Abstract
Overwhelming inflammation triggered by systemic infection in bacterial sepsis contributes to the pathology of this condition. Toll-like receptors (TLRs) are important in early septic inflammation. As a safeguard, the innate immune system has evolved to counter excessive inflammation through the induction of “tolerance.” In endotoxin tolerance, TLR signaling is inhibited and/or attenuated by multiple mechanisms that mitigate the ability of lipopolysaccharide (LPS) to activate critical kinases through TLR4. Here, we describe a novel mechanism. Protein kinase R (PKR), a kinase normally activated by a subset of TLRs, is rendered unresponsive to LPS in endotoxin-tolerized cells. In its naive state, PKR is subject to K63-linked ubiquitination (Ub), followed by K48-linked Ub, in response to LPS. In tolerance, the kinetics of this differential Ub is altered, resulting in a predominance of K48-linked chains, concomitant with a loss of PKR activation. These findings provide a novel mechanism by which a TLR-responsive kinase may be rendered inactive in tolerance.
IMPORTANCE
“Endotoxin tolerance” is a period of transient unresponsiveness to the lipopolysaccharide (LPS) outer membrane component of Gram-negative bacteria that is induced by prior exposure to LPS through Toll-like receptor 4 (TLR4). The loss of LPS-inducible cytokine production by macrophages from patients who have experienced Gram-negative sepsis is well documented, and the increased susceptibility of such patients to reinfection has been attributed to the development of endotoxin tolerance. Multiple mechanisms have been proffered to account for this attenuated response. Using the LPS-responsive kinase protein kinase R (PKR), we have identified differential K48 versus K63 ubiquitination as an additional molecular mechanism by which signal-transducing elements may be inactivated in a state of endotoxin tolerance. This work is highly significant because it links recent discoveries concerning the important role of ubiquitination of signaling molecules in regulating TLR signaling with the loss of LPS responsiveness in tolerance.
Introduction
The successful resolution of microbial infection in mammals initially requires a robust proinflammatory response that involves the synthesis and action of cytokines and chemokines, as well as agents with direct antimicrobial activities. These inflammatory mediators function by influencing and coordinating the behavior of a vast array of physiologic systems to respond appropriately to the individual infecting agent (1). While a potent and protective innate immune response is essential, the proinflammatory response must be tightly controlled to preclude excessive inflammation that may be an even greater threat to the host. In no situation is this perilous balance between the initiation and resolution of inflammation more important than in microbial sepsis. In septic patients, a disseminated bacterial infection leads to profound morbidity and mortality, resulting in over 200,000 deaths each year in the United States alone, at an estimated cost of treatment of billions of dollars (2). While sepsis is a major public health threat, no single treatment modality has yet emerged as effective in combating it. The pathobiology of sepsis has proven to be extremely complex but is believed to involve an initial acute phase of hyperinflammation initiated by elements of the innate immune system, including macrophages and neutrophils (3). This proximal innate response, in many instances, is initiated by a set of innate immune receptors, the Toll-like receptors (TLRs), which sense and respond to the unique chemistries of various microbial constituents. The molecular signatures of infection detected by differing TLRs are widely varied and include common structural components of Gram-positive bacteria, Gram-negative bacteria, viruses, and extracellular parasites. Direct or indirect ligation of TLRs by these conserved microbial structures initiates activation of multiple signal transduction cascades, communicated through a shared set of intracellular adapter proteins. Recruitment of specific kinases to the growing TLR-adapter receptor complex initiates induction of proinflammatory cytokines such as tumor necrosis factor alpha (TNF-α) and interleukin-1β (IL-1β), believed to be important in septic disease. Perhaps as a safeguard against the deleterious consequences that massive TLR ligation may elicit, as seen in sepsis, prolonged exposure of cells of the innate immune system (i.e., macrophages and neutrophils) to TLR ligands results in a transient state of refractoriness to subsequent stimulation that is known as “tolerance.”
Tolerance is considered important in vivo in human infections because circulating monocytes and macrophages from septic individuals display many of the same refractory phenotypes upon TLR agonist stimulation, as seen in in vitro restimulation experiments (4–7). In fact, the effects of sepsis-induced tolerance may persist for years following the clearance of the initial infection and may underlie the dramatically increased morbidity and mortality seen in postsepsis patient groups when compared to normal controls (8, 9).
Lipopolysaccharide (LPS)-induced tolerance does not, however, result in the global inhibition of TLR-induced inflammatory gene expression (10), and this observation led to the concept of macrophage “reprogramming” (11, 12) rather than “tolerance.” Since these early reports, the vast majority of studies that have sought to unravel the effects of tolerance/macrophage reprogramming have focused on the molecular mechanisms by which TLR signal transduction is altered or blocked in tolerance, and a few key aspects of this negative regulation have emerged. Specifically, it has been shown that the proximal TLR4-associated signal transduction complex is not as robustly assembled in response to LPS in tolerance (13). This may, in part, result from the fact that key posttranslational modifications, e.g., phosphorylation, to TLR4 do not occur in tolerized cells (14). Another critical mechanism by which signal transduction is inhibited in tolerance is the upregulation of negative regulators of signaling, e.g., IRAK-M. IRAK-M is a catalytically inactive member of the IRAK kinase family that prevents formation of IRAK-1–TRAF6 complexes downstream of TLR4 (15, 16). Contrary to the upregulation of IRAK-M, the critical signaling kinase, IRAK-1, is downregulated at the levels of protein and RNA in endotoxin-tolerant macrophages (17, 18). Direct remodeling of chromatin at target genes to prevent or potentiate transcriptional upregulation (19) has also been implicated as yet another mechanism by which tolerance is enforced. Interestingly, two reports have suggested that a ubiquitin ligase, SOCS-1, is involved in tolerance because tolerized SOCS-1-deficient macrophages display increased inflammatory cytokine production in comparison to that displayed by naive, tolerized macrophages (20). Nonetheless, despite the fact that endotoxin tolerance has been studied for more than 60 years, neither a clear understanding of its induction nor the impact of TLR tolerance on disease has emerged.
Protein kinase R (PKR) is a serine/threonine kinase best known as a regulator of protein translation activated by viral RNA in the cytosol (21–23). However, PKR has also been shown to be activated by LPS through TLR4 (24–26), although the precise role of PKR in mediating the response to endotoxin is not clear. In investigating the TLR4-mediated regulation of PKR, we discovered that PKR itself was a target of macrophage reprogramming and was negatively regulated in endotoxin-tolerized cells.
