Abstract
Background
Screening-CT is able to discover small peripheral lung nodules. The nature of these nodules is uncertain but it is reasonable that some of them, in particular the non solid ones, could represent precancerous lesions. A previous trial showed a reduction in size of peripheral nodules by inhaled budesonide in subjects with bronchial dysplasia.
Objective
The primary objective of the study was the evaluation of the effect of Budesonide as a chemopreventive agent for lung lesions. The primary endpoint was the modification of lung lesions at ld-CT scan (according to RECIST criteria) after one year of treatment in a person-specific analysis.
Methods
We performed a randomized, double-blind, placebo controlled trial to evaluate whether inhaled budesonide was able to reduce size and number of persistent, undetermined CT-detected lung nodules in high-risk asymptomatic subjects currently undergoing a five-year CT scan screening program at the European Institute of Oncology.
Results
Trial enrollment started in April 2006 and ended in July 2007 with the randomization of 202 current or former smokers with stable CT detected lung nodules set to receive budesonide 800µg or placebo twice-daily for 12 months.
Conclusion
Our trial represents the first phase II study of a chemopreventive intervention focusing on the peripheral lung, where the majority of lung cancers arise. The research was nested into a screening project with clear advantages in participant accrual and reduction of costs. This paper describes the rationale and design of the study, thus focusing on the methodology and operational aspects of the clinical trial. (Clinicaltrials.gov number. NCT00321893)
Keywords: budesonide, lung cancer, chemoprevention, low dose CT scan, screening
1. Introduction
Lung cancer is one of the most common cancers in humans. The annual incidence is steadily growing largely due to tobacco use. In addition to smoking cessation programs, chemoprevention may play a role in high risk individuals in as much as it has the potential to arrest or revert the cancerogenesis progression.
Budesonide is a widely used glucocorticoid for the treatment of asthma and it proved to inhibit all stages of lung carcinogenesis in various mouse models.[1–3] A long-term epidemiological trial on COPD suggested that patients treated with inhaled budesonide had a lower incidence of lung cancer, though the low number of events did not allow any definitive conclusion.[4]
In 2004 Lam et al.[5] conducted a phase IIb trial to determine the effect of inhaled budesonide in smokers with bronchial dysplasia, which is considered the main precursor of squamous cell carcinoma.[6] Subgroup analyses of the trial showed a favorable outcome in reducing the size of non calcified peripheral lung nodules detected by computed tomography (CT).
Spiral low-dose CT scan (ld-CT) of the chest is currently considered the most promising lung cancer screening modalities, thus effectively detecting early-stage and surgically resectable lung cancer in high-risk individuals.[7,8]
Unfortunately, the high rate of benign nodules and issues on making a differential diagnosis are critical factors that currently hamper introduction of large-scale screening programs. Small pulmonary nodules are in fact a frequent finding in the subjects submitted to ld-CT for early detection of lung cancer and have no clinical implications. Our data demonstrated an incidence of undetermined lung nodules in more than 50% of high risk individuals. The nature of these nodules remained uncertain but it is reasonable that some of them could represent precancerous lesions.
In particular, literature suggests that ground glass opacities (GGO), as compared to solid nodules, can represent either localized bronchoalveolar carcinoma without foci of active fibroblastic proliferation or atypical adenomatous hyperplasia[9], i.e. a known putative adenocarcinoma precursor lesion.[10] Kim et al. reported that of 53 persistent ground-glass opacities in 49 patients who underwent resection, 68% proved to be bronchoalveolar carcinoma, 7.5% were adenocarcinoma with predominant brochoalveolar components, 6% were atypical adenomatous hyperplasia, and 19% were nonspecific fibrosis or organizing pneumonia[11]. Similarly, Ohtsuka et al. reported that of 26 patients who underwent resection, bronchoalveolar carcinoma was diagnosed in 10 patients (38%), atypical adenomatous hyperplasia was diagnosed in 15 patients (58%), and focal scar was seen in 1 patient (4%)[12]. Based on this literature we decided to focus on persistent screen detected peripheral lung nodules as target markers to test inhaled budesonide by nesting the chemoprevention trial among the screening study. We conducted a randomized trial to evaluate if administration of Budesonide to individuals at high-risk for lung cancer with undetermined lung nodules at ld-CT was able to determine a shrinkage of lung nodules. Furthermore, in order to explore the hypothesis of a link between lung cancer and COPD - as suggested by literature publications[13] - we also evaluated the effect of budesonide on COPD both by spirometry and spiral CT. The present manuscript describes the design of the trial.
