SUMMARY
The IL-2/IL-2R interaction is essential for Treg cell development and homeostasis. Here we show that expression of IL-2Rβ chains that lack tyrosine residues important for the association of the adaptor Shc and STAT5 in IL-2Rβ-deficient mice resulted in production of a normal proportion of natural Treg cells that suppressed severe autoimmunity related with deficiency in IL-2/IL-2R. These mutant IL-2Rβ chains supported suboptimal and transient STAT5 activation that upregulated Foxp3 to normal levels in natural, but not induced, Treg cells. Nevertheless, gene expression profiling revealed many targets in peripheral natural Treg cells that were IL-2-dependent and a significant overlap between the Treg cell IL-2-dependent gene program and the Treg cell transcriptional signature. Collectively, these findings demonstrate that a critical, and perhaps minor, subset of IL-2-dependent targets is indexed to a low IL-2R signaling threshold and that a significant proportion of the Treg cell gene program is regulated by IL-2.
INTRODUCTION
The IL-2/IL-2R interaction is not only important for T effector cells but is also an essential regulator of Treg cell development and homeostasis (Malek, 2008). Indeed, the extreme systemic lethal autoimmunity associated with deficiency in IL-2, IL-2Rα (CD25), or IL-2Rβ (CD122) is primarily associated with defective Treg cell production (Almeida et al., 2002; Furtado et al., 2002; Malek et al., 2002). These mice harbor immature CD4+ Foxp3low CD25neg T cells that do not suppress peripheral autoreactive T cells (Bayer et al., 2007; Fontenot et al., 2005). IL-2 is critically required in the thymus to promote Treg development in an instructive manner, in part by upregulation of Foxp3 and CD25 (Burchill et al., 2008; Lio and Hsieh, 2008). IL-2 is also required for the peripheral homeostasis of Treg cells (Bayer et al., 2005; Setoguchi et al., 2005). Lower production of IL-2 defines one of the genetic factors rendering nonobese diabetic (NOD) mice susceptible to autoimmune disease that leads to impaired Treg cells (Yamanouchi et al., 2007). IL-2 is also necessary for the generation of Foxp3+ induced-Treg (iTreg) cells from naïve conventional peripheral T lymphocytes (Davidson et al., 2007; Zheng et al., 2007).
IL-2R signaling mechanisms have been most extensively studied in various IL-2-responsive cell lines that approximate activated effector T cells (Gaffen, 2001; Nelson and Willerford, 1998). This work has lead to a model where IL-2 signal transduction is initiated upon ligand-induced oligomerization of the IL-2R, consisting of IL-2Rα, IL-2Rβ and γc (CD132). This event brings the cytoplasmic tail of IL-2Rβ and γc in close proximity with their associated Jak-1 and Jak-3, respectively, allowing phosphorylation of 3 key tyrosines (Y) residues of IL-2Rβ. Phosphorylation of Y338 (Y341 in the mouse) permits recruitment of the adaptor Shc, which leads to activation of the MAPK and PI-3-kinase pathways, whereas phosphorylation of Y392 and Y510 (Y395 and Y498 in the mouse) preferentially and predominately recruits STAT5, which leads to activation of STAT5-dependent genes (Ascherman et al., 1997; Friedmann et al., 1996; Gaffen et al., 1996). This relationship is not strict and there appears to be some redundancy related to these tyrosine residues and pathways as STAT5 activation was associated with Y338 in some studies (Gaffen et al., 1996) and STAT5 activation has been reported to sustain PI-3-kinase activation independent of Shc (Lockyer et al., 2007). Importantly, optimal IL-2-dependent T cell growth factor activity in IL-2-reposnsive cell lines requires phosphorylation of all 3 tyrosines and signaling through these pathways.
In marked contrast to activated conventional T cells, mostly indirect evidence supports an important role for primarily IL-2-dependent activation of STAT5 in Treg cells. IL-2R signaling in Treg cells leads only to detectable STAT5 activation that is due to high PTEN levels (Bensinger et al., 2004; Walsh et al., 2006). In mice that lack STAT5, Treg cells are reduced (Antov et al., 2003; Burchill et al., 2003; Snow et al., 2003). Correspondingly, expression of constitutively active STAT5 increased Treg cell numbers when expressed in normal or Treg cell deficient mice (Burchill et al., 2003; Burchill et al., 2008). However, only one study has directly linked IL-2R-dependent activation of STAT5 and Treg cell production. This study showed that mature Treg cells developed in Rag2−/− chimeras after receiving IL-2Rβ−/− bone marrow that was transduced with an truncated IL-2Rβ chain that was designed to selectively activate STAT5 (Burchill et al., 2007). However, due to the small numbers of cells produced and the relatively short time frame of these experiments, signaling or suppressive activity by these Treg cells was not studied. Thus, the extent and exclusivity of STAT5 activation associated with this engineered IL-2Rβ chain and the durability of this type of signaling with respect to Treg cell homeostasis and function remain unclear.
Recent work has established a transcriptional signature for Treg cells that in part critically depends upon Foxp3 (Gavin et al., 2007; Hill et al., 2007; Lin et al., 2007). IL-2 has been suggested to be a key factor in sustaining the Foxp3 suppressive program in Treg cells (Gavin et al., 2007; Hill et al., 2007), but the extent that IL-2 controls the Treg gene signature has not been defined. Given this issue and the little that is known concerning IL-2R signaling mechanisms in Treg cells, the current study was undertaken to directly investigate the contribution of IL-2R signaling in Treg cell development, function, and homeostasis by examining the capacity of cytoplasmic domain mutant IL-2Rβ chains to support Treg cell production. The activity of mutant IL-2Rβ chains was also compared between Treg cells and conventional activated T lymphocytes. We find that Treg cells are effectively indexed to a low IL-2R signaling threshold and that a substantial fraction of the Treg cell transcriptional signature depends upon IL-2R signaling.
RESULTS
Mice expressing mutant IL-2R β-chains lack severe systemic autoimmunity
To directly examine the contribution of IL-2R signaling on primary T effector and Treg cells, Y341, or both Y395 and Y498, were mutated to phenylalanine or the H-region of the cytoplasmic domain was deleted to generate IL-2Rs that are predicted to predominantly activate Shc- or STAT5-dependent pathways, respectively (Figure 1A). Additionally, all 3 key tyrosine residues together were mutated to block these pathways. These constructs were expressed as transgenes in IL-2Rβ−/− mice in T lineage cells under the control of the CD2-mini gene. Typically 2 transgenic founders were identified and backcrossed to C57BL/6 IL-2Rβ−/− mice. As a control, IL-2Rβ−/− mice were also prepared that expressed transgenic wild-type (WT) IL-2Rβ. These mice are designated, 2RβY341, 2RβY395,498, 2RβY341,395,498, reflecting the Y→F mutations, 2RβΔH or 2RβWT, and all data reported represent transgenic mice on the IL-2Rβ−/− genetic background unless otherwise noted. Transgenic IL-2Rβs were largely T lineage restricted as IL-2Rβ was readily detected on essentially all thymocytes and mature T cells in the lymph nodes (LN) (Figure 1B) and spleen (not shown) but was absent on the non-T cell fraction in these peripheral immune tissues.
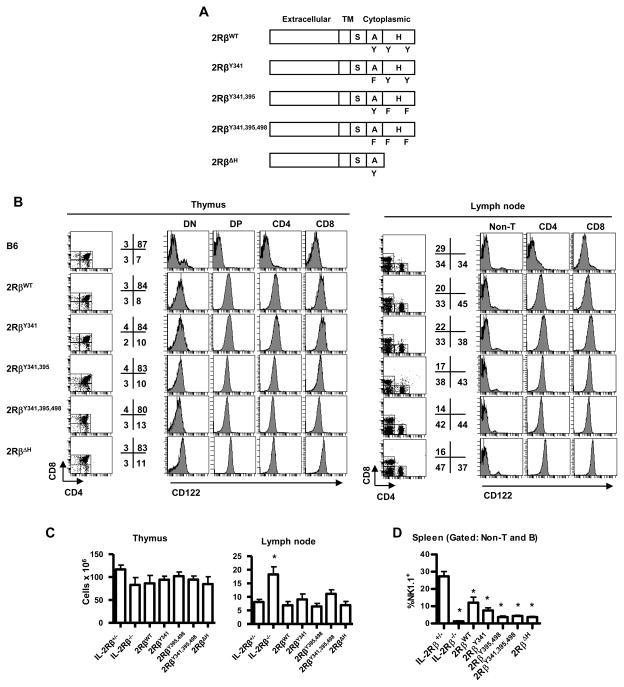
Figure 1. Characterization of lymphocyte cell populations within individual transgenic lines.
(A) Scheme represents mutant IL-2Rβ transgenes. Domains of IL-2Rβ are listed above the model, where TM refers to transmembrane. (B) IL-2Rβ expression by representative transgenic lines. Thymus and LNs were obtained from the indicated transgenic IL-2Rβ−/− mice and were assessed for CD122 (IL-2Rβ) expression after gating on the indicated populations of CD4+ and CD8+ cells. For reference, CD122 expression by normal WT C57BL/6 (B6) cells was included. DN and DP are CD4neg CD8neg or CD4+ CD8+ thymocytes, respectively. Data are representative of two founders/transgenic line. The numbers to the right of the dot plots represent the % of cells within the designated gated regions. (C) Thymic and LN cellularity and (D) splenic NK cells for the indicated transgenic lines. Data are mean ± SEM of ≥6 mice/group <16 wks of age.
