Abstract
Background
The induction of tolerance toward third-party solid organ grafts with allogeneic thymus tissue transplantation has not been previously demonstrated in human subjects.
Objective
Infants with complete DiGeorge anomaly (having neither thymus nor parathyroid function) were studied for conditions and mechanisms required for the development of tolerance to third-party solid organ tissues.
Methods
Four infants who met criteria received parental parathyroid with allogeneic thymus transplantation and were studied.
Results
Two of three survivors showed function of both grafts but subsequently lost parathyroid function. They demonstrated alloreactivity against the parathyroid donor in mixed lymphocyte cultures. For these 2 recipients, parathyroid donor HLA class II alleles were mismatched with the recipient and thymus. MHC class II tetramers confirmed the presence of recipient CD4+ T cells with specificity towards a mismatched parathyroid donor class II allele. The third survivor has persistent graft function and lacks alloreactivity towards the parathyroid donor. All parathyroid donor class II alleles were shared with either the recipient or the thymus graft, with minor differences between the parathyroid (HLA-DRB1*1104) and thymus (HLA-DRB1*1101). Tetramer analyses detected recipient T cells specific for the parathyroid HLA-DRB1*1104 allele. Alloreactivity towards the parathyroid donor was restored with low-doses of IL-2.
Conclusion
Tolerance toward parathyroid grafts in combined parental parathyroid and allogeneic thymus transplantation requires matching of thymus tissue to parathyroid HLA class II alleles to promote negative selection and suppression of recipient T cells that have alloreactivity toward the parathyroid grafts. This matching strategy may be applied toward tolerance induction in future combined thymus and solid organ transplantation efforts.
Keywords: Allorecognition, anergy, class II, DiGeorge, parathyroid, tetramers, thymus, tolerance, transplantation
Introduction
Solid organ transplantation offers hope for the treatment of many diseases but continues to face significant challenges in preventing rejection of the graft by the recipient1. Alloreactivity by T cells toward “foreign” HLA molecules presents one of the most significant mechanisms for rejection of transplanted allogeneic tissues2, 3. Recipient alloreactivity toward donor tissues may be modulated by positive and negative selection processes within the thymus.
Infants with complete DiGeorge anomaly (cDGA) offer an opportunity to study the role of the thymus in controlling allorecognition responses. DiGeorge anomaly results from abnormal embryonic development leading to possible defects extending from the first to sixth pharyngeal arches4. Affected individuals present at birth with a spectrum of malformations involving the heart, parathyroid glands, and thymus5–10. In “complete” DiGeorge anomaly, infants lack naïve (CD45RA+CD62L+)11 T cells due to athymia, resulting in a severe primary immunodeficiency that is usually fatal due to infection by 2 years of age. Allogeneic thymus transplantation leads to immunoreconstitution and increased survival12, 13. The thymus grafts provide an environment in which recipient thymocyte precursors undergo positive and negative selection and emerge in the circulation as functional naïve T cells. Although the transplanted thymus tissues are not HLA-matched to the subjects, the recipients demonstrate tolerance to the grafts14. While thymus transplantation has shown success in correcting the immune defects in subjects with cDGA12, hypocalcemia due to hypoparathyroidism remains an important cause of morbidity and mortality15.
Because of the importance of the thymus in the development of tolerance, we postulated that congenital athymia would provide a suitable model to assess the induction of tolerance to solid organ grafts when combined with thymus transplantation. We hypothesized that in cDGA subjects, we could achieve tolerance toward parental parathyroid grafts in transplant protocols using co-transplanted allogeneic thymus tissue.
To assess tolerance in the recipients, we used a combination of traditional and novel methods. Traditionally, mixed lymphocyte cultures (MLCs) have assessed alloreactive T cell proliferation toward donor cells due to HLA class II differences16–18, which appear to contribute more to rejection than HLA class I mismatches19. However, these and other immune assays that show a lack of alloreactivity towards the donor have at times been questioned as markers for tolerance due to perceived insufficient specificity1, 20. Newer technologies now offer the potential to directly visualize the presence of specific alloreactive T cells1, 21, 22. MHC tetramers consist of fluorescently labeled, multimeric MHC molecules of a defined specificity that can be loaded with oligopeptides23. As a result, tetramers of recipient MHC molecules containing donor HLA oligopeptides could identify the presence of recipient donor-specific alloreactive T cells.
Here we discuss these efforts to characterize tolerance and the factors associated with tolerance induction in recipients of allogeneic thymus tissue with solid organ transplantation.
Methods
Subjects
All subjects were enrolled in clinical trials under a research protocol approved by the Duke Institutional Review Board. Informed consent for these studies and procedures was obtained from the parents of all thymus donors and transplant recipients, the parathyroid donors, and healthy adult controls. All recipients met clinical and immunologic criteria for cDGA4–6, 8, 10, 12 with primary hypoparathyroidism (see Case Reports in the Online Repository). They required the initiation of calcium supplementation shortly after birth and had multiple intact parathyroid hormone (PTH) levels measured near or below the limit of detection prior to transplantation.
Donors
All thymus and parental parathyroid donors underwent donor screening as described12, 24, 25. The parathyroid donors had normal intact PTH levels and were as follows: the mother for Subjects 1, 3, and 4; and the father for Subject 2. For Subject 3, parathyroid transplantation was delayed by 37 days after thymus transplantation due to postponed collection of the donor parathyroid gland.
Thymus and parathyroid transplantation
Thymus tissue was processed as previously described12, 13, 26. All recipients were given rabbit antithymocyte globulin (RATGAM) and methylprednisolone prior to transplantation as used in protocols for cDGA subjects with immunosuppression12, 27.
The cultured allogeneic thymus tissues were transplanted into each recipient’s quadriceps muscles28. At the same time, the parathyroid donor underwent open exploration of the neck under general anesthesia in an adjacent operating room. After a parathyroid gland was located, the presence of parathyroid tissue was confirmed by histology. For further confirmation, a small amount of tissue was suspended in saline, yielding high levels of PTH on rapid testing (Elecsys PTH STAT Test, Roche, Switzerland). The gland was then removed and sectioned. The parathyroid tissues were placed into the quadriceps muscle of the subject adjacent to the area used for thymus transplantation.