2. Methods
2.1. Design overview
We conducted a randomized double-blinded phase IIb study in which high risk subjects received either budesonide 800 mg or placebo twice a day for one year using a turbuhaler device. Randomization was stratified according to sex, smoking status (current vs. former smokers) and kind of nodule (non solid and partially solid vs. solid nodules). Participants, investigators, and data managers were blinded to treatment allocation. The primary endpoint was the modification of lung lesions at ld-CT scan after one year of treatment (+/− 2 months) in a person-specific analysis (RECIST criteria). A total of 202 subjects were expected to be recruited in 12 months period; 101 per arm.
A schema of the trial design is presented in Figure 1.
Fig. 1.
Trial Design
2.2 Participants selection
Eligible participants were asymptomatic current or former smokers (people who quit smoking within the last 15 years) with a smoking history of more than 20 pack/years and older than 50 years, with persistent lung nodules of more than 4 mm detected at low dose CT scan of the second year on the screening trial. Subjects with solid nodules larger than 8 mm, should have a negative PET scan. Subjects with lung nodules with clearly benign morphological features at CT scan - i.e., homogenous calcification, solid nodules with regular and round or polygonal margins and distance from the pleura < 1 cm - or currently suffering from malignant disease or having had malignant disease within the last 5 years, or with a regular/chronic use of oral or inhaled corticosteroids, were excluded.
The candidates were contacted by phone by trained personnel illustrating the possibility of taking part in a chemoprevention trial and scheduling an appointment for an outpatient visit at the Institute. Randomization was performed within 2 months (i.e., 61 days) from the CT scan. Inclusion and exclusion criteria are here summarized in Figure 2.
Fig. 2.
Inclusion and exclusion criteria
2.3 Recruitment and retention strategy
Pre-Initiation phase
subjects with persistent lung nodules at second or third year of the screening program that were potentially eligible for the trial were indicated to the radiologists. While waiting for the CT scan, the potential candidate was given a leaflet about the possibility of being contacted for taking part in a prevention trial.
Active Recruitment phase
after the evaluation of CT scan by both the radiologist and the thoracic surgeon, the potential candidate was contacted by the staff of the Division of Cancer Prevention and Genetics for a first phone interview and the verification of all inclusion criteria. The trial design was explained and willingness to participate was asked. Candidates accepting to participate or interested in the study were invited for a visit at European Institute of Oncology (EIO) for informed consent and baseline visit.
Retention phase (which include adherence strategies)
a month after randomization, participants received a phone call to check whether the drug was correctly administered. Subjects were scheduled for periodic visits (at 6 months) during which a complete physical exam and lab tests (only at 6 months) were performed. A couple of weeks before the following scheduled appointment, participants were reminded about the visit, blood test and sputum sample and physical exam of month 12. Recruitment and retention effort were evaluated routinely by the site coordinator and the study staff.
2.4 Treatment groups
Patients who signed an informed consent and who met the eligibility criteria were randomly assigned to one of two groups for 12 months of treatment:
Group 1: Budesonide (800 micrograms/twice daily), administered by inhalation through a Turbuhaler® (Each Turbuhaler® contains a total of 200 doses of 400 µg of budesonide each).
Group 2: Budesonide-placebo. A similar appearing Turbuhaler® containing placebo was used daily (two puffs twice a day) by participants assigned to the placebo group.
Given that the only available data about budesonide in humans refer to a 800 µg twice daily dose,[5] and also that the incidence of side effects with once daily dosing versus twice daily dosing was not much different, and a good compliance was observed in the Lam’s study, we decided to use 800 µg/twice-daily with the intent of confirming Lam’s results. Toxicity was evaluated at each visit using the most recent version of the NCI toxicity criteria (CTCAE version 3.0, published 12/12/03). No run-in procedures took place in the trial. Known hypersensitivity to budesonide, lung tuberculosis, and other infection of the airways were the only contraindications. Other inhaled or systemic corticosteroids were avoided or limited during the year of treatment. Any use of systemic or inhaled steroids was clearly documented (time, doses, routes, indications) and strictly followed by the physician. All medications (prescription and over-the-counter), vitamin and mineral supplements, and/or herbs taken by the participant were documented on the concomitant medication CRF and included: start and stop date, dose and route of administration, and indication for use. Medications taken for a procedure (e.g., biopsy) were included. Patients were discouraged from taking unspecified medications.
2.5 Adherence / Compliance to treatment
Although drug concentration was not be measured in the blood, compliance was monitored in the following ways:
Patient Self-Report
patient's history - the most direct source of information - is the most widely employed measure of a patient's adherence to their medical regimen. However, patient self-reporting has been criticized as being too subjective, with patients tending to over-report their adherence by as much as two to fourfold. Positive information is helpful, but false negatives are common.