These transgenic mice on the IL-2Rβ−/− genetic backgrounds contained normal numbers of cells in the thymus (Figure 1C), spleen (not shown) and LNs (Figure 1C). The distribution of major T cells subsets was also largely normal in the thymus and periphery as defined by CD4 and CD8 (Figure 1B). One exception was a lower proportion and number of peripheral CD8+ T cells as reflected by increased CD4:CD8 ratio which was most striking for 2RβY395,498, 2RβY341,395,498, and 2RβΔH mice (Figure 1B, right), which in part likely reflects impaired IL-15 signaling through the mutant transgenic IL-2Rβ chains, resulting in decreased survival of CD8+ T cells (Surh et al., 2006). As expected NK cells were greatly reduced in the spleen (Figure 1D), as these cells largely depend on STAT5-dependent IL-15 signaling for their development in the bone marrow (Waldmann and Tagaya, 1999). Greater numbers of NK cells for the 2RβWT and the 2RβY341 mice may reflect some activity of these transgenes in the thymus, which is a secondary site of NK development (Di Santo and Vosshenrich, 2006). Thus, the expression of transgenic WT or mutant IL-2Rβ in all T lineage cells does not obviously alter the T cell compartment probably because CD25 is absent on the large majority of thymocytes and peripheral T cells and CD25 is required for responsiveness to IL-2.
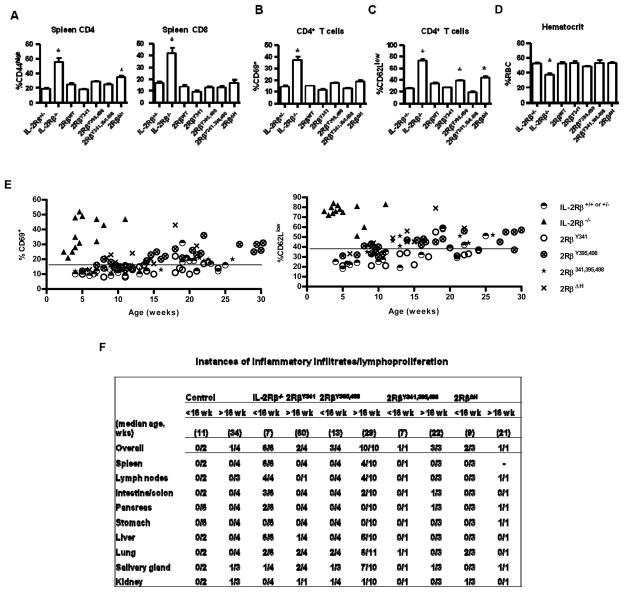
IL-2/IL-2R-deficient mice uniformly exhibit a rapid lethal autoimmune disease characterized by hyper-proliferative peripheral T cells with an activated phenotype (Sadlack et al., 1995; Sadlack et al., 1993; Suzuki et al., 1995; Willerford et al., 1995). When compared to C57BL/6 IL-2Rβ−/− mice, which usually die by 8–12 weeks of age, all these transgenic mice expressing mutant IL-2Rβ were effective breeders and overtly healthy (not shown), as long as the transgenic IL-2Rβ was expressed at a level equally or greater than that found on T cells in IL-2Rβ+/− mice. The 2RβY341 and 2Rβ395,498 lines were developed first and we have readily maintained some of these mice as long as 12–18 months without evidence of obvious disease. When specific characteristics associated with autoimmunity in IL-2Rβ−/− mice were evaluated for mice <16 weeks of age, each transgenic line contained largely normal LN cellularity (Figure 1C), and their peripheral T cells did not show an extensive activated phenotype after measuring CD44 expression for CD4 and CD8 T cells (Figure 2A) and CD69 and CD62L expression for CD4 (Figure 2B and 2C) and CD8 (not shown) T cells. This phenotypic profile indicates that these mice contain a largely normal complement of naïve peripheral T cells. Furthermore, all transgenic IL-2Rβ−/− mice were not anemic (Figure 2D). When compared to littermate controls, the CD4+ T cells from 2Rβ395,498 (Figure 2C) and 2RβΔ H (Figure 2A & 2C) mice exhibited a modest and statistically significant increase in the percentages of cells bearing CD44high and/or CD69. However, after 14–16 weeks of age, a trend was noted for increased percentages of CD69+ and CD62Llow CD4+ T cells for 2Rβ395,498 and 2RβΔH mice and for 2Rβ395,498, 2Rβ341,395,498, and 2RβΔH mice respectively, while this was rarely seen for 2RβY341 mice (Figure 2E). This finding is consistent with immune activation that is associated with autoimmunity.
Figure 2. Autoimmune status of transgenic IL-2Rβ−/− mice expressing mutant IL-2Rβ.
The indicated IL-2Rβ transgenic mice were assessed for peripheral T cells with an activated (A) CD44high, (B) CD69+, or (C) CD62Llow (C) phenotype or for (D) hemolytic anemia. Controls were IL-2Rβ+/− or IL-2Rβ−/− littermate mice. Data are mean ± SEM of ≥7 mice/group <16 wks of age, except for 2RβWT (n=3) (A-C) and representative of at least 5 mice/group in (D). Autoimmune status was also assessed as a function of age for the indicated transgenic mice by enumerating (E) the % of CD4+ T cells that were CD69+ or CD62Llow. The line in (E) represents the value for two standard deviations above of the mean for littermate control mice. (F) Hematoxylin-eosin fixed sections of the indictaed tissues were examined for inflammatory infiltrates.
Quantitative analysis of cell surface IL-2Rβ from Treg cells bearing the mutant IL-2R β-chains from these largely autoimmune free mice revealed levels of expression comparable or higher than in control IL-2Rβ+/− or IL-2Rβ+/+ mice (Supplemental Figure 1). We also identified two other 2RβY395,498 founders (#4 and #6), which expressed transgenic mutant IL-2Rβ at a lower level than found on Treg cells from IL-2Rβ+/− mice but at a higher level than found on the negative control, i.e. immature Treg cells in IL-2Rβ−/− mice. Progeny from these two founders (2Rβ395,498 founders 4 and 6) consistently exhibited symptoms of autoimmunity that were generally comparable to that found in autoimmune IL-2Rβ−/− animals (Supplemental Figure 2 and not shown). Data from these two founders were not included anywhere else in this report. Thus, at least for the 2RβY395,498 transgene, we found a dose-response relationship where protection from autoimmunity correlated with increased expression of mutant IL-2Rβ.
The histopathological changes in older (>16 wks) 2RβY395,498, 2RβY341,395,498, and 2RβΔH mice often showed perivascular lymphoplasmacytic inflammatory infiltrates in the lung and salivary gland (Fig. 2F), and less frequently in the liver, colon, stomach and pancreas. The severity of these infiltrates usually ranged from mild to moderate and representative examples of such infiltrates for the lung and salivary gland are shown in Supplemental Figure 3. Only the two oldest 2RβY341 mice showed infiltrates in the lung and salivary gland. These changes were more frequent and even appeared in some younger (< 16 wks) 2RβY395,498, 2RβY341,395,498, and 2RβΔH mice (Fig. 2F). In addition, inflammatory infiltrates in various non-lymphoid tissues were not usually accompanied by lymphoplasmatic hyperplasia in the spleen and LNs, suggesting that these former differences may precede obvious immune activation in secondary lymphoid tissues. In marked contrast, young untreated IL-2Rβ−/− mice readily showed extensive lymphoplasmatic hyperplasia in the spleen and LNs, lymphocytic infiltrates in the liver and severe colitis in the 3 oldest mice, but involvement of the salivary gland and lung was less frequent and found in the youngest IL-2Rβ−/− mice. Collectively, these functional and pathological analyses unexpectedly demonstrate that mutations of IL-2Rβ, which are predicted to severely impair IL-2R signal transduction, are associated with a long life-span and lack of severe autoimmunity.
Mutant IL-2R β-chains support the production of natural but not iTreg cells
IL-2Rβ−/− mice are characterized by containing a population of immature CD4+Foxp3lowCD25neg T cells that are found at a markedly reduced frequency, especially in the periphery, when compared to the mature CD4+Foxp3hiCD25+ population in normal WT mice (Bayer et al., 2007; Fontenot et al., 2005; Yu and Malek, 2006). We confirmed that there was a consistent 2-fold lower proportion (p=0.0088, t-test) of Foxp3low T cells in the thymus of IL-2Rβ−/− mice. For transgenic IL-2Rβ−/− mice expressing each mutant IL-2R β-chain, a largely normal proportion of mature CD4+Foxp3hiCD25+ Treg cells was found in the thymus and LN (Figure 3A and 3B), although the lower percentage of thymic immature Treg cells in the IL-2Rβ−/− mice did not reach statistical significance by the one way ANOVA. As the IL-2Rβ transgenic mutant mice contained normal thymic and peripheral cellularity (Figure 1C), these normal proportions translate to normal numbers of Treg cells. After gating the CD4+Foxp3+ T cells in the thymus and LNs, essentially all these Treg cells expressed transgenic IL-2Rβ at levels similar to control WT Treg cells (Figure. 3A).
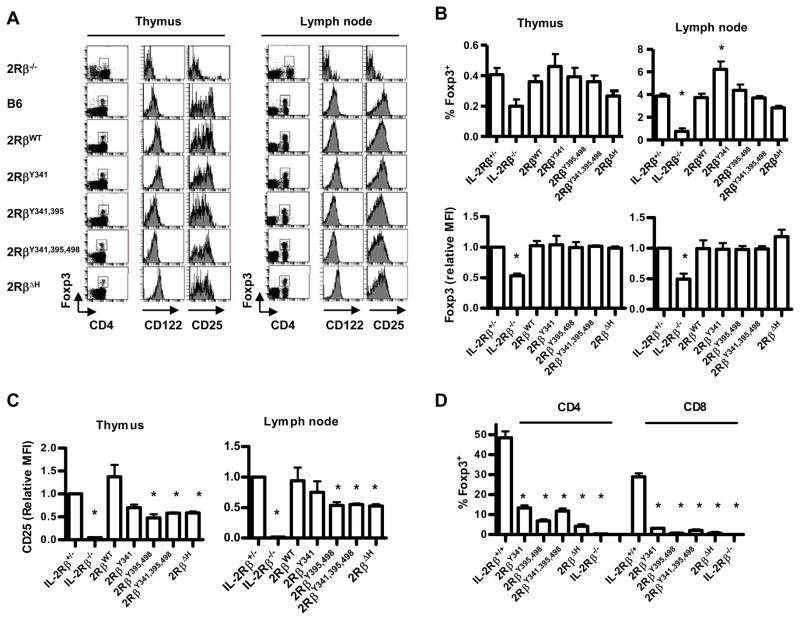
Figure 3. Natural and iTreg cells from mice expressing mutant IL-2Rβ.