Ubiquitination (Ub) plays an important role in regulating signal transduction downstream of TLR engagement. Both classical, proteolytic Ub, resulting from the attachment of ubiquitin molecules concatenated into chains by linking monomers one to the next via lysine 48 (K48), and noncanonical, nonproteolytic chains, utilizing lysine 63 (K63) linkages, are critical in TLR signaling (27, 28). The K63 Ub of signaling intermediates, such as IRAK-1 by TRAF6 and/or Pellino family members, facilitates the assembly of higher-order signaling complexes that lead to the activation of the transcription factor NF-κB (29, 30). Conversely, the K48 ligation of signaling elements, such as the adapter protein TIRAP/Mal, is thought to limit signaling (31). Differential Ub of the same molecule, leading to distinct signaling outcomes, was first coined “ubiquitin editing” by Lam and colleagues to describe quantitative changes in ubiquitination (32) and was subsequently adopted by Newton and colleagues to describe the regulation of IL-1-induced IRAK-1 (33). Interestingly, those authors also showed that Ub of signaling proteins may not be static or restricted to either K48 or K63 chains but may, in some instances, be a dynamic process that balances the expression of these two chain species, leading to fine-tuning of the innate response (33). The data presented herein support the involvement of “ubiquitin editing” in innate signaling and as a unique mechanism of PKR inactivation in endotoxin tolerance.
RESULTS
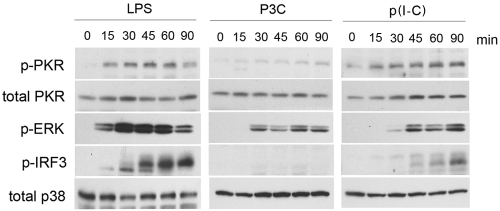
Given that the changes that occur in signal transduction pathways in TLR-tolerized or -reprogrammed macrophages are incompletely described, it is of importance to characterize more fully the regulation of TLR-responsive kinases in these states. The serine/threonine kinase PKR has been demonstrated by multiple investigators to be activated downstream of TLR4 engagement and has been shown to play a role in shaping the response to LPS (24–26). However, little work has been done on the capacity of TLRs other than TLR4 to activate PKR or on the molecular determinants of PKR activation by TLRs in general. To address this gap in our understanding, we initially sought to characterize the kinetics of PKR activity downstream of multiple TLRs in naive macrophages. Primary peritoneal macrophages were stimulated with ligands for TLRs that differentially utilize different adapter combinations (e.g., TLR2 [TIRAP/MyD88], TLR3 [TRIF only], or TLR4 [TIRAP/MyD88 and TRAM/TRIF]) over a 90-min time course, and the activation of PKR was measured by phosphospecific Western analysis (Fig. 1). We observed rapid activation of PKR in response to LPS, with kinetics of phosphorylation that closely mirrored that of the mitogen-activated protein kinase (MAPK) ERK1/2 (Fig. 1, left). Interestingly, we did not observe substantial PKR activation by the TLR2 ligand S-[2,3-bis(palmitoyloxy)-(2-RS)-propyl]-N-palmitoyl-(R)-Cys-Ser-Lys4-OH (P3C), and its ability to induce ERK phosphorylation was slightly delayed and less robust than that induced by LPS (Fig. 1, middle). The TLR3 ligand poly(I ⋅ C) [p(I ⋅ C)] also activated PKR (Fig. 1, right) but exhibited delayed and diminished ERK1/2 activation compared to that exhibited by LPS. As expected, the transcription factor IRF-3 was phosphorylated downstream of both TLR3 and -4 but not TLR2, with LPS being a stronger activator. Detection of equivalent levels of total PKR and p38 indicate equivalent levels of protein loading.
FIG 1 .
PKR is differentially activated by distinct TLRs. Primary thioglycolate-elicited macrophages were treated for the indicated times with either E. coli LPS (250 ng/ml), P3C (500 ng/ml), or p(I ⋅ C) (50 µg/ml). At the indicated times, whole-cell lysates were subjected to Western analysis and probed with antibodies directed against phosphorylated or total protein populations. These data are representative of 6 independent experiments.
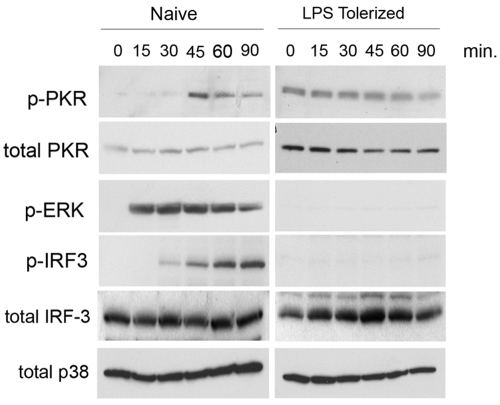
Since PKR activation has not been examined in TLR-reprogrammed macrophages, we next examined PKR activation in macrophages that were first incubated for 18 h with medium alone, or with the TLR4 agonist LPS, and then stimulated for a 90-min time course with a dose of Escherichia coli LPS identical to that used in Fig. 1 (250 ng/ml). We confirmed that PKR was activated by LPS stimulation in naive, medium-pretreated macrophages (Fig. 2, naive). Notably, total PKR protein levels were significantly elevated in LPS-reprogrammed macrophages, with a commensurate increase in basal phospho-PKR (p-PKR) levels (Fig. 2, LPS tolerized). However, restimulation of tolerant macrophages produced no LPS-inducible increase in PKR phosphorylation (Fig. 2). As it is possible that tolerant macrophages may simply exhibit delayed kinetics of PKR activation, additional time courses of activation to 180 min were carried out, with no LPS-mediated increase in PKR activity (data not shown). To compare the behavior of PKR under naive and reprogrammed conditions to that of other previously examined TLR-responsive signaling pathways, we also measured the activation levels of ERK1/2 and IRF-3. LPS pretreatment completely ablated LPS-induced ERK signaling, as previously reported (34) (Fig. 2). IRF-3 activation was similarly ablated in LPS-tolerized cells (Fig. 2).
FIG 2 .
PKR activation is differentially regulated in TLR-tolerized macrophages. Primary murine macrophages were treated overnight with medium alone (naive) or 10 ng/ml LPS (LPS tolerized). Following pretreatment, cells were washed and restimulated with LPS (250 ng/ml) for the indicated times. Whole-cell lysates were subjected to Western analysis and probed with antibodies directed against the indicated total or phosphospecific species. These data are representative of 3 independent experiments.
After this initial characterization of PKR responsiveness to TLRs in naive and TLR-reprogrammed macrophages, we sought to elucidate the mechanism by which LPS-induced PKR activation was lost in reprogrammed macrophages. We had reproducibly observed a modest decrease in PKR total protein levels in LPS-tolerized macrophages following subsequent restimulation with LPS, with kinetics that began close to the time of the peak of PKR activity in naive cells (approximately 45 min) (Fig. 2). This LPS-inducible loss of total PKR protein was also observed in the LPS-pretreated RAW 264.7 macrophage cell line (Fig. 3A). The similarity in kinetics between the loss of LPS-inducible PKR activation in tolerant cells between 30 and 90 min following LPS restimulation and the reduction of PKR total protein levels over the same period led us to hypothesize that induced degradation of PKR in tolerance may, in part, be responsible for the failure to observe PKR activation.
