a ATG morpholino injected embryos, fgf8 expression domain is expanded rostrally and extends into the midline and sometimes the (putative) somitic mesoderm (Fig. 3G–I). This suggests that the progression of the wavefront is much slower in ChCh and Sip1a compromised embryos. Slowing the wavefront is predicted to result in a broader zone of immature PSM. A similar phenomenon was also observed in Sip1a knockout mice (Maruhashi et al., 2005)
Rostral expansion of fgf8 expression domain at tailbud can be explained by local changes in cell number in the PSM. We tested whether rate of cell proliferation is also altered in ChCh and Sip1a compromised embryos using cell proliferation marker anti-phosphorylated histone H3 antibody. There is no significant difference between ChCh or Sip1a compromised embryos and their wild type siblings (n= 29, 31 and 19 respectively, data not shown). This indicates that rostral expansion of fgf8 expression domain is not due to altered cell proliferation.
The effects of ChCh knockdown on somite morphology are due to expanded FGF8 activity
In ChCh or Sip1a compromised embryos, fgf8 expression in the paraxial mesoderm is expanded rostrally and somites are narrower at the A/P axis and wider in the mediolateral axis. We hypothesized that the expansion in FGF8 expression in these embryos caused alterations in somite morphology. To test that hypothesis, we determined the effects of chch and sip1a knockdown on embryos with compromised FGF signaling. If rostral expansion of fgf8 is the cause of the somite phenotype observed in ChCh or Sip1a compromised embryos, then reduction of FGF8 activity will restore wild-type somite morphology. We employed two means to attenuate FGF activity, microinjection sprouty4 (spry4) mRNA and acerebellar (fgf8/ace) mutant embryos.
spry4 is a feedback induced antagonist of FGF signaling. FGF8 induces spry4 expression which in turn inhibits FGF activity (Furthauer et al., 2001). Microinjection of spry4 sense RNA weakly impedes FGF8 signaling in zebrafish (Furthauer et al., 2001). Injection of spry4 sense RNA into chch ATG morphants reduced the number of embryos with narrowed somites from 58% (ChCh ATG MO+lacZ) to 45% (ChCh ATG MO+spry4 RNA)) (p = 0.0063) (Supp. Fig. 5). Similarly, injection of spry4 sense RNA into sip1a ATG morphants reduced the number of embryos with narrowed somites from 93% (sip1a ATG MO+lacZ) to 66% (sip1a ATG MO+spry4 RNA)) (p = 0.0044) (Supp. Fig. 5). The ability of spry4 to restore somite morphology in only a subset of embryos likely reflect the poor stability of the injected RNA (Furthauer et al., 2001).
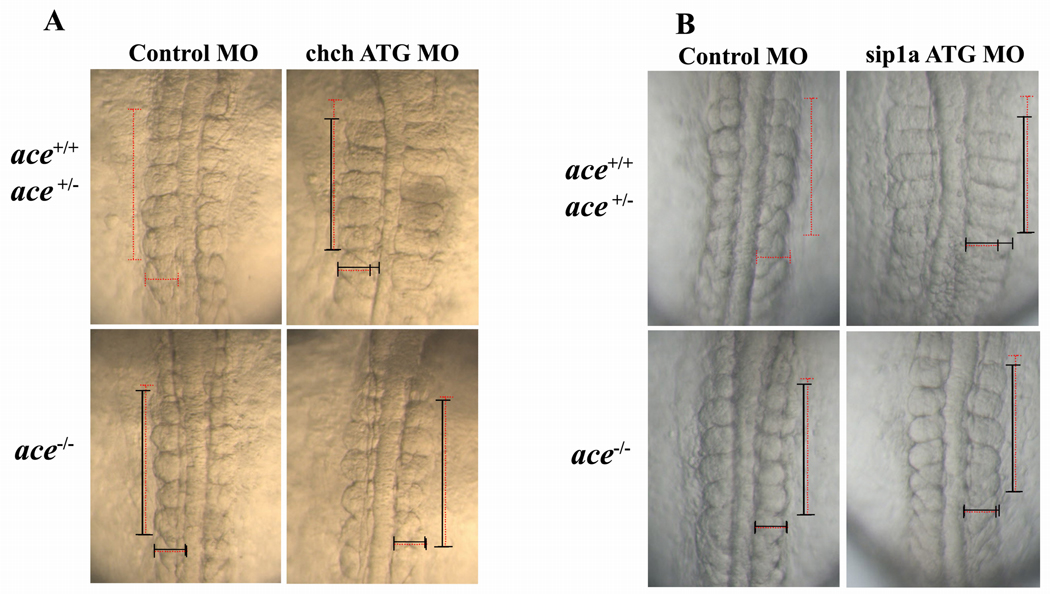
To circumvent this difficulty, we assayed the effects of blocking ChCh and Sip1a in ace/FGF8 mutants (Reifers et al., 1998; Draper et al., 2001). ace mutants show a loss of the isthmus and cerebellum, but do not have overt somite defects due to redundant FGF activity (Reifers et al., 1998; Sawada et al., 2001). Injection of chch ATG MO into ace homozygous embryos (somite length = 47.4 µm±2.4, n =15, p= 0.003) did not produce the alterations in somite morphology observed in control morpholino injected embryos (somite length = 48.1 µm±1.4, n= 20). In the same experiments, chch morpholino injection decreased somite length in wild type (somite length= 41.5 µm±4.6, n=32) and ace heterozygous siblings (somite length= 40.4 µm±2.4, n=29) (Fig. 4A).