Calendar completion
Each subject was given a 7-month calendar as a reminder of inhalation. Each subject was asked to cross the corresponding day of the calendar (1 cross for each inhalation, i.e. four crosses per day). Subjects were asked to fill the calendar and some additional space was left for patient’s notes. Each calendar was returned at the next scheduled visit.
Dose count
Each subject received a 3-month supply (at baseline and 3 month visits) or 6-month supply (at 6 month visit) of the drug or placebo and they were asked to return all full and empty Turbuhaler®, irrespective of their use. Doses were counted from turning the grip until the red mark was shown in the dose indicator window indicating that 20 doses were left in the inhaler. Compliance was measured as follows: number of doses taken (i.e., number of doses given-number of doses returned)/number of doses that should have been taken during that period of time. If inhalers were not returned, the compliance was calculated from the calendar. In case of discrepancy between the dose counts of the turbuhaler and the calendar, dose count was taken as the superseding compliance measure.
3. Clinical evaluation and procedures
The visit and data collection schedules are summarized in table 1. Complete physical exam, and safety lab tests were performed at 0, 6 and 12 months. Participants were asked to retest blood cortisol level should they have cortisol value below LLN at month 12. At month 3 an additional visit or phone contact depending on geographical accessibility was performed A phone call at month 13 (i.e., 1 month after the final CT scan) was performed to monitor safety and concomitant medications. Blood and sputum for biomarkers analysis were collected at 0, 6 and 12 months. Lung lesions were measured with ld-CT at 0 and 12 months.
Table 1.
Schedule of Events
| Evaluation/ Procedure |
Registration | Base line |
Random | Month 3 |
Month 6 |
Month 12 |
Taper- off Month 13 |
Early Termination |
|---|---|---|---|---|---|---|---|---|
| Informed Consent | X | |||||||
| Assess Eligibility | X(CT scan) | X | ||||||
| Medical History | X | |||||||
| Physical Exam | X | (X)a | X | X | X | |||
| Laboratory Tests | X | X | X | (X)c | X | |||
| Biomarkers (blood/sputum) | X | X | X | X | ||||
| Study Evaluations/Assessments | X | X | X | X | ||||
| Spirometry | X | X | ||||||
| Lung lesions evaluation | X | X | ||||||
| Concomitant Medications | X | X | X | X | X | X | ||
| Baseline symptoms | X | |||||||
| Dispense Study Agent | X | X | (X)b | |||||
| Collect study agent/Compliance | Xa/(X) | X | X | X | ||||
| Review Agent Diary/Record | X | X | X | X | ||||
| Adverse Events | X | X | X | X | X | |||
| Telephone Contact | (X) | X |
Notes:
Physical exam and vital signs were performed if Month 3 was not a phone contact
Tapering off period
One week after drug tapering off completion, cortisol level was repeated in participants with cortisol levels <LLN at month 12
4. Outcome measures
CT scan evaluation
Investigations were performed with low-dose technology, with a multidetector (8 or 16 slices) High Speed Advantage CT scanner (General Electric Corporation, Milwaukee, Wl, USA) with 140 Kvp, 30 Ma, pitch 1.75, 2.5 mm thickness, single breath, retro-reconstruction at 3mm interval and an effective dose equivalent to patient estimated to be 0.7mSv. Number, minor and maximum diameter, volume and type of lung nodules were registered before treatment and after 12 months.
Quantitative analysis of emphysema was based on attenuation values of CT numbers expressing Hounsfield Units (H.U.) calculated over the entire lung volume. Multiplannar reconstruction and 3-dimensional volume reconstruction were performed on the workstation (Advantage Windows 4.2, General Electric medical system). Lung parenchyma was isolated using a −200 H.U: threshold. Lung representation of voxel values were calculated on the voxel between −1023 H.U: and −200 H.U. Graphic representations of voxel values were calculated as well as minimum and maximum densities, volume and percentage of emphysema were automatically calculated.[14,15]
Laboratory endpoint measurement and respiratory function
Sputum and blood specimens were obtained from enrolled subjects at baseline and after 6 and 12 months of treatment for circulating biomarker measurement and gene methylation assessment.
The induction and treatment of sputum has been accomplished according to the method by Fahy et al.[16] Briefly, the method consisted in aerosol of 3% hypertonic saline solution for 20 minutes and collection of sputum in a previously weighted sterile container (50 ml). Before sputum collection, individuals were asked to rinse their oral cavity with saline solution and to clean their nose to avoid any sputum contamination with salival cells. Aerosol was delivered by an ultrasonic nebulizer producing particles with mean diameter of 2.5 micron at 1 ml/min.
