Abstract
Atrial fibrillation (AF) and obesity have both reached epidemic proportions. The impact of obesity on clinical outcomes in patients with established AF is unknown. We analyzed 2492 patients in the Atrial Fibrillation Follow-Up Investigation of Rhythm Management (AFFIRM) study. Body mass index (BMI) was evaluated as a categorical variable (normal: 18.5 to <25; overweight: 25 to <30; obese: ≥ 30). The rate of death from any cause was higher in the normal BMI group (5.8 per 100 patient-years) than in the overweight and obese groups (3.9 and 3.7, respectively). The cardiovascular death rate was highest in the normal BMI group (3.1 per 100 patient-years), lowest in the overweight group (1.5 per 100 patient-years), and intermediate in the obese group (2.1 per 100 patient-years). After adjustment for baseline factors, differences in the risk of death from any cause were no longer significant. However, overweight remained associated with a reduced risk of cardiovascular death (Hazard ratio 0.47, p=0.002). Obese patients were more likely to have an uncontrolled resting heart rate, but rhythm control strategy success was similar across BMI categories. In each of the BMI categories, the risk of death from any cause was similar for patients randomized to a rhythm or a rate control strategy. In conclusion, among patients with established AF, overweight and obesity do not adversely affect overall survival. Obesity does not appear to impact the relative benefit of a rate or rhythm control strategy.
Keywords: Atrial fibrillation, obesity, survival, cardioversion
Obesity, which is epidemic in the United States, is well-established as an independent risk factor for all-cause and cardiovascular mortality in the general population.1–4 However, among patients with certain established cardiovascular conditions, including coronary heart disease5 and heart failure,6 overweight and mild obesity are associated with a lower risk of death – the so-called “obesity paradox”.7,8 Recently, obesity has been demonstrated to be an independent risk factor for the development of atrial fibrillation (AF), a condition which is also rapidly increasing in prevalence.9,10 Given this interconnection between obesity and AF, we sought to determine the relation between obesity and clinical outcomes in patients with established AF. The Atrial Fibrillation Follow-Up Investigation of Rhythm Management (AFFIRM) study provided a large, well-characterized cohort of patients with high-risk AF in which to do so.
METHODS
Details of the AFFIRM protocol have been reported previously.11,12 Briefly, patients with AF requiring long-term treatment and with at least one risk factor for death or stroke were enrolled. Patients were randomized to either a rate-control strategy or a rhythm-control strategy. Mean follow-up was 3.5 years (range: 0 – 6 years).11 Resting heart rate control was assessed at the 1 year visit, and was considered adequate if ≤ 80 beats/minute. The classification of all fatal events and strokes were adjudicated by an independent clinical events committee. The Institutional Review Board of the University of Washington approved the protocol, and each clinical site obtained approval from its local Institutional Review Board. Every patient gave signed, informed consent to participate in the study. The study was supported by the National Heart, Lung, and Blood Institute (ClinicalTrials.gov identifier: NCT00000556).
Height and weight were recorded for AFFIRM participants at the baseline visit. The body mass index (BMI) was calculated by dividing weight in kilograms by the square of the height in meters. BMI was categorized according to the World Health Organization / National Institutes of Health classification scheme, with normal defined as 18.5 to <25; overweight, 25 to <30; and obese ≥ 30. The obese category was further subdivided into mildly obese (BMI 30 to <35), severely obese (BMI 35 to <40, and extremely obese (BMI ≥40).13 Underweight patients (BMI <18.5) were excluded from the analysis.
Baseline characteristics were compared across the 3 BMI groups using ANOVA. The primary endpoint was a comparison of death from any cause. Other clinical endpoints included cardiovascular death and stroke. The number of events per 100 person-years at risk was calculated for patients in each BMI group. Rate ratios were then calculated, comparing each BMI group with the other two groups. Corresponding P-values were calculated using Fisher's 2-tailed exact chi-square test. Cox proportional hazards modeling, using a stepwise regression procedure, was used to determine the independent relationship between BMI category and clinical events. Covariables that remained statistically significant and those that were deemed clinically relevant were included in the final model. These included: age; sex; a history of hypertension, diabetes mellitus, smoking, coronary artery disease, heart failure, or stroke; rhythm at randomization; left ventricular ejection fraction; and randomized treatment group.