Figure 4. Somite malformation in ChCh and Sip1a compromised embryos can be rescued by reduction of FGF8.
(A) Dorsal views of 10 somite stage and (B) 12 somite stage living embryos, anterior to the top. Somites are narrower at A/P axis, broader at mediolateral axis in wild type and ace heterozygous chch morphants, but not ace homozygous chch morphants (A). ace homozygous sip1a ATG morphants does not have somite phenotype but wild type and ace heterozygous sip1a morphants still have the somite defects. (B). Horizontal and vertical red dotted line spans the first five somites of the control injected embryo width and length for comparison to the first five somite of morphant embryos which is indicated with a black line. Embryos were genotyped for the ace allele after imaging.
Similarly, injection of sip1a ATG MO into ace homozygous embryos did not result in significant narrowing of somites in the A/P axis (somite length = 47.1 µm±2.4, n=9, p<0.0001) compared to control-injected embryos (somite length = 49.0 µm±2.1, n= 22). In the same experiments, somite length was decreased in sip1a ATG MO injected wild type (somite length = 40.1 µm±2.7, n= 17) and ace heterozygous siblings (somite length = 41.3 µm±2.3 n= 16).
These findings suggest that the consequences of chch knockdown on somite size are largely due to expansion of FGF signaling. However, in Sip1a compromised embryos, the overall phenotype is more severe than Chch compromised embryos and reduction of FGF signaling in these embryos is not sufficient to fully rescue the defects. Since Sip1 has wide range of functions that are distinct from known roles for ChCh including regulation of TGF-β and BMP pathways (Remacle et al., 1999; Verschueren et al., 1999; Postigo, 2003; Postigo et al., 2003; Nitta et al., 2004; van Grunsven et al., 2007) and cell fate determination (Remacle et al., 1999; Verschueren et al., 1999) some of the phenotypes observed in sip1a morphants may be due to FGF-independent activities of Sip1a.
Somite defects in ace mutants are unaltered by ChCh knockdown
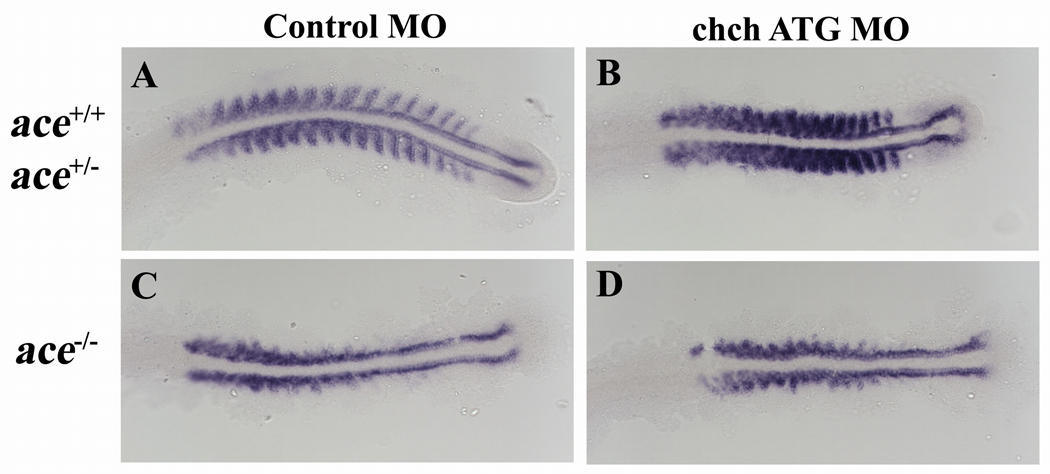
Previous studies have demonstrated the ChCh is an effector of FGF signaling. However, our results reveal that ChCh also represses fgf8 expression. FGF signaling in the somites is required to induce myoD expression and terminal differentiation of subset of fast muscle cells. ace mutants have reduced somatic myoD expression (Groves et al., 2005). If FGF8 functions downstream of ChCh in somitogenesis, somite defects present in ace mutants will not be altered by chch knockdown. Therefore, we performed RNA in-situ hybridization with myoD probe on ChCh compromised ace mutant embryos. As expected, we observed that lateral myoD expression in the somites is lost in ace mutant embryos, but adaxial cell expression was unaffected (Groves et al., 2005) (Fig. 5C). We observed similar reduction of myoD expression in ChCh compromised ace−/− embryos as in control MO injected ace−/− embryos (Fig. 5D). This observation is consistent with the model that FGF8 acts downstream of ChCh during somite formation.
Figure 5. Somite defects in ace mutants are unaltered by ChCh knockdown.
Flat mount RNA in situ hybridization of myoD in 17 somite stage ChCh compromised wild type, ace+/− and ace−/− embryos(A–D). All views are dorsal; anterior to the left. Lateral myoD expression in the somites is lost in ace mutant embryos (C). myoD expression pattern in ChCh compromised ace−/− embryos is similar to control MO injected ace−/− siblings (D).