All subjects underwent Pulmonary Function Tests (PFTs), single breath DLCO (Carbone Monoxide Lung Diffusion) and percutaneous arterial saturation. Lung volumes and flow rate were measured in the sitting position according with the American Thoracic Society recommendations[17]. Values were expressed as absolute and a percentage of predicted normal values. The single breath DLCO was measured by infrared CO analyzer and corrected for barometric pressure and temperature according to the American Thoracic Society recommendations. Results were expressed as percentage of the predicted values forced expiratory flow in 1 second (FEV1%) [18]. Percutaneous arterial saturation was assessed by a continuous pulse oximeter.
5. Toxicity and dose modification
Subjects were asked to maintain the full dose throughout the treatment period. However, in case of toxicity, dose modification was applied and recorded.
Toxicity was evaluated at each visit using the most recent version of the NCI toxicity criteria (CTCAE version 3.0, published 12/12/03). If grade 1 toxicity occurred, the patient was to be maintained on full dose. Toxicity was checked depending on clinical relevance. In case of grade 2 toxicity or higher, the dose was changed according to the potential relationship to study drug as listed in the table 2. Since a prolonged use of the drug might cause suppression of adrenal function which could appear after therapy cessation, drug interruption had to be gradual. After treatment completion each participant was provided with a new turbuhaler containing the same drug/placebo as provided during treatment period - i.e., placebo participant got placebo, while budesonide participant got budesonide -. Subjects were instructed to taper-off the study drug by 50% on the first week (one inhalation in the morning and one inhalation in the evening) and by an additional 50% reduction on the second week (1 inhalation in the evening). Drug was then stopped. After an additional week of wash out, each participant with a 12 month cortisol level below the LNL was asked to repeat blood cortisol test.
Table 2.
Dose Reduction Algorithm
| Toxicity | Attribution to study drug | ||||
|---|---|---|---|---|---|
| grade | Unrelated | Unlikely | Possible | Probable | Definite |
| 1 | C | C | C | C | C |
| 2 | C | C | S-R0 | S-R1 | S-R1 |
| 3 | S-R2 | S-R2 | S-R1 | W | W |
| 4 | S-R2 | S-R1 | W | W | W |
C = Continue drug
S-R0 = Stop drug until toxicity reaches grade 1 or lower, then restart drug at full dose (800µg/twice daily). If toxicity resumes, stop drug again until toxicity reaches grade 1 or lower, and then restart at 50% of dose (400µg/twice daily) and maintain the reduced dose.
S-R1 = Stop drug until toxicity reaches grade 1 or lower, then reduce drug by 50% (400µg/twice daily) and maintain the reduced dose.
S-R2 = Stop drug until toxicity reaches grade 1 or lower, then reduce drug by 50% (400µg/twice daily or matched placebo) for 1 month. If toxicity is no more than grade 1, restart drug at full dose (800µg/twice daily). If toxicity resumes after reintroduction of full dose, stop drug again until toxicity reaches grade 1 or lower and then reduce drug by 50% (400µg/twice daily) and maintain this dose. If toxicity does not resolve with initial dose reduction, a second dose reduction of 200µg/twice daily is acceptable and dose will be maintained at this level for the duration of the trial. After the second dose reduction, no further reductions will be permitted and patients with returning toxicities will be removed from the study.
6. Statistical consideration
6.1 Criteria for evaluation and endpoint definition
The primary endpoint was the shrinkage of lung nodules in a per-subject analysis according to RECIST Criteria[19] measured by two observers. A partial response was considered a reduction of 30% or more of the longest diameter for any single nodules larger than 5 mm. For single nodules with longest diameter equal to or less than 5 mm, thought to be less precisely measurable, complete disappearance was considered as a treatment response. In case of multiple lesions a success of treatment was considered a complete or partial response - according to RECIST criteria -. In detail: a complete response when disappearance of all target lesions occurred; partial response when at least a 30% decrease in the sum of the longest diameter of target lesions, taking as reference the baseline longest diameter , was obtained; progression disease when the sum of the longest diameter of target lesions increase at least a 20% (to take into account of measurement error), taking as reference the smallest sum longest diameter recorded since the treatment started or the appearance of one or more new lesions; stable disease: neither sufficient shrinkage to qualify for partial response nor sufficient increase to qualify for progressive disease, taking as reference the smallest sum longest diameter since the treatment started.
6.2 Sample size justification
The planned sample size was 202 subjects (101 per arm), in order to show a treatment effect in 20% of subjects in the budesonide arm, i.e., a nodule shrinkage in 30% in the treated arm versus 10% in the placebo arm (α = 0.05, 1–β = 0.90, chi-square two-sided test). These assumptions were based on previous data on a pilot early detection study with low-dose CT, where the rate of spontaneous regression of undetermined lung nodules in the follow-up was as high as 10%.[20] The sample size was adjusted considering a 10% of non-informative drop-out rate. Accrual rate was expected to be 17 subjects/month. Considering 20% of acceptance rate of the pool of eligible subjects at the CT scan, the trial was proposed to 85 subjects every month (20 cases a week).