Routine immune and clinical assessments
Immune phenotyping by flow cytometry, lymphocyte proliferative responses to phytohemagglutinin and tetanus toxoid, and MLCs were performed according to standard protocols12, 27. All MLCs were performed after 10% of the recipients’ circulating T cells had the naïve phenotype (CD45RA+CD62L+). Peripheral blood mononuclear cell (PBMC) proliferative responses were assessed by counts per minute (cpm) of 3H-thymidine incorporation.
Intact PTH levels were measured according to the standard practices of the clinical laboratories at our institution and the referring institutions. The lower limits of normal for intact PTH levels at all facilities ranged from 10–15 pg/ml. The limit of detection for samples tested at our institution was 5 pg/ml.
High resolution typing for HLA-A, -B, -C, -DRB1, and -DQB1 alleles was obtained as a part of routine clinical testing. HLA-DQA1 and HLA-DPB1 analyses were performed by the Duke Clinical Transplantation Immunology Laboratory (CTIL) using a Luminex (Austin, TX) reverse sequence specific oligonucleotide multiplex bead assay and Invitrogen (Carlsbad, CA) sequence specific primer kit, respectively. Standard panel reactive HLA antibody screening was conducted by the Duke CTIL for Subjects 1, 3, and 4 (at 3.3, 2.5, and 2.2 years after transplantation, respectively).
Assessments for alloantigen-specific T cells
MHC class II tetramers were created to assess for recipient allospecific T cells by flow cytometry. “Positive” tetramers consisted of recipient HLA-DR molecules loaded with parental parathyroid donor oligopeptides from the HLA-DRB1 allele not shared with the recipient. Positive tetramers were constructed (Beckman Coulter tetramer synthesis facility, Fullerton, CA) for Subject 1 (amino acids 62–82 of HLA-DRB1*0701 bound within HLA-DRB1*0101 tetramers) and Subject 4 (amino acids 71–90 of HLA-DRB1*1104 loaded into HLA-DRB1*0301 tetramers). Tetramers could not be successfully generated for Subject 3. For “negative” tetramers, used to assess the specificities of the HLA oligopeptides, the tetramer molecules contained an antigenically irrelevant oligopeptide from the human class II-associated invariant chain peptide (CLIP) instead29. A detailed description of the tetramer synthesis process is provided in the Online Repository.
Tetramer staining of subject PBMCs was performed following the manufacturer’s (Beckman Coulter) instructions. In brief, 106 PBMCs were incubated with either positive or negative phycoerythrin-conjugated tetramers for 90 minutes at 37°C. Two percent mouse serum (Jackson ImmunoResearch Laboratories, West Grove, PA) was added for another 30 minutes at 37°C to block non-specific staining of surface antibodies. Surface antibodies to CD3 (allophycocyanin [APC]-Cy7-conjugated, BD Biosciences, San Jose, CA) and CD4 (fluorescein isothiocyanate-conjugated, Beckman Coulter) were applied. An additional mix of APC-conjugated antibodies to CD8, CD13, CD14 (all from BD Biosciences), CD16 (BioLegend, San Diego, CA), CD19, and CD56 (both from Beckman Coulter) was added to label non-relevant cell populations. The cells were then further incubated for 20 minutes and washed prior to data acquisition in the flow cytometer. The flow cytometry data were analyzed by gating for APC-negative, CD3+ cells. Then, CD4+ cells were chosen and plotted vs. tetramer-positive cells. For these experiments, PBMCs were obtained from Subject 1 at 4.3 years post-transplantation, from Subject 4 at 3.3 years post-transplantation, and from healthy adult controls.
Assessments for anergy
To test for anergy, IL-2 (Invitrogen BioSource, Carlsbad, CA) was added to Subject 4 PBMCs in all conditions parallel to a standard MLC assay: to recipient PBMCs co-cultured with irradiated autologous PBMCs; to recipient PBMCs co-cultured with irradiated parathyroid donor PBMCs; to recipient PBMCs co-cultured with irradiated pooled allogeneic PBMCs; and to recipient PBMCs placed in culture medium without irradiated cells. The cells were cultured in triplicate for 6 days. The concentration of IL-2 was optimized at 2 U/ml. This concentration was chosen after titration experiments were performed to identify the concentration that resulted in minimal proliferation when added to recipient PBMCs in culture medium without irradiated stimulator cells.
Statistical analyses
Differences in proliferative responses were tested for statistical significance using two-tailed Student’s t tests. The raw proliferation data (in cpm) were log-transformed prior to performing the statistical analyses. Significant statistical differences were defined by p values <0.05. Highly significant differences were demonstrated by p values <0.005. Results were then plotted as bar graphs depicting mean values and standard error bars using GraphPad Prism Software version 5.00 (San Diego, CA).
Results
Subjects
Four subjects met criteria for cDGA and primary hypoparathyroidism. Subjects 1, 2, and 4 received combined allogeneic thymus and parental parathyroid transplantation at 3.6, 3.4, and 4.3 months of life, respectively. Subject 3 underwent allogeneic thymus transplantation at 8.7 months of age followed by parental parathyroid transplantation 1.3 months later. All subjects were given RATGAM prior to transplantation. None of the subjects received immunosuppression past 6 months post-transplantation12, 27, 30.
The surgical procedures were tolerated well by the recipients and parathyroid donors with no immediate adverse events directly related to either thymus or parathyroid transplantation. Subject 2 died at 9.6 months after transplantation from chronic pulmonary disease due to mechanical ventilation dependence resulting from congenital thoracic anomalies.
Assessments of graft function
All recipients developed circulating naïve T cells in similar fashion to other cDGA recipients after allogeneic thymus transplantation12. The 3 surviving subjects also produced normal T cell proliferative responses to phytohemagglutinin and tetanus toxoid.