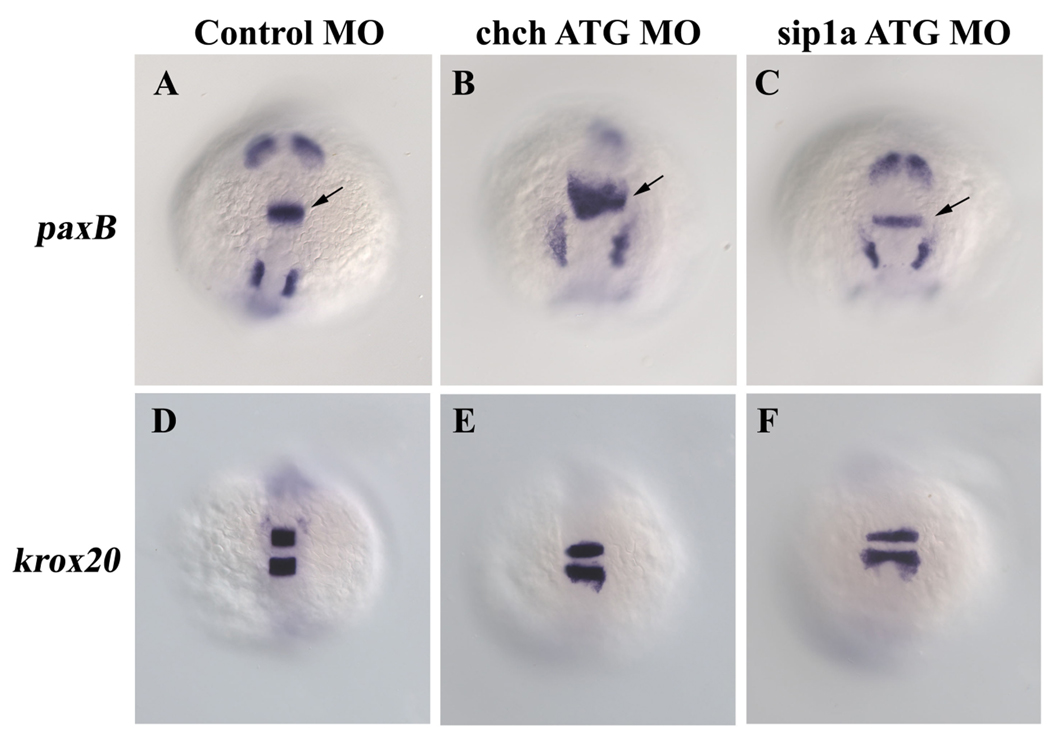
To determine whether ChCh and Sip1a modulate FGF signaling in other tissues, we examined the consequence of knockdown of ChCh and Sip1a in the isthmus, which is a well-characterized site of FGF activity. We also observed that the expression of the FGF target gene pax2a is expanded anteriorly in the isthmus in chch morphants, but not in sip1a morphants (Fig. 6A–C). While the hindbrain is wider in ChCh and Sip1a compromised embryos (probably due to convergence defects), a similar alteration was not observed in krox20 expression (Fig. 6D–F). These results suggest that negative regulation of FGF8 by ChCh is not limited to the mesoderm, but that the ChCh has a broader function in modulating FGF signaling.
Figure 6. Repression of FGF8 by ChCh is not limited to the mesoderm.
Whole mount RNA in situ hybridization of pax2a and krox20 in 10-somite stage ChCh and Sip1a compromised embryos (A–F). All views are dorsal; anterior to the top. FGF target gene pax2a is expanded anteriorly in the isthmus in chch morphants, but not in sip1a morphants (A–C). The anterior extent of krox20 expression is not altered by chch or sip1a knockdown (D–F). Arrows denote isthmus.
Discussion
ChCh and Sip1 modulate FGF-dependent processes and act as a switch between mesodermal and neural inducing activities of FGF in chick, Xenopus and zebrafish (Sheng et al., 2003; Londin et al., 2007b). Although their regulatory properties and function during gastrulation have been studied extensively, very little is known about the requirements for ChCh and Sip1a at other stages. In zebrafish, both genes are expressed in the developing mesoderm (Londin et al., 2007a; Delalande et al., 2008). Previous studies using SIP1 knockout mice and sip1 morphant zebrafish embryos did not clearly identify the function of Sip1 in somitogenesis (Maruhashi et al., 2005; Delalande et al., 2008).
In the present study, we characterized the function of ChCh and Sip1a in zebrafish somitogenesis. Our data revealed that chch and sip1a knockdown resulted in embryos with somites that are less extended thru A/P axis while over-extended in the mediolateral axis. In addition, cyclic expression of her1 and her7 is maintained in formed somites in these embryos. We observed that these defects correlated with an anterior expansion of FGF8 expression in the tailbud. In ChCh morphants, the defects in somite morphology could almost entirely suppressed by blocking FGF8, while the same treatment only partially restored the overall defects in sip1a morphants. Taken together, these findings demonstrate that ChCh and Sip1a regulate somitogenesis by mediating the position of the FGF8 mediated wavefront in the zebrafish PSM.
