Abstract
Objective
Recent literature has emphasized the simultaneous assessment of multiple physiological stress response systems in an effort to identify biobehavioral risk factors of psychopathology in maltreated populations. The current study assessed whether an asymmetrical stress response, marked by activation in one system and a blunted response in another system, predicted higher levels of psychopathology over time.
Methods
Data were collected from an ongoing, prospective study of females with a substantiated history of childhood sexual abuse (n = 52) and a non-abused comparison group (n = 77). Childhood sexual abuse was determined at the initial study visit. Vagal tone and cortisol were measured 7 years later to assess physiological response to a laboratory stressor across these systems. Depressive symptoms and antisocial behaviors were assessed 6 years after the completion of the laboratory stressor.
Results
Structural equation modeling indicated that a prior history of childhood sexual abuse predicted an asymmetrical physiological response to stress in late adolescence. In turn, this asymmetrical response predicted both higher levels of depression and antisocial behaviors in young adulthood.
Conclusions
Childhood sexual abuse may sensitize females to respond to moderate daily stressors in a manner that places them at higher risk for experiencing depressive symptom and antisocial behaviors over time.
Practice implications
The management of mild to moderate stress in the everyday lives of maltreated females may be a particularly useful point of intervention in order to protect against later psychopathology
Introduction
Childhood maltreatment, including physical abuse, sexual abuse, and neglect, has been linked to a number of adverse developmental outcomes in childhood, adolescence and young adulthood. Developmental correlates of childhood maltreatment include increased aggression, emotion dysregulation, anxiety, depression, and post-traumatic stress disorder (Cicchetti & Rogosch, 2001a; Kaufman et al., 1997; Paolucci, Genuis, & Violato, 2001; Shields & Cicchetti, 1998; Shipman & Zeman, 2001; Trickett & Schellenbach, 1998). Despite this substantial research connecting childhood maltreatment to negative outcomes, not all maltreated children develop subsequent psychopathology (Binder, McNiel, & Goldstone, 1996; Kendall-Tackett, Williams, & Finkelhor, 1993). This variability in outcome represents a considerable opportunity to gain understanding of specific risk and protective factors that may shape the development of psychopathology in maltreated populations.
Toward this end, researchers have given considerable attention to identifying biobehavioral markers of risk in maltreated populations to help explain individual differences in the development of psychopathology. The autonomic nervous system (ANS) and hypothalamic-pituitary-adrenal (HPA) axis have been targeted predominantly because they are the two main physiological systems activated during environmental stress including childhood adversity and maltreatment. In healthy individuals, the sympathetic branch of the ANS is activated through the locus coeruleus which elevates production of norepinephrine and stimulates the adrenal medulla to release epinephrine. Catecholamines such as norepinephrine and epinephrine are ultimately responsible for increasing blood glucose, heart rate, and blood pressure that aid the body in resolving a stressor (Cacioppo, 1994). Vagal influence over cardiac activity, which is responsible for modulating increased sympathetic activity through the hypothalamus and amygdala, often withdraws during stressor situations (Porges, 2003). Hence, the process of a reduction in vagal influence observed in stressor situations has been referred to as vagal suppression or withdrawal. Activation of the HPA axis begins when a stressor stimulates the corticotrophin-releasing hormone (CRH) in the hypothalamus, leading to secretion of the adrenocorticotropic hormone (ACTH) in the anterior pituitary, and resulting in increased concentrations of cortisol produced by the adrenal cortex (Chrousos & Gold, 1992).
When the stressor is resolved, the hypothalamus and anterior pituitary regulate cortisol concentrations by suppressing production of CRH and ACTH in a process known as the negative-feedback loop (Munck, Guyre, & Holbrook, 1984). Despite being separate arms of the body’s stress response, the ANS and HPA are designed to assist the individual under stressful conditions, are influenced by similar brain structures, possess similar physiologic functions such as “fight or flight,” and contain regulatory capacities that modulate heightened physiological activity.
One potential risk associated with exposure to severe and chronic stress such as childhood maltreatment is that the inordinate stress may impair ANS or HPA functioning as well as the interplay that occurs between these two systems. Exposure to childhood maltreatment has been related to persistently high levels of catecholamine activity (DeBellis et al., 1999; DeBellis et al., 1994; Perry & Murburg, 1994), heightened sympathetic activity under novel stress situations (Heim, Newport et al., 2000; Luecken, 1998; Orr et al., 1998), and vagal withdrawal in those people experiencing symptoms of post-traumatic stress disorder (Sack, Hopper, & Lamprecht, 2004). Similarly, severe childhood stress has been associated with high concentrations of cortisol (Carrion et al., 2002; Cicchetti & Rogosch, 2001b; Delahanty, Nugent, Christopher, & Walsh, 2005; Pfeffer, Altemus, Heo, & Jiang, 2007), disrupted diurnal rhythms in cortisol production (Gunnar & Vazquez, 2001; King, Mandansky, King, Fletcher, & Brewer, 2001), and blunted cortisol response in stress reactivity paradigms (Carpenter et al., 2007; Hart, Gunnar, & Cicchetti, 1995). Thus, exposure to severe or chronic forms of stress, such as childhood maltreatment, may result in the dysregulation of both ANS and HPA functioning, each of which has been associated with various forms of psychopathology (Dietrich et al., 2007; Goodyer, Herbert, Moor, & Altham, 1991; Mezzacappa et al., 1997; Pajer, Gardner, Rubin, Perel, & Neal, 2001; Raine, Venables, & Williams, 1990). Recent efforts to further explicate potential biobehavioral pathways to the development of psychopathology have called for the simultaneous assessment of both ANS and HPA activity as opposed to a single system approach. For example, Bauer and colleagues (Bauer, Quas, & Boyce, 2002) outline two models that may improve the manner by which physiological biomarkers and risk for psychopathology are pursued. The first such model is an additive or symmetrical model where the extreme ends of the continuum in both ANS and HPA activity places children and adolescents at greater risk for psychopathology. According to this additive model, individuals who experience negligible stimulation in both ANS and HPA systems, as well as those who experience extreme stimulation in both these systems, are at increased risk for the development of psychopathology. Prior evidence has supported this view claiming that understimulation in these systems may be an aversive physiological state prompting aggressive and antisocial behavior (Raine et al., 1990), and where overstimulation in these systems may prompt internalizing behaviors (Cicchetti & Rogosch, 2001a; Mezzacappa et al., 1997), all with the aim of regulating or coping with these physiological states. Research to date has supported such symmetrical models of psychopathology where understimulation in both sympathetic and cortisol response (Gordis, Granger, Susman, & Trickett, 2006) and overstimulation in both sympathetic and cortisol response (El-Sheikh, Erath, Buckhalt, Granger, & Mize, 2008) were associated with higher rates of behavior problems.
