Abstract
An improved chemical synthesis of N-2((2S)-2-(3,5-difluorophenyl)-2-hydroxyethanoyl)-N1-((7S)-5-methyl-6-oxo-6,7-dihydro-5H-dibenzo[b,d]azepin-7-yl)-L-alaninamide (LY411,575, 9a), a known γ-secretase inhibitor, is described. The key synthetic steps, which used no chiral chromatography in the entire sequence, involved 1) improved microwave-assisted synthesis of a seven-membered lactam (±)-(5,7-dihydro-6H-dibenz-[b,d]azepin-6-one 2, and, 2) convenient isolation of pure LY411575 from a mixture of four diastereomers by simple flash silica gel chromatography. Starting from the resolved aminolactams 5a and 5b, all four diastereomers were produced in enantiomerically pure form.
Keywords: LY411575, diastereomer, Microwave, chiral chromatography, R-lactic acid
Alzheimer’s disease has claimed 27 million individuals worldwide, more than 4 million in the United States alone.1a,b The presence of amyloid-β-containing peptide plaques in the brain is the hallmark of this senile disease. It is known that, of the two Aβ-peptides (1-40 and 1-42), the longer form has a stronger tendency to deposit insoluble plaques in the AD brain.1c,2 Considerable genetic, biophysical, and toxicological data emerging from studies of familial forms of Alzheimer’s disease (AD), transgenic animal modeling of amyloid β-protein precursor (APP) and presenilin (PS) mutants, toxicological and biophysical studies have strongly suggested a pathogenic role in AD for Aβ42.3 This discovery immediately points to a possibility of developing drugs for AD that may target Aβ42. Several strategies can be pursued to stop or slow down the progression of the disease by preventing the formation or intracellular deposition of 1-42 Aβ-peptide. One of the prominent approaches to do this is to develop inhibitors of γ-secretase4, an intramembrane aspartic protease best represented by a macromolecular complex comprised of Presenilin-1 (PS1), Pen-2, Aph-1, and Nicastrin, and is known to endoproteolyze APP as well as several other transmembrane proteins.5 One of these inhibitors, LY411575 (9a, Figure 1) that has shown promise for significantly reducing brain and CSF levels of the Aβ-peptides through its γ-secretase activity in recent years was developed by Eli Lilly.6
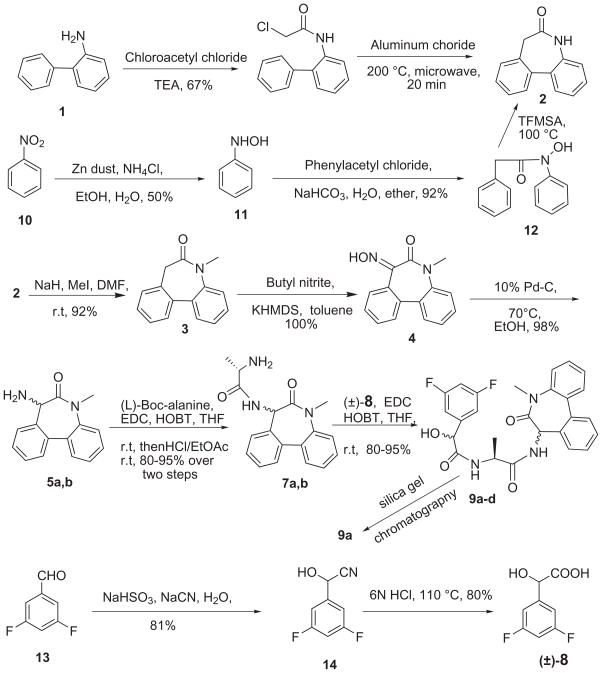
Scheme 1.
Synthesis of LY 411575 from racemic diphenyl lactam 5
To the best of our knowledge, LY411575 is not available commercially. Details of its chemical synthesis were reported in a patent.6 However, in our hands this synthesis did not lend itself to generating multigram quantities of LY411575 needed for our ongoing pharmacological studies on the downregulation of Aβ (1-42) peptide levels in animal models. This was due to (1) difficulties in achieving efficient synthetic quantities of the dibenzazepinone 2 (Scheme 1), (2) reported necessity of generating enantiomerically pure (S)-3,5-difluoromandelic acid, and (3) required chiral resolution of the two diastereomers of the Boc-derivative of the dibenzazepinone-alaninamide amines 7 by preparative chiral HPLC which worked for small scale preparations only. For our multigram drug needs, we needed to make the synthesis concise by devising a short and efficient synthesis of the dibenzazepinone 2 (Scheme 1), as well as adopting a synthetic strategy by eliminating the need for resolutions on chiral HPLC columns.