(A) CD4+ Foxp3+ T cells within the thymus and LNs of the indicated mice were examined for CD25 (IL-2Rα) and CD122 (IL-2Rβ) expression. (B) % (n ≥5 mice/group) and levels of Foxp3 (n≥3 mice/group) and (C) the level of CD25 (≥3 mice/group) for Foxp3+ T cells in the thymus and LNs. Levels are expressed as mean fluorescent intensity (MFI). The MFI of staining by control IL-2Rβ+/− was normalized to a value of 1 and used to compare staining by T cells bearing mutant IL-2Rβ. (D) Production of Foxp3+ T cells after culture of spleen cells from the indicated mice (n≥2) with TGFβ and IL-2. Data are mean ± SEM for mice <16 wks of age.
A consequence of restoring IL-2R signaling within the immature Treg cells contained in IL-2Rβ-deficient mice is upregulation of Foxp3 and CD25 (Bayer et al., 2007). For thymic and peripheral Treg cells, each mutant IL-2R β-chain supported Foxp3 levels, as assessed by median fluorescent intensity (MFI), to a level comparable to that in control WT mice (Figure 3B). Each mutant IL-2R β-chain also readily supported induction of CD25 on the CD4+Foxp3+ T cells in the thymus and periphery (Figure 3A) and this induction is striking in comparison to the minimal level of CD25 associated with immature Treg cells within IL-2Rβ−/− mice. However, each mutant IL-2R β-chain supported lower levels of CD25 on Treg cells, which usually reached statistical significance, when compared to CD25 levels on control WT Treg cells (Figure 3C). Collectively, these data indicated that mutations of all critical IL-2Rβ tyrosine residues still resulted in sufficient IL-2R signal transduction to readily support Treg cell development and peripheral homeostasis, although they do not fully support upregulation of CD25.
IL-2 in concert with TGFβ is required for production of CD4+ Foxp3+ iTreg cells and leads to the induction of Foxp3 by CD8+ T cells (Ahmadzadeh et al., 2007; Davidson et al., 2007; Zheng et al., 2007). Therefore, the capacity of mutant IL-2R β-chains to support development of iTreg cells was tested after the culture of spleen cells with anti-CD3 in the presence of exogenous IL-2 and TGFβ (Fig. 3D). When these activated T cells were examined 48 hr later for Foxp3 expression, 30–50% of control WT CD4+ and CD8+ T cells expressed Foxp3. In marked contrast, at least 5-fold fewer Foxp3+ cells were found in cultures from T cells expressing mutant IL-2R β-chains. The higher % of CD4+ Foxp3+ T cells may in part reflect survival of natural Treg cells within the spleen as there was essential no induction of Foxp3 for CD8+ T cells bearing mutant IL-2R β-chains. Overall, these data indicate that mutations of key tyrosine residues for IL-2R signaling readily support development, maintenance, and homeostasis of natural Treg cells while poorly supporting production of iTreg cells.
IL-2R signaling by Treg cells bearing mutant IL-2Rβ
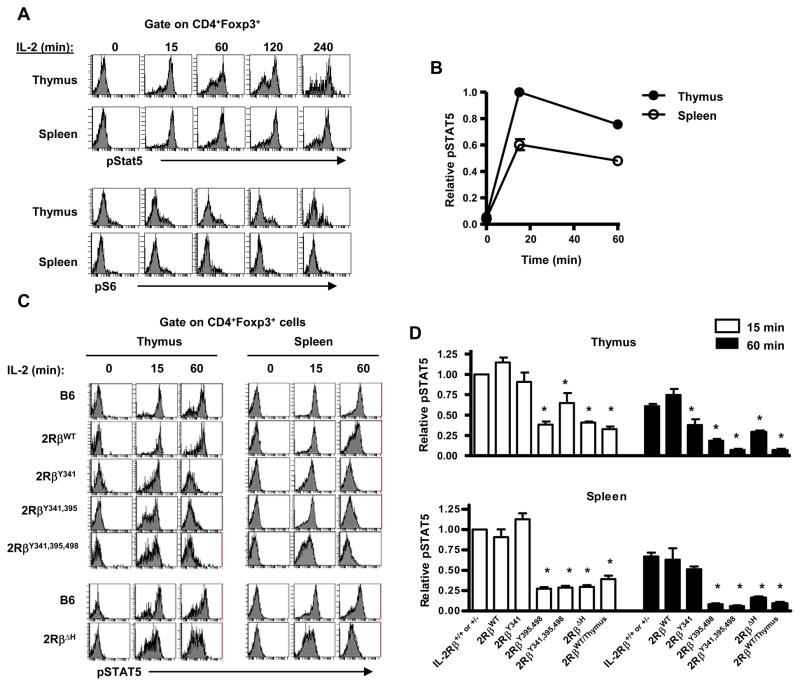
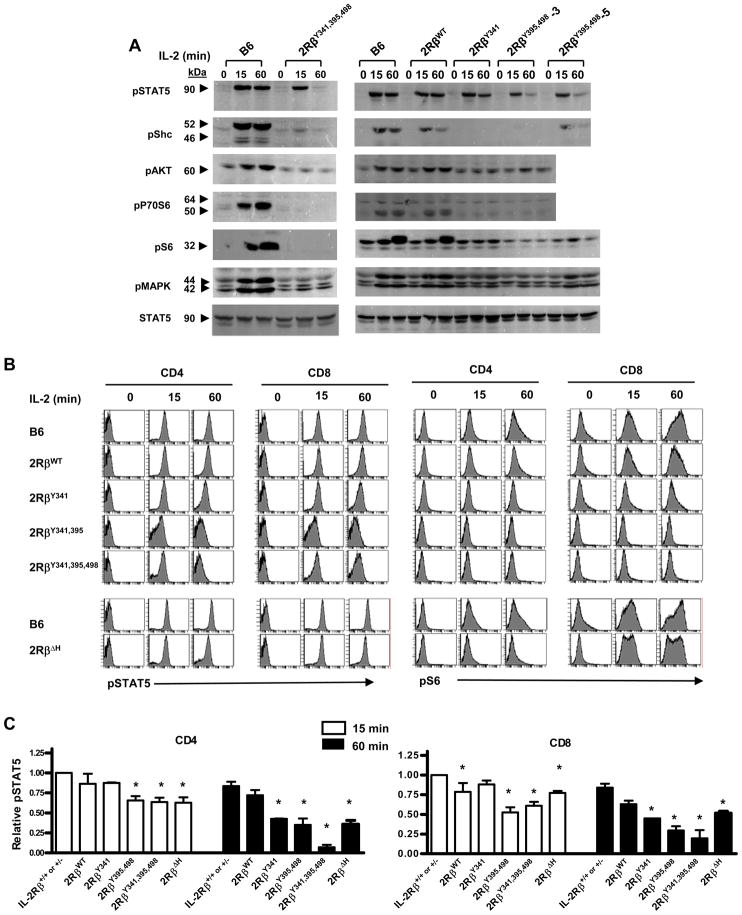
STAT5 activation is critical for Treg cell production (Antov et al., 2003; Burchill et al., 2003; Burchill et al., 2007; Snow et al., 2003). We initially confirmed that IL-2 readily induced sustained STAT5 phosphorylation in thymic and peripheral Treg cells in control WT mice while minimally inducing pS6, a down-stream target of the PI-3-kinase/Akt pathway (Figure 4A). This latter result was expected due to high PTEN levels in Treg cells (Walsh et al., 2006). Quantitative analysis of pSTAT5 revealed that thymic Treg cells activated nearly twofold more pSTAT5 than peripheral Treg cells indicating that these Treg cells are more responsive to IL-2 (Figure 4B). With respect to pSTAT5 activation by Treg cells expressing mutant IL-2Rβ chains, 15 min after IL-2 treatment, thymic and peripheral Treg cells expressing 2RβY341 induced near normal pSTAT5 while it was readily measurable but significantly reduced in Treg cells expressing the other mutant IL-2Rβ chains (Figure 4C, D). When Treg cells were activated with IL-2 for 60 minutes, pSTAT5 was further reduced in comparison to control littermate Treg cells, especially for 2RβY395,498, 2RβY341,395,498, and 2RβΔH. Analysis of 2RβWT/Thymus mice also revealed weak transient STAT5 activation for their thymocytes and peripheral Treg cells (Figure 4D). 2RβWT/Thymus mice represent our previously well-characterized model where the proximal lck promoter was used to drive transgenic WT IL-2Rβ preferentially to the thymus which efficiently prevented autoimmunity when expressed in IL-2Rβ-deficient mice (Malek et al., 2000; Malek et al., 2002). Collectively, these findings indicate that weak transient STAT5 activation supported by mutant IL-2Rβ chains is sufficient to develop and maintain of a population of Treg cells that show considerable efficacy to suppress autoimmune disease and suggest that these tyrosine residues may normally function to sustain activation of STAT5 in Treg cells.
Figure 4. Signaling by CD4+Foxp3+ T cells bearing mutant IL-2Rβ.
Thymocytes and spleen cells from the indicated mice were cultured in medium for 30 min and then stimulated with an excess of IL-2 (10 ng/ml) for the indicated time. (A) Representative pSTAT5 and pS6 staining by normal B6 CD4+Foxp3+ T cells. (B) Summary of all experiments for pSTAT5 activation by WT Treg cells where the MFI of pSTAT5 staining at 15 min by thymic Treg cells was normalize to a value of 1. (C) Representative comparison of IL-2-dependent pSTAT5 by Treg cells from B6 and mutant IL-2Rβ transgenic mice. (D) Summary of all comparisons where the MFI of pSTAT5 staining at 15 min by control IL-2Rβ+/+ or +/− was normalize to a value of 1 and used to compare staining by Treg cells bearing mutant IL-2Rβ. Data are the mean ± SEM of 2–5 mice/group <16 wks of age.