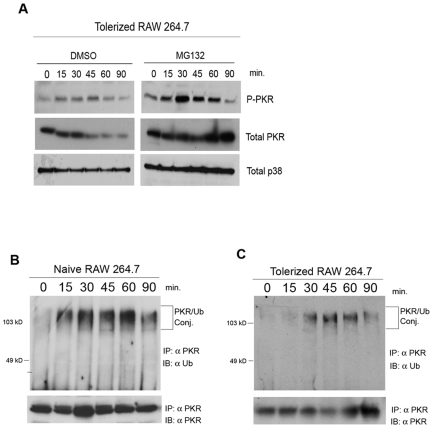
FIG 3 .
PKR is inducibly ubiquitinated in tolerized macrophages, and tolerance depends upon a functional proteosome. (A) RAW 264.7 cells tolerized overnight with LPS (10 ng/ml) were pretreated for 30 min with either vehicle only (DMSO) or MG132 (25 µM). Cells were subsequently stimulated with LPS (250 ng/ml) for the indicated times. Whole-cell lysates were subjected to Western analysis and probed with the indicated antibodies. (B and C) Naive (B) or LPS-tolerized (C) RAW 264.7 cells were stimulated with LPS (250 ng/ml), and cells were lysed at the indicated times. PKR was immunoprecipitated from cell lysates, and immunoprecipitating complexes were subjected to SDS-PAGE. Separated complexes were sequentially probed with a monoclonal antibody directed against ubiquitin and a monoclonal antibody against PKR. Data are representative of 3 independent experiments. IB, immunoblotting.
Given that there is rapidly expanding literature demonstrating a role for ubiquitin/proteosome-mediated proteolysis in regulating TLR signaling (27, 35), we next explored a role for the proteosome in inhibiting PKR activation in tolerance. In initial experiments, two populations of RAW 264.7 cells were tolerized overnight with 10 ng/ml E. coli LPS. Eighteen hours later, one set was pretreated for 30 min with vehicle alone (dimethyl sulfoxide [DMSO]) as a control, and the other was pretreated for 30 min with the proteosome inhibitor MG132 at 25 μM. After pretreatment with vehicle or MG132, both macrophage populations were washed and restimulated with LPS (250 ng/ml), and cell lysates were harvested at the indicated time points (Fig. 3A). As expected from the results of Fig. 2, activation of PKR, measured by phosphorylation, was weak in the vehicle-treated, LPS-tolerized cells, and the level of total PKR was markedly reduced after 45 min of LPS treatment. Strikingly, proteosome inhibition by MG132 restored LPS-induced PKR activation in endotoxin-tolerized macrophages and prevented the inducible loss of PKR protein (Fig. 3A, top and middle, respectively). Proteosome inhibition also appeared to increase the kinetics by which PKR is inactivated (Fig. 3A), as judged by the ratio of total PKR to phosphorylated PKR at 90 min. This may be due to a more rapid negative feedback resulting from greater PKR signaling in the MG132-treated cells. This result is consistent with a role for ubiquitin-mediated proteolysis in preventing PKR activation in endotoxin tolerance.
Since a role for differential Ub in endotoxin tolerance had not been described previously, we further explored this possibility. PKR activity has not heretofore been shown to be regulated by Ub. Therefore, we initially sought to assay more directly for LPS-induced ubiquitination of PKR. To do this, endogenous PKR was immunoprecipitated from lysates of medium-pretreated (naive) or LPS-pretreated (tolerized) RAW 264.7 cells after primary or secondary stimulation with LPS, respectively. Immunoprecipitates were washed with buffer containing 2 M urea to prevent contamination by nonspecifically binding ubiquitinated proteins, subjected to gel electrophoresis, and immunoblotted with monoclonal antibody directed against ubiquitin (Fig. 3B and C). Basal levels of polyubiquitinated PKR were barely detectable in naive RAW 264.7 macrophages, but rapid and robust high-molecular-weight, ubiquitinated PKR was detected 15 min after LPS stimulation and persisted until 90 min (Fig. 3B). In contrast, in the LPS-tolerized macrophages, LPS-inducible Ub of PKR was delayed in kinetics and lessened in intensity in comparison to naive cells (Fig. 3C). Total immunoprecipitable PKR protein levels were not significantly altered over time in naive cells (Fig. 3B and C). This result was surprising because our previous observation that proteosome inhibition could restore activation in tolerance (Fig. 3A) had led us to expect that PKR would be inducibly ubiquitinated to a greater degree in tolerant cells, leading to subsequent degradation and, thus, explaining the failure in observing PKR activation in tolerized macrophages. The fact that PKR displays greater total ubiquitination in naive cells led us to hypothesize that there may be qualitative differences in the types of ubiquitin chains attached to PKR in naive versus tolerant states rather than merely quantitative differences.