The synthesis of dibenzazepinone 2 (Scheme 1) was originally reported by Stollé cyclization of the 2-chloroacetyl derivative of 2-phenylaniline with AlCl3 at elevated temperatures.7 However, the authors reported that they had prepared 7-phenyloxindole (a 5-membered Stollé ring closure to indole instead of 7-membered lactam). This assignment of structure was revised by Brown to dibenzazepinone 2.8 This reaction, in our hands, however, produced intractable black tars from which the desired lactam was obtained by cumbersome silica gel chromatography only in 18–20% yield. More recently, alternative syntheses of the same lactam 2 have been reported. Thus, Baudoin and collaborators have prepared small quantities of the lactam 2 by a two-step reaction sequence by Palladium-catalyzed borylation-Suzuki reaction (45% overall yield).9 A four-step sequence of generating lactam 2 was reported in which nitrobenzene was partially reduced by Zn to phenylhydroxylamine that, after derivatizing to a hydroxylamide, was cyclized by heating it with neat trifluoromethanesufonic acid as solvent.10 We prepared lactam 2 by this route (Scheme 1) and found that the reaction sequence could not be easily scaled up and that the overall yields of the lactam were low. More recently, Fuwa have reported a general method of generating several macrocyclic lactams (including the lactam 2) which were obtained in six steps. In this scheme, 2-bromoaniline and 2-iodobenzyl cyanide were coupled in a key step via borylation-Suzuki-Miyaura protocol to a biphenyl methylene cyanide that was subsequently carried to lactam 2 in five steps with the final step being a Staudinger-aza-Wittig lactam ring closure.11 A four step synthesis of the lactam 2 beginning with 2-nitro-2′-biphenylcarboxylic acid was also known in the literature that give 4–6 grams of the lactam but requires the use of large quantities (ca. one mole) of hazardous diazomethane.12
Recently, a large number of high temperature reactions have been carried out using affordable microwave technology.13 Compared to the thermal reaction conditions, the microwave energy seems to facilitate high temperature reactions often selectively producing cleaner desired products in higher yield by minimizing side products, and considerably reducing reaction times. Since we were interested in producing multigram quantities of the lactam 2, we focused on using the shortest and least expensive method to produce large quantities of the lactam 2. To this end, we chose to employ microwave methodology to improve upon our poor yield of the lactam obtained in our thermal reaction runs. Thus, the intramolecular Friedel-Craft cyclization of the 2-chloroacetyl derivative of 2-phenylaniline was attempted under AlCl3 catalysis with or without solvent under different temperature and microwave wattage conditions. The best yields of the 7-membered biphenyl lactam 2 were obtained by irradiating a dry mixture of the lactam precursor with anhydrous AlCl3 for 20 min at 200 °C. The black tars were still obtained as in the thermal reaction but seemed to be more amenable to effective extraction with n-butanol. After a short path silica gel chromatography, the desired lactam was readily crystallized in 40% isolated yield. When an aprotic, high boiling solvent, such as N,N-dimethylformamide and N-methylpyrrolidone was used, only 7-phenyloxindole (Stollé condensation product) was obtained in high yield. Next, the lactam 2 was N-methylated to lactam 3 in DMF at room temperature in 92% yield. Oximation of 3 with butyl nitrite and potassium hexamethyldisilazide to 4 followed by catalytic hydrogenation at 70 °C afforded the racemic aminobiphenyl lactam 5 in essentially quantitative yield (Scheme 1).