The second model proposed by Bauer and colleagues is described as an interactive model where a response to stress is observed in one system and an understimulated or blunted response is observed in another. The interactive model is particularly important as activation in one system combined with a blunted response in another may represent global impairment in the body’s physiological response to stress, thereby limiting an individual’s physiological resources to effectively cope with the demands of a stressor. While the exact physiological mechanisms responsible for an asymmetric response remain unknown, there are several findings that shed light on why an asymmetrical response pattern may occur in maltreated individuals. First, the attenuation hypothesis (Susman, 2006) suggests that after a period of hypersecretion of cortisol in the HPA axis, this system down-regulates cortisol via decreased biosynthesis of hormones in the HPA axis, down-regulation of pituitary receptors, and increased negative feedback sensitivity (Fries, Hesse, Hellhammer, & Hellhammer, 2005; Heim, Ehlert, & Hellhammer, 2000). While severe or chronic stress may induce potential changes in shared brain structures, such as the hypothalamus and amygdala, across the HPA axis and ANS, this same attenuation process may not occur in the ANS. For instance, ANS functioning had continued sensitivity to repeated exposure to a laboratory stressor whereas HPA axis functioning declined over time (Schommer, Hellhammer, & Kirschbaum, 2003). Thus, ANS and HPA axis functioning may exhibit differential patterns of habituation to stress in that ANS response remains sensitive to stressors whereas cortisol response attenuates over time. Although previous research has linked ANS and HPA axis functioning to various forms of psychopathology (Pajer et al., 2001; Sack et al., 2004), the extent to which this asymmetrical pattern is related to levels of psychopathology, above and beyond single system indicators, is unknown.
As such, this study is an empirical test of Bauer et al.’s (2002) interactive model and its utility in making predictions about subsequent psychopathology. In the current study, an asymmetrical response profile was defined as consisting of a withdrawal of vagal influence over cardiac activity and a blunted cortisol response to a laboratory stressor. This type of asymmetrical response is similar to previous research in that it assesses cortisol reactivity as an indication of HPA axis activity while differing by using vagal withdrawal, instead of salivary alpha-amylase or skin conductance, as an indication of ANS activity. Vagal withdrawal may be a particularly beneficial index as it is purported to measure the physiological regulation of stress and emotions (Porges, 2003). To date, much of the literature explicating the relationship between childhood maltreatment and psychopathology has used retrospective methods in cross-sectional designs. However, prospective assessment of childhood maltreatment in a repeated measures design with an appropriate non-abused comparison condition adds needed experimental rigor and control that can strengthen conclusions about the long-term effects of maltreatment on relevant psychological and physiological outcomes. The current study is one such attempt to assess the effects of an asymmetrical physiological response to stress as a potential biobehavioral pathway to the development of subsequent psychopathology in sexually abused and comparison females followed prospectively for 13 years. By homogenizing the maltreatment experience of our sample, generalization to the experience of sexual abuse can be more readily made, whereas previous studies have included a conglomeration of maltreatment types without parsing the effects of one form of maltreatment over the others. However, because multiple forms of maltreatment tend to co-occur and since there is not ample sampling of alternative forms of abuse in the current analysis, inferences are made about the potential for asymmetry in a group of females with truncated variability with regard to maltreatment type. The 2 main aims were as follows: 1) determine whether sexual abuse in childhood predicted adolescent ANS/HPA asymmetry, specifically parasympathetic withdrawal combined with blunted cortisol reactivity, during a laboratory stressor paradigm, and 2) assess whether this asymmetrical response predicted higher levels of depressive symptoms and antisocial behaviors in young adulthood.
Method
Sample
Participants (N = 187) were enrolled in a longitudinal study examining the developmental effects of childhood sexual abuse. Sexually abused participants were referred from child protective service (CPS) agencies in the Washington, DC metropolitan area and were required to meet the following criteria: (a) CPS-substantiated contact sexual abuse including genital contact and/or penetration; (b) enrollment in the study within 6 months of the disclosure of abuse; (c) perpetration by a family member; (d) participation of a non-abusing caregiver in the research study; and (e) participant age ranging between 6–16 years. Comparison participants were recruited through community advertisements and screened for prior CPS involvement. Comparison participants with a documented history of sexual abuse were not included in the study. Sexually abused and comparison participants were similar on age, race, socioeconomic status (SES), family constellation (1 or 2 parent households), and residing zip codes. See Table 1 for demographic information across abused and comparison participants.
Table 1.
Demographic Information and Means for Main Effect Variables Across Sexually Abused and Comparison Females.
| Sexually Abused Mean (SD) or n | Comparison Mean (SD) or n | |
|---|---|---|
| Late Adolescence (N=144) | ||
| Age | 18.54 (3.62) | 17.76 (3.34) |
| Race | ||
| White | 36 | 42 |
| African-American | 20 | 40 |
| Asian | 1 | 0 |
| Hispanic | 3 | 2 |
| SES | 37.65 (12.25) | 37.49 (11.17) |
| AUC | 0.01 (.16) | 0.09 (.26)* |
| Vw | 0.09 (.60) | −0.02 (.51) |
| YSR – Internalizing | 17.14 (7.92) | 15.08 (7.85) |
| YSR – Externalizing | 18.46 (8.98) | 15.08 (9.37)* |
| Young Adulthood (N =129) | ||
| Age | 24.94 (3.46) | 24.13 (3.06) |
| BDI | 11.47 (10.82) | 9.30 (7.24) |
| ABF | 26.64 (30.56) | 29.03 (27.89) |
= p < .05. SES = Hollingshead ratings of socioeconomic status; AUC = Area under the curve for cortisol reactivity; Vw = Vagal withdrawal; YSR = Youth Self-report; BDI = Beck Depression Inventory; ABF = Antisocial Behavior Form.
The sample was assessed at 6 time points throughout development. Data for the current set of analyses were gleaned from 3 of these time-points: the first assessment (childhood) when sexual abuse status was determined and the mean age was 11.06 (SD = 2.98), the fourth assessment (late adolescence) when measurement of physiological response to stress was assessed and the mean age was 18.09 (SD = 3.47), and the sixth assessment (young adulthood) when the most recent assessment of psychopathology was obtained and the mean age was 24.46 (SD = 3.24).