Functional activity of mutant IL-2Rβ chains on effector T cells
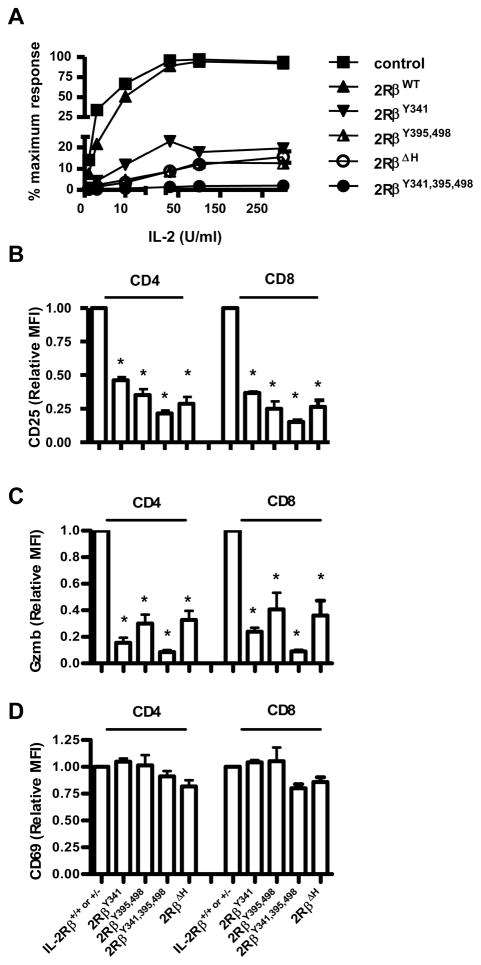
The ability of the mutant IL-2Rβ chains to support Treg cell development and function raised the question concerning their activity on effector T cells. Therefore, spleen cells were cultured with anti-CD3 and the resulting T cell blasts were washed and re-cultured with IL-2 to test their proliferative capacity to IL-2 (Figure 5A). T blasts bearing mutant IL-2Rβ showed marked impairment in IL-2-dependent proliferation, with greater impairment with increasing the number of Y→F mutations. This result is consistent with a number of studies that have explored the role of IL-2Rβ signal transduction leading to T cell growth in IL-2-dependent cell lines (Gaffen, 2001). These anti-CD3 activated T cells were also directly examined for expression of two other IL-2-dependent activities, i.e. upregulation of CD25 (Figure 5B) and expression of granzyme B (Figure 5C). In each case, the anti-CD3 activated T cells bearing mutant IL-2Rβ chains expressed lower levels of these molecules reflecting impaired IL-2R signaling in response to endogenous IL-2. The lowest expression was always associated with 2Rβ341,395,498 mutation. As a control, induction of CD69 was similar for all anti-CD3-activated T cells bearing mutant IL-2Rβ (Figure 5D). Collectively, these findings confirm the importance of these tyrosine residues and the H-region in IL-2R signaling by conventional effector T lymphocytes.
Figure 5. Proliferative and functional responses by T blasts bearing mutant IL-2Rβ.
Spleen cells from the indicated mice were culture with anti-CD3 for 48 hr. (A) The activated T cells were washed and re-cultured in the indicated level of IL-2 for 24 hr. 3H-thymidine was added during the last 4 hr of culture. Each data point represents the mean of at least 3 separate experiments, where the highest response was normalized to 100%. The activated CD4+ and CD8+ spleen cells were stained for (B) CD25, (C) granzyme B (Gzmb), or (D) CD69. The MFI of staining by control IL-2Rβ+/+ or +/− was normalized to a value of 1 and used to compare staining by T cells bearing mutant IL-2Rβ. Data are the mean ± SEM of ≥3 mice/group <16 wks of age.
IL-2R signaling by effector T cells bearing mutant IL-2Rβ
Western blot analysis was also performed for a number of defined signaling intermediates in the IL-2 signal transduction pathway. For these experiments, anti-CD3 T cell blasts were cultured overnight in IL-4 and rested for 4 hours before re-stimulation with IL-2 as this aided detection of down-stream targets of the adaptor Shc, i.e. phosphorlyated (p)-MAPK, pAKT, p70S6 kinase, pS6, which are also induced by TCR signaling. IL-2-dependent signaling by T blasts from C57BL/6 and 2RβY341,395,498 mice was initially compared. With respect to activation of STAT5, strong sustained activation of pSTAT5 was noted for the C57BL/6 T blasts while the activation of pSTAT5 by 2Rβ341,395,498 T blasts was initially strong, but not sustained as evident 60 min after stimulation by IL-2 (Figure 6A, left). In contrast, IL-2-dependent activation of pShc, pATK, pP70S6 kinase, pS6, or pMAPK was strong and sustained only for the C57BL/6 control T blasts. A slightly different pattern of results was noted for T blasts from 2Rβ341 and 2Rβ395, 498 mice. Again, pSTAT5 was readily detected 15 min after IL-2 stimulation for both mutant IL-2Rβ chains (Figure 6A, right). With respect to the 2RβY341, pSTAT5 was somewhat more sustained than seen for IL-2Rβ395,498, but there was no obvious activation of any other phospho-proteins in the IL-2 pathway. In contrast, 2RβY395,498 mutation supported some activation of pShc, that was most obvious in founder #5 with higher expression of the 2RβY395,498 transgene, and 2RβY395,498 also supported detectable, but weak, activation of signaling molecules ascribed to the adaptor Shc, i.e. pMAPK, and pS6. As a control, IL-2 driven signaling by 2RβWT T blasts was essentially comparable to that seen for C57BL/6 T blasts.
Figure 6. IL-2-dependent signaling by T blasts bearing mutant IL-2Rβ.
(A) Spleen cells from the indicated mice were cultured with anti-CD3 for 48 hr. The T cells were isolated by magnetic bead sorting using anti-Thy-1.2, cultured in IL-4 for 24 hr, and then washed and “rested” in medium for 4 hr. These T cells were cultured with IL-2 for the indicated time and cytoplasmic extracts were prepared. Western blots were probed with mAbs to the indicated phospho-tyrosine proteins. mAb to unmodified STAT5 served as a loading control. Data are representative of 2 experiments. (B) The indicated anti-CD3 activated spleen cells were cultured in medium for 4 hr and then stimulated with IL-2 for the indicated time followed by staining for pSTAT5 and pS6. (C) Summary of all experiments where the MFI of pSTAT5 staining at 15 min by control IL-2Rβ+/+ or +/− was normalized to a value of 1 and used to compare staining by T cells bearing mutant IL-2Rβ. Data are the mean ± SEM of ≥3 mice/group <16 wks of age.
mAbs specific for pSTAT5 and pS6 were used to examine IL-2R signaling by CD4 and CD8 T cells. With respect to pSTAT5, these data closely approximated the findings from the Western blots and confirm the transient nature of STAT5 phosphorylation by each of the mutant IL-2Rβ chains (Fig. 6B). Thus, weak transient tyrosine-independent STAT5 activation is not a property unique to Treg cells. Furthermore, the quantitative pattern of pSTAT5 was comparable for both activated CD4 and CD8 T cells (Fig. 6C). In contrast, pS6 was more strikingly upregulated in CD8+ T cells and this was partially maintained in CD8+ T cells bearing IL-2RβΔH, but not any of the tyrosine mutations, implicating a role for the H-region in negative regulation of IL-2R signaling. The finding of greater IL-2-dependent activation of PI-3-kinase/Akt pathway by the CD8+ T cells provides a potential molecular explanation for our consistent observation that CD8+ T cells rapidly outnumber CD4+ T cells in anti-CD3 stimulated mixed cultures in vitro (unpublished data) and that CD8+ T cells showed a greater dependence on IL-2 for expansion after superantigen stimulation of T cells in vivo (Jin et al., 2006). Collectively, these data indicate that initial STAT5 activation readily occurs without a strict requirement for any of the 3 key functional tyrosine residues in the cytoplasmic tail of IL-2Rβ and suggest that these tyrosine residues may function to sustain activation of STAT5 and the MAPK and PI-3K/Akt pathways in effector T cells.
Gene expression profiling of Treg and T effector cells with impaired IL-2R
To further evaluate the role of IL-2R signal in Treg cells, Affymetrix gene expression profiles were obtained from CD4+CD25high Treg cells directly isolated from the spleen of C57BL/6 WT or 2RβWT/Thymus mice by FACS sorting. These cells were always >99% CD4+CD25high and typically are at least 95% Foxp3+ cells. To compare these findings to the gene expression profile for T effector cells, the spleens were also activated with anti-CD3 for 48 hr and then the activated CD4+CD25+ T cells were isolated to >99% purity by FACS sorting. The 2RβWT/Thymus T mice were chosen for this analysis because their Treg cells showed transient activation of STAT5 that was similar to 2Rβ395,498 and 2Rβ341,395,498 Treg cells (Fig. 4D) and this permitted direct comparison to the extensively characterized IL-2-dependent 2RβWT/Thymus effector cells, including previous gene expression analysis (Gong and Malek, 2007). These experiments represented 3 independent biological replicates, and for the Treg cells, a pool from at least 2 mice/sample.