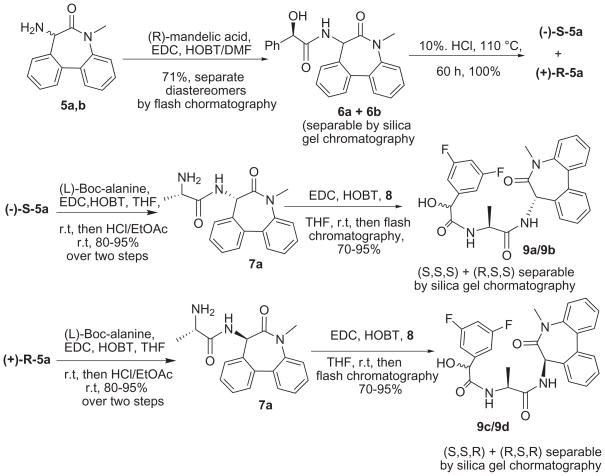
Initially, we decided to carry the racemic aminolactam 5 through the end of LY411575 synthesis, hoping that the desired isomer might be isolated from a mixture of the four diastereomers by silica gel chromatography rather than by two inconvenient chiral HPLC separations. To this end, the racemic aminolactam 5 was coupled with Boc-(L)-alanine, but the two diastereomers could not be separated by silica gel. The chiral HPLC, as reported,6a with larger batches of mixtures met with limited success. Despite these disappointing results we decided to go forward and complete the synthesis of LY411575 with a remote hope that silica gel chromatography of the final four diastereomers of LY411575 might be of some help in isolating the desired enantiomerically pure diastereomer LY411575 in adequate quantities (Scheme 1). The butoxycarbonyl (Boc) group of the lactam alaninamide was removed under mild acidic conditions and the resulting amine diastereomeric mixture 7 was coupled to racemic 3,5-difluoromandelic acid to generate a mixture of four diastereomers. The mixture could be partially analyzed on the basis of its 1H-NMR spectra, which gave four clear resonance signals each for N-methyl and C-methyl proton. More significantly, and much to our delight, the desired enantiomer LY411575 was readily isolated from this complex mixture of four diastereomers in a reasonable yield.
The absolute configuration (S,S,S) of the major isolated isomer was immediately suspected to be that of LY411575 by its expected activity in well-characterized γ-secretase assays. However, to confirm its configuration positively and to evaluate the other three diastereomers of LY411575 for γ-secretase activity, it was felt necessary to synthesize and isolate all four diastereomers in enantiomerically pure form. We had initially attempted to react separately the resolved 3,5-difluoromandelic acid enantiomers with the diastereomeric amide 7 (Scheme 1) with the hope of isolating LY411575 from the two resulting diastereomers by silica gel chromatography. However, these diastereomers could not be separated in pure form either by flash chromatography or reverse phase HPLC. This necessitated resolving the racemic amines 5 in order to achieve silica gel separation of LY411575 from the other diastereomer in the final step of synthesis To this end, chiral resolution of the racemic lactam amine 5 was attempted by crystallization from its diastereomeric salts, as well as by converting it to diastereomeric amides, with some optically pure acids, such as tartaric acid, camphor sulfonic acid, malic acid, lactic acid and mandelic acid. While diastereomeric salt formation was not significantly successful with any of the chiral acids tried, the diastereomeric amides 6a,b with R-mandelic acid could be easily separated by silica gel chromatography (Scheme 2). Fortunately, the R-mandelic acid component of each diastereomer was successfully and selectively removed from the chirally pure amines in high yield without any evidence of attendant hydrolysis of the lactam ring by a 60 hour reflux with 10% hydrochloric acid. In this way, pure S- and R-5-amino-7-methyl-5,7-dihydro-6H-dibenz-[b,d]azepin-6-one 5a and 5b were isolated and characterized by converting them to their crystalline HCl-salt form. The absolute configuration of each enantiomeric amine was determined by comparing the γ-secretase activity of the final isomers of LY411575 (vide supra).
Scheme 2.
Synthesis of diastereomers of LY411575
In summary, a highly convenient and useful synthesis of a well-known γ-secretase inhibitor LY411575 is devised that is capable of rapidly producing multigram quantities of the drug without resorting to chiral chromatography or HPLC at any step of the synthetic sequence. Starting from the aminolactam (±)-5, the overall yield of isolated, enantiomerically pure, LY411575 was 13.6% (See experimental data for alll compounds in the Supplementary material).