In the rate control arm, we assessed the mean resting heart rate and the proportion of patients with uncontrolled resting heart rate (>80 beats per minute (bpm)) at the 1 year visit among patients remaining in AF. In the rhythm control arm, we assessed the proportion of patients never achieving sinus rhythm, the proportion of patients with at least 1 episode of AF during the study period, and the proportion of electrical cardioversions that were successful in restoring sinus rhythm. All analyses were conducted using SAS, version 8.2 (SAS Institute, Cary, North Carolina). A 2-sided p-value <0.05 was considered to represent statistical signficance.
RESULTS
BMI was available for 2518 of 4060 patients enrolled in AFFIRM. After exclusion of underweight patients (n=26), the cohort for this analysis consisted of 2492 patients (61.4% of the total AFFIRM population). Baseline characteristics among patients with and without missing BMI data were generally similar, although patients with missing BMI data were significantly more likely to have a history of coronary artery disease (40% vs. 37%) and heart failure (26% vs. 21%) and were less likely to be enrolled following a first episode of AF (31% vs. 38%).
The mean BMI was 29.0 ± 5.9. The normal BMI, overweight, and obese groups included 637 (25.5%), 965 (38.7%), and 890 patients (35.7%), respectively. Among the patients in the obese group, 556 were mildly obese (22.3% of total), 207 were severely obese (8.3% of total), and 127 were extremely obese (5.1% of total). Significant differences between the BMI groups were noted with respect to numerous baseline comorbidities and medications (Table 1).
Table 1.
Baseline Characteristics according to Body Mass Index Category
| Variable | Body Mass Index (kg/m2) | P value | ||
|---|---|---|---|---|
| 18.5 to <25 (n=637) |
25 to <30 (n=965) |
≥30 (n=890) |
||
| Age, mean (SD), years | 72.4 ± 7.1 | 70.7 ± 7.5 | 66.4 ± 8.4 | <0.001 |
| Body mass index, mean (SD) (kg/m2) | 22.7 ± 1.6 | 27.5 ± 1.5 | 35.2 ± 5.1 | <0.001 |
| Female | 321 (50%) | 305 (32%) | 353 (40%) | <0.001 |
| Rate control group | 310 (49%) | 499 (52%) | 440 (49%) | 0.4 |
| Hypertension | 392 (62%) | 658 (68%) | 724 (81%) | <0.001 |
| Diabetes mellitus | 73 (12%) | 163 (17%) | 264 (30%) | <0.001 |
| Smoking | 86 (14%) | 99 (10%) | 104 (12%) | 0.1 |
| History of coronary artery disease | 201 (32%) | 404 (42%) | 319 (36%) | <0.001 |
| Prior MI | 87 (14%) | 185 (19%) | 141 (16%) | 0.01 |
| Prior heart failure | 124 (20%) | 190 (20%) | 217 (24%) | 0.02 |
| Prior stroke or TIA | 98 (15%) | 123 (13%) | 93 (11%) | 0.02 |
| First episode of AF | 214 (34%) | 358 (37%) | 943 (38%) | 0.005 |
| AF at randomization | 288 (46%) | 444 (46%) | 396 (45%) | 0.8 |
| Systolic blood pressure, mean (SD) (mmHg) | 136 ± 18.5 | 134 ± 18.8 | 134 ± 18.6 | 0.07 |
| Ejection fraction, mean (SD) (%) | 55.9 ± 7.9 | 55.7 ± 7.3 | 55.3 ± 7.8 | 0.5 |
| Left atrial dimension >4cm | 424 (67%) | 702 (73%) | 716 (76%) | <0.001 |
| Warfarin | 555 (87%) | 844 (87%) | 771 (87%) | 0.9 |
| ACE inhibitor | 208 (33%) | 371 (38%) | 411 (46%) | <0.001 |
| Beta blocker | 266 (42%) | 451 (47%) | 404 (45%) | 0.1 |
| Calcium channel blocker | 142 (23%) | 195 (21%) | 234 (27%) | 0.06 |
| Digoxin | 286 (47%) | 351 (39%) | 375 (44%) | 0.005 |
| Amiodarone | 122 (19%) | 188 (19%) | 178 (20%) | 0.6 |
| Sotalol | 104 (16%) | 147 (15%) | 152 (17%) | 0.5 |
| Class I antiarrhythmic drug | 93 (15%) | 108 (12%) | 90 (11%) | 0.02 |
Clinical event rates are presented in Table 2. There was no significant difference in the rate of stroke among patients in any of the BMI categories. Death from any cause occurred in 304 patients (12.2%) during the study period. The observed death rate was significantly higher among patients in the normal BMI group (5.8 deaths per 100 patient-years) than among those in the overweight (3.9 deaths per 100 patient-years, p=0.005) and obese groups (3.7 deaths per 100 patient-years, p=0.002). Among patients in the obese group, the death rate was 3.5 per 100 patient-years among mildly obese patients, 4.4 per 100 patient-years among severely obese patients, and 3.3 per 100 patient-years among extremely obese patients (p=NS for comparisons among these groups).