Expanded FGF8 expression in PSM would be predicted to alter the FGF gradient that regulates somite length (Sawada et al., 2001). However, our finding that somite width was also altered by the enhanced FGF8 expression was surprising. Because expression of dominant-negative ChCh blocks FGF mediated mesoderm induction in animal cap assays (Sheng et al., 2003), ChCh is generally thought of as a positive effector of FGF signaling. ChCh is induced in response to FGF in chick, xenopus and zebrafish (Sheng et al., 2003; Londin et al., 2007a) and our results demonstrate that ChCh represses expression of FGF8, implying that it functions in a negative feedback loop to repress FGF signaling. In a broad context, this relationship is consistent with functions of ChCh in cell movement. Previous studies have shown that ChCh impedes cell movement (Sheng et al., 2003; Londin et al., 2007b). While FGFs have complex roles in cell migration, in many tissues FGF promotes cell migration (Sun et al., 1999; Ciruna and Rossant, 2001; Yang et al., 2002). Therefore it is conceivable that in the effects of ChCh on cell movement are due to blocking, rather then promoting FGF signaling. Further studies are needed to elucidate the precise mechanisms by which ChCh modulates FGF signal transduction.
The somite phenotype observed in sip1a ATG morphants is stronger than chch ATG morphants and although somite phenotype can be rescued by blocking FGF activity, the overall defects cannot be fully restored. Thus far, Sip1 is the only described transcriptional target of ChCh. In several situations, overexpression of sip1 is sufficient to compensate for ChCh deficits (Sheng et al., 2003; Snir et al., 2006). However, Sip1 has a wide range of activities that are likely to be ChCh independent. These include regulation of TGFβ pathways (Remacle et al., 1999; Verschueren et al., 1999; Postigo, 2003; Postigo et al., 2003), expression of BMP4 (Nitta et al., 2004; van Grunsven et al., 2007), mesodermal gene expression (Remacle et al., 1999; Verschueren et al., 1999) and E-cadherin transcription (Remacle et al., 1999; Comijn et al., 2001).
The functional differences between the alternatively spliced forms of zebrafish Sip1a remains unclear. The previously described alternatively spliced form of sip1a lacks a zinc finger (Delalande et al., 2008). Because we identified a structurally similar form of Sip1b (Supp. Fig. 2), we reasoned that the two forms have unique functions. To test for distinct activities during somitogenesis, we used splice morpholinos to block production of each form while leaving the other intact. Our approach demonstrated the effectiveness of morpholinos to specifically eliminate alternative spliced message. However, we were unable to detect somite defects or any other patterning defects in the respective Sip1a form-specific morphants. This suggests that each form can compensate for loss of the other during somitogenesis or that protein derived from maternal mRNA is sufficient to compensate for the loss of wild-type zygotic mRNA.
In conclusion, we studied the functions of ChCh and Sip1a during zebrafish somitogenesis and found that ChCh and Sip1a modulate somite morphogenesis by repressing FGF8 expression at the PSM. Significantly, we determined that Fgf8 is downstream of ChCh, suggesting a negative feedback loop between chch/sip1a and fgf8. Our data also demonstrates that regulation of FGF signaling by ChCh is not limited to the PSM. FGF signaling has diverse functions in a many biological processes and investigation of the vertebrate EST databases reveals that ChCh transcripts are detected in low levels in a wide array of tissues (data not shown). It will therefore be important for future studies to determine the importance of modulation of FGF signaling by ChCh in these contexts.
Experimental procedures
Adult fish and embryo maintenance
Adult zebrafish strains and embryos obtained from natural crossings were maintained at 28.5°C. Developmental stages of the embryos were determined according to Kimmel et. al. (Kimmel et al., 1995).
Expression constructs, mRNA synthesis and morpholinos
spry4 ORF was amplified from 10 somite stage wild type total first strand cDNA using GTTCTAGAGGCTCGAGGAAGGTCCTGCAAACCAT/TCTTTTTGCAGGATCCTGAGGAACACGACCTACA primer pair. Amplified fragment is then cloned to pCS2+ at BamHI and XhoI sites. Capped sense mRNA was synthesized using mMESSAGE mMACHINE Kit (Ambion).
The sequences of the morpholinos used are
chch ATG MO 5'- CAGTATAGTCCAGATCAGAAGACGC -3’,
chch ATG MO2 5’- GCTTCTGGACACAACCGGTACACAT -3’(Londin et al., 2007b).
sip1a and sip1b ATG and splice morpholinos are kindly provided by Iain T. Shepherd (Delalande et al., 2008).
sip1a splice variant targeting MO2 5’- GTCTAAATGTGATATACCTGTGC -3’
sip1a splice variant targeting MO3 5’- CGCGTACATACCACTTTCAGTCTTC -3’
Primers used to monitor the efficiency of the splice variant targeting MO are:
MO2: ATGTACGCGTGTGACTTGTG / CATTTGTCGCACTGGTAAGG
MO3: TTAAGAAGACTGAAAGTGGAAAGC / CATTTGTCGCACTGGTAAG
Standard control oligo (Gene Tools) is used as control. mRNA and morpholino solutions were diluted to desired concentration with 0.2M KCl supplemented with phenol red. Typically, 500 pg of spry4 mRNA, 10–15ng of ChCh ATG morpholino1 and ChCh ATG morpholino2, 2–4ng of sip1a ATG, 10ng of sip1a splice morpholino, 5ng of sip1b ATG, 1ng of sip1b splice morpholino is injected to one- to two-cell stage embryos.