6.3 Randomization, stratification and statistical analysis
In order to keep at minimum the imbalance in treatments a stratified blocked randomization strategy was used thus considering only the relevant prognostic factors. Subjects were stratified according to gender, smoking habits (current vs. former smokers) and nodule characteristics (solid vs. GGO, represented by semisolid or non solid nodules). If a subject had both solid and GGO nodules, the patient was stratified as GGO. In Lam’s study a slightly better outcome was indeed demonstrated in females and former smokers. Regarding the nodule type a GGO was expected to be more frequently a precancerous lesions (a more specific target of treatment) than solid nodules. A blocked randomization was done within each stratum.
All randomized patients who received at least one dose were included in the study analysis with an intent-to-treat approach. An efficacy analysis was carried out after excluding non-compliers and subjects who dropped-out and who refused treatment after randomization. Subjects were considered compliant with at least 50% of the drug taken. On a more conservative approach, patients who dropped-out or had missing final CT scans were considered in the failure group. Both the intent-to-treat and efficacy analyses were conducted on a per subject basis. An adjusted chi-square test was used to compare response rates in the two arms. Multiple logistic regression analysis was used in order to determine the simultaneous effects of various potential risk factors on disease progression (e.g. age, smoking history).
Summary statistics by timetables with mean, median, standard deviation, min and max for change in size for target and non-target lesions and sputum biomarkers were produced for the secondary endpoints statistical analysis. Furthermore patterns for missing data were checked for randomness (e.g. MCAR, MAR, MNAR) and repeated measures analysis were performed after checking for the most appropriate covariance model. In case of non-normality non-parametric alternatives were considered. For all measurements of response, the 95% confidence intervals were also provided. No interim analysis was planned.
7. Results
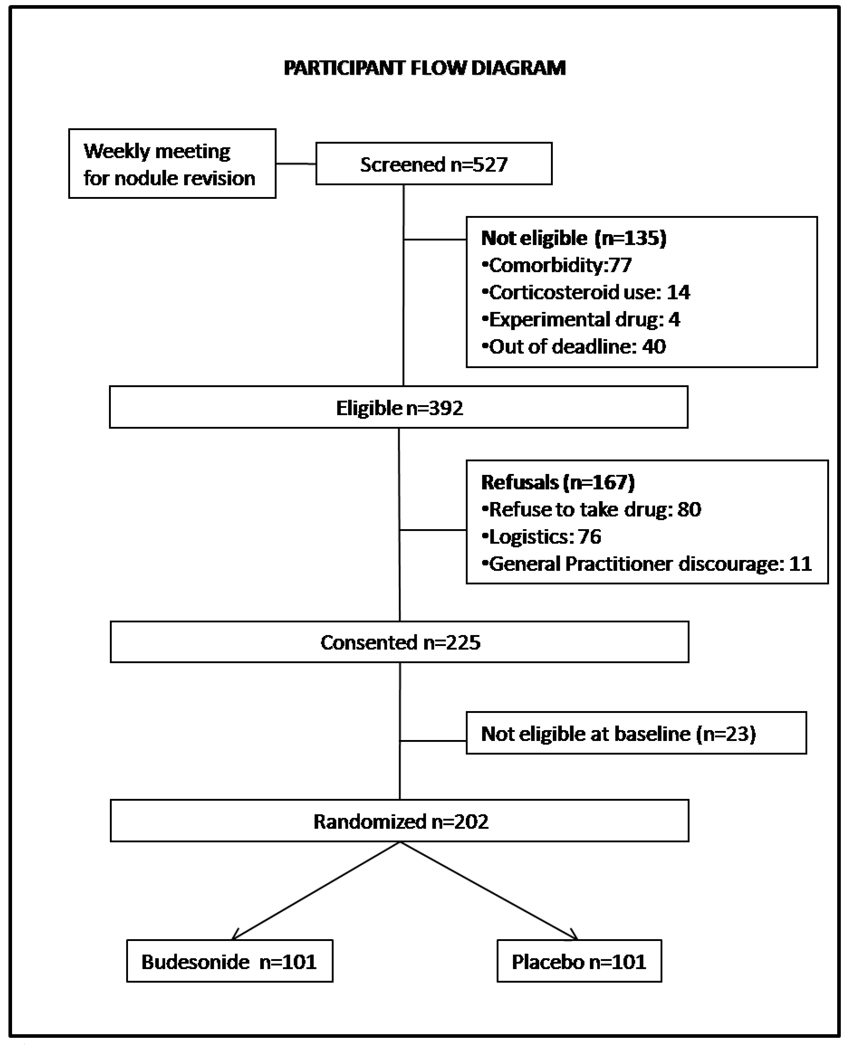
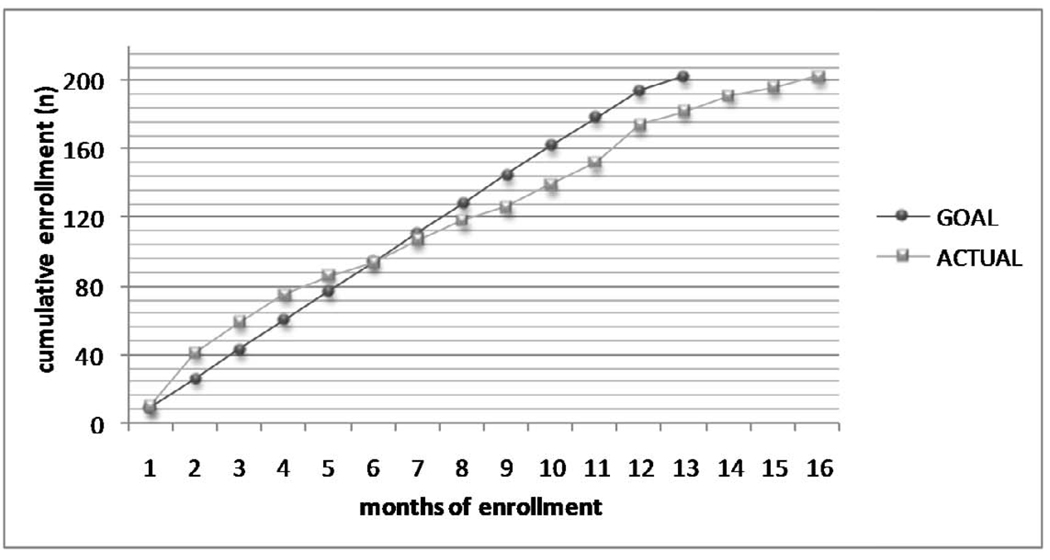