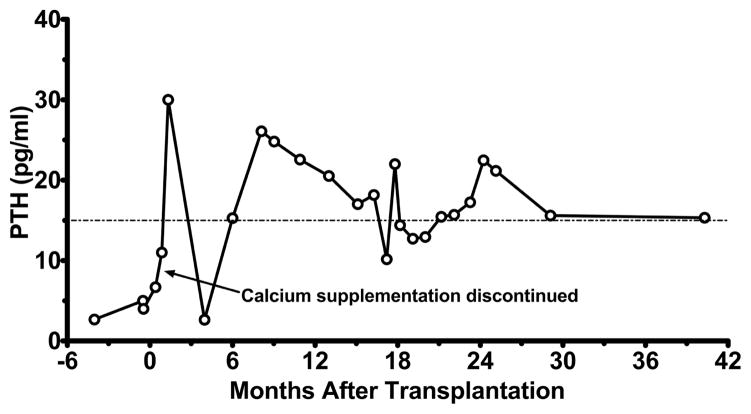
All cDGA subjects developed normal PTH levels and were able to discontinue calcium supplementation after parathyroid transplantation. Fluctuations in intact PTH levels were observed, consistent with the known difficulties in processing serum PTH samples31, 32. Subject 4 has maintained normal serum calcium levels and persistent PTH production within the normal range (Figure 1) since calcium supplementation was discontinued, indicating that the parathyroid graft has continued to function over 4 years after transplantation. Subjects 1 and 3, on the other hand, lost parathyroid graft function at 13 and 9 months after parathyroid transplantation, respectively, and require calcium supplementation for hypocalcemia (Figure E2).
Figure 1.
Parathyroid graft function in Subject 4 after combined parental parathyroid with allogeneic thymus transplantation. Calcium supplementation was weaned off by 15 days after transplantation. The subject remains off calcium supplementation. Intact PTH levels are shown vs. time after parathyroid transplantation. The lower limit of normal (15 pg/ml) is demarcated.
Assessments of allorecognition towards the parathyroid graft
To assess allorecognition-mediated rejection as a potential mechanism for loss of parathyroid graft function, HLA typing of the recipients and donors was performed. Matching between the recipient, parathyroid donor, and thymus donor at the HLA class I loci was not necessary for long-term parathyroid graft function (Table E2). However, HLA class II mismatches between the parathyroid donors and the alleles of the recipients and thymus donors were observed in the 2 surviving subjects who lost parathyroid function (Subjects 1 and 3). These mismatched parathyroid donor alleles offer potential targets for allorecognition-mediated rejection (see boxes in Table I). On the other hand, all of the HLA class II alleles for the parathyroid donor for Subject 4 (who has long-term parathyroid graft function) matched alleles in either the recipient or the thymus graft (although DRB1*1101 of the thymus differs from the DRB1*1104 of the parathyroid by 1 substitution in the first 100 amino acids, G to V at position 86). Thus, matching of the thymus tissue to the parathyroid graft for HLA class II alleles not shared between the parathyroid and Subject 4 was associated with long-term parathyroid graft function.
Table I.
HLA class II typing of recipients, their parathyroid (PT) donors, and their thymus donors.
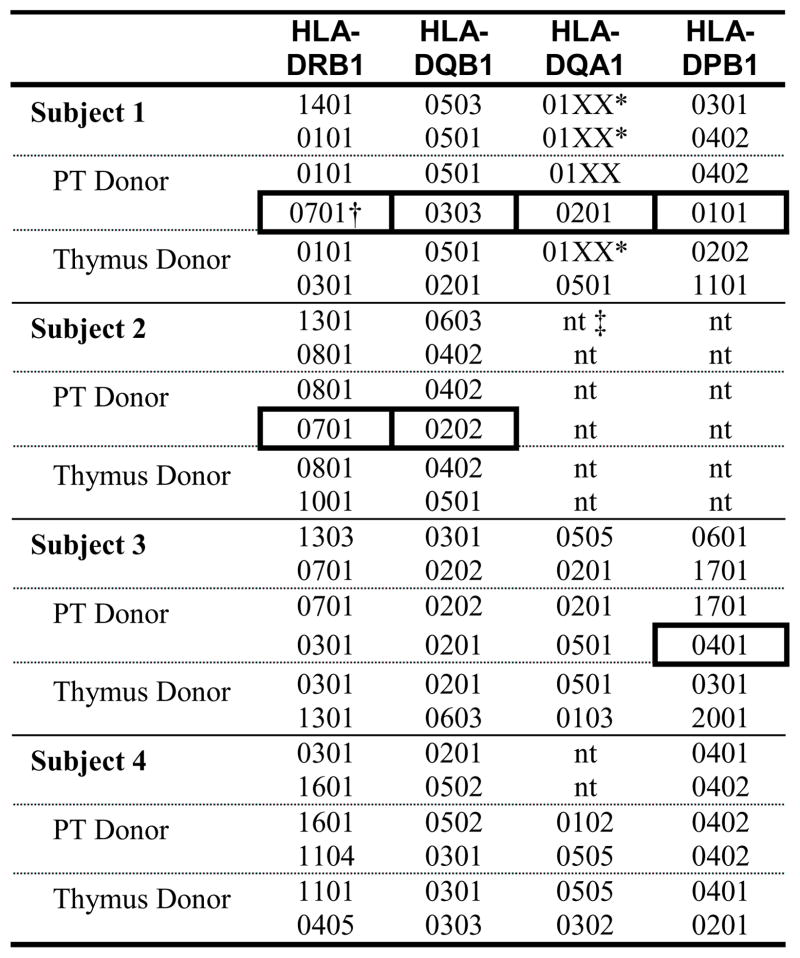 |
Only low-resolution typing available for this allele.
Parathyroid donor HLA class II alleles not shared with the recipient or the thymus donor are highlighted with boxes.
Abbreviation: nt, not tested.