Table 2.
Rate of Clinical Events according to Body Mass Index Category
| Normal (n=637) |
Overweight (n=965) |
Obese (n=890) |
|
|---|---|---|---|
| Death from any cause per 100 person-years (95% CI) | 5.8 (4.8–7.1) | 3.9 (3.3–4.7)* | 3.7 (3.0–4.5)* |
| Cardiovascular death per 100 person-years (95% CI) | 3.1 (2.4–4.0) | 1.5 (1.1–2.0)* | 2.1 (1.6–2–8) |
| Stroke per 100 person-years (95% CI) | 1.1 (0.7–1.8) | 1.2 (0.8–1.7) | 1.2 (0.8–1.7) |
All deaths were accounted for, but the cause of death was unknown for 27 patients (8.8% of deaths).
Event rates per 100 person-years for AFFIRM patients with missing BMI data (n=1542): death from any cause: 5.4 (4.9–6.4); cardiovascular death: 2.8 (2.4–3.2); stroke: 1.3 (1.0–1.6).
All comparisons between the overweight and obese groups were non-significant.
P<0.05 for comparison with normal BMI group.
Cardiovascular death occurred in 148 patients (5.9%). The observed cardiovascular death rate was highest among patients in the normal BMI group (3.1 per 100 patient-years), lowest among those in the overweight group (1.5 per 100 patient-years, p=0.001 vs. normal BMI group), and intermediate among those in the obese group (2.1 per 100 patient-years, p=0.07 vs. normal BMI group). Among patients in the obese group, the cardiovascular death rate was 1.9 per 100 patient-years among mildly obese patients, 2.7 per 100 patient-years among severely obese patients, and 2.2 per 100 patient-years among extremely obese patients (p=NS for comparisons among these groups).
Results of multivariable Cox proportional hazards models are presented in Table 3. After adjustment for relevant covariables, neither overweight nor obesity was significantly associated with the risk of death from any cause. Overweight was significantly associated with a lower risk of cardiovascular death (Hazard Ratio (HR) 0.47, p=0.002).
Table 3.
Multivariate analysis of death from any cause and cardiovascular death.