Of the 187 participants with a designated sexual abuse status (abused = 1, comparison = 0), 163 attended the late adolescence assessment. Thirteen of these participants were excluded because they were originally assigned to the comparison group but later disclosed experiencing an instance of sexual abuse. Six additional participants were excluded because they had cortisol sample concentration three standard deviations above the sample mean. Hence, the total sample size available at the late adolescence assessment was 144. Of these 144 subjects, 129 (abused: n = 52, non-abused: n = 77) attended the young adulthood assessment.
Procedure
All procedures for this study were approved by local institutional review boards where the data were collected. Parents/legal guardians provided consent for participants under the age of 18. Participants over the age of 18 provided their own consent. Data on each participant’s sexual abuse status was obtained from official protective service records at the childhood assessment and retained for subsequent data analysis.
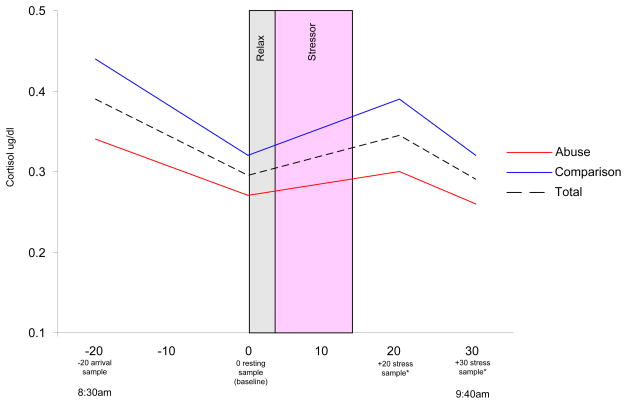
All physiological data obtained in this study were gathered during the late adolescence assessment. All late adolescence assessments began at approximately 8:30 am. Once consent/assent was obtained, participants provided an initial saliva sample (−20 minute sample). Twenty minutes later a second saliva sample was obtained and used as a baseline sample (0 minute sample). Participants then started an electrocardiogram (ECG) in order to track peak R waves and inter-beat intervals during both a relaxation condition and a subsequent cognitive stressor (see Figure 1). The relaxation period involved participants sitting quietly while listening to music (Enya) via headphones and viewing a computer screen-saver (Afterdark™ fish screen saver) for 5 minutes. After this relaxation period, participants completed a 10-minute cognitive stressor (a timed, mental rotation task) requiring the accurate determination of whether two rotated, novel stimuli were the same objects or whether one was an inverted image of the other. Incorrect responses were followed by a loud, aversive noise. Five ceiling items were imbedded at the end of the task to ensure that all participants endured some incorrect responses. Because this study was examining a vulnerable population, extreme care was taken to ensure no one person had any significant or lingering negative effects due to completion of the stressor. Trained interviewers assessed participant’s reaction during and after the stressor to ensure no one was significantly or psychologically distressed. No participant reported any discomfort by completing the cognitive stressor. Participants then provided a third (+20 minute sample) and fourth (+30 minute sample) saliva sample 20 and 30 minutes, respectively, after their 0 minute baseline sample (see Figure 1).
Figure 1.
Procedures and cortisol response by abuse status and combined total.
* = Adjusted for Diurnal Decline.
Psychological functioning was assessed at both the late adolescence and young adult assessments. Measures of internalizing and externalizing behaviors were administered at the late adolescence assessment as part of a battery of self-report questionnaires. Similarly, measures of depression and antisocial behaviors were administered at the young adulthood assessment.
Measures
Physiological
Cortisol
All saliva samples were stored at −70°C until they were assayed in duplicate using a highly-sensitive enzyme immunoassay by Salimetrics Laboratories (State College, PA). The test used 25 ml of saliva per determination, has a lower limit sensitivity of .003 μg/dl, standard curve range from .007–1.8 μg/dl, and average intra- and inter-assay coefficients of variation 5.10% and 8.20%, respectively. Cortisol concentrations for each saliva sample are represented in μg/dl. Area under the curve (AUC; Pruessner, Kirschbaum, Meinlschmid, & Hellhammer, 2003) was used to assess cortisol reactivity by abuse status for samples 0, +20, and +30 minutes samples. Consistent with the diurnal profile or morning samples of cortisol (Rosemalen et al., 2005; Susman et al., 2007), a significant decline in cortisol concentrations occurring between the −20 sample and the 0 sample twenty minutes later, t141 = 6.49, p < .001, was found. Because the late adolescence assessments occurred in the morning hours, it was necessary to adjust the post-stressor samples in order to discern the true impact of the stressor in light of this naturally occurring diurnal decline. To characterize each individual’s rate of diurnal decline, values obtained at sample 0 were subtracted from the −20 sample. This difference score was indicative of participants’ 20 minute rate of decline (D). This number was then used to adjust the +20 and +30 samples taken after the cognitive stressor. Since the +20 sample occurred 20 minutes after the baseline sample, each individual’s D was added to the +20 sample. The +30 sample occurred 10 minutes subsequent to the +20 sample so the +30 sample was adjusted according to a 10 minute diurnal decline rate (or D/2). Values used in the AUC equation consisted of sample 0, the adjusted +20 sample, and the adjusted +30 sample (See Figure 1). Two participants had missing data on cortisol concentrations and were excluded from analyses resulting in a total available sample size for AUC analyses of 142.
Vagal tone and vagal withdrawal
Electrodes were placed on the chest and abdomen of each participant during the ECG. R waves and inter-beat intervals (IBI) were recorded using the MXEdit 2.01 Vagal Tone Monitor (Delta-Biometrics, Bethesda, MD). Artifacts were visually inspected and edited by one of the authors (J. N.) who was a certified vagal tone editor before IBI data were analyzed. Vagal tone was obtained using a moving polynomial method that derived an estimate of the respiratory sinus arrhythmia in each respective experimental condition based on 30 second epochs of IBI data. The resulting metric is an indicator of vagal influence over cardiac activity for each participant in the five-minute relaxation period (Vrelax) and the ten-minute cognitive stressor (Vstressor). Vagal withdrawal (Vw) was quantified as the change in vagal tone from the relaxation condition to the cognitive stressor condition (Vw = Vrelax − Vstressor). This difference score serves as an indication of the reduction in parasympathetic influence over cardiac activity experienced as a result of the stressor. Cardiac data collected at the late adolescence assessment was lost on 31 participants due to computer failure. This reduced the total number of subjects with available Vw analyses to 113.