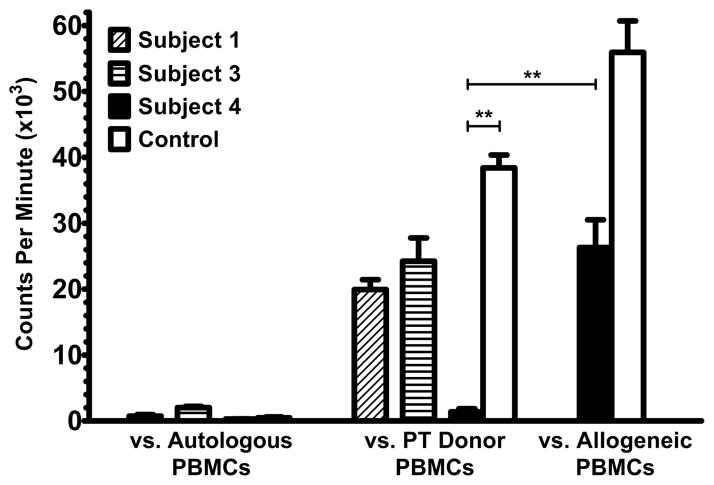
The subjects were tested for allorecognition toward the parathyroid donors in MLCs. PBMCs from Subject 1 and Subject 3 proliferated strongly against irradiated parathyroid donor PBMCs when tested at 6.7 and 8.5 months after transplantation (Figure 2). In contrast, Subject 4 showed no significant proliferative responses toward the parathyroid donor at 15.1 months post-transplantation compared to recipient responses toward allogeneic cells. Healthy adult control PBMCs responded strongly to irradiated PBMCs from the parathyroid donor for Subject 4. These findings for Subject 4 have been replicated in a total of 6 experiments to beyond 40 months post-transplantation (data not shown). Thus, the presence of allorecognition toward the parathyroid donor observed in the MLCs was associated with the loss of parathyroid graft function, and the absence of allorecognition toward the parathyroid donor was associated with long-term parathyroid graft function. Additional studies after the recipients successfully discontinued intravenous immunoglobulin replacement showed that no subjects have anti-HLA class I or class II antibodies, arguing against rejection by antibody-mediated allorecognition mechanisms.
Figure 2.
Mixed lymphocyte cultures. Non-irradiated responder PBMCs were co-cultured with irradiated autologous or parathyroid (PT) donor PBMCs. Results represent cpm of 3H-thymidine incorporation. For Subject 4, healthy adult control PBMC responses to irradiated PBMCs from the PT donor for Subject 4 and responses by Subject 4 and the control to pooled allogeneic PBMCs are shown for comparison. **p < 0.005.
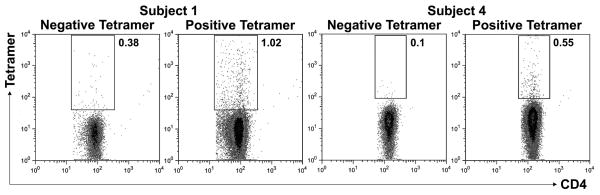
We then sought to confirm the presence of recipient donor-specific alloreactive T cells. Parathyroid donor HLA-DRB1 oligopeptides were loaded into recipient-type MHC class II tetramer molecules to test for the presence of alloantigen-specific CD4+ T cells. For Subject 1, tetramers were generated using parathyroid donor HLA-DRB1*0701 oligopeptides bound within recipient-type HLA-DRB1*0101 tetramer molecules. The HLA-DRB1*0701 oligopeptides used for Subject 1 were previously shown to stimulate alloreactive T cells33. Subject 3 could not be assessed because oligopeptides from the only HLA class II mismatch (HLA-DPB1*0401) did not bind stably within recipient-type HLA-DRB1*0701 molecules. For Subject 4, HLA-DRB1*1104 oligopeptides (amino acid positions 71–90) were loaded into recipient-type HLA-DRB1*0301 tetramers. We identified the presence of over 1% Subject 1 CD4+ T cells that bound to the tetramer containing the parathyroid donor oligopeptide at 4.3 years after transplantation (Figure 3). In Subject 4 at 3.3 years after transplantation, a smaller percentage of CD4+ T cells showed affinity for the tetramer loaded with the parathyroid donor HLA-DRB1*1104 peptide. Importantly, binding of the tetramers to T cells from control individuals was not observed (Figure E3).
Figure 3.
Tetramer analyses. Subjects 1 and 4 were tested for CD4+ T cells with allorecognition toward parathyroid donor oligopeptides (from the HLA-DRB1 allele not shared with the recipient) presented within recipient-type MHC class II tetramers (“positive”). “Negative” tetramers contained an antigenically irrelevant peptide. Percentages of tetramer-positive cells are shown, gated on CD4+ T cells.
Assessments of peripheral tolerance mechanisms
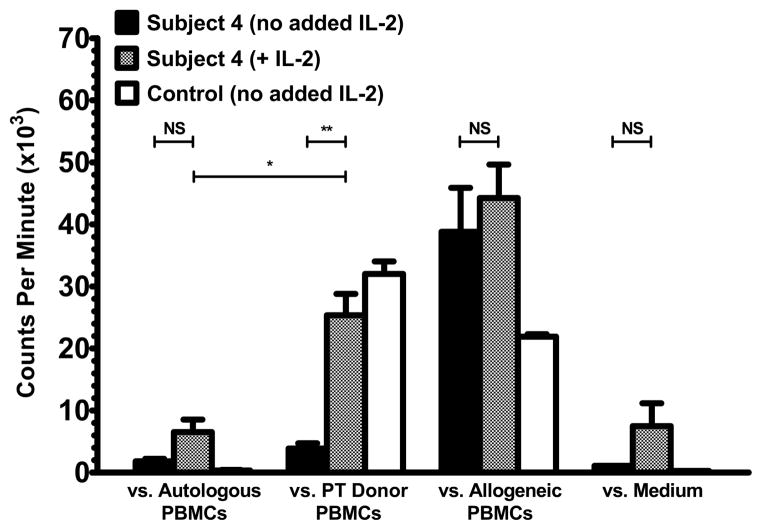
We studied mechanisms of peripheral tolerance responsible for the suppression of alloreactivity toward the parathyroid donor in Subject 4. In assessments for anergy, a low dose of IL-2 (2 U/mL) restored alloreactivity by Subject 4 PBMCs (obtained 3.3 years post-transplantation) towards the parathyroid donor cells (Figure 4). The response of the Subject 4 PBMCs to the parathyroid donor was also statistically greater than the autologous response. Proliferation of the recipient PBMCs to irradiated autologous PBMCs and proliferation of the recipient PBMCs in culture medium without irradiated cells remained low after the addition of IL-2. These findings for Subject 4 were confirmed by a subsequent analysis. The results suggest that some Subject 4 T cells with allorecognition towards the parathyroid donor have been rendered anergic to maintain tolerance towards the parathyroid graft. In separate experiments, we did not observe a role for regulatory T cells in suppressing alloreactivity by Subject 4 T cells toward the parathyroid donor when tested at 17 months after transplantation (Figure E4).