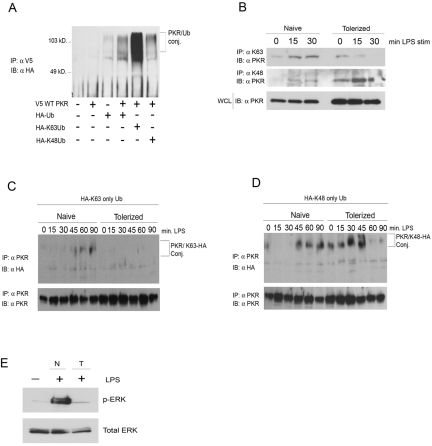
Recent studies have suggested that TLR signaling intermediates are modified by ubiquitin chains through K48 or K63 linkages that lead to negative or positive regulation, respectively. To delineate if PKR is differentially modified by these ubiquitin chain types in naive versus tolerized cells, we first utilized an overexpression system in the HEK293T cell line. V5-tagged wild-type (WT) PKR was overexpressed in HEK293T cells, along with expression vectors for WT hemagglutinin (HA)-tagged ubiquitin (HA-Ub) or HA-tagged ubiquitin bearing a single lysine at either position 48 or 63 (HA-K48 only or HA-K63 only). Twenty-four hours later, V5-PKR was precipitated from whole-cell lysates, and the immunoprecipitates were subjected to Western blot analysis with anti-HA monoclonal antibody. A high-molecular-weight, ubiquitinated PKR species was detected only when immunoprecipitated PKR was coexpressed with HA-tagged ubiquitin (Fig. 4A). Significant Ub of PKR upon cotransfection with each of our ubiquitin constructs was observed, indicating that PKR can be modified by both K48 and K63 chains and holding out the possibility that PKR activity may be both positively and negatively regulated by Ub. Much of the work that has been done to describe a role for K48 or K63 ubiquitin chains in signaling has been done with experimental systems involving overexpression of potential targets and/or ligases. Such systems have yielded significant insights but carry with them potential for artifactual interactions. In an effort to circumvent such issues, we initially sought to demonstrate LPS-dependent K48 and/or K63 ubiquitination of PKR in cell lines. Naive or LPS-tolerant RAW 264.7 cells were stimulated with LPS, and proteins from whole-cell lysates specifically modified by K63 Ub chains were immunoprecipitated, utilizing a monoclonal antibody specific for K63 chains. Immunoprecipitates were resolved by polyacrylamide gel electrophoresis (PAGE) and probed with a monoclonal antibody against PKR (Fig. 4B). LPS induced rapid K63 modification of PKR in naive RAW cells but not in LPS-tolerant cells. Interestingly, the anti-K63 Ub monoclonal antibody precipitated PKR as a single species, perhaps indicating that in response to LPS, PKR undergoes a single K63 modification. As there is no commercially available monoclonal antibody that will specifically precipitate K48 chains, we initially attempted to transfect our HA-tagged K48-only or K63-only expression constructs into RAW 264.7 cells and immunoprecipitate endogenous PKR following LPS stimulation to observe HA tag modification. These experiments were unsuccessful, presumably due to the extremely low transfectability of this cell line (data not shown). As an alternate approach, we established an experimental system utilizing a readily transfectable derivative of the HeLa cell line that stably expresses TLR4 and MD-2 (MAT4). This cell line has been previously reported to respond authentically to stimulation with LPS (36), and in our hands, this cell line exhibits a loss of LPS-dependent ERK activation following prolonged exposure to LPS, a hallmark of tolerance (Fig. 4E). To assess the role of lysine-specific Ub in this context, MAT4 cells were transiently transfected with the mutant construct that encodes the HA-K63-only ubiquitin and then stimulated overnight with either medium alone or medium supplemented with LPS. Following overnight incubation, MAT4 cells were washed and then restimulated with LPS over a 90-min time course, PKR was immunoprecipitated at 15-min intervals, and the immunoprecipitates were subjected to Western analysis with anti-HA monoclonal antibody. In naive cells, we observed a transient K63-specific Ub of PKR, with kinetics that closely mirrored that of PKR phosphorylation (Fig. 4C). Remarkably, this ubiquitin modification was undetectable by this assay in LPS-tolerized cells, indicating for the first time an alteration of the Ub of a TLR signaling element in LPS-tolerant cells. The identical experiment carried out using the HA-K48 ubiquitin mutant revealed a K48 ubiquitination of PKR in naive MAT4 cells (Fig. 4D). Differentially, K48 ubiquitination of PKR was enhanced in both kinetics and intensity in the LPS-tolerized MAT4 cells (Fig. 4D).
FIG 4 .
PKR undergoes K63 and K48 ubiquitination in response to LPS. (A) HEK293T cells were transfected with cDNA constructs expressing V5-tagged wild-type (WT) PKR and/or HA-tagged WT ubiquitin, HA K63-only Ub, or HA K48-only Ub. Twenty-four hours later, cells were lysed, and PKR was immunoprecipitated from whole-cell lysates. Immunoprecipitates were subjected to SDS-PAGE and probed with monoclonal antibody against HA. (B) Raw 264.7 cells were incubated overnight with medium alone or 100 ng/ml of LPS. Cells were washed and restimulated with 250 ng/ml LPS for the indicated times. Immunoprecipitation was carried out with anti-K63 Ub chain monoclonal antibody. (C and D) MAT4 cells were transfected with cDNA constructs expressing either K63-only HA-tagged Ub (C) or K48-only HA-tagged Ub (D) and immediately treated overnight with medium alone or 100 ng/ml LPS. Eighteen hours following primary treatment, cells were washed and stimulated with 250 ng/ml LPS, PKR was immunoprecipitated from whole-cell lysates, and immunoprecipitates were subjected to SDS-PAGE, followed by probing with anti-HA monoclonal antibody. (E) Naive (N) or LPS-tolerized (T) MAT4 cells were restimulated with 250 ng/ml LPS, and phospho-ERK levels were assayed by Western blotting. These data are representative of 4 independent experiments.
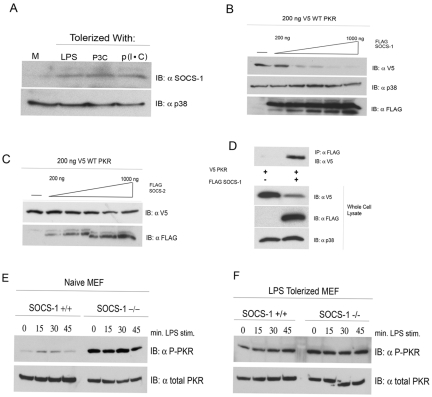
Because our data strongly suggest that ubiquitin-mediated proteolysis plays a role in the inactivation of PKR in LPS-tolerized cells, we next sought to ascertain the identity of the K48 ligase that is responsible. Among the LPS-inducible K48 ligases, the SOCS family of ubiquitin ligases has been linked extensively to the negative regulation of cytokine and inflammatory processes (37). In particular, SOCS-1 is upregulated by LPS and has been reported to catalyze the destruction of proximal elements of TLR signal complexes (31). In addition, SOCS-1-deficient mice have been reported to be impaired in their capacity to induce endotoxin tolerance (20, 38), although others have reached different conclusions in regard to a role for SOCS-1 as a mediator of tolerance (39). We examined the steady-state levels of SOCS-1 by Western analysis of both naive macrophages and macrophages that had been rendered tolerant by preincubation with medium only or with LPS, P3C, or p(I ⋅ C). In the naive state, SOCS-1 protein levels were comparatively low (Fig. 5A). However, in tolerant macrophages, SOCS-1 levels were significantly elevated regardless of stimulus used (Fig. 5A). We therefore investigated the possibility that PKR and SOCS-1 interact functionally in vitro, resulting in K48 Ub of PKR. To test this hypothesis, PKR was overexpressed in HEK293T cells, along with increasing concentrations of a SOCS-1 expression vector. We observed a striking dose-dependent reduction in basal PKR protein expression levels in response to an increase in SOCS-1 following 24 h of incubation (Fig. 5B, top). This was not the result of widespread nonspecific degradation of TLR4-responsive kinases because endogenous levels of the TLR-responsive MAPK p38 were not reduced, even at the highest levels of transfected SOCS-1 (Fig. 5A, bottom). Since overexpression of K48 ligases can, in some instances, lead to a loss of target specificity, we repeated this experiment using a closely related family member, SOCS-2, in lieu of SOCS-1. Importantly, SOCS-2 overexpression had a negligible effect on the basal level of PKR, even at the highest dose (Fig. 5C). Since the effects of SOCS-1 on PKR protein levels may be the result of regulation of an intermediate element, we evaluated the potential for SOCS-1 and PKR to interact physically in our overexpression system. V5-tagged WT PKR was transfected into HEK293T cells without or with concomitant transfection of FLAG-tagged SOCS-1, and immunoprecipitations were carried out with anti-FLAG monoclonal antibody. PKR was immunoprecipitated only in the presence of cotransfected SOCS-1 (Fig. 5D, top). Western analysis of whole-cell lysates revealed the expected reduction in PKR expression levels when coexpressed with SOCS-1 (Fig. 5D, bottom). The capacity of SOCS-1 to interact physically with and catalyze degradation of PKR in HEK293T cells supports the possibility that SOCS-1 regulates PKR protein levels during the TLR4-mediated response to LPS. To assay for the potential in vivo significance of SOCS-1 in regulating PKR activity, we obtained SOCS-1 knockout mouse embryonic fibroblasts (MEFs) and ascertained LPS-responsive PKR activation in naive and LPS-tolerized cells. Stimulation of wild-type, naive MEFs produced a modest and transient activation of PKR (Fig. 5E). Remarkably, PKR activity was dramatically enhanced and maximal in unstimulated SOCS-1−/− MEFs and could not be further enhanced by LPS treatment at any dose used, demonstrating a role for SOCS-1 in regulating PKR activity (Fig. 5E). In LPS-tolerized MEFs, however, PKR activity could not be stimulated by LPS in either the SOCS-1+/+ or SOCS-1−/− genotype, suggesting that a combination of mechanisms play a role in enforcing PKR tolerance (Fig. 5F).