| Variable | Hazard ratio (95% CI) | p value |
|---|---|---|
| Death from any cause: | ||
| Overweight | 0.74 (0.53 – 1.03) | 0.07 |
| Obese | 0.82 (0.57 – 1.18) | 0.3 |
| Age (per year) | 1.06 (1.04 – 1.08) | <0.001 |
| Female gender | 0.85 (0.63 – 1.15) | 0.3 |
| Hypertension | 1.29 (0.94 – 1.75) | 0.12 |
| Diabetes | 1.99 (1.48 – 2.66) | <0.001 |
| Smoking | 1.84 (1.27 – 2.65) | 0.001 |
| Coronary artery disease | 1.82 (1.37 – 2.42) | <0.001 |
| Congestive heart failure | 2.05 (1.53 – 2.76) | <0.001 |
| Stroke | 1.69 (1.20 – 2.39) | 0.003 |
| Ejection fraction <50% | 1.17 (1.01 – 1.36) | 0.04 |
| AF at randomization | 0.82 (0.63 – 1.07) | 0.14 |
| Rhythm control arm | 1.23 (0.94 – 1.6) | 0.14 |
| Cardiovascular death: | ||
| Overweight | 0.47 (0.29 – 0.76) | 0.002 |
| Obese | 0.69 (0.42 – 1.14) | 0.15 |
| Age (per year) | 1.04 (1.01 – 1.07) | 0.005 |
| Female gender | 1.12 (0.74 – 1.71) | 0.6 |
| Hypertension | 1.36 (0.86 – 2.16) | 0.2 |
| Diabetes | 2.36 (1.57 – 3.57) | <0.001 |
| Smoking | 1.55 (0.92 – 2.63) | 0.10 |
| Coronary artery disease | 1.80 (1.20 – 2.72) | 0.005 |
| Congestive heart failure | 2.56 (1.69 – 3.89) | <0.001 |
| Stroke | 1.97 (1.19 – 3.24) | 0.008 |
| Ejection fraction <50% | 1.41 (1.16 – 1.72) | <0.001 |
| AF at randomization | 0.80 (0.55 – 1.17) | 0.3 |
| Rhythm control arm | 1.14 (0.78 – 1.66) | 0.5 |
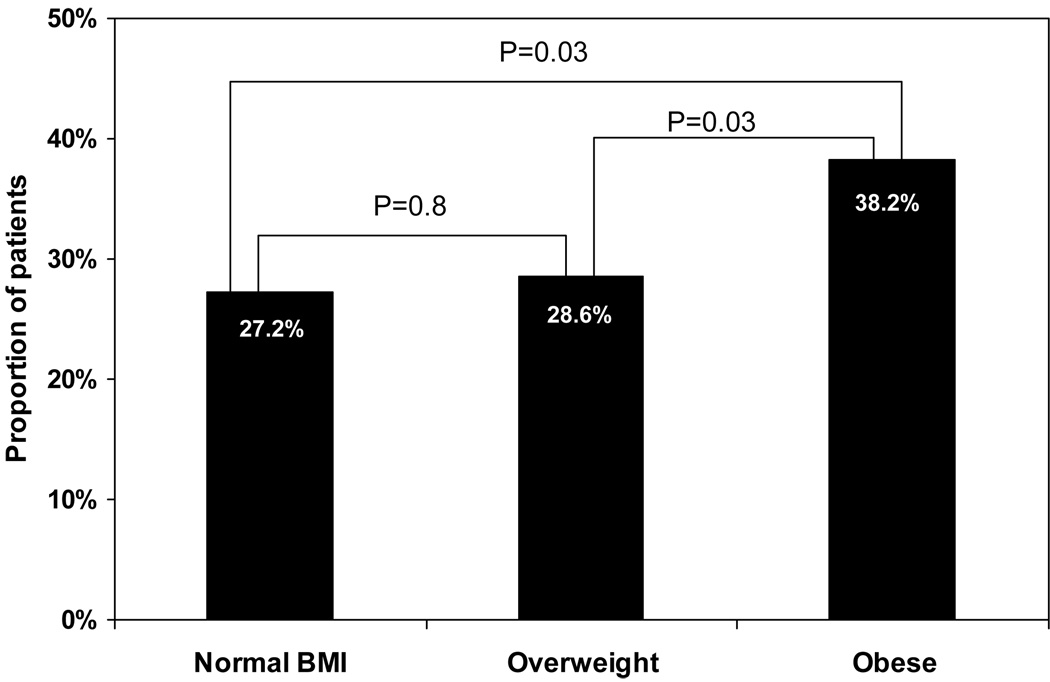
Complete data were available for 1249 patients randomized to a rate control strategy. There were 522 patients (42%) in AF at the time of the baseline visit, and the mean resting heart rate was similar among patients in each of the BMI groups (normal: 80.7 ± 14.8 bpm; overweight: 80.0 ± 13.4 bpm; obese: 80.7 ± 14.3 bpm, p=0.9). At the 1 year visit, the number of patients in AF increased to 642, comprising 56% of patients with available 1 year follow-up. The proportion of patients receiving beta blockers and calcium channel blockers at 1 year was similar in each of the BMI groups, while digoxin use was significantly lower among overweight patients (normal BMI: 59.2%, overweight: 44.1%; obese: 53.7%, p<0.01). For patients in AF, the mean resting heart rate at 1 year was significantly higher among obese patients (78.7 ± 13.2 bpm) than among patients in the normal BMI group (75.1 ± 12.8 bpm, p=0.02) and tended to be higher than among overweight patients (76.1 ± 12.5 bpm, p=0.06). The proportion of patients in AF with uncontrolled resting heart rate (>80 bpm) at 1 year was significantly higher among obese patients than among patients in each of the other BMI groups (Figure). After adjustment for baseline differences and rate control medication use at 1 year, obese patients remained more likely to have an uncontrolled resting heart rate when compared to patients in the normal BMI group (HR 1.9, 95% CI 1.1–3.1, p=0.01). Few patients in AF had a resting heart rate >100bpm at the 1 year visit: 5 in the normal BMI group (3.4%), 9 in the overweight group (3.4%), and 11 in the obese group (4.6%) (p=NS for all comparisons).