Asymmetrical response profile
Consistent with previous literature (Hart et al., 1995; Sack et al., 2004), an indicator of asymmetrical stress response was created using median splits of both Vw and cortisol AUC response. Participants (n = 28) who experienced an above the median value of Vw and below the median value of AUC received a score of “1,” indicating they had an asymmetric physiological response to stress. All others (n = 84) were given a score of “0” on this variable.
Psychosocial
Youth Self-Report
The Youth Self-Report (YSR; Achenbach, 1991) is a widely used self-report measure assessing a broad range of individual functioning categorized into 2 broad dimensions: 1) internalizing behaviors such as anxiety, depression, and withdrawal, and 2) externalizing behaviors such as aggression, hyperactivity, and delinquency. The YSR has shown excellent internal consistency (Cronbach’s α = .95) and discrimination between clinical and non-clinical populations (Achenbach, 1991). Internal consistency estimates for the Internalizing and Externalizing scales at the late adolescence assessment are α = .85 and α = .88, respectively. The YSR was administered at the late adolescence assessment to determine self-reported levels of internalizing and externalizing behaviors.
Beck Depression Inventory-II
The Beck Depression Inventory-II (BDI; Beck, Steer, & Brown, 1996) is a 21-item measure assessing symptoms of major depressive disorder according to the Diagnostic and Statistical Manual for Mental Disorders-IV (American Psychiatric Association, 1994). The BDI-II is a widely-used measure with excellent internal consistency (α = .93) and good concurrent validity (r = .71) with other measures of depression (Beck, Steer, Ball, & Ranieri, 1996; Beck, Steer, & Brown, 1996). Internal consistency of the BDI at the young adulthood assessment was excellent (α = .90) with 76% of respondents in the minimally depressed range, 13% in the mildly depressed range, 6% in the moderately depressed range, and 6% in the severely depressed range. The BDI-II was administered to assess mood functioning in the 2 weeks prior to the young adulthood assessment.
Antisocial Behaviors Form
The Antisocial Behaviors Form (ABF; Noll, 2006) was created for purposes of this study and is a self-report measure assessing symptoms of antisocial personality disorder according to the DSM-IV. The ABF consists of participation in 22 antisocial acts involving unlawful behavior, physical aggression and deceit since the age of 18. Participants indicate the frequency in which they have engaged in specific antisocial behaviors on a seven-point Likert scale ranging from “Never” (0) to “More than 10 times” (6). Example items include “Have you purposely destroyed or defaced property?” and “Have you harassed or badgered others with the intent of causing them significant distress?” Items are then weighted by the level of remorse expressed after engaging in an antisocial act from ranging from “Very sorry” (0) to “Not sorry” (4). Higher scores on the ABF indicate that a participant engaged in an antisocial act and a lack of remorse felt after engaging in the act, a hallmark feature of antisocial personality disorder. The ABF at the young adult assessment had good internal consistency (Cronbach’s α = .87) and convergent validity (r = .32 – .42) with other measures of aggressive behaviors (e.g., Margolin, Burman, John, & O’Brien, 2000).
Results
As can be seen in Table 1, sexually abused and comparison participants were similar on many variables measured at the late adolescence and young adulthood assessments. For instance, there were no significant differences on age, race, or socioeconomic status between the two groups. However, there were significant differences observed between groups at the late adolescence assessment on mean AUC, F(1, 140) = 3.99, p < .05, and self-reported externalizing behaviors, F(1, 132) = 4.41, p < .05. These differences indicate that participants with a history of child sexual abuse were significantly more likely to have a blunted cortisol AUC response (see also Figure 1) and higher levels of externalizing behaviors when compared to participants without such a history. There were no significant differences between abused and comparison participants on age or scores on the BDI and ABF at the young adulthood assessment.
Structural Equation Modeling via LISREL v8.54 (Joreskog & Sorbom, 1993; Joreskog & Sorbom, 2001) was used to test a comprehensive, longitudinal model estimating the predictive relationships among childhood sexual abuse, asymmetrical physiological response in adolescence, and levels of depression and antisocial behaviors in young adulthood while controlling for the effects of age, minority status, and previous levels of internalizing and externalizing behaviors. This model also included Vw and AUC values as main effect predictors of depression and antisocial behaviors to further evaluate the contribution of an asymmetric response profile above and beyond the main effects of each individual physiological system. Finally, previous literature has indicated that resting values on physiological measures are predictive of levels of reactivity during stressor paradigms (Benjamin, 1963). To address this issue, the structural model included Vrelax in order to control for the influence of initial values of vagal tone on an individual’s Vw during the stressor. Zero-order inter-correlations between all variables included in the structural model are included in Table 2. The longitudinal structural model is presented in Figure 2. Factor loadings for single-indicator variables were fixed to one and error paths were set to zero. To approximate latent space for variables where reliability estimates were obtainable (Raykov, 1997), factor loading paths were fixed to α and error terms were fixed to 1 – α.
Table 2.
Zero-order Inter-correlations among Variables Included in Structural Model1
| Variable | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Age | 1.00 | ||||||||||
| 2. Minority | −.24** | 1.00 | |||||||||
| 3. Abuse | .12 | .10 | 1.00 | ||||||||
| 4. Internalizing | .05 | −.02 | .13 | 1.00 | |||||||
| 5. Externalizing | −.12 | .15 | .18* | .60*** | 1.00 | ||||||
| 6. AUC | .09 | .10 | −.17* | .06 | .14 | 1.00 | |||||
| 7. Vrelax | −.16 | −.13 | .01 | −.19* | −.04 | −.14 | 1.00 | ||||
| 8. Vw | −.05 | .18* | .09 | .10 | .00 | .04 | .20* | 1.00 | |||
| 9. Asymmetry | .06 | .08 | .24** | .10 | −.03 | −.32*** | .15 | .42*** | 1.00 | ||
| 10. BDI | .06 | .02 | .12 | .30*** | .22* | .08 | .07 | .13 | .19 | 1.00 | |
| 11. ABF | .00 | .14 | −.04 | .03 | .29*** | −.01 | .07 | −.04 | .19 | .57 | 1.00 |
Minority: White=0, Minority=1; Abuse: Comparison=0, Sexually Abused=1; AUC: Area under the curve for cortisol reactivity; Vrelax = Vagal tone during relaxation condition; Vw = Vagal withdrawal; BDI = Beck Depression Inventory; ABF = Antisocial Behavior Form.
= Point-biserial correlations presented for dichotomous and continuous variables; Phi coefficients presented for correlations between dichotomous variables; Pearson’s Product Moment presented for correlations between continuous variables.