FIG 5 .
SOCS-1 physically interacts with and negatively regulates PKR. (A) Primary peritoneal macrophages were stimulated for 18 h with medium (M), LPS (10 ng/ml), P3C (100 ng/ml), or p(I ⋅ C) (10 µg/ml). Cells were harvested, and the levels of SOCS-1 protein were examined by Western analysis. (B and C) HEK293T cells were transfected with 200 ng V5-tagged WT PKR and either empty vector or an increasing amount of cDNA expressing FLAG-tagged SOCS-1 (B) or SOCS-2 (C). Twenty-four hours following transfection, whole-cell lysates were subjected to Western analysis with antibodies against the indicated species. (D) HEK293T cells transfected with V5-PKR alone or in conjunction with FLAG-tagged SOCS-1. Cell lysates were immunoprecipitated with anti-FLAG antibody and separated by SDS-PAGE, followed by Western blotting with the indicated antibodies. These data are representative of 3 independent experiments. (E and F) WT and SOCS-1−/− MEFs were cultured overnight in medium alone (E) or in medium supplemented with LPS (100 ng/ml) (F). Following 18 h of treatment, cells were washed and restimulated with LPS (250 ng/ml) for the indicated times. Whole-cell lysates were resolved by SDS-PAGE and probed with antibodies against phosphorylated or total PKR. These data are representative of 3 independent experiments.
DISCUSSION
While the clinical importance of sepsis as a major public health issue has long been recognized, our understanding of the molecular biology of this condition has significantly lagged behind, and despite repeated efforts, new palliative options for treatment have not been forthcoming. This may, in part, result from the fact that sepsis reflects a complex interplay between the host immune response and the invading pathogen. Host macrophages activated through innate immune surveillance receptors, such as TLRs, are instrumental in the pathology of sepsis, as evidenced by the adaptive phenomenon of TLR-induced macrophage reprogramming. Indeed, the initiation of a tolerized state of the innate immune system is not limited exclusively to TLRs, as an analogous, although distinct, tolerance results after prolonged ligation of other signaling receptors, including the IL-1 receptor (13) and NOD-like receptors (NLRs) (40). In an attempt to expand our understanding of signaling in tolerance/reprogramming, we have discovered that PKR, shown previously to be activated by LPS, is an additional signaling element that is subject to tolerance. Although the canonical model for PKR activation requires its interaction with exogenous RNAs not known to be produced downstream of TLR ligation, multiple studies have reported roles for PKR in the cellular response to LPS. Specifically, PKR was shown to interact directly with the TLR4 adapter TIRAP/Mal and to exhibit a partial dependence on MyD88 for activation through TLR4 (24). Delineation of the precise TLR-inducible signaling complexes leading to PKR activation will be a compelling avenue for investigation and should expand our understanding of TLR signaling. One candidate intermediary for linking TLRs to PKR activation is the PKR-interacting protein PACT/RAX, known to activate PKR under diverse conditions of cellular stress independently of RNA (41, 42). Our results show that PKR activation is not a feature of all TLRs, as evidenced by the fact that PKR is activated by TLR4 and TLR3 but not by TLR2. We also show that total PKR levels are elevated in LPS-tolerant murine macrophages but not in MEFs, a fact that may reflect the far greater LPS sensitivity of macrophages compared to that of embryonic fibroblasts.
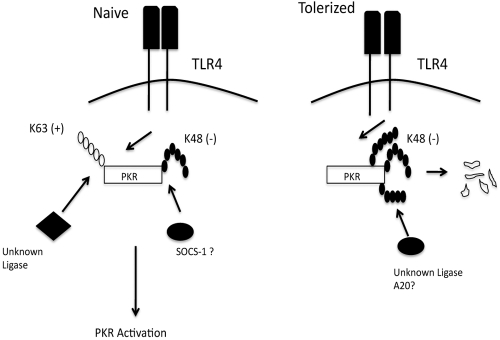
Our observations are the first to provide a link between posttranslational modification of signaling elements by Ub and the differential regulation of signaling in tolerance (see Fig. 6). Given the significant and expanding role played by Ub in regulating TLR signaling, it is highly unlikely that PKR is the only such element to undergo negatively regulating shifts in patterns of Ub in tolerance/reprogramming. As such, we provide evidence for a new paradigm in tolerance that may conceivably work in conjunction with other recently described mechanisms. For example, Medzhitov and colleagues recently described selective chromatin remodeling in endotoxin-tolerized macrophages as a means to render some promoters “tolerizable” while leaving others responsive to LPS (19). It is conceivable that attenuating signaling by altering the balance of K63 Ub versus K48 Ub of key downstream signaling molecules reduces TLR signal strength, further exacerbating the effect of chromatin remodeling. In a previous report by Newton and colleagues, sequential waves of K63 Ub, followed by K48 ubiquitin modifications, were induced on IRAK1, following ligation of the IL-1 receptor (33). The present work is, to our knowledge, only the second example of rapid ubiquitin editing/switching of Ub in innate signaling and the first example in the context of TLRs (33). Whether a deubiquitinating enzyme such as A20 plays any role in ubiquitin switching in the context of PKR remains to be determined. Our data also expand the concept of ubiquitin editing to show that it is not necessarily a static balance but, rather, that the ratios of K63 to K48 chains added to a given kinase during signaling may shift with cellular context, a shift that may have profound consequences on the behavior of signal-transducing intermediates and, thus, the immune response. Additional studies will be required to determine if an altered K63 versus K48 balance, as seen in macrophage reprogramming, is also important in other scenarios beyond endotoxin tolerance.