Figure.
Proportion of patients in atrial fibrillation with uncontrolled resting heart rate at 1 year, according to body mass index category.
Complete data were available for 1243 patients randomized to a rhythm control strategy. The proportion of patients never achieving sinus rhythm was similar among patients with normal BMI (4.0%), overweight patients (4.8%), and obese patients (5.8%) (p=NS). In addition, there was no significant difference in the proportion of patients with at least 1 episode of AF during the study period (66.1%, 67.3%, and 67.1%, respectively, p=NS).
Electrical cardioversion was attempted on 602 occasions in 400 patients. The proportion of patients that had at least 1 successful cardioversion was similar among patients with normal BMI (90.7%), overweight patients (85.2%), and obese patients (83.0%) (p=NS), as was the proportion of first attempted cardioversions that were successful (87.2%, 83.2%, and 77.6%, respectively, p=NS).
In each of the BMI categories, the risk of death from any cause was similar for patients randomized to a rhythm control strategy or a rate control strategy. The adjusted HR was 0.92 (95% CI 0.56–1.51, p=0.7) among patients with normal BMI, 1.45 (95% CI 0.93–2.23, p=0.10) among overweight patients, and 1.35 (95% CI 0.84–2.17, p=0.2) among obese patients. A test for interaction between BMI category and randomized treatment strategy with respect to the risk of death was not significant (p >0.1).
DISCUSSION
Despite the large number of patients with both obesity and AF, the literature regarding the relationship between these 2 epidemics is sparse. Obesity is an important risk factor for the development of AF.9,10,14 Among patients with established AF, obesity is associated with progression from paroxysmal to permanent AF,15 and obesity does not appear to affect outcomes following radiofrequency catheter ablation of AF.16 To our knowledge, however, ours is the first study to assess the impact of obesity on morbidity and mortality among patients with established AF.
In our study, patients in the overweight and obese BMI categories comprised a remarkable 74% of the study population. Although obesity is associated with an increased risk of overall and cardiovascular mortality in the general population,1–4 we found that among patients with established AF overweight and obesity - even severe and extreme obesity - were not associated with a higher risk of death, and overweight was independently associated with a substantially reduced risk of death from cardiovascular causes. We also demonstrated that strict rate control was more difficult to achieve in obese patients, but that there was no relation between BMI category and the ability to maintain rhythm control. Perhaps most importantly from a clinical standpoint, the relative benefit of a rate control versus a rhythm control strategy was similar among each of the BMI groups.
The “obesity paradox” - an association between overweight and obesity and a more favorable prognosis – is poorly understood but has been observed consistently among patients with established cardiovascular disease, including chronic coronary heart disease,5 acute myocardial infarction,17,18 acute19 and chronic heart failure,6,8,17,20 and peripheral arterial disease.21 In this first study to address this issue among patients with established AF, our findings were generally in line with previous studies: overweight and obesity were not associated with an increased risk of death from any cause and overweight was strongly associated with a reduced risk of cardiovascular death.
It is possible that the relation between increasing BMI and improved outcomes is not physiologic but rather is completely explained by confounding.6 In our study, however, after comprehensive adjustment for baseline factors, overweight remained significantly associated with a markedly reduced risk of cardiovascular death. Hence, differences in baseline risk do not appear to completely explain this phenomenon.