Figure 4.
Assessments for anergic alloreactive T cells. Subject 4 and control PBMCs were cultured with irradiated PBMCs (autologous, parathyroid (PT) donor, or allogeneic) or in medium without irradiated cells. In parallel, IL-2 was added to Subject 4 PBMCs in all four conditions. Results (incorporated 3H-thymidine) are shown at 3.3 years after transplantation. NS, p > 0.05; *p < 0.05; **p < 0.005.
Discussion
Combined parental parathyroid with allogeneic thymus transplantation in 4 infants with cDGA led to parathyroid and thymus graft function in all recipients. Of the 3 survivors, 2 (Subjects 1 and 3) subsequently lost parathyroid graft function. The other survivor (Subject 4) continues to demonstrate parathyroid function without long-term immunosuppression. Endogenous PTH production in Subject 4 (who had no parathyroid function from birth) remains unlikely. Subject 4 was only 4.8 months of age when calcium supplementation was discontinued. Of 35 infants with cDGA who received calcium supplementation before allogeneic thymus transplantation (excluding the subjects who received parathyroid transplants) and who have survived to at least 6 months of age, only 1 was successfully weaned off of calcium supplementation below 6 months of age as accomplished for Subject 4. This infant had a PTH level of 12 pg/ml while calcium supplementation was being given, in contrast to the 4 subjects in this report who all had PTH levels ≤ 6 pg/ml before receiving the parathyroid grafts. Thus, this 1 case that demonstrated almost normal endogenous PTH production prior to discontinuing calcium supplementation clearly differs from our observations in Subject 4.
We hypothesized that loss of parathyroid function occurred due to rejection of the grafts by alloreactive recipient T cells and that long-term function resulted from tolerance. HLA typing revealed potential parathyroid donor HLA class II targets for rejection in the 2 recipients who lost parathyroid graft function but not in the recipient who has continued parathyroid function. Furthermore, the recipients who lost parathyroid function also showed alloreactivity toward their parathyroid donors in MLCs, while the subject with persistent function demonstrated tolerance. We were able to observe the presence of recipient T cells with specificity toward parathyroid donor HLA class II alloantigens using MHC class II tetramers. For Subject 1, who appears to have rejected the parathyroid graft, the presence of one of many potential populations of recipient T cells with parathyroid donor alloantigen specificity was confirmed. For Subject 4, who demonstrates persistent graft function and tolerance toward the parathyroid donor in MLCs, a small percentage of tetramer-positive cells was observed. These cells may have escaped deletion in the thymus graft because they have specificity for HLA-DRB1*1104 and not the HLA-DRB1*1101 on the thymus graft epithelial cells.
To our knowledge, we report the first use of MHC class II tetramers for direct observation of T cells with alloantigen specificity at the single-cell level. We found that only certain oligopeptides bound within the MHC class II clefts of the designated tetramer molecules. Of note, our empiric method for creating the tetramers also allowed us to map the relevant T cell epitopes within the first 100 amino acids for HLA-DRB1*0701 (amino acids 62–82) when presented by HLA-DRB1*0101, and HLA-DRB1*1104 (amino acids 71–90) when presented by HLA-DRB1*0301. For Subject 3, none of the parathyroid donor oligopeptides tested bound to HLA-DRB1*0701 tetramers. The peptides within the first 100 amino acids of HLA-DPB1*0401 do not appear to be presented to T cells within HLA-DRB1*0701 molecules of antigen presenting cells. Instead, more C-terminal peptides from HLA-DPB1*0401 may be presented by HLA-DRB1*0701. Likely, one or more of the HLA-DPB1*0401 oligopeptides binds to HLA-DRB1*1303 or other Subject 3 HLA class II molecules (HLA-DQ or -DP) for which tetramers are not currently available. This approach for screening the tetramers for binding to the various oligopeptides – although effective – was resource-consuming, which may limit its general use at this time. Finally, as exemplified by Subject 4, who has detectable tetramer-positive CD4+ T cells yet remains tolerant toward the parathyroid graft, tetramer assays performed together with functional assessments for allorecognition (such as MLCs) enhance evaluation of alloreactivity in allograft recipients.
We considered potential peripheral mechanisms that could confer tolerance in Subject 4. Low-dose IL-2 restored proliferation by Subject 4 PBMCs toward the parathyroid donor, indicating a role for anergy34–37 as a tolerance mechanism. An attractive unifying theory, incorporating the presence of the tetramer-positive cells, suggests that the cells may have been rendered anergic because the affinity of these cells for HLA-DRB1*1104 could lead to rejection of the similar HLA-DRB1*1101 on the thymus graft. We have not confirmed this hypothesis due to blood volume constraints in Subject 4 that preclude isolating sufficient numbers of the tetramer-positive cells (in Figure 3) to assess anergy (as in Figure 4). In separate experiments, we were unable to observe a role for CD25+ regulatory T cells in maintaining tolerance by Subject 4 toward the parathyroid graft at 17 months after transplantation.