Whole mount in-situ hybridization and immunohistochemistry
In situ hybridizations were performed according to Thisse et. al. (Thisse et al., 1993). Digoxigenin labeled probes for in situ hybridization was synthesized using T7, T3 or Sp6 RNA polymerase (Roche). Hybridized probes were detected using NBT/BCIP system (Roche). Stained embryos were re-fixed in 4% PFA and either stored in 100% methanol or cleared in Benzyl benzoate/benzyl alcohol solution (2:1) and mounted in Canada balsam/methyl salicylate (2.5% v/v) or flat mounted in 70% glycerol. Embryos were viewed with Zeiss Axioplan microscope, digitally photographed with Zeiss Axiocam camera. Images were processed and assembled with Zeiss Axiovision and Adobe Photoshop.
Genotyping acerebellar (ace) mutants
chch and sip1a ATG MO injected embryos obtained from ace heterozygous incross were genotyped following imaging. PCR primers to genotype ace embryos are GCCAAGCTTATAGTAGAGAC/ AAGTGATGACTTTTTCAGATA. Following PCR, products were cut with EcoRV which digests the mutant but not wild-type alleles.
Supplementary Material
Acknowledgements
We thank Bernadette Holdener, Jerry Thomsen and members of their laboratories for helpful suggestions; Laura Mentzer for technical support; Heena Rana and Andrew Taibi for fish care. We are also grateful to the many labs that provided reagents including fish stocks, constructs and probes. This work was supported by NIH grant RO1HD043998 (HS).
References
- Amaya E, Musci TJ, Kirschner MW. Expression of a dominant negative mutant of the FGF receptor disrupts mesoderm formation in Xenopus embryos. Cell. 1991;66:257–270. doi: 10.1016/0092-8674(91)90616-7. [DOI] [PubMed] [Google Scholar]
- Aulehla A, Wehrle C, Brand-Saberi B, Kemler R, Gossler A, Kanzler B, Herrmann BG. Wnt3a plays a major role in the segmentation clock controlling somitogenesis. Dev Cell. 2003;4:395–406. doi: 10.1016/s1534-5807(03)00055-8. [DOI] [PubMed] [Google Scholar]
- Bessho Y, Sakata R, Komatsu S, Shiota K, Yamada S, Kageyama R. Dynamic expression and essential functions of Hes7 in somite segmentation. Genes Dev. 2001;15:2642–2647. doi: 10.1101/gad.930601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borland CZ, Schutzman JL, Stern MJ. Fibroblast growth factor signaling in Caenorhabditis elegans. Bioessays. 2001;23:1120–1130. doi: 10.1002/bies.10007. [DOI] [PubMed] [Google Scholar]
- Bottcher RT, Niehrs C. Fibroblast growth factor signaling during early vertebrate development. Endocr Rev. 2005;26:63–77. doi: 10.1210/er.2003-0040. [DOI] [PubMed] [Google Scholar]
- Cinquin O. Understanding the somitogenesis clock: what's missing? Mech Dev. 2007;124:501–517. doi: 10.1016/j.mod.2007.06.004. [DOI] [PubMed] [Google Scholar]
- Ciruna B, Rossant J. FGF signaling regulates mesoderm cell fate specification and morphogenetic movement at the primitive streak. Dev Cell. 2001;1:37–49. doi: 10.1016/s1534-5807(01)00017-x. [DOI] [PubMed] [Google Scholar]
- Comijn J, Berx G, Vermassen P, Verschueren K, van Grunsven L, Bruyneel E, Mareel M, Huylebroeck D, van Roy F. The two-handed E box binding zinc finger protein SIP1 downregulates E-cadherin and induces invasion. Mol Cell. 2001;7:1267–1278. doi: 10.1016/s1097-2765(01)00260-x. [DOI] [PubMed] [Google Scholar]
- Cooke J. Somite abnormalities caused by short heat shocks to pre-neurula stages of Xenopus laevis. J Embryol Exp Morphol. 1978;45:283–294. [PubMed] [Google Scholar]
- Cooke J, Zeeman EC. A clock and wavefront model for control of the number of repeated structures during animal morphogenesis. J Theor Biol. 1976;58:455–476. doi: 10.1016/s0022-5193(76)80131-2. [DOI] [PubMed] [Google Scholar]
- Coumoul X, Deng CX. Roles of FGF receptors in mammalian development and congenital diseases. Birth Defects Res C Embryo Today. 2003;69:286–304. doi: 10.1002/bdrc.10025. [DOI] [PubMed] [Google Scholar]
- Dale JK, Maroto M, Dequeant ML, Malapert P, McGrew M, Pourquie O. Periodic notch inhibition by lunatic fringe underlies the chick segmentation clock. Nature. 2003;421:275–278. doi: 10.1038/nature01244. [DOI] [PubMed] [Google Scholar]