Some authorities have suggested that the obesity paradox implies the presence of poor nutritional status and/or cachexia among patients with a lower BMI, and that this leads to the association between a higher BMI and a reduced mortality risk.5,8,22 Indeed, it is a consistent finding that underweight patients have a substantially increased mortality risk.8,23 However, in our study we excluded underweight patients yet still observed a lower risk of cardiovascular death among patients in the overweight group.
It is possible that the presence of additional body mass, although predisposing to the development of cardiovascular disease and therefore premature death, is truly protective once cardiovascular disease has become established. Overweight and obese patients may have greater metabolic reserve than lean individuals, allowing for a greater tolerance for metabolic stress.6 Adipose tissue produces soluble tumor necrosis factor α (TNF α) receptors, which may play an anti-inflammatory, cardioprotective role.24 The higher blood pressure levels seen in overweight and obese patients may allow for greater up-titration of therapies such as angiotensin converting enzyme inhibitors and beta blockers, drugs that are likely to be life-extending in many patients with AF.6,25 Each of these potential mechanisms, however, remains speculative.
Finally, it is possible that the current classification of “overweight” is suboptimal. In the present study, as well as in general population studies, there is little evidence that a BMI in the overweight range is associated with excess mortality.1,2 With this in mind, and in the absence of definitive data regarding the impact of purposeful weight loss in patients with AF (or in other disease states exhibiting an obesity paradox), the approach to elevated BMI remains uncertain. Therefore, aggressive modification of conventional risk factors and an increase in moderate physical activity should remain the focus of lifestyle interventions.
With respect to the treatment of AF, we found that the ability to successfully perform electrical cardioversion, and the long-term success of a rhythm control strategy, were similar irrespective of BMI category. In contrast, the likelihood of obtaining adequate heart rate control was substantially lower in obese patients. Importantly, however, we found similar outcomes for rate and rhythm control strategies in each of the BMI categories. Therefore, our results suggest that the decision to pursue either a rate or rhythm control strategy in an individual patient should be based on factors other than the BMI.
This analysis has several potential limitations that require consideration. First, baseline data on BMI were missing for a considerable number of AFFIRM patients, and there was a modest imbalance in several baseline characteristics and in the clinical event rates in this group. Because the distribution of BMI in those patients with missing data is unknown, the potential effect on our results is uncertain. Second, data on BMI were collected only at the baseline visit. We are therefore unable to explore the relationship between changes in BMI over time and clinical outcomes. Third, despite making comprehensive adjustments for baseline differences between patients in each of the BMI categories, residual confounding might remain. Finally, although BMI is the most commonly used measure of obesity, it does not directly distinguish between adipose and lean tissue or central and peripheral adiposity.6
Acknowledgments
The AFFIRM study was supported by the National Heart, Lung, and Blood Institute. The present analysis was supported by the Cardiovascular Research Institute, Washington Hospital Center, Washington DC.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors report no financial relationships and no conflicts of interest.