Our results compare favorably to other human parathyroid allotransplantation efforts. Long-term function of allogeneic parathyroid grafts has been reported in 2 patients who first received allogeneic renal grafts and were able to discontinue immunosuppression, Their parathyroid grafts were fully or partially matched to the kidney donors (HLA class II typing was not reported in the second case)38, 39. Other approaches have focused upon the use of tissue manipulation and special culture techniques to reduce HLA class II expression (ubiquitous in parathyroid stroma40) in the parathyroid grafts rather than to induce tolerance41–43. Even after these methods, prolonged parathyroid graft function has been poor (55% of grafts retain function beyond 2 months).43 Other attempts forgoing the use of long-term immunosuppression without inducing tolerance toward the parathyroid tissues have not resulted in prolonged graft function or the ability to discontinue calcium supplementation beyond 1 year after transplantation44–46.
Overall, our findings suggest that allogeneic thymus tissue can be used to induce tolerance to solid organ grafts in infants with cDGA. Based upon our data in 4 recipients of parental parathyroid and allogeneic thymus grafts, we hypothesize that HLA class II matching between the thymus and solid organ tissues is important for inducing tolerance towards the solid organ graft. We believe that the solid organ graft HLA class II alleles must be expressed in the thymus graft, either by the thymus graft medullary epithelial cells or recipient antigen presenting cells (e.g., dendritic cells). Any T cells that have alloreactivity toward these alleles yet escape negative selection in the thymus graft are peripherally suppressed. Matching of the HLA class I alleles does not appear to be necessary for tolerance. This model suggests the need for additional studies to assess the potential for using this matching strategy to induce tolerance to solid organ grafts in combination with allogeneic thymus transplantation in children with cDGA. Further application of this knowledge toward immunocompetent recipients may be challenging, likely requiring depletion of the recipient T cells and suppression or elimination of the recipient thymic function prior to transplantation. Our studies may also support the argument39 for the development of pre-typed banks of tissues, including both thymus and solid organ tissues, that can be used for transplantation. The tissues of the appropriate HLA types could then be selected to promote thymus graft-mediated induction of tolerance toward the solid organ tissue.
Key Messages.
Allogeneic thymus tissue may be used for tolerance induction to third-party solid organ grafts in athymic patients.
Tolerance requires elimination or suppression of recipient allorecognition responses toward solid organ donor HLA class II alleles; HLA class I alleles do not appear to play an important role in tolerance versus rejection.
Subsets of recipient alloreactive CD4+ T cells can be identified using custom-synthesized MHC class II tetramers.
Acknowledgments
This work was funded by the American Academy of Allergy, Asthma, and Immunology 2006 Third-Year Fellow-in-Training Research and 2008 Senior Allergy/Immunology Fellow Transition Awards; and NIH grants R01 AI 047040, R21 AI 060967, M03 RR 30 (General Clinical Research Center, National Center for Research Resources), T32 AI 007062-28A2, and 2 K12 HD043494 06. Dr. Bonilla has grant support from Talecris Biotherapeutics, Inc. MLM is a member of the Duke Comprehensive Cancer Center.
The authors thank Drs. Samuel R. Fisher and James A. Blumenthal for screening the parathyroid donor candidates. The authors appreciate the contributions of Dr. J. Michael Cook of the Duke Comprehensive Cancer Center flow cytometry facility and of Marilyn J. Alexieff, Jie Li, Chia-San Hsieh, Julie E. Cox, and Michele E. Cox for processing specimens and for regulatory support. We acknowledge the effort of Angelica DeOliveira in the Duke Clinical Transplantation Immunology Laboratory for HLA typing of HLA-DQ and HLA-DP alleles. The authors thank the Duke Pediatric Allergy and Immunology faculty and fellows for clinical care of the subjects. We express our gratitude to Dr. Brian P. Vickery for critical review of the manuscript prior to submission. Finally, we acknowledge Dr. Prescott Atkinson (University of Alabama at Birmingham) for referral of one transplant recipient.
Non-Standard Abbreviations
- APC
allophycocyanin
- cDGA
complete DiGeorge anomaly
- CLIP
class II-associated invariant chain peptide
- CPM
counts per minute
- CTIL
clinical transplantation immunology laboratory
- MLC
mixed lymphocyte culture
- PBMC
peripheral blood mononuclear cell
- PTH
parathyroid hormone
- RATGAM
rabbit antithymocyte globulin
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Zarkhin V, Sarwal MM. Microarrays: Monitoring for Transplant Tolerance and Mechanistic Insights. Clin Lab Med. 2008;28:385–410. doi: 10.1016/j.cll.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 2.Sherman LA, Chattopadhyay S. The Molecular Basis of Allorecognition. Ann Rev Immunol. 1993;11:385–402. doi: 10.1146/annurev.iy.11.040193.002125. [DOI] [PubMed] [Google Scholar]