Among the 4821 subjects who underwent the second yearly low dose CT screening in the EIO CT screening trial, a total of 527 subjects were evaluated for protocol inclusion and 443 were eligible according to nodule characteristics. The flow diagram of study of the randomized trial is shown in Figure 3. Two hundred and two were randomized in a 16 month period (from April 2006 – July 2007) with an average accrual of 13 subjects per month (figure 4). Men and women were 153 and 49 respectively; 167 participants were current smokers, 35 were former. The total number of detected nodules was 279 (solid 77%, partially solid 16% and non solid 9%). There was no difference in the distribution of subtype nodules between the two groups (data not shown). Overall 198 subjects completed the 12 months study and will be included in the analysis. Three subjects were lost to follow up and one participant withdraw consent to the trial (drop out: 2%). Compliance was similar in the two groups with 84.6% of the population receiving at least half of the study drug dose (83.1% in the Budesonide arm and 86% in the placebo arm, p=0.697). Most common side effects related to budesonide (mainly grade 1) were oral infections (thrush), voice changes and cough.
Fig. 3.
Number of subject for each phase of the randomized trial
Fig. 4.
Accrual (from April 2006 – July 2007)
8. Discussion
Well established clinical trial models are mandatory to provide preliminary evidence of efficacy in humans and their development represents a major issue to proceed to definitive phase III trials for the progress in preventing lung cancer. Prior chemoprevention trials addressed central squamous carcinogenesis using of bronchial dysplasia as outcome measure, resulting the peripheral lung inaccessible to study.[5,6,21].
The current trial represents the first phase II study of a chemopreventive intervention specifically focusing on the peripheral lung nodules, where the majority of lung cancers arise.
The effect of inhaled budesonide on lung nodules had in fact already been analyzed by Lam and colleagues[5]. However the shrinkage of nodules was analyzed retrospectively as a secondary endpoint. As a consequence, compared to our current study, only eleven subjects in the budesonide group and nineteen subjects in the placebo group were analyzed; the nodules identified by CT scanning were mainly very small (<4 mm), frequently new, and only rarely non-solid. Such new nodules may well represent acute inflammation that resolves spontaneously or with inhaled corticosteroids. In order to exclude from our intervention trial the small inflammatory lesions that resolve spontaneously, we selected only persistent nodules over two successive yearly CT scans. Treatment duration is another important difference (six months vs. one year). All these features make our trial unique.