= p < .001;
= p < .01;
= p < .05.
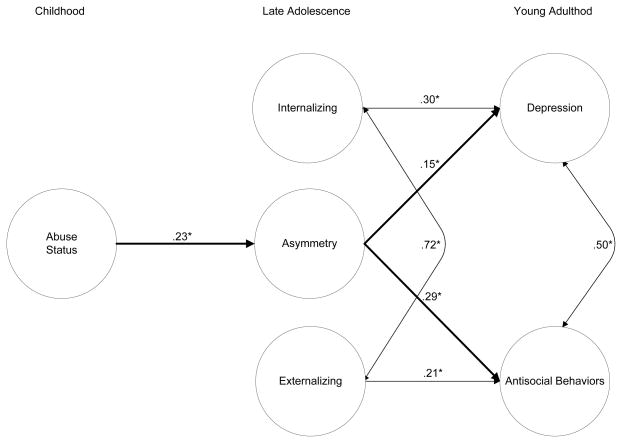
Figure 2.
Structural equation model for sexual abuse status, physiological asymmetry, and depressive and antisocial functioning. χ2(18) = 46.87, p < .001; GFI = .94; RMR = .07. Age, minority status, vagal tone during relaxation, and main effects for vagal withdrawal and area under the curve for cortisol were included as covariates in this model. Correlations among exogenous variables were freely estimated (see Table 2). Estimates are based on pairwise associations generated via PRELIS with N’s as follows: Internalizing and Externalizing (N = 142), Asymmetry (N=113) and Depression and Antisocial Behaviors (N = 129).
Overall, the model depicted in Figure 2 fit the data well with a strong goodness of fit index (GFI = .94) and low residual values (RMR = .07). Although these are not shown in Figure 2, correlations among exogenous variables were allowed to freely correlate (see zero-order estimates in Table 2). Examination of the model revealed that sexual abuse during childhood significantly predicted an asymmetrical physiological response to a novel stressor at the late adolescence assessment, β = .23, p < .05. This result suggests that sexual abuse in childhood was closely associated with a simultaneous vagal withdrawal and blunted cortisol AUC response to a cognitive stressor 7 years after the documented sexual abuse. This result held when main effects for Vw and AUC were also included in the model (not shown in Figure 2).
Results also show that an asymmetric response profile assessed in adolescence significantly predicted higher scores on the BDI, β = .15, p < .05, and on the ABF, β = .29, p < .05, in young adulthood while controlling for prior levels of internalizing and externalizing behaviors. These results lend support to the assessment of both vagal and cortisol responses to stress over individual systems alone and that, via the pathway through this asymmetry, childhood sexual abuse victims are at risk for subsequent psychopathology in the forms of depression and antisocial behaviors in young adulthood.
Discussion
Based on recent calls for the assessment of multiple physiological systems to help explain the development of psychopathology (Bauer et al., 2002), the purpose of this study was to test a biobehavioral model examining the influence of an asymmetrical physiological stress response informed by previous research linking vagal withdrawal and blunted cortisol responses with internalizing and externalizing behaviors. To test this model in a prospective manner, these processes were assessed in a sample of females who experienced substantiated childhood sexual abuse and were followed longitudinally through adolescence and into young adulthood. This design allowed for predictions about the effects of childhood sexual abuse on late adolescent asymmetrical physiological response and, in turn, the effects of an asymmetrical response on the development of psychopathology in young adulthood. This was accomplished in a comprehensive statistical model that controlled for age, minority status, prior internalizing and externalizing behaviors, resting vagal tone, and the main effect contributions of each physiological system. This hypothesized model fit the data well suggesting that the study of asymmetrical biomarkers as significant determinants of psychopathology in abused populations might hold promise.
These findings indicated that childhood sexual abuse significantly predicted an asymmetrical physiological response to a novel laboratory stressor approximately 6 years after the disclosure of the abuse incident. This finding seems particularly important in that it supports models that have examined the impact of severe and chronic stress on the functioning of physiological systems. In light of previous research (Carpenter et al., 2007; Hart et al., 1995), the finding that abuse is related to lower overall cortisol reactivity is not particularly surprising nor is it particularly additive to current literature. It has been proposed that this impairment may come about through a process where the HPA axis adapts to sustained periods of cortisol hypersecretion by down-regulating cortisol secretion following a stressor (Susman, 2006). The resulting hyposecretion is proposed to have an adaptive function physiologically as prolonged exposure to cortisol has deleterious effects on brain structures such as the hippocampus and frontal cortex as well as cardiovascular and immunological functioning (Bremner & Vermetten, 2001; De Bellis & Duchibhatla, 2006; McEwen & Wingfield, 2003; Raison & Miller, 2003; Sapolsky, Romero, & Munck, 2000). Hence, the attenuation hypothesis (Susman, 2006) and the concept of allostatic load (McEwen, 2007) assert that organisms strive to regulate physiological response to prolonged stress to prevent physical harm to the organism, thereby resulting in a blunting of any one particular system such as the HPA axis.
However, the results also suggest that a blunting of the cortisol response, in simultaneous combination with vagal withdrawal, may be a particularly problematic stress-system combination in light of future psychological development. The results from this study showed that an asymmetrical physiological response of vagal withdrawal and blunted cortisol AUC response in late adolescence was predictive of higher levels of depressive symptoms and antisocial behaviors in young adulthood even after controlling for demographic variables, prior psychological functioning, and the main effect contributions of each individual physiological system. Thus, even though the blunting of one particular system may hold benefit for certain organic structures and functions in terms of allostatic load, it may in fact increase one’s risk of psychopathology. This finding is particularly salient as our index of asymmetry predicted both internalizing (depression) and externalizing (antisocial) behaviors suggesting that the assessment of both ANS and HPA activity simultaneously adds to our ability to predict psychopathology more generally. This is important due to the fact that internalizing and externalizing behaviors often co-occur and the etiologies as well as the developmental consequences of the two are often difficult to disentangle. Additional research in this area is needed to further explicate such varying effects of HPA impairment and associated ANS responses.