- Dale KJ, Pourquie O. A clock-work somite. Bioessays. 2000;22:72–83. doi: 10.1002/(SICI)1521-1878(200001)22:1<72::AID-BIES12>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Delalande JM, Guyote ME, Smith CM, Shepherd IT. Zebrafish sip1a and sip1b are essential for normal axial and neural patterning. Dev Dyn. 2008;237:1060–1069. doi: 10.1002/dvdy.21485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delfini MC, Dubrulle J, Malapert P, Chal J, Pourquie O. Control of the segmentation process by graded MAPK/ERK activation in the chick embryo. Proc Natl Acad Sci U S A. 2005;102:11343–11348. doi: 10.1073/pnas.0502933102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draper BW, Morcos PA, Kimmel CB. Inhibition of zebrafish fgf8 pre-mRNA splicing with morpholino oligos: a quantifiable method for gene knockdown. Genesis. 2001;30:154–156. doi: 10.1002/gene.1053. [DOI] [PubMed] [Google Scholar]
- Dubrulle J, McGrew MJ, Pourquie O. FGF signaling controls somite boundary position and regulates segmentation clock control of spatiotemporal Hox gene activation. Cell. 2001;106:219–232. doi: 10.1016/s0092-8674(01)00437-8. [DOI] [PubMed] [Google Scholar]
- Durbin L, Brennan C, Shiomi K, Cooke J, Barrios A, Shanmugalingam S, Guthrie B, Lindberg R, Holder N. Eph signaling is required for segmentation and differentiation of the somites. Genes Dev. 1998;12:3096–3109. doi: 10.1101/gad.12.19.3096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsberg H, Crozet F, Brown NA. Waves of mouse Lunatic fringe expression, in four-hour cycles at two-hour intervals, precede somite boundary formation. Curr Biol. 1998;8:1027–1030. doi: 10.1016/s0960-9822(07)00424-1. [DOI] [PubMed] [Google Scholar]
- Furthauer M, Reifers F, Brand M, Thisse B, Thisse C. sprouty4 acts in vivo as a feedback-induced antagonist of FGF signaling in zebrafish. Development. 2001;128:2175–2186. doi: 10.1242/dev.128.12.2175. [DOI] [PubMed] [Google Scholar]
- Furthauer M, Thisse C, Thisse B. A role for FGF-8 in the dorsoventral patterning of the zebrafish gastrula. Development. 1997;124:4253–4264. doi: 10.1242/dev.124.21.4253. [DOI] [PubMed] [Google Scholar]
- Furthauer M, Van Celst J, Thisse C, Thisse B. Fgf signalling controls the dorsoventral patterning of the zebrafish embryo. Development. 2004;131:2853–2864. doi: 10.1242/dev.01156. [DOI] [PubMed] [Google Scholar]
- Gates MA, Kim L, Egan ES, Cardozo T, Sirotkin HI, Dougan ST, Lashkari D, Abagyan R, Schier AF, Talbot WS. A genetic linkage map for zebrafish: comparative analysis and localization of genes and expressed sequences. Genome Res. 1999;9:334–347. [PubMed] [Google Scholar]
- Giudicelli F, Lewis J. The vertebrate segmentation clock. Curr Opin Genet Dev. 2004;14:407–414. doi: 10.1016/j.gde.2004.06.014. [DOI] [PubMed] [Google Scholar]
- Groves JA, Hammond CL, Hughes SM. Fgf8 drives myogenic progression of a novel lateral fast muscle fibre population in zebrafish. Development. 2005;132:4211–4222. doi: 10.1242/dev.01958. [DOI] [PubMed] [Google Scholar]
- Holley SA, Geisler R, Nusslein-Volhard C. Control of her1 expression during zebrafish somitogenesis by a delta-dependent oscillator and an independent wave-front activity. Genes Dev. 2000;14:1678–1690. [PMC free article] [PubMed] [Google Scholar]
- Holley SA, Julich D, Rauch GJ, Geisler R, Nusslein-Volhard C. her1 and the notch pathway function within the oscillator mechanism that regulates zebrafish somitogenesis. Development. 2002;129:1175–1183. doi: 10.1242/dev.129.5.1175. [DOI] [PubMed] [Google Scholar]
- Huang H, Lu FI, Jia S, Meng S, Cao Y, Wang Y, Ma W, Yin K, Wen Z, Peng J, Thisse C, Thisse B, Meng A. Amotl2 is essential for cell movements in zebrafish embryo and regulates c-Src translocation. Development. 2007;134:979–988. doi: 10.1242/dev.02782. [DOI] [PubMed] [Google Scholar]
- Jiang YJ, Aerne BL, Smithers L, Haddon C, Ish-Horowicz D, Lewis J. Notch signalling and the synchronization of the somite segmentation clock. Nature. 2000;408:475–479. doi: 10.1038/35044091. [DOI] [PubMed] [Google Scholar]
- Kimelman D, Maas A. Induction of dorsal and ventral mesoderm by ectopically expressed Xenopus basic fibroblast growth factor. Development. 1992;114:261–269. doi: 10.1242/dev.114.1.261. [DOI] [PubMed] [Google Scholar]
- Kimmel CB, Ballard WW, Kimmel SR, Ullmann B, Schilling TF. Stages of embryonic development of the zebrafish. Dev Dyn. 1995;203:253–310. doi: 10.1002/aja.1002030302. [DOI] [PubMed] [Google Scholar]
- Krens SF, He S, Lamers GE, Meijer AH, Bakkers J, Schmidt T, Spaink HP, Snaar-Jagalska BE. Distinct functions for ERK1 and ERK2 in cell migration processes during zebrafish gastrulation. Dev Biol. 2008;319:370–383. doi: 10.1016/j.ydbio.2008.04.032. [DOI] [PubMed] [Google Scholar]