References
- 1.Pischon T, Boeing H, Hoffmann K, Bergmann M, Schulze MB, Overvad K, van der Schouw YT, Spencer E, Moons KG, Tjonneland A, Halkjaer J, Jensen MK, Stegger J, Clavel-Chapelon F, Boutron-Ruault MC, Chajes V, Linseisen J, Kaaks R, Trichopoulou A, Trichopoulos D, Bamia C, Sieri S, Palli D, Tumino R, Vineis P, Panico S, Peeters PH, May AM, Bueno-de-Mesquita HB, van Duijnhoven FJ, Hallmans G, Weinehall L, Manjer J, Hedblad B, Lund E, Agudo A, Arriola L, Barricarte A, Navarro C, Martinez C, Quiros JR, Key T, Bingham S, Khaw KT, Boffetta P, Jenab M, Ferrari P, Riboli E. General and abdominal adiposity and risk of death in Europe. N Engl J Med. 2008;359:2105–2120. doi: 10.1056/NEJMoa0801891. [DOI] [PubMed] [Google Scholar]
- 2.McGee DL. Body mass index and mortality: a meta-analysis based on person-level data from twenty-six observational studies. Ann Epidemiol. 2005;15:87–97. doi: 10.1016/j.annepidem.2004.05.012. [DOI] [PubMed] [Google Scholar]
- 3.Manson JE, Willett WC, Stampfer MJ, Colditz GA, Hunter DJ, Hankinson SE, Hennekens CH, Speizer FE. Body weight and mortality among women. N Engl J Med. 1995;333:677–685. doi: 10.1056/NEJM199509143331101. [DOI] [PubMed] [Google Scholar]
- 4.Calle EE, Thun MJ, Petrelli JM, Rodriguez C, Heath CW., Jr Body-mass index and mortality in a prospective cohort of U.S. adults. N Engl J Med. 1999;341:1097–1105. doi: 10.1056/NEJM199910073411501. [DOI] [PubMed] [Google Scholar]
- 5.Romero-Corral A, Montori VM, Somers VK, Korinek J, Thomas RJ, Allison TG, Mookadam F, Lopez-Jimenez F. Association of bodyweight with total mortality and with cardiovascular events in coronary artery disease: a systematic review of cohort studies. Lancet. 2006;368:666–678. doi: 10.1016/S0140-6736(06)69251-9. [DOI] [PubMed] [Google Scholar]
- 6.Oreopoulos A, Padwal R, Kalantar-Zadeh K, Fonarow GC, Norris CM, McAlister FA. Body mass index and mortality in heart failure: a meta-analysis. Am Heart J. 2008;156:13–22. doi: 10.1016/j.ahj.2008.02.014. [DOI] [PubMed] [Google Scholar]
- 7.Artham SM, Lavie CJ, Milani RV, Ventura HO. The obesity paradox: impact of obesity on the prevalence and prognosis of cardiovascular diseases. Postgrad Med. 2008;120:34–41. doi: 10.3810/pgm.2008.07.1788. [DOI] [PubMed] [Google Scholar]
- 8.Kalantar-Zadeh K, Block G, Horwich T, Fonarow GC. Reverse epidemiology of conventional cardiovascular risk factors in patients with chronic heart failure. J Am Coll Cardiol. 2004;43:1439–1444. doi: 10.1016/j.jacc.2003.11.039. [DOI] [PubMed] [Google Scholar]
- 9.Wang TJ, Parise H, Levy D, D'Agostino RB, Sr, Wolf PA, Vasan RS, Benjamin EJ. Obesity and the risk of new-onset atrial fibrillation. JAMA. 2004;292:2471–2477. doi: 10.1001/jama.292.20.2471. [DOI] [PubMed] [Google Scholar]
- 10.Wanahita N, Messerli FH, Bangalore S, Gami AS, Somers VK, Steinberg JS. Atrial fibrillation and obesity--results of a meta-analysis. Am Heart J. 2008;155:310–315. doi: 10.1016/j.ahj.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 11.Wyse DG, Waldo AL, DiMarco JP, Domanski MJ, Rosenberg Y, Schron EB, Kellen JC, Greene HL, Mickel MC, Dalquist JE, Corley SD. A comparison of rate control and rhythm control in patients with atrial fibrillation. N Engl J Med. 2002;347:1825–1833. doi: 10.1056/NEJMoa021328. [DOI] [PubMed] [Google Scholar]
- 12.Atrial fibrillation follow-up investigation of rhythm management -- the AFFIRM study design. The Planning and Steering Committees of the AFFIRM study for the NHLBI AFFIRM investigators. Am J Cardiol. 1997;79:1198–1202. [PubMed] [Google Scholar]
- 13.Physical status: the use and interpretation of anthropometry. Report of a WHO Expert Committee. World Health Organ Tech Rep Ser. 1995;854:1–452. [PubMed]