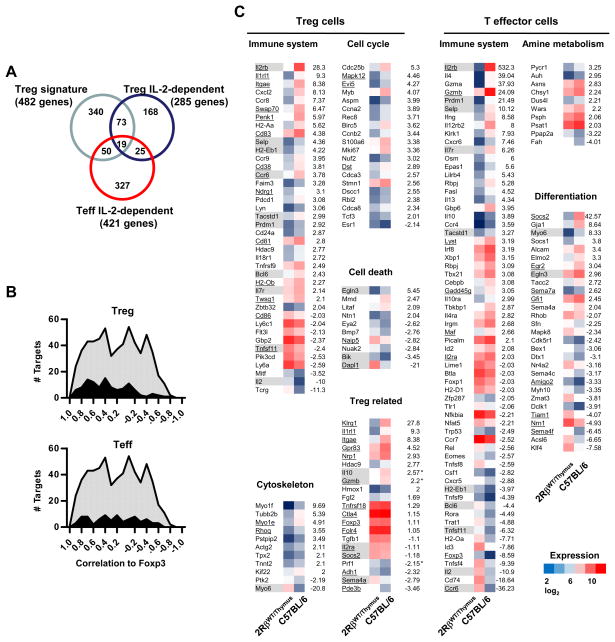
These analyses indicate that 353 (254 up and 99 down) and 553 (205 up and 348 down) Affymetrix targets were IL-2-dependent by ≥2-fold (p<0.05, t-test), representing 285 and 421 distinct genes for Treg and T effector cells, respectively (Fig. 7A). These gene lists for Treg and T effector cells are shown in Supplemental Tables 1 and 2. As 2RβWT/Thymus Treg cell development and function is outwardly normal, we were somewhat surprised by the substantial number of IL-2-dependent genes in the peripheral Treg cells. This finding indicates that there are many targets in 2RβWT/Thymus Treg cells that are not properly expressed with minimal IL-2R signaling.
Figure 7. IL-2-dependent mRNA in Treg and T effector cells.
(A) IL-2-dependent genes (>2-fold) from Treg and CD4+ T effector cells that overlap with each other and the Treg cell gene signature. (B) IL-2 dependent targets (dark curves) that overlap with the Treg cell transcriptional signature (light shaded curves). The overlapping Treg (upper graph) and T effector (lower graph) targets vs. all targets of the Treg transcriptional signature plotted as a function of their correlation to Foxp3 expression as reported by (Hill et al., 2007). (C) Functional groups of IL-2-dependent (>2-fold) differentially expressed genes by Treg and CD4+ T effector cells. Shaded genes represent those transcripts that overlap between IL-2-dependent Treg and T effector cells. Underlined genes were identified in the Treg cell transcriptional signature.
The Treg cell transcriptional signature was determined by gene expression profiling of a variety of Treg-like cells obtained directly ex vivo or after activation in vitro (Hill et al., 2007). By varying Foxp3 expression in this analysis, the Treg signature was shown to contain only a subset of genes that correlated with Foxp3 expression. Part of the Treg signature was shown to be distinctively influenced by IL-2, TGFβ, and T cell activation in general. Comparison of our gene lists with this recently published Treg cell transcriptional signature (Hill et al., 2007) revealed a striking overlap in gene expression profiles (Fig. 7A). 19.1% (92 of 482) and 14.3% (69 of 482) of the IL-2-dependent genes in Treg and T effector cells, respectively, overlapped with the Treg transcriptional signature. These two sets of IL-2-dependent targets, which overlap with the Treg cell transcriptional signature, were largely distinct. Currently, 29,122 mouse genes have been annotated based on nucleotide sequence by Mouse Genome Informatics (MGI). The 482 genes of the Treg transcription signature represent 1.65% of the total number of genes. Thus, the 19.1% and 14.3% overlap with Treg (p=2.5 × 10−53) and T effector (p=6.1 × 10−37) genes is highly significant (Fisher exact test) and is consistent with IL-2 critically regulating important characteristics of Treg cells. Furthermore, 10.5% (44 of 421) of IL-2-dependent Treg genes overlap with IL-2-dependent T effector genes and reciprocally 15.4% (44 of 285) of IL-2-dependent T effector genes overlap with Treg cells, which is slightly lower than when compared to the Treg transcriptional signature. This degree of overlap was also highly significant (p at least 9.1 × 10−22; Fisher exact test) considering these sets of genes represent 0.98% and 1.44% of all mouse genes. This finding suggests a mechanism of important overlapping, but mainly distinctive, processes that depend on IL-2R signaling in Treg and T effector cells and these distinctive IL-2-dependent processes are both reflected in the Treg transcriptional signature.
We plotted the past data (Hill et al., 2007) of the Treg transcriptional signature that represent 603 Affymetrix targets as a function to their published correlation to Foxp3 expression (Figure 7B, both graphs, light shaded curves). From 30–40 targets span nearly the entire range of from high to low correlation to Foxp3. We also then similarly plotted the IL-2-dependent targets from Treg (Figure 7B, upper graph, dark curves) and T effector cells (Figure 7B, lower graph, dark curve) that overlapped with the Treg cell transcriptional signature. A greater proportion of the IL-2-dependent Affymetrix targets in Treg cells (56.3%) more closely correlated (≥0.4) with Foxp3 expression of the Treg transcriptional signature than for IL-2-dependent genes associated with T effector cells (42.8%), the latter which showed a uniform distribution of IL-2-dependent targets in relationship to their correlation to Foxp3. This result is also consistent with unique aspects the of IL-2-dependent Treg and T effector gene programs intersecting with the Treg cell transcriptional signature.
With respect to Gene Ontology classification, IL-2-dependent targets of Treg and T effector cells were over-represented by genes with known function in the immune system (Z-scores: 3.58, Treg cells upregulated targets; 6.91 and 4.17, T effector cells up and down-regulated targets, respectively) (Figure. 7C). Those targets that were shared between Treg and T effector cells are indicated by shading whereas those targets that overlapped with the Treg cell transcriptional signature are underlined (Figure 7C). Unique to T effectors cells was the overexpression of IL-2-dependent up-regulated targets in amine metabolism (Z-score 3.88) and cell differentiation (z-score 4.06). Treg cells showed unique over-representation of IL-2-dependent up-regulated targets involved in cytoskeleton (Z-score 3.86) and cell cycle (Z-score, 5.29) and down-regulated targets for cell death (Z-scores, 3.16). The latter two groups are consistent with a possible direct role of IL-2 in contributing to homeostatic proliferation by peripheral Treg cells. Several well-characterized Treg related targets such as Foxp3, Tgfb1, CD25, Tnfrsf 18 (GITR), Socs2, and Fgl2 were expressed at near normal levels in 2RβWT/Thymus. This finding likely explains the generally effective development and function of these Treg cells. As we showed that both Foxp3 and CD25 are dependent upon IL-2 signaling (Fig. 3), it is likely these and perhaps other Treg-related targets, e.g. Socs2, are effectively regulated by lower IL-2R signaling associated with 2RβWT/Thymus and the other mutant IL-2Rβ chains. Collectively, these data indicate that a substantial portion of the gene program associated with Treg cells depends on IL-2, but this list does not include several important IL-2-dependent targets that are readily responsive to low IL-2R signaling.
DISCUSSION
Here we demonstrate that both thymic Treg cell development and their peripheral homeostasis effectively occur by IL-2Rs that harbor mutations of key tyrosine residues in the cytoplasmic tail of IL-2Rβ that are required for optimal signal transduction. Mutation of all three of these tyrosine residues still led to weak transient STAT5 activation, indicating that IL-2R signaling was not completely abrogated. Thus, these data demonstrate that key IL-2-dependent targets in Treg cells are index to a low IL-2R signaling threshold. One of these targets is likely Foxp3 because Foxp3 expression depends in part on STAT5 activation (Burchill et al., 2007; Yao et al., 2007; Zorn et al., 2006) and we show that Foxp3 was upregulated to normal levels in thymic and peripheral Treg cells bearing mutant IL-2Rβ chains.
The indexing of Treg cells to a low IL-2R signaling threshold provides an explanation concerning how Treg cells effectively utilize an essential cytokine that is transiently and perhaps minimally expressed. For example, there are very few IL-2 producing cells in the thymus and such cells are not abundant in peripheral immune tissues (D’Souza and Lefrancois, 2004; Sojka et al., 2004; Yang-Snyder and Rothenberg, 1998). More specifically to Treg cells, one of their suppressive mechanisms is to inhibit IL-2 production by autoreactive or effector T cells (Thornton and Shevach, 2000). Thus, Treg cells likely exist in an IL-2 poor environment, yet IL-2 is essential for thymic maturation and subsequent homeostasis in the periphery. Treg cells, therefore, appear to adapt to low IL-2 through fully inducing and maintaining Foxp3 by a minimal requirement for IL-2-dependent pSTAT5 activation. As such, this mechanism links Foxp3, which enforces and maintains the Treg cell suppressive program (Gavin et al., 2007; Lin et al., 2007), to a low threshold for IL-2R signal transduction.
The effectiveness of a low IL-2R signaling threshold for Treg cells has important implications in immunotherapy that targets the IL-2/IL-2R. First, immunotherapy based on anti-IL-2R blockade is unlikely to fully inhibit IL-2 binding to the IL-2R or even approach the degree of IL-2R signaling inhibition associated with our IL-2Rβ mutants that readily support and maintain Treg cells in vivo. Thus, Treg cells are likely to be substantially resistant to approaches that aim to inhibit T cell immunity by directly blocking the IL-2R. Indeed, many clinical protocols have utilized humanized anti-IL-2R mAb (anti-Tac, Daclizumab, Zenapac) (Waldmann, 2007) without noting side effects consistent with impaired Treg cells, e.g. T cell activation or autoimmunity. Second, Treg cells are predicted to be relatively responsive to IL-2-based therapy and administering low dose IL-2 might represent a means to enhance Treg cell function in vivo. In this regard, treatment of NOD mice with a low concentration of an agonist IL-2/anti-IL-2 complex prevented autoimmune diabetes by enhancing Treg cell survival and function (Tang et al., 2008).