FIG 6 .
Schematic summary of results. Model representing differential PKR ubiquitination in naive or LPS-tolerized macrophages and the consequences for LPS-induced PKR activity.
Finally, we provide evidence for PKR being an additional target for SOCS-1, thereby expanding its role as a critical regulator of TLR4 signaling. Loss of SOCS-1 results in constitutive PKR hyperactivity in naive cells, even in the absence of LPS stimulation. Why a loss of SOCS-1 results in dramatically and constitutively elevated levels of phosphorylated PKR, but not total PKR, is not entirely clear. One possible explanation is that SOCS-1 interacts only with and negatively regulates the phosphorylated/activated pool of PKR protein. This hypothesis is supported by the published observation that SOCS-1 requires its SH2 domain to interact with phosphotyrosine residues on target proteins to function as a ligase. It is also important to point out that while the residue detected by the phosphospecific antibody we have used strongly correlates with PKR activity, formal measurement of kinase activity in SOCS-1 knockout MEFs has not been done. Nevertheless, the elimination of SOCS-1 alone did not restore LPS responsiveness in tolerant MEFs. This is perhaps not entirely surprising, as we also observed a loss of LPS-dependent K63 Ub in tolerance, which may be a redundant mechanism to negatively govern PKR
MATERIALS AND METHODS
Cells and reagents.
Primary peritoneal macrophages were prepared as described previously (43). Briefly, 3 ml of 3% sterile fluid thioglycolate (Remel) was injected intraperitoneally (i.p.) into 6- to 8-week-old, wild-type (WT) C57BL/6J mice (Jackson Laboratories, Bar Harbor, ME). Four days later, macrophages were harvested by peritoneal lavage with sterile saline.
HEK293T (ATCC, Manassas, VA) cells were cultured in Dulbecco modified Eagle medium (DMEM; BioWhittaker) supplemented with 10% fetal bovine serum (FBS), 2 mM l-glutamine, 100 U/ml penicillin, and 100 µg/ml streptomycin. The RAW 264.7 macrophage-like cell line (ATCC) was cultured in RPMI 1640 (BioWhittaker) supplemented with 10% FBS, 2 mM l-glutamine, 100 U/ml penicillin, and 100 µg/ml streptomycin. The MAT4 cell line that stably expresses human TLR4 and MD-2 (a kind gift from Liwu Li, Virginia Tech University, Blacksburg, VA) was maintained in DMEM supplemented as described for HEK293T cells. Wild-type and SOCS-1 knockout fibroblasts were a kind gift from Atsushi Okumura (University of Pennsylvania Veterinary School) and were maintained in DMEM.
Protein-free, phenol-water-extracted Escherichia coli K235 LPS was prepared as described elsewhere (43). The TLR2 ligand S-[2,3-bis(palmitoyloxy)-(2-RS)-propyl]-N-palmitoyl-(R)-Cys–Ser–Lys4-OH (P3C) was obtained from EMC Microcollections (Tübingen, Germany). The TLR3 agonist poly(I ⋅ C) was obtained from Sigma-Aldrich (St. Louis, MO). MG132 was purchased from Calbiochem (Gibbstown, NJ) and was resuspended in DMSO, according to the manufacturer’s instructions. Monoclonal antibody to PKR (clone B-10) and polyclonal antibody to SOCS-1 were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Phosphospecific anti-PKR antibody was purchased from BioSource (Carlsbad, CA). Antiubiquitin (clone PD4) and anti-p38 antibodies were obtained from Cell Signaling (Danvers, MA). Anti-HA monoclonal antibody was purchased from Roche (Indianapolis, IN). Anti-V5 monoclonal antibody was obtained from Invitrogen (Carlsbad, CA). Anti-K63 monoclonal antibody was obtained from Biomol (Plymouth, PA). Expression vectors encoding HA-tagged WT, K63-only, and K48-only ubiquitin were kindly provided by Yixian Zheng (Carnegie Institute of Washington) and were described elsewhere (44). A V5-tagged PKR plasmid was a kind gift from Ganesh Sen (Lerner Research Institute, Cleveland Clinic). Expression plasmids for FLAG-tagged SOCS-1 and SOCS-2 were kind gifts from Raymond Donnelly (FDA, Bethesda, MD).
Stimulation and induction of tolerance in primary macrophages.
For primary stimulation experiments, primary peritoneal macrophages were plated at a density of 4 × 106 cells per well in a six-well plate and treated with TLR ligands for the indicated time periods at the following final concentrations: LPS, 250 ng/ml; P3C, 500 ng/ml; and p(I ⋅ C), 50 µg/ml. Doses of individual TLR ligands were selected after optimizing the experimental conditions. Cells were harvested in lysis buffer (20 mM HEPES, 1.0% Triton X-100, 0.1% sodium dodecyl sulfate [SDS], 150 mM NaCl, 10 mM NaF, 1 mM phenylmethylsulfonyl fluoride [PMSF]), and the lysates were used for Western analyses with described antibodies.
For experiments involving tolerance, macrophages were incubated for 18 h with medium, 100 ng/ml LPS, 100 ng/ml P3C, or 10 µg/ml p(I ⋅ C), washed extensively with phosphate-buffered saline (PBS), and restimulated with 250 ng/ml LPS for the indicated times.
Transfection of HEK293T cells and Western blot analysis.
Whole-cell lysates from treated HEK293T cells, RAW 264.7 cells, or primary murine macrophages were obtained after the cells were washed twice in PBS by the addition of lysis buffer (20 mM HEPES, 1.0% Triton X-100, 0.1% SDS, 150 mM NaCl, 10 mM NaF, 1 mM PMSF) and subsequent incubation at 4°C. Cell lysates were separated by electrophoresis in a denaturing SDS-PAGE gel and by subsequent transfer to a polyvinylidene difluoride (PVDF) membrane. Blots were incubated overnight in relevant primary antibodies at 4°C, washed 3 times with PBS, and then incubated with the appropriate horseradish peroxidase (HRP)-conjugated secondary antibody (Jackson Immunochemicals, ME). Blots were developed following incubation in ECL Plus Western blotting detection reagent (Amersham Biosciences, Piscataway, NJ).
Ubiquitin immunoprecipitations.