The real identity of these nodules cannot be established without histological examination. However, if previous authors [11,12] considered these GGOs sufficiently suspicious to warrant surgery, it is possible that even smaller nodules, such as those identified in the context of our study of CT screening might represent less advanced cancers than those described in the literature.
Even though there are other causes for small lung nodules beside adenomatous hyperplasia, the aim of testing the potential activity of Budesonide on sub-solid nodules (partially solid and non solid) is an important issue, especially if we consider that these lesions represent a frequent finding of CT screening and have a higher probability to progress to lung malignancies. [11,22] In addition, some of these lesions may represent multifocal indolent bronchoalveolar adenocarcinoma[23] for which local systemic treatment is not always successful.
Our study was nested into a screening project with clear advantages in terms of participant accrual and reduction of costs. The study methodology was successful with a single center accrual of 13 cases per month showing that the screened population was highly motivated to participate into a chemoprevention trial.
Retention rate and compliance were extremely high. Treatment was well tolerated with the vast majority of adverse events being grade 1, according to the most recent version of the National Cancer Institute toxicity criteria (CTCAE version 3.0, published 12.12.03). The only significantly drug-related events were altered taste, voice change and cortisol suppression. No serious adverse event was considered drug related.
We estimate that the impact of our randomized trial on the outcomes of the screening study will be minimal, even in case of treatment success. The small number of subjects treated with budesonide (100), compared with the population of the COSMOS Study (more than 5000 subjects) being the main reason. However, a therapeutic success will lead us to develop new studies, possibly focusing exclusively on a subgroup of participants with such nodules.
Further studies are in fact needed to improve risk assessment, integrating demographic, CT, and eventually molecular information to optimize the identification of individuals with the highest short term lung cancer risk who may benefit the most from chemopreventive interventions.
The detailed results of the trial will be published shortly.
Acknowledgments
The trial was supported by the National Cancer Institute (grant number: N01-CN-35159). Drug and placebo ere provided at no cost by AstraZeneca, Sweden.
We thank the data manager Raffaella Bertolotti of the COSMOS Study, the staff of the Division of Radiology of the European Institute of Oncology and Margherita Omesso for writing assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Pereira MA, Li Y, Gunning WT, Kramer PM, Al Yaqoub F, Lubet RA, et al. Prevention of mouse lung tumors by budesonide and its modulation of biomarkers. Carcinogenesis. 2002;23:1185–1192. doi: 10.1093/carcin/23.7.1185. [DOI] [PubMed] [Google Scholar]
- 2.Wang Y, Zhang Z, Kastens E, Lubet RA, You M. Mice with alterations in both p53 and Ink4a/Arf display a striking increase in lung tumor multiplicity and progression: differential chemopreventive effect of budesonide in wild-type and mutant A/J mice. Cancer Res. 2003;63:4389–4395. [PubMed] [Google Scholar]
- 3.Alyaqoub FS, Tao L, Kramer PM, Steele VE, Lubet RA, Gunning WT, et al. Prevention of mouse lung tumors and modulation of DNA methylation by combined treatment with budesonide and R115777 (Zarnestra MT) Carcinogenesis. 2007;28:124–129. doi: 10.1093/carcin/bgl136. [DOI] [PubMed] [Google Scholar]
- 4.Parimon T, Chien JW, Bryson CL, McDonell MB, Udris EM, Au DH. Inhaled corticosteroids and risk of lung cancer among patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2007;175:712–719. doi: 10.1164/rccm.200608-1125OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lam S, leRiche JC, McWilliams A, MacAulay C, Dyachkova Y, Szabo E, et al. A randomized phase IIb trial of pulmicort turbuhaler (budesonide) in people with dysplasia of the bronchial epithelium. Clin Cancer Res. 2004;10:6502–6511. doi: 10.1158/1078-0432.CCR-04-0686. [DOI] [PubMed] [Google Scholar]