Our study shows that chronic low IL-2R signaling is remarkably effective in producing an effective population of Treg cells. Nevertheless, there are important biological consequences of such impaired IL-2R function. Notably, we consistently observed an increase in an activated T cell phenotype and inflammatory infiltrates, most often in the lung and salivary gland, in progeny of older mice that expressed mutant IL-2R β-chains. These types of abnormality were also reported for IL-2−/− and IL-2Rα−/− mice (Sharma et al., 2006) and were also seen in several younger IL-2Rβ−/− mice, as an apparent early consequence of their systemic autoimmunity. Thus, impaired chronic IL-2R signaling over a long time-frame leads to symptoms consistent with organ-specific autoimmunity, which in our model appears analogous to Sjögren’s syndrome. In a related manner, decreased IL-2 production has been associated with the Idd3 locus of NOD mice that impairs Treg cell function and contributes to autoimmune diabetes (Yamanouchi et al., 2007). These findings also raise the possibility that the recent association of polymorphisms in human CD25 with susceptibility to several autoimmune diseases might ultimately be reflected by chronic lower IL-2R signaling.
An interesting issue is whether immune stimulation of mice that contain Treg cells with impaired IL-2R signaling may predispose one to severe autoimmunity. In this regard, 2RβWT/Thymus mice, whose Treg cells also exhibited weak transient STAT5 due to the very low expression of the IL-2Rβ chain, did not show obvious clinical symptoms when challenged with vaccinia virus, allografts, nominal antigen, or superantigen even though they mounted largely normal and effective immune responses (Jin et al., 2006; Yu et al., 2003). Whether other forms of immune challenges and/or inflammatory signals exacerbate the autoimmune symptoms associated with older mice harboring Treg cells with impaired IL-2R signaling remains to be determined.
Past work is consistent with a model where Treg cells depend primarily on IL-2-dependent activation of STAT5 and our study is consistent with that view. However, the basis by which IL-2R-dependent STAT5-activation successfully supports Treg cells production remains poorly understood. Another important aspect of this study is that we show that Treg cells utilize a unique mechanism to activate STAT5 for their development and homeostasis but this signaling is insufficient to support IL-2-dependent T cell growth by conventional T cells or development of iTreg cells in vitro. Weak transient STAT5 activation occurred after mutations of 3 critical tyrosine residues in the cytoplasmic tail of IL-2Rβ and this IL-2R readily supported Treg cell production in vivo. Deletion of the distal 123 amino acids of the cytoplasmic tail of IL-2Rβ, i.e. the H-region, including the two dominant tyrosine residues for STAT5 docking, also supported transient STAT5 activation and Treg cell production. These results demonstrates that initial STAT5 activation does not depend upon some other undefined interaction within the H-region of IL-2Rβ and provides a mechanistic explanation for past work that showed that domain deletions of the A- or H-regions of the IL-2Rβ cytoplasmic tail also prevented autoimmune symptoms after expression in IL-2Rβ−/− mice (Fujii et al., 1998). Overall these findings are consistent with a model where the initial IL-2R-dependent activation of STAT5 is independent of docking with these 3 tyrosine resides and may be independent of docking with the cytoplasmic tail of IL-2Rβ. Nevertheless, phosphorylation of these tyrosine residues ultimately promotes docking of STAT5 and other signaling molecules, such as the adapter Shc, to assembly a complex for sustained IL-2R signal transduction. The basis for IL-2Rβ tyrosine-independent activation of STAT5 remains to be determined. One possibility is a tyrosine-independent H-region-independent association of STAT5 with IL-2Rβ. Alternatively, past studies examining gp160 in cell lines that over-expressed receptor related components provided evidence for direct Jak-1/STAT5 activation (Fujitani et al., 1997) and perhaps this is also operative for the IL-2R.
Gene expression profiling of WT vs. 2RβWT/Thymus peripheral Treg cells demonstrated that a substantial number of targets remain IL-2 dependent. Thus, the capacity of weak transient STAT5 activation to readily support largely normal Treg cell development and homeostasis is likely due to the sensitivity of a few key targets, e.g. Foxp3 as discussed above, to this low IL-2R signal transduction rather than a global control of the IL-2 program in Treg cells by low threshold IL-2R signaling. The types of genes dependent upon IL-2 included those related to the immune system and cell cycle. This latter finding corresponds to past work where we showed that WT peripheral Treg cells exhibited substantially greater BrdU incorporation and proliferation than 2RβWT/Thymus Treg cells (Bayer et al., 2007) and is consistent with the view that IL-2 is normally an important mediator of Treg cell homeostasis. Past gene profiling of IL-2-dependent genes in Treg cells also reached a similar conclusion related to Treg cell growth and the cell cycle (Fontenot et al., 2005). However, many of the key IL-2-dependent targets identified previously were not found in our gene array analysis. We believe that this is accounted for in the distinct protocols utilized. We examined Treg cells from autoimmune-free mice with chronic long-term impaired IL-2R signaling. Past work evaluated IL-2 dependent targets after autoimmune IL-2-deficient mice received a brief acute exposure to IL-2 (Fontenot et al., 2005).
Direct assessment of IL-2R signal transduction in CD4+ Treg and T effector cells closely approximate each other. However, the IL-2-dependent targets in these cell populations were largely distinctive, but with a high significant overlap (10–15%). This latter finding is consistent with IL-2 controlling several common processes in these two cell types. Even more striking was the overlap (20%) of IL-2-dependent transcripts in Treg cells with the recently published Treg cell transcriptional signature (Hill et al., 2007). Thus, our study places on firm ground the importance of IL-2 in regulating a significant portion of the Treg cell gene program.
METHODS
Mice
C57BL/6 mice were obtained from the Jackson Laboratories (Bar harbor, ME). C57BL/6 IL-2R β-deficient mice were previously described (Suzuki et al., 1995) and maintained in our laboratory as autoimmune-free breeding stock by adoptive transfer of CD4+CD25+ Treg or CD4+ T cells at birth (Malek et al., 2002). 2RβWT/Thymus represents IL-2Rβ−/− mice that express thymus-targeted transgenic WT IL-2Rβ have been previously described (Malek et al., 2000; Malek et al., 2002). To produce other transgenic mice, full length mouse IL-2Rβ cDNA was subcloned into pENTR (Invitrogen, Carlsbad, CA). As required, this cDNA was subjected to site directed mutagenesis using the QuikChange site directed mutagenesis kit (Stratagene, La Jolla, CA) to change the indicated tyrosine codons to phenylalanine or to introduce a stop codon at position 390 for the ΔH mutation (Fig. 1A), where position 1 represents the first amino acid after removal of the 24 amino acid signal peptide. All mutations were verified by DNA sequencing. The CD2 mini-gene cassette vector p29Δ2(Sal−), kindly provided by Paul Love and Al Singer, was modified to replace the EcoR I/Sal I fragment with the Gateway Cassette (Intvitrogen) to permit directional recombination based subcloning of each cDNA within pENTR. The purified transgenic expression cassette was microinjected into (B6 × SJL)F2 oocytes. Transgenic founders were identified by PCR and backcrossed 3–5 generations to C57BL/6 IL-2Rβ−/− mice. Mice were housed in laminar flow racks and were maintained in viral-antigen-free conditions. Animal studies were approved by the Institutional Animal Care and Use Committee at the University of Miami.
Cell purification, cell culture and functional assays
CD4+ CD25+ Treg cells were purified by first depletion of B cells and CD8+ T cells followed by magnetic bead based positive selection of CD25+ T cells as previously described (Malek et al., 2002). These cells were typically at least 90% CD4+ CD25+ T cells. For the microarray analysis, these cells were subjected to further purification by FACS sorting for CD4+ CD25high T cells using a BDAria cell sorter. To prepare activated T cells, spleen cells (2 × 106 cells/well) were cultured with anti-CD3 in 24 well culture plates for 48 hr as previously described (Malek et al., 2001). To measure intracellular granzyme B, prior to staining these activated T cells were re-cultured at 1 × 106 cells/well in 24 well plates for 4 hr with PMA (50 ng/ml) and ionomycin (1 μM) in the presence of brefeldin A (GolgiPLug, BD-Biosciences). To assess IL-2-dependent proliferation, the anti-CD3 activated spleen cells were washed 3x and re-cultured (2 × 104 cell/well) for 24 hr with the indicated concentration of IL-2 (Peprotech) in 96 well plates for 24 hr, where 3H-thymidine was added for the final 4 hr of culture (Malek et al., 2001). For Western blotting, T cells from the anti-CD3 activated spleen cells were purified by positive selection using anti-Thy-1.2 magnetic beads (Miltenyi Biotec). These purified T cells were cultured overnight at 1 × 106 cells/ml in T25 flasks in 5–10 ml of medium containing mouse IL-4 (10 ng/ml). For iTreg cells, spleen cells were cultured with anti-CD3, TGFβ (5 ng/ml; Peprotech) and IL-2 (10 ng/ml) for 48 hr.
FACS analysis
For surface staining, cells were subjected to two-step staining by incubation with FITC-CD4, Cychrome-CD8 and either biotin-CD122, -CD44, -CD25, -CD69, or -CD62L followed by PE-streptavidin. Alternatively, multi-color staining for Treg cells was obtained according to instructions by the manufacturer (eBiosciences) for intracellular staining of Foxp3 by incubation with FITC-CD4, PerCP-CD8, PECy7-CD25, PE-Foxp3, and biotin-CD122 followed by APC-strepavidin. NK cells were enumerated after incubation of spleen or LN cells with PE-NK1.1, FITC-B220 and biotin-TCRβ followed by CyChrome streptavidin. Intracellular granzyme B was performed as previously described (Gong and Malek, 2007) by incubation with FITC-CD4, CyChrome CD8, and PE-granzyme B. Intracellular staining for pSTAT5 or pS6 kinase was performed essentially as previously described (Bayer et al., 2007). In brief, after stimulation with IL-2, the cells were fixed in in 1.6% paraformaldehye and 100% methanol, and stained by incubation with PE-CD4, PerCP-CD8, PacBlue-Foxp3, as required, and either AlexaFluor 647 anti-STAT5 (pY694, BD Pharmingen) or AlexaFluor 488 anti-pS6 (Cell Signaling). Cells were analyzed on a Becton Dickinson LSR1 or LSR2 using CellQuest and Diva software. Typically at least 50,000–100,000 events were collected per sample
Western blots
Cell extracts (100 × 106 cells/ml) were prepared using M-PERB protein extraction reagent with Halt™ protease and phosphatase inhibitor cocktail (Pierce). Western blotting was performed on reducing 10% SDS-PAGE gels after 2 × 106 cell equivalents were loaded per lane as previously described (Yu and Malek, 2001). The blots were blocked and then incubated with mAbs (all from Cell Signaling) to pSTAT5, pShc, pAkt, pP70S6, pS6, pMapk and unphosphoryaled STAT5, as a loading control. After washing, the blots were then incubated with goat anti-rabbit HRP (Cell Signaling). Bands were developed by incubation of the blots with ECL chemiluminescence reagent (GE-Healthcare Bio-sciences Corp.) according to the manufacturer’s instructions.