Acknowledgments
The financial support provided for this synthetic project by National Institutes of Health (NIH Grant no. P01 AG20206) is gratefully acknowledged.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and notes
- 1.(a) Wimo A, Jonsson L, Winblad B. Dement Geriatr Cogn Disord. 2006;21:175–81. doi: 10.1159/000090733. [DOI] [PubMed] [Google Scholar]; (b) Hebert LE, Scherr PA, Bienias JL, et al. Arch Neurol. 2003;60:1119–1122. doi: 10.1001/archneur.60.8.1119. [DOI] [PubMed] [Google Scholar]; (c) Selkoe DJ. Physiol Rev. 2001;81:741–766. doi: 10.1152/physrev.2001.81.2.741. [DOI] [PubMed] [Google Scholar]
- 2.Price DL, Sisodia SS. Annu Rev Neurosci. 1998;21:479–505. doi: 10.1146/annurev.neuro.21.1.479. [DOI] [PubMed] [Google Scholar]
- 3.(a) Younkin SG. J Physiol, Paris. 1998;92:289–292. doi: 10.1016/s0928-4257(98)80035-1. [DOI] [PubMed] [Google Scholar]; (b) Golde TE. J Neurochem. 2006;99:689–707. doi: 10.1111/j.1471-4159.2006.04211.x. [DOI] [PubMed] [Google Scholar]
- 4.(a) Wolfe MS, Xia WM, Ostaszewski BL, Diehl TS, Kimberly WT, Selkoe DJ. Nature. 1999;398:513–517. doi: 10.1038/19077. [DOI] [PubMed] [Google Scholar]; (b) Takasugi N, Tomita T, Hayashi I, Tsuruoka M, Niimura M, Takahashi Y, Thinakaran G, Iwatsubo T. Nature. 1998;422:438–441. doi: 10.1038/nature01506. [DOI] [PubMed] [Google Scholar]; (c) De Strooper B. Neuron. 2003;38:9–12. doi: 10.1016/s0896-6273(03)00205-8. [DOI] [PubMed] [Google Scholar]; (d) De Strooper B, Annaert W, Cupers P, Saftig P, Craessaerts K, Mumm JS, Schroeter EH, Schrijvers V, Wolfe MS, Ray WJ, et al. Nature. 1999;398:518–522. doi: 10.1038/19083. [DOI] [PubMed] [Google Scholar]
- 5.Li YM. Mol Interven. 2001;1:198–207. [PubMed] [Google Scholar]
- 6.(a) Wu JT, Tung JS, Thorsett ED, Pleiss MA, Nissen JS, Neitz J, Latimer LH, John V, Freedman S, Britton TC, Audia JE, Reel JK, Mabry TE, Dressman BA, Cwi CL, Droste JJ, Henry SS, McDaniel SL, Scott WL, Stucky RD. WO 9828268 A 2. Porter, WPCT Int Appl. 1998:889.; (b) Audia JE, Hyslop PA, Nissen JS, Thompson RC, Tung JS, Tanner LI. WO 00/19210. 2000
- 7.(a) Wiesner K, Valenta C, Manson AJ, Stonner FW. J Am Chem Soc. 1955;77:675–683. [Google Scholar]; (b) Dewar MJS, Kaneko C, Bhattacharjee MK. J Am Chem Soc. 1962;84:4884–4887. [Google Scholar]
- 8.Brown R, Roger FC, Butcher M. Tetrahedron Lett. 1971;8:667–670. [Google Scholar]
- 9.Baudoin O, Cesario M, Guenard D, Gueritte F. J Org Chem. 2002;67:1, 1199–1207. doi: 10.1021/jo0160726.For a variation of this method, see: Best JD, Jay MT, Otu F, Ma J, Nadin A, Ellis S, Lewis HD, Pattison C, Reilly M, Harrison T, Shearman MS, Williamson TL, Atack J. J Pharmacol Exp Ther. 2005;313:902–908. doi: 10.1124/jpet.104.081174.
- 10.Endo Y, Ohta T, Shudo K, Okamoto T. Heterocycles. 1977;8:367–370. [Google Scholar]
- 11.Fuwa H, Okamura Y, Morohashi Y, Tomita T, Iwatsubo T, Kan T, Fukuyama T, Natsugari H. Tetrahedron Lett. 2004;45:2323–2326. [Google Scholar]
- 12.Muth CW, Sung WL, Papanastassiou ZB. J Am Chem Soc. 1955;77:3393–3395. [Google Scholar]
- 13.(a) Gedye R, Smith F, Westaway K, Ali H, Baldisera L. Tetrahedron Lett. 1986;27:279–282. [Google Scholar]; (b) Stuerga D, Gonon K, Lallemant M. Tetrahedron. 1993;49:6229–6234. [Google Scholar]; (c) Lidström P, Tinerney J, Wathey B, Westman J. Tetrahedron. 2001;57:9225–9283. [Google Scholar]