The implications of this study are considered in light of several limitations. First, though the effects of childhood sexual abuse are likely considerable for both males and females, we were not able to address this since our sample consists entirely of females. Future research with male populations will clarify whether or not an asymmetrical profile occurs for maltreated males as well as females and if this profile is linked to levels of psychopathology. Second, the current study used a sample of females who experienced childhood sexual abuse. Although documented cases of childhood sexual abuse were used, sexual abuse in childhood often co-occurs with other forms of maltreatment and thus the present analysis cannot rule out whether the results are due solely to childhood sexual abuse or a combination of maltreatment experiences. Third, median splits were used to create the index of asymmetry used in this study. While median splits are likely the most precise test of Bauer et al.’s (2002) interactive model, the use of median splits does regard someone with a score near the median in the same category as someone well above or below the median. Clear clinical cutoffs would be an ideal means for categorizing asymmetrical response; however, in the absence of such cutoffs, median splits represent an appropriate method for the current study without an apparent limitation on power. Fourth, only one asymmetrical response model was tested in this study.
Though previous literature and theory led to the current definition of physiological asymmetry, it is possible that other physiological response profiles hold as much potential in explaining the development of psychopathology. As mentioned earlier, there are studies that have documented the effects of a symmetrical response profile in predicting poorer psychological outcomes (El-Sheikh et al., 2008). It may also be true that other profiles of asymmetrical response, such as low sympathetic response and high cortisol response, predict future psychopathology. Future research in biobehavioral markers of risk for psychopathology will benefit from improved methodology that consists of simultaneous assessment of multiple physiological systems in prospective, longitudinal designs.
Overall, this study adds to a growing literature demonstrating that the assessment of interrelated physiological systems such as the ANS and HPA adds a unique contribution to the prediction of psychological functioning above and beyond the impact of any individual system alone. One implication of these findings is that the assessment of both ANS and HPA responses to stress might be important in the comprehensive understanding of subsequent psychopathology. Assessing both systems allows for an opportunity to determine the interactive effects of these systems thus enhancing predictive models of biobehavioral pathways explaining the development of psychopathology.
Results from this study suggest that sexual abuse may increases risk for displaying an asymmetrical stress response profile which, in turn, may place victims at risk for future psychopathology. Practitioners who work with these victims should be aware that abuse survivors might experience a range of clinical phenomena including anxiety, depression, aggression, antisocial behaviors and that these conditions and their associated symptoms may represent alternative means of coping with physiologic stressors whether they are the stressors of every day life or the stressors of traumatic reminders. Along with the assessment, monitoring and treatment of victims’ mood and behavioral functioning, it is recommended that treatment of childhood abuse might also focus on developing skills for coping with common stressors as well as traumatic reminders. Monitoring and treatment should be extended, or at least revisited, throughout development as traumatic reminders often appear at various points in development as issues reminiscent of the initial trauma become developmentally salient (Finkelhor & Browne, 1985). This may be particularly important during the adolescent years when issues surrounding dating, romantic attachments, and coital initiation become important developmental milestones that require adaptive coping. This is also a period when patterns of alcohol and substance usage are adopted and can serve to ward off the anxiety symptoms associated with traumatic reminders. Such usage can facilitate or lead to a host of behaviors that are precursors to later problematic and antisocial behaviors. Hence, comprehensive, developmental approaches to treatment may help prevent maladaptive coping that is indicative of psychopathology.
Acknowledgments
This research was supported by the National Institutes of Health (R01 MH048330; R03 HD045346; T32DK063929), Department of Health and Human Services (ACYF 90CA1686l), W. T. Grant Foundation, and Smith Richardson Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Achenbach T. Manual for the youth self-report and 1991 profile. Burlington, VT: University of Vermont; 1991. [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4. Washington, DC: American Psychiatric Press; 1994. [Google Scholar]
- Bauer AM, Quas JA, Boyce WT. Associations between physiological reactivity and children’s behavior: Advantages of a multisystem approach. Journal of Developmental & Behavioral Pediatrics. 2002;23(2):102–113. doi: 10.1097/00004703-200204000-00007. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Ball R, Ranieri WF. Comparison of Beck Depression Inventories-IA and -II in Psychiatric Outpatients. Journal of Personality Assessment. 1996;67(3):588. doi: 10.1207/s15327752jpa6703_13. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Brown GK. Manual for the Beck Depression Inventory-II. San Antonio, TX: Psychological Corporation; 1996. [Google Scholar]
- Benjamin LS. Statistical treatment of the law of initial values (LIV) in autonomic research: A review and recommendation. Psychosomatic Medicine. 1963;25(6):556–566. doi: 10.1097/00006842-196311000-00005. [DOI] [PubMed] [Google Scholar]
- Binder RL, McNiel DE, Goldstone RL. Is adaptive coping possible for adult survivors of childhood sexual abuse? Psychiatric Services. 1996;47(2):186–188. doi: 10.1176/ps.47.2.186. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Vermetten E. Stress and development: behavioral and biological consequences. Development and Psychopathology. 2001;13(3):473–489. doi: 10.1017/s0954579401003042. [DOI] [PubMed] [Google Scholar]