DNA microarray analysis
Total mRNA was isolated by TRIzol reagent and further purified using RNAeasy Minikit (Qiagen). RNA quality and quantity were assessed by analysis using a Agilent 2100 BioAnalyzer. A single round of linear probe amplification with 1–2 μg of total RNA (Affymetrix) was used for each RNA sample. Probe preparation and microarray analysis were performed at Expression Analysis (Durham, NC) using the 430 2.0 Affymetrix chip that contains oligonucleotides to 39,000 mouse transcriptions covering the entire expressed mouse genome. Fluorescent images were detected in a GeneChip Scanner 3000 using GCOS 1.3 software (Affymetrix) to extract the expression data. An estimate of signal for each transcript was calculated and then normalized using the GCRMA method of the Bioconductor R package. Two group comparisons of the transformed data were performed for 3 biological replicate samples by paired t-tests. Genes expressed >2.0-fold up or down (p<0.05) between the C57BL/6 WT and 2RβWT/Thymus were considered differentially expressed genes.
Statistical analysis
Unless otherwise noted, all statistical analysis of the data in Figures 1–6 were performed by a one way ANOVA using Tukey’s multiple comparison test. Statistically significant differences (p<0.05) for comparison to IL-2Rβ+/+ or +/− littermate controls are designated by * in the graphs.
Supplementary Material
Acknowledgments
We thank Danny Barzana and Ben Boyter for technical assistance, Maritza Inza, Laura Romero and the Sylvester Comprehensive Cancer Center Transgenic Mouse Facility for production of the transgenic mice, and Guoyan Cheng for comments on the manuscript. Our work is supported by grants R01 CA45957 and P01 CA109094 from the National Institutes of Health.
Footnotes
Supplemental data include 3 figures and 2 tables.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahmadzadeh M, Antony PA, Rosenberg SA. IL-2 and IL-15 each mediate de novo induction of FOXP3 expression in human tumor antigen-specific CD8 T cells. J Immunother. 2007;30:294–302. doi: 10.1097/CJI.0b013e3180336787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida AR, Legrand N, Papiernik M, Freitas AA. Homeostasis of peripheral CD4+ T cells: IL-2R α and IL-2 shape a population of regulatory cells that controls CD4+ T cell numbers. J Immunol. 2002;169:4850–4860. doi: 10.4049/jimmunol.169.9.4850. [DOI] [PubMed] [Google Scholar]
- Antov A, Yang L, Vig M, Baltimore D, Van Parijs L. Essential role for STAT5 signaling in CD25+CD4+ regulatory T cell homeostasis and the maintenance of self-tolerance. J Immunol. 2003;171:3435–3441. doi: 10.4049/jimmunol.171.7.3435. [DOI] [PubMed] [Google Scholar]
- Ascherman DP, Migone TS, Friedmann MC, Leonard WJ. Interleukin-2 (IL-2)-mediated induction of the IL-2 receptor α chain gene. Critical role of two functionally redundant tyrosine residues in the IL-2 receptor beta chain cytoplasmic domain and suggestion that these residues mediate more than Stat5 activation. JBiol Chem. 1997;272:8704–8709. doi: 10.1074/jbc.272.13.8704. [DOI] [PubMed] [Google Scholar]
- Bayer AL, Yu A, Adeegbe D, Malek TR. Essential role for interleukin-2 for CD4+CD25+ T regulatory cell development during the neonatal period. J Exp Med. 2005;201:769–777. doi: 10.1084/jem.20041179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer AL, Yu A, Malek TR. Function of the IL-2R for thymic and peripheral CD4+CD25+ Foxp3+ T regulatory cells. J Immunol. 2007;178:4062–4071. doi: 10.4049/jimmunol.178.7.4062. [DOI] [PubMed] [Google Scholar]
- Bensinger SJ, Walsh PT, Zhang J, Carroll M, Parsons R, Rathmell JC, Thompson CB, Burchill MA, Farrar MA, Turka LA. Distinct IL-2 receptor signaling pattern in CD4+CD25+ regulatory T cells. J Immunol. 2004;172:5287–5296. doi: 10.4049/jimmunol.172.9.5287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burchill MA, Goetz CA, Prlic M, O’Neil JJ, Harmon IR, Bensinger SJ, Turka LA, Brennan P, Jameson SC, Farrar MA. Distinct effects of STAT5 activation on CD4+ and CD8+ T cell homeostasis: development of CD4+CD25+ regulatory T cells versus CD8+ memory T cells. J Immunol. 2003;171:5853–5864. doi: 10.4049/jimmunol.171.11.5853. [DOI] [PubMed] [Google Scholar]
- Burchill MA, Yang J, Vang KB, Moon JJ, Chu HH, Lio CW, Vegoe AL, Hsieh CS, Jenkins MK, Farrar MA. Linked T cell receptor and cytokine signaling govern the development of the regulatory T cell repertoire. Immunity. 2008;28:112–121. doi: 10.1016/j.immuni.2007.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burchill MA, Yang J, Vogtenhuber C, Blazar BR, Farrar MA. IL-2 receptor beta-dependent STAT5 activation is required for the development of Foxp3+ regulatory T cells. J Immunol. 2007;178:280–290. doi: 10.4049/jimmunol.178.1.280. [DOI] [PubMed] [Google Scholar]
- D’Souza WN, Lefrancois L. Frontline: An in-depth evaluation of the production of IL-2 by antigen-specific CD8 T cells in vivo. Eur J Immunol. 2004;34:2977–2985. doi: 10.1002/eji.200425485. [DOI] [PubMed] [Google Scholar]
- Davidson TS, DiPaolo RJ, Andersson J, Shevach EM. Cutting Edge: IL-2 is essential for TGF-β-mediated induction of Foxp3+ T regulatory cells. J Immunol. 2007;178:4022–4026. doi: 10.4049/jimmunol.178.7.4022. [DOI] [PubMed] [Google Scholar]
- Di Santo JP, Vosshenrich CA. Bone marrow versus thymic pathways of natural killer cell development. Immunol Rev. 2006;214:35–46. doi: 10.1111/j.1600-065X.2006.00461.x. [DOI] [PubMed] [Google Scholar]
- Fontenot JD, Rasmussen JP, Gavin MA, Rudensky AY. A function for interleukin 2 in Foxp3-expressing regulatory T cells. Nat Immunol. 2005;6:1142–1151. doi: 10.1038/ni1263. [DOI] [PubMed] [Google Scholar]
- Friedmann MC, Migone TS, Russell SM, Leonard WJ. Different interleukin 2 receptor β-chain tyrosines couple to at least two signaling pathways and synergistically mediate interleukin 2-induced proliferation. Proc Natl Acad Sci U S A. 1996;93:2077–2082. doi: 10.1073/pnas.93.5.2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii H, Ogasawara K, Otsuka H, Suzuki M, Yamamura K, Yokochi T, Miyazaki T, Suzuki H, Mak TW, Taki S, Taniguchi T. Functional dissection of the cytoplasmic subregions of the IL-2 receptor βγ chain in primary lymphocyte populations. EMBO J. 1998;17:6551–6557. doi: 10.1093/emboj/17.22.6551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujitani Y, Hibi M, Fukada T, Takahashi-Tezuka M, Yoshida H, Yamaguchi T, Sugiyama K, Yamanaka Y, Nakajima K, Hirano T. An alternative pathway for STAT activation that is mediated by the direct interaction between JAK and STAT. Oncogene. 1997;14:751–761. doi: 10.1038/sj.onc.1200907. [DOI] [PubMed] [Google Scholar]
- Furtado GC, Curotto de Lafaille MA, Kutchukhidze N, Lafaille JJ. Interleukin 2 signaling is required for CD4+ regulatory T cell function. J Exp Med. 2002;196:851–857. doi: 10.1084/jem.20020190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaffen SL. Signaling domains of the interleukin 2 receptor. Cytokine. 2001;14:63–77. doi: 10.1006/cyto.2001.0862. [DOI] [PubMed] [Google Scholar]
- Gaffen SL, Lai SY, Ha M, Liu X, Hennighausen L, Greene WC, Goldsmith MA. Distinct tyrosine residues within the interleukin-2 receptor β chain drive signal transduction specificity, redundancy, and diversity. J Biol Chem. 1996;271:21381–21390. doi: 10.1074/jbc.271.35.21381. [DOI] [PubMed] [Google Scholar]
- Gavin MA, Rasmussen JP, Fontenot JD, Vasta V, Manganiello VC, Beavo JA, Rudensky AY. Foxp3-dependent programme of regulatory T-cell differentiation. Nature. 2007;445:771–775. doi: 10.1038/nature05543. [DOI] [PubMed] [Google Scholar]
- Gong D, Malek TR. Cytokine-dependent Blimp-1 expression in activated T cells inhibits IL-2 production. J Immunol. 2007;178:242–252. doi: 10.4049/jimmunol.178.1.242. [DOI] [PubMed] [Google Scholar]
- Hill JA, Feuerer M, Tash K, Haxhinasto S, Perez J, Melamed R, Mathis D, Benoist C. Foxp3 transcription-factor-dependent and -independent regulation of the regulatory T cell transcriptional signature. Immunity. 2007;27:786–800. doi: 10.1016/j.immuni.2007.09.010. [DOI] [PubMed] [Google Scholar]