- 3.Sykes M. Mechanisms of Transplantation Tolerance in Animals and Humans. Transplantation. 2009;87(9S):S67–S9. doi: 10.1097/TP.0b013e3181a2a6b8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thomas RA, Landing BH, Wells TR. Embryologic and other developmental considerations of thirty-eight possible variants of the DiGeorge Anomaly (DGA) Am J Med Genet Suppl. 1987;3:43–66. doi: 10.1002/ajmg.1320280508. [DOI] [PubMed] [Google Scholar]
- 5.DiGeorge AM. Discussion of Cooper MD, Peterson RDA, Good RA. A new concept of cellular basis of immunity. J Pediatr. 1965;67:907. [Google Scholar]
- 6.Conley ME, Beckwith JB, Mancer JFK, Tenckhoff L. The Spectrum of the DiGeorge Syndrome. J Pediatr. 1979;94:883–90. doi: 10.1016/s0022-3476(79)80207-3. [DOI] [PubMed] [Google Scholar]
- 7.Hong R. Reconstitution of T-cell deficiency by thymic hormone or thymus transplantation therapy. Clin Immunol Immunopathol. 1986;40:136–41. doi: 10.1016/0090-1229(86)90077-2. [DOI] [PubMed] [Google Scholar]
- 8.Müller W, Peter H, Wilken M, Juppner H, Kallfelz HC, Krohn HP, et al. The DiGeorge syndrome. I. Clinical evaluation and course of partial and complete forms of the syndrome. Eur J Pediatr. 1988;147:496–502. doi: 10.1007/BF00441974. [DOI] [PubMed] [Google Scholar]
- 9.Hong R. The DiGeorge Anomaly. Immunodefic Rev. 1991;3:1–14. [PubMed] [Google Scholar]
- 10.Markert ML, Hummell DS, Rosenblatt HM, Schiff SE, Harville TO, Williams LW, et al. Complete DiGeorge Syndrome: Persistence of Profound Immunodeficiency. J Pediatr. 1998;132:15–21. doi: 10.1016/s0022-3476(98)70478-0. [DOI] [PubMed] [Google Scholar]
- 11.Picker LJ, Treer JR, Ferguson-Darnell B, Collins PA, Buck D, Terstappen LW. Control of lymphocyte recirculation in man. I. Differential regulation of the peripheral lymph node homing receptor L-selection on T cells during the virgin to memory cell transition. J Immunol. 1993;150:1105–21. [PubMed] [Google Scholar]
- 12.Markert ML, Devlin BH, Alexieff MJ, Li J, McCarthy EA, Gupton SE, et al. Review of 54 patients with complete DiGeorge anomaly enrolled in protocols for thymus transplantation: outcome of 44 consecutive transplants. Blood. 2007;109:4539–47. doi: 10.1182/blood-2006-10-048652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Markert ML, Devlin BH, McCarthy EA, Chinn IK, Hale LP. Thymus Transplantation. In: Lavani C, Moran CA, Morandi U, Schoenhuber R, editors. Thymus Gland Pathology: Clinical, Diagnostic and Therapeutic Features. Milan: Springer-Verlag Italia; 2008. pp. 255–67. [Google Scholar]
- 14.Chinn IK, Devlin BH, Li Y-J, Markert ML. Long-term tolerance to allogeneic thymus transplants in complete DiGeorge anomaly. Clin Immunol. 2008;126:277–81. doi: 10.1016/j.clim.2007.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Markert ML, Sarzotti M, Ozaki DA, Sempowski GD, Rhein ME, Hale LP, et al. Thymus transplantation in complete DiGeorge syndrome: immunologic and safety evaluations in 12 patients. Blood. 2003;102:1121–30. doi: 10.1182/blood-2002-08-2545. [DOI] [PubMed] [Google Scholar]
- 16.Schwarz MR. The Mixed Lymphocyte Reaction: An In Vitro Test For Tolerance. J Exp Med. 1968;127:879–90. doi: 10.1084/jem.127.5.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.MacLaurin BP. Thymus Origin of Lymphocytes Reacting and Stimulating Reaction in Mixed Lymphocyte Cultures - Studies in the Rat. Clin Exp Immunol. 1972;10:649–59. [PMC free article] [PubMed] [Google Scholar]
- 18.Jeras M. The role of in vitro alloreactive T-cell functional tests in the selection of HLA matched and mismatched haematopoietic stem cell donors. Transpl Immunol. 2002;10:205–14. doi: 10.1016/s0966-3274(02)00067-9. [DOI] [PubMed] [Google Scholar]
- 19.Morris PJ, Johnson RJ, Fuggle SV, Belger MA, Briggs JD. Analysis of factors that affect outcome of primary cadaveric renal transplantation in the UK. Lancet. 1999;354:1147–52. doi: 10.1016/s0140-6736(99)01104-6. [DOI] [PubMed] [Google Scholar]
- 20.Silvers WK, Lubaroff DM, Wilson DB, Fox D. Mixed Lymphocyte Reactions and Tissue Transplantation Tolerance. Science. 1970;167:1264–6. doi: 10.1126/science.167.3922.1264. [DOI] [PubMed] [Google Scholar]
- 21.Benitez F, Najafian N. Novel Noninvasive Assays to Predict Transplantation Rejection and Tolerance: Enumeration of Cytokine-Producing Alloreactive T Cells. Clin Lab Med. 2008;28:365–73. doi: 10.1016/j.cll.2008.07.002. [DOI] [PubMed] [Google Scholar]
- 22.Ekong UD, Miller SD, O'Gorman MRG. In vitro assays of allosensitization. Pediatr Transplant. 2009;13:25–34. doi: 10.1111/j.1399-3046.2008.01042.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kwok WW, Ptacek NA, Liu AW, Buckner JH. Use of class II tetramers for identification of CD4+ T cells. J Immunol Methods. 2002;268:71–81. doi: 10.1016/s0022-1759(02)00201-6. [DOI] [PubMed] [Google Scholar]
- 24.Zorn E, Nelson EA, Mohseni M, Porcheray F, Kim H, Litsa D, et al. IL-2 regulates FOXP3 expression in human CD4+CD25+ regulatory T cells through a STAT-dependent mechanism and induces the expansion of these cells in vivo. Blood. 2006;108:1571–9. doi: 10.1182/blood-2006-02-004747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Title 21, Code of Federal Regulations 1271, Subchapter L. Human Cells, Tissues, and Cellular and Tissue-Based Products. Food and Drug Administration; 2006. [Google Scholar]