To determine the ubiquitination status of PKR in RAW 264.7 cells, immunoprecipitation (IP) was performed as follows. A total of 3 × 106 cells were plated per well in a 6-well tissue culture plate and treated with medium only or 100 ng/ml E. coli LPS. Eighteen hours later, cells were washed twice in PBS, and the medium was replaced. Cells were restimulated with 250 ng/ml E. coli LPS, and individual wells were harvested at various time points in lysis buffer (20 mM HEPES, 1% Triton X-100, 0.1% SDS, 150 mM NaCl). Total lysates were precleared with protein A gel (Sigma) for 30 min, and PKR was immunoprecipitated with 1 µg of anti-PKR monoclonal antibody (clone B10) and additional protein A gel for 2 h at 4°C. Immune complexes were washed 3 times with 1 ml of lysis buffer, followed by being washed once in lysis buffer supplemented with 1 M urea (Sigma). Immunoprecipitated proteins were separated by SDS-PAGE, transferred to PVDF membranes, and probed for the presence of ubiquitinated PKR using antiubiquitin monoclonal antibody. For analysis of ubiquitination in HEK293T, 7.5 × 105 cells were plated in 6-well plates and transfected the following morning with constructs expressing tagged PKR and/or constructs expressing HA-tagged ubiquitin. Twenty-four hours after transfection, cells were lysed in lysis buffer and precipitated with 1 µg of anti-V5 antibody. Immunoprecipitates were probed with anti-HA monoclonal antibody.
Transfection of and immunoprecipitation from MAT4 cells.
MAT4 cells were plated at a density of 5 × 105 cells per well in a six-well dish and transfected 24 h later with 1 µg of empty vector or vector that expresses either HA-K63-only or HA-K48-only ubiquitin. Eight hours after the end of transfection, cells were stimulated with medium alone or medium containing 100 ng/ml LPS for an additional 18 h. Cells were then washed 3 times in PBS and stimulated with 250 ng/ml LPS for the indicated times. Samples from each time point were immunoprecipitated in lysis buffer with anti-PKR monoclonal antibody, and immunoprecipitates were separated by SDS-PAGE. Blots were probed with anti-HA monoclonal antibodies as described above.
ACKNOWLEDGMENTS
We thank Peter Murray, Evan Parganas, and James Ihle for critical assistance with mouse experiments, Raymond Donnelly and Ganesh Sen for contributing expression constructs, and Liwu Li and Atsushi Okumura for providing MAT4- and SOCS-1-deficient cells, respectively. Additionally, we thank Bret Hassel for critical evaluation of the manuscript.
This work was supported in part by grants from the National Institutes of Health to S.N.V. (AI-18797) and N.Q. (GM50870).
Footnotes
Citation Perkins, D. J., N. Qureshi, and S. N. Vogel. 2010. A Toll-like receptor-responsive kinase, protein kinase R, is inactivated in endotoxin tolerance through differential K63/K48 ubiquitination. mBio 1(5):e00239-10. doi:10.1128/mBio.00239-10.
REFERENCES
- 1. Medzhitov R. 2008. Origin and physiological roles of inflammation. Nature 454:428–435 [DOI] [PubMed] [Google Scholar]
- 2. Remick D. G. 2003. Cytokine therapeutics for the treatment of sepsis: why has nothing worked? Curr. Pharm. Des. 9:75–82 [DOI] [PubMed] [Google Scholar]
- 3. West M. A., Heagy W. 2002. Endotoxin tolerance: a review. Crit. Care Med. 30:S64–S73 [PubMed] [Google Scholar]
- 4. Docke W. D., Randow F., Syrbe U., Krausch D., Asadullah K., Reinke P., Volk H. D., Kox W. 1997. Monocyte deactivation in septic patients: restoration by IFN-gamma treatment. Nat. Med. 3:678–681 [DOI] [PubMed] [Google Scholar]
- 5. Munoz C., Carlet J., Fitting C., Misset B., Bleriot J. P., Cavaillon J. M. 1991. Dysregulation of in vitro cytokine production by monocytes during sepsis. J. Clin. Invest. 88:1747–1754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Flach R., Majetschak M., Heukamp T., Jennissen V., Flohe S., Borgermann J., Obertacke U., Schade F. U. 1999. Relation of ex vivo stimulated blood cytokine synthesis to post-traumatic sepsis. Cytokine 11:173–178 [DOI] [PubMed] [Google Scholar]
- 7. Shahbazian L. M., Jeevanandam M., Petersen S. R. 1999. Release of proinflammatory cytokines by mitogen-stimulated peripheral blood mononuclear cells from critically ill multiple-trauma victims. Metabolism 48:1397–1401 [DOI] [PubMed] [Google Scholar]
- 8. Medvedev A. E., Sabroe I., Hasday J. D., Vogel S. N. 2006. Tolerance to microbial TLR ligands: molecular mechanisms and relevance to disease. J. Endotoxin Res. 12:133–150 [DOI] [PubMed] [Google Scholar]
- 9. Rittirsch D., Flierl M. A., Ward P. A. 2008. Harmful molecular mechanisms in sepsis. Nat. Rev. Immunol. 8:776–787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Henricson B. E., Manthey C. L., Perera P. Y., Hamilton T. A., Vogel S. N. 1993. Dissociation of lipopolysaccharide (LPS)-inducible gene expression in murine macrophages pretreated with smooth LPS versus monophosphoryl lipid A. Infect. Immun. 61:2325–2333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Shnyra A., Brewington R., Alipio A., Amura C., Morrison D. C. 1998. Reprogramming of lipopolysaccharide-primed macrophages is controlled by a counterbalanced production of IL-10 and IL-12. J. Immunol. 160:3729–3736 [PubMed] [Google Scholar]
- 12. Hirohashi N., Morrison D. C. 1996. Low-dose lipopolysaccharide (LPS) pretreatment of mouse macrophages modulates LPS-dependent interleukin-6 production in vitro. Infect. Immun. 64:1011–1015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Medvedev A. E., Lentschat A., Wahl L. M., Golenbock D. T., Vogel S. N. 2002. Dysregulation of LPS-induced Toll-like receptor 4-MyD88 complex formation and IL-1 receptor-associated kinase 1 activation in endotoxin-tolerant cells. J. Immunol. 169:5209–5216 [DOI] [PubMed] [Google Scholar]
- 14. Medvedev A. E., Piao W., Shoenfelt J., Rhee S. H., Chen H., Basu S., Wahl L. M., Fenton M. J., Vogel S. N. 2007. Role of TLR4 tyrosine phosphorylation in signal transduction and endotoxin tolerance. J. Biol. Chem. 282:16042–16053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kobayashi K., Hernandez L. D., Galan J. E., Janeway C. A., Jr., Medzhitov R. , Flavell R. A. 2002. IRAK-M is a negative regulator of Toll-like receptor signaling. Cell 110:191–202 [DOI] [PubMed] [Google Scholar]