- Jin H, Gong D, Adeegbe D, Bayer AL, Rolle C, Yu A, Malek TR. Quantitative assessment concerning the contribution of IL-2Rβ for superantigen-mediated T cell responses in vivo. Int Immunol. 2006;18:565–572. doi: 10.1093/intimm/dxh398. [DOI] [PubMed] [Google Scholar]
- Lin W, Haribhai D, Relland LM, Truong N, Carlson MR, Williams CB, Chatila TA. Regulatory T cell development in the absence of functional Foxp3. Nat Immunol. 2007;8:359–368. doi: 10.1038/ni1445. [DOI] [PubMed] [Google Scholar]
- Lio CW, Hsieh CS. A two-step process for thymic regulatory T cell development. Immunity. 2008;28:100–111. doi: 10.1016/j.immuni.2007.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockyer HM, Tran E, Nelson BH. STAT5 is essential for Akt/p70S6 kinase activity during IL-2-induced lymphocyte proliferation. J Immunol. 2007;179:5301–5308. doi: 10.4049/jimmunol.179.8.5301. [DOI] [PubMed] [Google Scholar]
- Malek TR. The biology of interleukin-2. Annu Rev Immunol. 2008;26:453–479. doi: 10.1146/annurev.immunol.26.021607.090357. [DOI] [PubMed] [Google Scholar]
- Malek TR, Porter BO, Codias EK, Scibelli P, Yu A. Normal lymphoid homeostasis and lack of lethal autoimmunity in mice containing mature T cells with severely impaired IL-2 receptors. J Immunol. 2000;164:2905–2914. doi: 10.4049/jimmunol.164.6.2905. [DOI] [PubMed] [Google Scholar]
- Malek TR, Yu A, Scibelli P, Lichtenheld MG, Codias EK. Broad programming by IL-2 receptor signaling for extended growth to multiple cytokines and functional maturation of antigen-activated T cells. J Immunol. 2001;166:1675–1683. doi: 10.4049/jimmunol.166.3.1675. [DOI] [PubMed] [Google Scholar]
- Malek TR, Yu A, Vincek V, Scibelli P, Kong L. CD4 regulatory T cells prevent lethal autoimmunity in IL-2Rβ-deficient mice. Implications for the nonredundant function of IL-2. Immunity. 2002;17:167–178. doi: 10.1016/s1074-7613(02)00367-9. [DOI] [PubMed] [Google Scholar]
- Nelson BH, Willerford DM. Biology of the interleukin-2 receptor. Adv Immunol. 1998;70:1–81. doi: 10.1016/s0065-2776(08)60386-7. [DOI] [PubMed] [Google Scholar]
- Sadlack B, Lohler J, Schorle H, Klebb G, Haber H, Sickel E, Noelle RJ, Horak I. Generalized autoimmune disease in interleukin-2-deficient mice is triggered by an uncontrolled activation and proliferation of CD4+ T cells. Eur J Immunol. 1995;25:3053–3059. doi: 10.1002/eji.1830251111. [DOI] [PubMed] [Google Scholar]
- Sadlack B, Merz H, Schorle H, Schimpl A, Feller AC, Horak I. Ulcerative colitis-like disease in mice with a disrupted interleukin-2 gene. Cell. 1993;75:253–261. doi: 10.1016/0092-8674(93)80067-o. [DOI] [PubMed] [Google Scholar]
- Setoguchi R, Hori S, Takahashi T, Sakaguchi S. Homeostatic maintenance of natural Foxp3+ CD25+ CD4+ regulatory T cells by interleukin (IL)-2 and induction of autoimmune disease by IL-2 neutralization. J Exp Med. 2005;201:723–735. doi: 10.1084/jem.20041982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma R, Zheng L, Guo X, Fu SM, Ju ST, Jarjour WN. Novel animal models for Sjogren’s syndrome: expression and transfer of salivary gland dysfunction from regulatory T cell-deficient mice. J Autoimmunity. 2006;27:289–296. doi: 10.1016/j.jaut.2006.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snow JW, Abraham N, Ma MC, Herndier BG, Pastuszak AW, Goldsmith MA. Loss of tolerance and autoimmunity affecting multiple organs in STAT5A/5B-deficient mice. J Immunol. 2003;171:5042–5050. doi: 10.4049/jimmunol.171.10.5042. [DOI] [PubMed] [Google Scholar]
- Sojka DK, Bruniquel D, Schwartz RH, Singh NJ. IL-2 secretion by CD4+ T cells in vivo is rapid, transient, and influenced by TCR-specific competition. J Immunol. 2004;172:6136–6143. doi: 10.4049/jimmunol.172.10.6136. [DOI] [PubMed] [Google Scholar]
- Surh CD, Boyman O, Purton JF, Sprent J. Homeostasis of memory T cells. Immunol Rev. 2006;211:154–163. doi: 10.1111/j.0105-2896.2006.00401.x. [DOI] [PubMed] [Google Scholar]
- Suzuki H, Kundig TM, Furlonger C, Wakeham A, Timms E, Matsuyama T, Schmits R, Simard JJL, Ohashi PS, Griesser H, et al. Deregulated T cell activation and autoimmunity in mice lacking interleukin-2 receptor β. Science. 1995;268:1472–1476. doi: 10.1126/science.7770771. [DOI] [PubMed] [Google Scholar]
- Tang Q, Adams JY, Penaranda C, Melli K, Piaggio E, Sgouroudis E, Piccirillo CA, Salomon BL, Bluestone JA. Central role of defective interleukin-2 production in the triggering of islet autoimmune destruction. Immunity. 2008;28:687–697. doi: 10.1016/j.immuni.2008.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornton AM, Shevach EM. Suppressor effector function of CD4+CD25+ immunoregulatory T cells is antigen nonspecific. J Immunol. 2000;164:183–190. doi: 10.4049/jimmunol.164.1.183. [DOI] [PubMed] [Google Scholar]
- Waldmann TA. Daclizumab (anti-Tac, Zenapax) in the treatment of leukemia/lymphoma. Oncogene. 2007;26:3699–3703. doi: 10.1038/sj.onc.1210368. [DOI] [PubMed] [Google Scholar]
- Waldmann TA, Tagaya Y. The multifaceted regulation of interleukin-15 expression and the role of this cytokine in NK cell differentiation and host response to intracellular pathogens. Annu Rev Immunol. 1999;17:19–49. doi: 10.1146/annurev.immunol.17.1.19. [DOI] [PubMed] [Google Scholar]
- Walsh PT, Buckler JL, Zhang J, Gelman AE, Dalton NM, Taylor DK, Bensinger SJ, Hancock WW, Turka LA. PTEN inhibits IL-2 receptor-mediated expansion of CD4+ CD25+ Tregs. J Clin Invest. 2006;116:2521–2531. doi: 10.1172/JCI28057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willerford DM, Chen J, Ferry JA, Davidson L, Ma A, Alt FW. Interleukin-2 receptor α chain regulates the size and content of the peripheral lymphoid compartment. Immunity. 1995;3:521–530. doi: 10.1016/1074-7613(95)90180-9. [DOI] [PubMed] [Google Scholar]
- Yamanouchi J, Rainbow D, Serra P, Howlett S, Hunter K, Garner VE, Gonzalez-Munoz A, Clark J, Veijola R, Cubbon R, et al. Interleukin-2 gene variation impairs regulatory T cell function and causes autoimmunity. Nat Genet. 2007;39:329–337. doi: 10.1038/ng1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang-Snyder JA, Rothenberg EV. Spontaneous expression of interleukin-2 in vivo in specific tissues of young mice. Dev Immunol. 1998;5:223–245. doi: 10.1155/1998/12421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao Z, Kanno Y, Kerenyi M, Stephens G, Durant L, Watford WT, Laurence A, Robinson GW, Shevach EM, Moriggl R, et al. Nonredundant roles for Stat5a/b in directly regulating Foxp3. Blood. 2007;109:4368–4375. doi: 10.1182/blood-2006-11-055756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu A, Malek TR. The proteasome regulates receptor-mediated endocytosis of interleukin-2. J Biol Chem. 2001;276:381–385. doi: 10.1074/jbc.M007991200. [DOI] [PubMed] [Google Scholar]
- Yu A, Malek TR. Selective availability of IL-2 is a major determinant controlling the production of CD4+CD25+Foxp3+ T regulatory cells. J Immunol. 2006;177:5115–5121. doi: 10.4049/jimmunol.177.8.5115. [DOI] [PubMed] [Google Scholar]
- Yu A, Zhou J, Marten N, Bergmann CC, Mammolenti M, Levy RB, Malek TR. Efficient induction of primary and secondary T cell-dependent immune responses in vivo in the absence of functional IL-2 and IL-15 receptors. J Immunol. 2003;170:236–242. doi: 10.4049/jimmunol.170.1.236. [DOI] [PubMed] [Google Scholar]
- Zheng SG, Wang J, Wang P, Gray JD, Horwitz DA. IL-2 is essential for TGF-β to convert naive CD4+CD25− cells to CD25+Foxp3+ regulatory T cells and for expansion of these cells. J Immunol. 2007;178:2018–2027. doi: 10.4049/jimmunol.178.4.2018. [DOI] [PubMed] [Google Scholar]
- Zorn E, Nelson EA, Mohseni M, Porcheray F, Kim H, Litsa D, Bellucci R, Raderschall E, Canning C, Soiffer RJ, et al. IL-2 regulates FOXP3 expression in human CD4+CD25+ regulatory T cells through a STAT-dependent mechanism and induces the expansion of these cells in vivo. Blood. 2006;108:1571–1579. doi: 10.1182/blood-2006-02-004747. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.