- 26.Markert ML, Kostyu DD, Ward FE, McLaughlin TM, Watson TJ, Buckley RH, et al. Successful formation of a chimeric human thymus allograft following transplantation of cultured postnatal human thymus. J Immunol. 1997;158:998–1005. [PubMed] [Google Scholar]
- 27.Markert ML, Alexieff MJ, Li J, Sarzotti M, Ozaki DA, Devlin BH, et al. Postnatal thymus transplantation with immunosuppression as treatment for DiGeorge syndrome. Blood. 2004;104:2574–81. doi: 10.1182/blood-2003-08-2984. [DOI] [PubMed] [Google Scholar]
- 28.Rice HE, Skinner MA, Mahaffey SM, Oldham KT, Ing RJ, Hale LP, et al. Thymic transplantation for complete DiGeorge syndrome: Medical and surgical considerations. J Pediatr Surg. 2004;39:1607–15. doi: 10.1016/j.jpedsurg.2004.07.020. [DOI] [PubMed] [Google Scholar]
- 29.Day CL, Seth NP, Lucas M, Appel H, Gauthier L, Lauer GM, et al. Ex vivo analysis of human memory CD4 T cells specific for hepatitis C virus using MHC class II tetramers. J Clin Invest. 2003;112:831–42. doi: 10.1172/JCI18509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Markert ML, Alexieff MJ, Li J, Sarzotti M, Ozaki DA, Devlin BH, et al. Complete DiGeorge syndrome: Development of rash, lymphadenopathy, and oligoclonal T cells in 5 cases. J Allergy Clin Immunol. 2004;113:734–41. doi: 10.1016/j.jaci.2004.01.766. [DOI] [PubMed] [Google Scholar]
- 31.Martin KJ, Gonzalez EA. The evolution of assays for parathyroid hormone. Curr Opin Nephrol Hypertens. 2001;10:569–74. doi: 10.1097/00041552-200109000-00004. [DOI] [PubMed] [Google Scholar]
- 32.Remijn JA, Beukhof JR, Dikkeschei LD. Large Fluctuations in Parathyroid Hormone Concentrations After Autotransplantation of Parathyroid Tissue in the Forearm. Clin Chem. 2007;53:534–5. doi: 10.1373/clinchem.2006.083667. [DOI] [PubMed] [Google Scholar]
- 33.Najafian N, Salama AD, Fedoseyeva EV, Benichou G, Sayegh MH. Enzyme-Linked Immunosorbent Spot Assay Analysis of Peripheral Blood Lymphocyte Reactivity to Donor HLA-DR Peptides: Potential Novel Assay for Prediction of Outcomes for Renal Transplant Recipients. J Am Soc Nephrol. 2002;13:252–9. doi: 10.1681/ASN.V131252. [DOI] [PubMed] [Google Scholar]
- 34.Essery G, Feldmann M, Lamb JR. Interleukin-2 can prevent and reverse antigen-induced unresponsiveness in cloned human T lymphocytes. Immunology. 1988;64:413–7. [PMC free article] [PubMed] [Google Scholar]
- 35.Blumenthal RL, Campbell DE, Hwang P, DeKruyff RH, Frankel LR, Umetsu DT. Human alveolar macrophages induce functional inactivation in antigen-specific CD4 T cells. J Allergy Clin Immunol. 2001;107:258–64. doi: 10.1067/mai.2001.112845. [DOI] [PubMed] [Google Scholar]
- 36.Schwartz RH. T Cell Anergy. Ann Rev Immunol. 2003;21:305–34. doi: 10.1146/annurev.immunol.21.120601.141110. [DOI] [PubMed] [Google Scholar]
- 37.Fathman CG, Lineberry NB. Molecular mechanisms of CD4+ T-cell anergy. Nat Rev Immunol. 2007;7:599–609. doi: 10.1038/nri2131. [DOI] [PubMed] [Google Scholar]
- 38.Ross AJ, Dale JK, Gunnells JC, Wells SA. Parathyroid transplantation: Fate of a long-term allograft in man. Surgery. 1979;85:382–4. [PubMed] [Google Scholar]
- 39.Alfrey EJ, Perloff LJ, Asplund MW, Dafoe DC, Grossman RA, Bromberg JS, et al. Normocalcemia thirteen years after successful parathyroid allografting in a recipient of a renal transplant. Surgery. 1992;111:234–6. [PubMed] [Google Scholar]
- 40.Bjerneroth G, Juhlin C, Rastad J, Akerstrom G, Klareskog L. MHC Class I and II Antigen Expression on Parathyroid Cells and Prospects for their Allogeneic Transplantation. Transplantation. 1993;56:717–21. doi: 10.1097/00007890-199309000-00040. [DOI] [PubMed] [Google Scholar]
- 41.Sollinger HW, Mack E, Cook K, Belzer FO. Allotransplantation of Human Parathyroid Tissue Without Immunosuppression. Transplantation. 1983;36:599–602. doi: 10.1097/00007890-198336060-00001. [DOI] [PubMed] [Google Scholar]
- 42.Hasse C, Klock G, Schlosser A, Zimmermann U, Rothmund M. Parathyroid allotransplantation without immunosuppression. Lancet. 1997;350:1296–7. doi: 10.1016/S0140-6736(05)62473-7. [DOI] [PubMed] [Google Scholar]
- 43.Nawrot I, Wozniewicz B, Tolloczko T, Sawicki A, Gorski A, Chudzinski W, et al. Allotransplantation of Cultured Parathyroid Progenitor Cells Without Immunosuppression: Clinical Results. Transplantation. 2007;83:734–40. doi: 10.1097/01.tp.0000258601.17505.9d. [DOI] [PubMed] [Google Scholar]
- 44.Feind CR, Weber CJ, Derenoncourt F, Williams GA, Hardy MA, Reemtsma K. Survival and allotransplantation of cultured human parathyroids. Transplant P. 1979;11:1011–6. [PubMed] [Google Scholar]
- 45.Tolloczko T, Woziniewicz B, Sawicki A, Nawrot I, Migaj M, Zabitkowska T, et al. Cultured Parathyroid Cell Transplantation Without Immunosuppression in the Treatment of Surgical Hypoparathyroidism. Transplant P. 1994;26:1901–2. [PubMed] [Google Scholar]
- 46.Kunori T, Tsuchiya T, Itoh J, Watabe S, Arai M, Satomi T, et al. Improvement of Postoperative Hypocalcemia by Repeated Allotransplantation of Parathyroid Tissue without Anti-Rejection Therapy. Tohoku J Exp Med. 1991;165:33–40. doi: 10.1620/tjem.165.33. [DOI] [PubMed] [Google Scholar]