- 16. van ’t Veer C., van den Pangaart P. S., van Zoelen M. A., de Kruif M., Birjmohun R. S., Stroes E. S., de Vos A. F., van der Poll T. 2007. Induction of IRAK-M is associated with lipopolysaccharide tolerance in a human endotoxemia model. J. Immunol. 179:7110–7120 [DOI] [PubMed] [Google Scholar]
- 17. Li L., Cousart S., Hu J., McCall C. E. 2000. Characterization of interleukin-1 receptor-associated kinase in normal and endotoxin-tolerant cells. J. Biol. Chem. 275:23340–23345 [DOI] [PubMed] [Google Scholar]
- 18. Yamin T. T., Miller D. K. 1997. The interleukin-1 receptor-associated kinase is degraded by proteasomes following its phosphorylation. J. Biol. Chem. 272:21540–21547 [DOI] [PubMed] [Google Scholar]
- 19. Foster S. L., Hargreaves D. C., Medzhitov R. 2007. Gene-specific control of inflammation by TLR-induced chromatin modifications. Nature 447:972–978 [DOI] [PubMed] [Google Scholar]
- 20. Nakagawa R., Naka T., Tsutsui H., Fujimoto M., Kimura A., Abe T., Seki E., Sato S., Takeuchi O., Takeda K., Akira S., Yamanishi K., Kawase I., Nakanishi K., Kishimoto T. 2002. SOCS-1 participates in negative regulation of LPS responses. Immunity 17:677–687 [DOI] [PubMed] [Google Scholar]
- 21. Cole J. L. 2007. Activation of PKR: an open and shut case? Trends Biochem. Sci. 32:57–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gale M., Jr., Tan S. L. , Katze M. G. 2000. Translational control of viral gene expression in eukaryotes. Microbiol. Mol. Biol. Rev. 64:239–280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sadler A. J., Williams B. R. 2007. Structure and function of the protein kinase R. Curr. Top. Microbiol. Immunol. 316:253–292 [DOI] [PubMed] [Google Scholar]
- 24. Horng T., Barton G. M., Medzhitov R. 2001. TIRAP: an adapter molecule in the Toll signaling pathway. Nat. Immunol. 2:835–841 [DOI] [PubMed] [Google Scholar]
- 25. Goh K. C., deVeer M. J., Williams B. R. 2000. The protein kinase PKR is required for p38 MAPK activation and the innate immune response to bacterial endotoxin. EMBO J. 19:4292–4297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hsu L. C., Park J. M., Zhang K., Luo J. L., Maeda S., Kaufman R. J., Eckmann L., Guiney D. G., Karin M. 2004. The protein kinase PKR is required for macrophage apoptosis after activation of Toll-like receptor 4. Nature 428:341–345 [DOI] [PubMed] [Google Scholar]
- 27. Bhoj V. G., Chen Z. J. 2009. Ubiquitylation in innate and adaptive immunity. Nature 458:430–437 [DOI] [PubMed] [Google Scholar]
- 28. Chen Z. J., Sun L. J. 2009. Nonproteolytic functions of ubiquitin in cell signaling. Mol. Cell 33:275–286 [DOI] [PubMed] [Google Scholar]
- 29. Moynagh P. N. 2009. The Pellino family: IRAK E3 ligases with emerging roles in innate immune signalling. Trends Immunol. 30:33–42 [DOI] [PubMed] [Google Scholar]
- 30. Pineda G., Ea C. K., Chen Z. J. 2007. Ubiquitination and TRAF signaling. Adv. Exp. Med. Biol. 597:80–92 [DOI] [PubMed] [Google Scholar]
- 31. Mansell A., Smith R., Doyle S. L., Gray P., Fenner J. E., Crack P. J., Nicholson S. E., Hilton D. J., O’Neill L. A., Hertzog P. J. 2006. Suppressor of cytokine signaling 1 negatively regulates Toll-like receptor signaling by mediating Mal degradation. Nat. Immunol. 7:148–155 [DOI] [PubMed] [Google Scholar]
- 32. Lam Y. A., Xu W., DeMartino G. N., Cohen R. E. 1997. Editing of ubiquitin conjugates by an isopeptidase in the 26S proteasome. Nature 385:737–740 [DOI] [PubMed] [Google Scholar]
- 33. Newton K., Matsumoto M. L., Wertz I. E., Kirkpatrick D. S., Lill J. R., Tan J., Dugger D., Gordon N., Sidhu S. S., Fellouse F. A., Komuves L., French D. M., Ferrando R. E., Lam C., Compaan D., Yu C., Bosanac I., Hymowitz S. G., Kelley R. F., Dixit V. M. 2008. Ubiquitin chain editing revealed by polyubiquitin linkage-specific antibodies. Cell 134:668–678 [DOI] [PubMed] [Google Scholar]
- 34. Sato S., Takeuchi O., Fujita T., Tomizawa H., Takeda K., Akira S. 2002. A variety of microbial components induce tolerance to lipopolysaccharide by differentially affecting MyD88-dependent and -independent pathways. Int. Immunol. 14:783–791 [DOI] [PubMed] [Google Scholar]
- 35. O’Neill L. A. 2009. Regulation of signaling by non-degradative ubiquitination. J. Biol. Chem. 284:8209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wysocka J., Reilly P. T., Herr W. 2001. Loss of HCF-1-chromatin association precedes temperature-induced growth arrest of tsBN67 cells. Mol. Cell. Biol. 21:3820–3829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. O’Shea J. J., Murray P. J. 2008. Cytokine signaling modules in inflammatory responses. Immunity 28:477–487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kinjyo I., Hanada T., Inagaki-Ohara K., Mori H., Aki D., Ohishi M., Yoshida H., Kubo M., Yoshimura A. 2002. SOCS1/JAB is a negative regulator of LPS-induced macrophage activation. Immunity 17:583–591 [DOI] [PubMed] [Google Scholar]
- 39. Gingras S., Parganas E., de Pauw A., Ihle J. N., Murray P. J. 2004. Re-examination of the role of suppressor of cytokine signaling 1 (SOCS1) in the regulation of Toll-like receptor signaling. J. Biol. Chem. 279:54702–54707 [DOI] [PubMed] [Google Scholar]
- 40. Kim Y. G., Park J. H., Shaw M. H., Franchi L., Inohara N., Nunez G. 2008. The cytosolic sensors Nod1 and Nod2 are critical for bacterial recognition and host defense after exposure to Toll-like receptor ligands. Immunity 28:246–257 [DOI] [PubMed] [Google Scholar]
- 41. Bennett R. L., Blalock W. L., Abtahi D. M., Pan Y., Moyer S. A., May W. S. 2006. RAX, the PKR activator, sensitizes cells to inflammatory cytokines, serum withdrawal, chemotherapy, and viral infection. Blood 108:821–829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Patel R. C., Sen G. C. 1998. PACT, a protein activator of the interferon-induced protein kinase, PKR. EMBO J. 17:4379–4390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. McIntire F. C., Sievert H. W., Barlow G. H., Finley R. A., Lee A. Y. 1967. Chemical, physical, biological properties of a lipopolysaccharide from Escherichia coli K-235. Biochemistry 6:2363–2372 [DOI] [PubMed] [Google Scholar]
- 44. Vong Q. P., Cao K., Li H. Y., Iglesias P. A., Zheng Y. 2005. Chromosome alignment and segregation regulated by ubiquitination of survivin. Science 310:1499–1504 [DOI] [PubMed] [Google Scholar]