- Cacioppo JT. Social neuroscience: Autonomic, neuroendocrine, and immune responses to stress. Psychophysiology. 1994;31(2):113–128. doi: 10.1111/j.1469-8986.1994.tb01032.x. [DOI] [PubMed] [Google Scholar]
- Carpenter LL, Carvalho JP, Tyrka AR, Wier LM, Mello AF, Mello MF, Anderson GM, Wilkinson CW, Price LH. Decreased adrenocorticotropic hormone and cortisol responses to stress in healthy adults reporting significant childhood maltreatment. Biological Psychiatry. 2007;62(10):1080–1087. doi: 10.1016/j.biopsych.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrion VG, Weems CF, Ray RD, Glaser B, Hessl D, Reiss AL. Diurnal salivary cortisol in pediatric posttraumatic stress disorder. Biological Psychiatry. 2002;51(7):575–582. doi: 10.1016/s0006-3223(01)01310-5. [DOI] [PubMed] [Google Scholar]
- Chrousos GP, Gold PW. The concepts of stress and stress system disorders. Overview of physical and behavioral homeostasis. The Journal Of The American Medical Association. 1992;267(9):1244–1252. [PubMed] [Google Scholar]
- Cicchetti D, Rogosch F. The impact of child maltreatment and psychopathology on neuroendocrine functioning. Development and Psychopathology. 2001a;13(4):783–804. [PubMed] [Google Scholar]
- Cicchetti D, Rogosch FA. Diverse patterns of neuroendocrine activity in maltreated children. Development and Psychopathology. 2001b;13(3):677–693. doi: 10.1017/s0954579401003145. [DOI] [PubMed] [Google Scholar]
- De Bellis MD, Duchibhatla M. Cerebellar volumes in pediatric maltreatment-related posttraumatic stress disorder. Biological Psychiatry. 2006;60:697–703. doi: 10.1016/j.biopsych.2006.04.035. [DOI] [PubMed] [Google Scholar]
- DeBellis MD, Baum AS, Birmaher B, Keshavan MS, Eccard CH, Boring AM, Jenkins FJ, Ryan ND. Developmental traumatology. Part I: Biological stress systems. Biological Psychiatry. 1999;45(10):1259–1270. doi: 10.1016/s0006-3223(99)00044-x. [DOI] [PubMed] [Google Scholar]
- DeBellis MD, Chrousos GP, Dorn LD, Burke L, Helmers K, Kling MA, Trickett PK, Putnam FW. Hypothalamic-pituitary-adrenal axis dysregulation in sexually abused girls. Journal of Clinical Endocrinology and Metabolism. 1994;78(2):249–255. doi: 10.1210/jcem.78.2.8106608. [DOI] [PubMed] [Google Scholar]
- Delahanty DL, Nugent NR, Christopher NC, Walsh M. Initial urinary epinephrine and cortisol levels predict acute PTSD symptoms in child trauma victims. Psychoneuroendocrinology. 2005;30(2):121–128. doi: 10.1016/j.psyneuen.2004.06.004. [DOI] [PubMed] [Google Scholar]
- Dietrich A, Riese H, Sondeijker F, Greaves-Lord K, van Roon AM, Ormel J, Neeleman J, Rosmalen JG. Externalizing and internalizing problems in relation to autonomic function: A population-based study in preadolescents. Journal of the American Academy of Child & Adolescent Psychiatry. 2007;46(3):378–386. doi: 10.1097/CHI.0b013e31802b91ea. [DOI] [PubMed] [Google Scholar]
- El-Sheikh M, Erath SA, Buckhalt JA, Granger DA, Mize J. Cortisol and children’s adjustment: The moderating role of sympathetic nervous system activity. Journal of Abnormal Child Psychology. 2008;36(4):601–611. doi: 10.1007/s10802-007-9204-6. [DOI] [PubMed] [Google Scholar]
- Finkelhor D, Browne A. The traumatic impact of child sexual abuse: A conceptualization. American Journal of Orthopsychiatry. 1985;55(4):530–541. doi: 10.1111/j.1939-0025.1985.tb02703.x. [DOI] [PubMed] [Google Scholar]
- Fries E, Hesse J, Hellhammer J, Hellhammer DH. A new view on hypocortisolism. Psychoneuroendocrinology. 2005;30(10):1010–1016. doi: 10.1016/j.psyneuen.2005.04.006. [DOI] [PubMed] [Google Scholar]
- Goodyer I, Herbert J, Moor S, Altham P. Cortisol hypersecretion in depressed school-aged children and adolescents. Psychiatry Research. 1991;37(3):237–244. doi: 10.1016/0165-1781(91)90060-3. [DOI] [PubMed] [Google Scholar]
- Gordis EB, Granger DA, Susman EJ, Trickett PK. Asymmetry between salivary cortisol and alpha-amylase reactivity to stress: relation to aggressive behavior in adolescents. Psychoneuroendocrinology. 2006;31(8):976–987. doi: 10.1016/j.psyneuen.2006.05.010. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, Vazquez DM. Low cortisol and a flattening of expected daytime rhythm: potential indices of risk in human development. Development and Psychopathology. 2001;13(3):515–538. doi: 10.1017/s0954579401003066. [DOI] [PubMed] [Google Scholar]
- Hart J, Gunnar M, Cicchetti D. Salivary cortisol in maltreated children: Evidence of relations between neuroendocrine activity and social competence. Development and Psychopathology. 1995;7(1):11–26. [Google Scholar]
- Heim C, Ehlert U, Hellhammer DH. The potential role of hypocortisolism in the pathophysiology of stress-related bodily disorders. Psychoneuroendocrinology. 2000;25(1):1–35. doi: 10.1016/s0306-4530(99)00035-9. [DOI] [PubMed] [Google Scholar]
- Heim C, Newport DJ, Heit S, Graham YP, Wilcox M, Bonsall R, Miller AH, Nemeroff CB. Pituitary-adrenal and autonomic responses to stress in women after sexual and physical abuse in childhood. Journal of the American Medical Association. 2000;284(5):592–597. doi: 10.1001/jama.284.5.592. [DOI] [PubMed] [Google Scholar]
- Joreskog KG, Sorbom D. LISREL 8: Structural equation modeling with the SIMPLIS command language. Chicago, IL, Hillsdale, NJ, USA; England: Scientific Software International Lawrence Erlbaum Associates, Inc; 1993. [Google Scholar]
- Joreskog KG, Sorbom D. LISREL 8: User’s reference guide. Lincolnwood, IL: Scientific Software International, Inc; 2001. [Google Scholar]
- Kaufman J, Birmaher B, Perel J, Dahl RE, Moreci P, Nelson B, Wells W, Ryan ND. The corticotropin-releasing hormone challenge in depressed abused, depressed nonabused, and normal control children. Biological Psychiatry. 1997;42(8):669– 679. doi: 10.1016/s0006-3223(96)00470-2. [DOI] [PubMed] [Google Scholar]
- Kendall-Tackett KA, Williams LM, Finkelhor D. Impact of sexual abuse on children: A review and synthesis of recent empirical studies. Psychological Bulletin. 1993;113(1):164–180. doi: 10.1037/0033-2909.113.1.164. [DOI] [PubMed] [Google Scholar]
- King JA, Mandansky D, King S, Fletcher KE, Brewer J. Early sexual abuse and low cortisol. Psychiatry and Clinical Neurosciences. 2001;55(1):71–74. doi: 10.1046/j.1440-1819.2001.00787.x. [DOI] [PubMed] [Google Scholar]