- 6.Lee JS, Lippman SM, Benner SE, Lee JJ, Ro JY, Lukeman JM, et al. Randomized placebo-controlled trial of isotretinoin in chemoprevention of bronchial squamous metaplasia. J Clin Oncol. 1994;12:937–945. doi: 10.1200/JCO.1994.12.5.937. [DOI] [PubMed] [Google Scholar]
- 7.Henschke CI, McCauley DI, Yankelevitz DF, Naidich DP, McGuinness G, Miettinen OS, et al. Early Lung Cancer Action Project: overall design and findings from baseline screening. Lancet. 1999;354:99–105. doi: 10.1016/S0140-6736(99)06093-6. [DOI] [PubMed] [Google Scholar]
- 8.Veronesi G, Bellomi M, Mulshine JL, Pelosi G, Scanagatta P, Paganelli G, et al. Lung cancer screening with low-dose computed tomography: a non-invasive diagnostic protocol for baseline lung nodules. Lung Cancer. 2008;61:340–349. doi: 10.1016/j.lungcan.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 9.Yamada S, Kohno T. Video-assisted thoracic surgery for pure ground-glass opacities 2 cm or less in diameter. Ann Thorac Surg. 2004;77:1911–1915. doi: 10.1016/j.athoracsur.2003.12.040. [DOI] [PubMed] [Google Scholar]
- 10.Kerr KM. Pulmonary preinvasive neoplasia. J Clin Pathol. 2001;54:257–271. doi: 10.1136/jcp.54.4.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim HY, Shim YM, Lee KS, Han J, Yi CA, Kim YK. Persistent pulmonary nodular ground-glass opacity at thin-section CT: histopathologic comparisons. Radiology. 2007;245:267–275. doi: 10.1148/radiol.2451061682. [DOI] [PubMed] [Google Scholar]
- 12.Ohtsuka T, Watanabe K, Kaji M, Naruke T, Suemasu K. A clinicopathological study of resected pulmonary nodules with focal pure ground-glass opacity. Eur J Cardiothorac Surg. 2006;30:160–163. doi: 10.1016/j.ejcts.2006.03.058. [DOI] [PubMed] [Google Scholar]
- 13.Punturieri A, Szabo E, Croxton TL, Shapiro SD, Dubinett SM. Lung cancer and chronic obstructive pulmonary disease: needs and opportunities for integrated research. J Natl Cancer Inst. 2009;101:554–559. doi: 10.1093/jnci/djp023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Crausman RS, Lynch DA, Mortenson RL, King TE, Jr, Irvin CG, Hale VA, et al. Quantitative CT predicts the severity of physiologic dysfunction in patients with lymphangioleiomyomatosis. Chest. 1996;109:131–137. doi: 10.1378/chest.109.1.131. [DOI] [PubMed] [Google Scholar]
- 15.Paciocco G, Uslenghi E, Bianchi A, Mazzarella G, Roviaro GC, Vecchi G, et al. Diffuse cystic lung diseases: correlation between radiologic and functional status. Chest. 2004;125:135–142. doi: 10.1378/chest.125.1.135. [DOI] [PubMed] [Google Scholar]
- 16.Fahy JV, Steiger DJ, Liu J, Basbaum CB, Finkbeiner WE, Boushey HA. Markers of mucus secretion and DNA levels in induced sputum from asthmatic and from healthy subjects. Am Rev Respir Dis. 1993;147:1132–1137. doi: 10.1164/ajrccm/147.5.1132. [DOI] [PubMed] [Google Scholar]
- 17.American Thoracic Society. Standardization of Spirometry, 1994 Update. Am J Respir Crit Care Med. 1995;152:1107–1136. doi: 10.1164/ajrccm.152.3.7663792. [DOI] [PubMed] [Google Scholar]
- 18.American Thoracic Society. Single-breath carbon monoxide diffusing capacity (transfer factor). Recommendations for a standard technique--1995 update. Am J Respir Crit Care Med. 1995;152:2185–2198. doi: 10.1164/ajrccm.152.6.8520796. [DOI] [PubMed] [Google Scholar]
- 19.Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 20.Bellomi M, Veronesi G, Rampinelli C, Ferretti S, De FE, Maisonneuve P. Evolution of lung nodules < or =5 mm detected with low-dose CT in asymptomatic smokers. Br J Radiol. 2007;80:708–712. doi: 10.1259/bjr/46019726. [DOI] [PubMed] [Google Scholar]
- 21.van den Berg RM, Teertstra HJ, van ZN, van TH, Visser C, Pasic A, et al. CT detected indeterminate pulmonary nodules in a chemoprevention trial of fluticasone. Lung Cancer. 2008;60:57–61. doi: 10.1016/j.lungcan.2007.09.007. [DOI] [PubMed] [Google Scholar]
- 22.Lee HJ, Goo JM, Lee CH, Park CM, Kim KG, Park EA, et al. Predictive CT findings of malignancy in ground-glass nodules on thin-section chest CT: the effects on radiologist performance. Eur Radiol. 2009;19:552–560. doi: 10.1007/s00330-008-1188-2. [DOI] [PubMed] [Google Scholar]
- 23.Pelosi G, Sonzogni A, Veronesi G, De CE, Maisonneuve P, Spaggiari L, et al. Pathologic and molecular features of screening low-dose computed tomography (LDCT)-detected lung cancer: a baseline and 2-year repeat study. Lung Cancer. 2008;62:202–214. doi: 10.1016/j.lungcan.2008.03.012. [DOI] [PubMed] [Google Scholar]