- 14.Watanabe H, Tanabe N, Watanabe T, Darbar D, Roden DM, Sasaki S, Aizawa Y. Metabolic syndrome and risk of development of atrial fibrillation: the Niigata preventive medicine study. Circulation. 2008;117:1255–1260. doi: 10.1161/CIRCULATIONAHA.107.744466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tsang TS, Barnes ME, Miyasaka Y, Cha SS, Bailey KR, Verzosa GC, Seward JB, Gersh BJ. Obesity as a risk factor for the progression of paroxysmal to permanent atrial fibrillation: a longitudinal cohort study of 21 years. Eur Heart J. 2008;29:2227–2233. doi: 10.1093/eurheartj/ehn324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jongnarangsin K, Chugh A, Good E, Mukerji S, Dey S, Crawford T, Sarrazin JF, Kuhne M, Chalfoun N, Wells D, Boonyapisit W, Pelosi F, Jr, Bogun F, Morady F, Oral H. Body mass index, obstructive sleep apnea, and outcomes of catheter ablation of atrial fibrillation. J Cardiovasc Electrophysiol. 2008;19:668–672. doi: 10.1111/j.1540-8167.2008.01118.x. [DOI] [PubMed] [Google Scholar]
- 17.Abdulla J, Kober L, Abildstrom SZ, Christensen E, James WP, Torp-Pedersen C. Impact of obesity as a mortality predictor in high-risk patients with myocardial infarction or chronic heart failure: a pooled analysis of five registries. Eur Heart J. 2008;29:594–601. doi: 10.1093/eurheartj/ehn010. [DOI] [PubMed] [Google Scholar]
- 18.Mehta RH, Califf RM, Garg J, White HD, Van de Werf F, Armstrong PW, Pieper KS, Topol EJ, Granger CB. The impact of anthropomorphic indices on clinical outcomes in patients with acute ST-elevation myocardial infarction. Eur Heart J. 2007;28:415–424. doi: 10.1093/eurheartj/ehl329. [DOI] [PubMed] [Google Scholar]
- 19.Fonarow GC, Srikanthan P, Costanzo MR, Cintron GB, Lopatin M. An obesity paradox in acute heart failure: analysis of body mass index and inhospital mortality for 108,927 patients in the Acute Decompensated Heart Failure National Registry. Am Heart J. 2007;153:74–81. doi: 10.1016/j.ahj.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 20.Curtis JP, Selter JG, Wang Y, Rathore SS, Jovin IS, Jadbabaie F, Kosiborod M, Portnay EL, Sokol SI, Bader F, Krumholz HM. The obesity paradox: body mass index and outcomes in patients with heart failure. Arch Intern Med. 2005;165:55–61. doi: 10.1001/archinte.165.1.55. [DOI] [PubMed] [Google Scholar]
- 21.Galal W, van Gestel YR, Hoeks SE, Sin DD, Winkel TA, Bax JJ, Verhagen H, Awara AM, Klein J, van Domburg RT, Poldermans D. The obesity paradox in patients with peripheral arterial disease. Chest. 2008;134:925–930. doi: 10.1378/chest.08-0418. [DOI] [PubMed] [Google Scholar]
- 22.Anker SD, Ponikowski P, Varney S, Chua TP, Clark AL, Webb-Peploe KM, Harrington D, Kox WJ, Poole-Wilson PA, Coats AJ. Wasting as independent risk factor for mortality in chronic heart failure. Lancet. 1997;349:1050–1053. doi: 10.1016/S0140-6736(96)07015-8. [DOI] [PubMed] [Google Scholar]
- 23.Simoons ML, Bonneux L. Obesity, cardiology, and beyond. J Am Coll Cardiol. 2008;52:986–987. doi: 10.1016/j.jacc.2008.05.052. [DOI] [PubMed] [Google Scholar]
- 24.Mohamed-Ali V, Goodrick S, Bulmer K, Holly JM, Yudkin JS, Coppack SW. Production of soluble tumor necrosis factor receptors by human subcutaneous adipose tissue in vivo. Am J Physiol. 1999;277:E971–E975. doi: 10.1152/ajpendo.1999.277.6.E971. [DOI] [PubMed] [Google Scholar]
- 25.Anker SD, Negassa A, Coats AJ, Afzal R, Poole-Wilson PA, Cohn JN, Yusuf S. Prognostic importance of weight loss in chronic heart failure and the effect of treatment with angiotensin-converting-enzyme inhibitors: an observational study. Lancet. 2003;361:1077–1083. doi: 10.1016/S0140-6736(03)12892-9. [DOI] [PubMed] [Google Scholar]