- Luecken LJ. Childhood attachment and loss experiences affect adult cardiovascular and cortisol function. Psychosomatic Medicine. 1998;60(6):765–772. doi: 10.1097/00006842-199811000-00021. [DOI] [PubMed] [Google Scholar]
- Margolin G, Burman B, John RS, O’Brien M. Domestic Conflict Index. Los Angeles, CA: University of Southern California; 2000. [Google Scholar]
- McEwen BS. Physiology and neurobiology of stress and adaptation: Central role of the brain. Physiological Reviews. 2007;87(3):873–904. doi: 10.1152/physrev.00041.2006. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Wingfield JC. The concept of allostasis in biology and biomedicine. Hormones and Behavior. 2003;43(1):2–15. doi: 10.1016/s0018-506x(02)00024-7. [DOI] [PubMed] [Google Scholar]
- Mezzacappa E, Tremblay RE, Kindlon D, Saul JP, Arseneault L, Seguin J, Pihl RO, Earls F. Anxiety, antisocial behavior, and heart rate regulation in adolescent males. Journal Of Child Psychology And Psychiatry, And Allied Disciplines. 1997;38(4):457–469. doi: 10.1111/j.1469-7610.1997.tb01531.x. [DOI] [PubMed] [Google Scholar]
- Munck A, Guyre PM, Holbrook NJ. Physiological functions of glucocorticoids in stress and their relation to pharmacological actions. Endocrinology Review. 1984;5(1):25–44. doi: 10.1210/edrv-5-1-25. [DOI] [PubMed] [Google Scholar]
- Noll J. Antisocial Behavior Form. Cincinnati, OH: Cincinnati Children’s Hospital Medical Center; 2006. [Google Scholar]
- Orr SP, Lasko NB, Metzger LJ, Berry NJ, Ahern CE, Pitman RK. Psychophysiologic assessment of women with posttraumatic stress disorder resulting from childhood sexual abuse. Journal of Consulting and Clinical Psychology. 1998;66(6):906–913. doi: 10.1037//0022-006x.66.6.906. [DOI] [PubMed] [Google Scholar]
- Pajer K, Gardner W, Rubin RT, Perel J, Neal S. Decreased cortisol levels in adolescent girls with conduct disorder. Archives of General Psychiatry. 2001;58(3):297–302. doi: 10.1001/archpsyc.58.3.297. [DOI] [PubMed] [Google Scholar]
- Paolucci EO, Genuis ML, Violato C. A meta-analysis of the published research on the effects of child sexual abuse. Journal of Psychology. 2001;135(1):17–36. doi: 10.1080/00223980109603677. [DOI] [PubMed] [Google Scholar]
- Perry BD, Murburg MM. Catecholamine function in posttraumatic stress disorder: Emerging concepts. Washington, DC: American Psychiatric Association; 1994. Neurobiological sequelae of childhood trauma: PTSD in children; pp. 233–255. [Google Scholar]
- Pfeffer CR, Altemus M, Heo M, Jiang H. Salivary cortisol and psychopathology in children bereaved by the September 11, 2001 terror attacks. Biological Psychiatry. 2007;61(8):957–965. doi: 10.1016/j.biopsych.2006.07.037. [DOI] [PubMed] [Google Scholar]
- Porges SW. The Polyvagal Theory: Phylogenetic contributions to social behavior. Physiology & Behavior. 2003;79(3):503–513. doi: 10.1016/s0031-9384(03)00156-2. [DOI] [PubMed] [Google Scholar]
- Pruessner JC, Kirschbaum C, Meinlschmid G, Hellhammer DH. Two formulas for computation of the area under the curve represent measures of total hormone concentration versus time-dependent change. Psychoneuroendocrinology. 2003;28(7):916–931. doi: 10.1016/s0306-4530(02)00108-7. [DOI] [PubMed] [Google Scholar]
- Raine A, Venables PH, Williams M. Relationships between central and autonomic measures of arousal at age 15 years and criminality at age 24 years. Archives of General Psychiatry. 1990;47(11):1003–1007. doi: 10.1001/archpsyc.1990.01810230019003. [DOI] [PubMed] [Google Scholar]
- Raison CL, Miller AH. When not enough is too much: The role of insufficient glucocorticoid signaling in the pathophysiology of stress-related disorders. American Journal of Psychiatry. 2003;160(9):1554–1565. doi: 10.1176/appi.ajp.160.9.1554. [DOI] [PubMed] [Google Scholar]
- Raykov T. Equivalent structural equation models and group equality constraints. Multivariate Behavioral Research. 1997;32(2):95. doi: 10.1207/s15327906mbr3202_1. [DOI] [PubMed] [Google Scholar]
- Rosemalen JG, Oldehinkel AJ, Ormel J, de Winter AF, Buitelaar JK, Verhulst FC. Determinants of salivary cortisol levels in 10–12 year old children; A population-based study of individual differences. Psychoneuroendocrinology. 2005;30(5):483–495. doi: 10.1016/j.psyneuen.2004.12.007. [DOI] [PubMed] [Google Scholar]
- Sack M, Hopper JW, Lamprecht F. Low respiratory sinus arrhythmia and prolonged psychophysiological arousal in posttraumatic stress disorder: Heart rate dynamics and individual differences in arousal regulation. Biological Psychiatry. 2004;55(3):284–290. doi: 10.1016/s0006-3223(03)00677-2. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM, Romero LM, Munck AU. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocrinology Review. 2000;21(1):55–89. doi: 10.1210/edrv.21.1.0389. [DOI] [PubMed] [Google Scholar]
- Schommer NC, Hellhammer DH, Kirschbaum C. Dissociation between reactivity of the hypothalamus-pituitary-adrenal axis and the sympathetic-adrenal-medullary system to repeated psychosocial stress. Psychosomatic Medicine. 2003;65(3):450–460. doi: 10.1097/01.psy.0000035721.12441.17. [DOI] [PubMed] [Google Scholar]
- Shields A, Cicchetti D. Reactive aggression among maltreated children: The contributions of attention and emotion dysregulation. Journal of Clinical Child Psychology. 1998;27(4):381–395. doi: 10.1207/s15374424jccp2704_2. [DOI] [PubMed] [Google Scholar]
- Shipman KL, Zeman J. Socialization of children’s emotion regulation in mother-child dyads: A developmental psychopathology perspective. Development and Psychopathology. 2001;13(2):317–336. doi: 10.1017/s0954579401002073. [DOI] [PubMed] [Google Scholar]
- Susman EJ. Psychobiology of persistent antisocial behavior: Stress, early vulnerabilities and the attenuation hypothesis. Neuroscience and Biobehavioral Reviews. 2006;30(3):376–389. doi: 10.1016/j.neubiorev.2005.08.002. [DOI] [PubMed] [Google Scholar]
- Susman EJ, Dockray S, Schiefelbein VL, Herwehe S, Heaton JA, Dorn LD. Morningness/eveningness, morning-to-afternoon cortisol ratio, and antisocial behavior problems during puberty. Developmental Psychology. 2007;43(4):811–822. doi: 10.1037/0012-1649.43.4.811. [DOI] [PubMed] [Google Scholar]
- Trickett PK, Schellenbach C. Violence against children in the family and community. Washington, DC: American Psychological Association; 1998. [Google Scholar]