- LaBonne C, Whitman M. Mesoderm induction by activin requires FGF-mediated intracellular signals. Development. 1994;120:463–472. doi: 10.1242/dev.120.2.463. [DOI] [PubMed] [Google Scholar]
- Lee BM, Buck-Koehntop BA, Martinez-Yamout MA, Dyson HJ, Wright PE. Embryonic neural inducing factor churchill is not a DNA-binding zinc finger protein: solution structure reveals a solvent-exposed beta-sheet and zinc binuclear cluster. J Mol Biol. 2007;371:1274–1289. doi: 10.1016/j.jmb.2007.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Londin ER, Mentzer L, Gates KP, Sirotkin HI. Expression and regulation of the zinc finger transcription factor Churchill during zebrafish development. Gene Expr Patterns. 2007a;7:645–650. doi: 10.1016/j.modgep.2007.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Londin ER, Mentzer L, Sirotkin HI. Churchill regulates cell movement and mesoderm specification by repressing Nodal signaling. BMC Dev Biol. 2007b;7:120. doi: 10.1186/1471-213X-7-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruhashi M, Van De Putte T, Huylebroeck D, Kondoh H, Higashi Y. Involvement of SIP1 in positioning of somite boundaries in the mouse embryo. Dev Dyn. 2005;234:332–338. doi: 10.1002/dvdy.20546. [DOI] [PubMed] [Google Scholar]
- McGrew MJ, Dale JK, Fraboulet S, Pourquie O. The lunatic fringe gene is a target of the molecular clock linked to somite segmentation in avian embryos. Curr Biol. 1998;8:979–982. doi: 10.1016/s0960-9822(98)70401-4. [DOI] [PubMed] [Google Scholar]
- Nitta KR, Tanegashima K, Takahashi S, Asashima M. XSIP1 is essential for early neural gene expression and neural differentiation by suppression of BMP signaling. Dev Biol. 2004;275:258–267. doi: 10.1016/j.ydbio.2004.08.010. [DOI] [PubMed] [Google Scholar]
- Oates AC, Ho RK. Hairy/E(spl)-related (Her) genes are central components of the segmentation oscillator and display redundancy with the Delta/Notch signaling pathway in the formation of anterior segmental boundaries in the zebrafish. Development. 2002;129:2929–2946. doi: 10.1242/dev.129.12.2929. [DOI] [PubMed] [Google Scholar]
- Palmeirim I, Henrique D, Ish-Horowicz D, Pourquie O. Avian hairy gene expression identifies a molecular clock linked to vertebrate segmentation and somitogenesis. Cell. 1997;91:639–648. doi: 10.1016/s0092-8674(00)80451-1. [DOI] [PubMed] [Google Scholar]
- Postigo AA. Opposing functions of ZEB proteins in the regulation of the TGFbeta/BMP signaling pathway. Embo J. 2003;22:2443–2452. doi: 10.1093/emboj/cdg225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postigo AA, Depp JL, Taylor JJ, Kroll KL. Regulation of Smad signaling through a differential recruitment of coactivators and corepressors by ZEB proteins. Embo J. 2003;22:2453–2462. doi: 10.1093/emboj/cdg226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pourquie O. Vertebrate somitogenesis. Annu Rev Cell Dev Biol. 2001;17:311–350. doi: 10.1146/annurev.cellbio.17.1.311. [DOI] [PubMed] [Google Scholar]
- Pourquie O. The segmentation clock: converting embryonic time into spatial pattern. Science. 2003;301:328–330. doi: 10.1126/science.1085887. [DOI] [PubMed] [Google Scholar]
- Prince VE, Holley SA, Bally-Cuif L, Prabhakaran B, Oates AC, Ho RK, Vogt TF. Zebrafish lunatic fringe demarcates segmental boundaries. Mech Dev. 2001;105:175–180. doi: 10.1016/s0925-4773(01)00398-7. [DOI] [PubMed] [Google Scholar]
- Qiu X, Xu H, Haddon C, Lewis J, Jiang YJ. Sequence and embryonic expression of three zebrafish fringe genes: lunatic fringe, radical fringe, and manic fringe. Dev Dyn. 2004;231:621–630. doi: 10.1002/dvdy.20155. [DOI] [PubMed] [Google Scholar]
- Reifers F, Bohli H, Walsh EC, Crossley PH, Stainier DY, Brand M. Fgf8 is mutated in zebrafish acerebellar (ace) mutants and is required for maintenance of midbrain-hindbrain boundary development and somitogenesis. Development. 1998;125:2381–2395. doi: 10.1242/dev.125.13.2381. [DOI] [PubMed] [Google Scholar]
- Remacle JE, Kraft H, Lerchner W, Wuytens G, Collart C, Verschueren K, Smith JC, Huylebroeck D. New mode of DNA binding of multi-zinc finger transcription factors: deltaEF1 family members bind with two hands to two target sites. Embo J. 1999;18:5073–5084. doi: 10.1093/emboj/18.18.5073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saga Y, Takeda H. The making of the somite: molecular events in vertebrate segmentation. Nat Rev Genet. 2001;2:835–845. doi: 10.1038/35098552. [DOI] [PubMed] [Google Scholar]
- Sato Y, Yasuda K, Takahashi Y. Morphological boundary forms by a novel inductive event mediated by Lunatic fringe and Notch during somitic segmentation. Development. 2002;129:3633–3644. doi: 10.1242/dev.129.15.3633. [DOI] [PubMed] [Google Scholar]
- Sawada A, Shinya M, Jiang YJ, Kawakami A, Kuroiwa A, Takeda H. Fgf/MAPK signalling is a crucial positional cue in somite boundary formation. Development. 2001;128:4873–4880. doi: 10.1242/dev.128.23.4873. [DOI] [PubMed] [Google Scholar]
- Serth K, Schuster-Gossler K, Cordes R, Gossler A. Transcriptional oscillation of lunatic fringe is essential for somitogenesis. Genes Dev. 2003;17:912–925. doi: 10.1101/gad.250603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng G, dos Reis M, Stern CD. Churchill, a zinc finger transcriptional activator, regulates the transition between gastrulation and neurulation. Cell. 2003;115:603–613. doi: 10.1016/s0092-8674(03)00927-9. [DOI] [PubMed] [Google Scholar]
- Snir M, Ofir R, Elias S, Frank D. Xenopus laevis POU91 protein, an Oct3/4 homologue, regulates competence transitions from mesoderm to neural cell fates. Embo J. 2006;25:3664–3674. doi: 10.1038/sj.emboj.7601238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X, Meyers EN, Lewandoski M, Martin GR. Targeted disruption of Fgf8 causes failure of cell migration in the gastrulating mouse embryo. Genes Dev. 1999;13:1834–1846. doi: 10.1101/gad.13.14.1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland D, Samakovlis C, Krasnow MA. branchless encodes a Drosophila FGF homolog that controls tracheal cell migration and the pattern of branching. Cell. 1996;87:1091–1101. doi: 10.1016/s0092-8674(00)81803-6. [DOI] [PubMed] [Google Scholar]
- Takada S, Stark KL, Shea MJ, Vassileva G, McMahon JA, McMahon AP. Wnt-3a regulates somite and tailbud formation in the mouse embryo. Genes Dev. 1994;8:174–189. doi: 10.1101/gad.8.2.174. [DOI] [PubMed] [Google Scholar]
- Thisse B, Thisse C. Functions and regulations of fibroblast growth factor signaling during embryonic development. Dev Biol. 2005;287:390–402. doi: 10.1016/j.ydbio.2005.09.011. [DOI] [PubMed] [Google Scholar]
- Thisse C, Thisse B, Schilling TF, Postlethwait JH. Structure of the zebrafish snail1 gene and its expression in wild-type, spadetail and no tail mutant embryos. Development. 1993;119:1203–1215. doi: 10.1242/dev.119.4.1203. [DOI] [PubMed] [Google Scholar]
- van Grunsven LA, Taelman V, Michiels C, Verstappen G, Souopgui J, Nichane M, Moens E, Opdecamp K, Vanhomwegen J, Kricha S, Huylebroeck D, Bellefroid EJ. XSip1 neuralizing activity involves the co-repressor CtBP and occurs through BMP dependent and independent mechanisms. Dev Biol. 2007;306:34–49. doi: 10.1016/j.ydbio.2007.02.045. [DOI] [PubMed] [Google Scholar]
- Verschueren K, Remacle JE, Collart C, Kraft H, Baker BS, Tylzanowski P, Nelles L, Wuytens G, Su MT, Bodmer R, Smith JC, Huylebroeck D. SIP1, a novel zinc finger/homeodomain repressor, interacts with Smad proteins and binds to 5'-CACCT sequences in candidate target genes. J Biol Chem. 1999;274:20489–20498. doi: 10.1074/jbc.274.29.20489. [DOI] [PubMed] [Google Scholar]
- Wahl MB, Deng C, Lewandoski M, Pourquie O. FGF signaling acts upstream of the NOTCH and WNT signaling pathways to control segmentation clock oscillations in mouse somitogenesis. Development. 2007;134:4033–4041. doi: 10.1242/dev.009167. [DOI] [PubMed] [Google Scholar]
- Weinberg ES, Allende ML, Kelly CS, Abdelhamid A, Murakami T, Andermann P, Doerre OG, Grunwald DJ, Riggleman B. Developmental regulation of zebrafish MyoD in wild-type, no tail and spadetail embryos. Development. 1996;122:271–280. doi: 10.1242/dev.122.1.271. [DOI] [PubMed] [Google Scholar]
- Yamamoto A, Amacher SL, Kim SH, Geissert D, Kimmel CB, De Robertis EM. Zebrafish paraxial protocadherin is a downstream target of spadetail involved in morphogenesis of gastrula mesoderm. Development. 1998;125:3389–3397. doi: 10.1242/dev.125.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Dormann D, Munsterberg AE, Weijer CJ. Cell movement patterns during gastrulation in the chick are controlled by positive and negative chemotaxis mediated by FGF4 and FGF8. Dev Cell. 2002;3:425–437. doi: 10.1016/s1534-5807(02)00256-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.