Abstract
The decay of eukaryotic mRNA is triggered mainly by deadenylation, which leads to decapping and degradation from the 5′ end of an mRNA. Poly(A)-binding protein has been proposed to inhibit the decapping process and to stabilize mRNA by blocking the recruitment of mRNA to the P-bodies where mRNA degradation takes place after stimulation of translation initiation. In contrast, several lines of evidence show that poly(A)-binding protein (Pab1p) has distinct functions in mRNA decay and translation in yeast. To address the translation-independent function of Pab1p in inhibition of decapping, we examined the contribution of Pab1p to the stability of non-translated mRNAs, an AUG codon-less mRNA or an mRNA containing a stable stem-loop structure at the 5′-UTR. Tethering of Pab1p stabilized non-translated mRNAs, and this stabilization did not require either the eIF4G-interacting domain of Pab1p or the Pab1p-interacting domain of eIF4G. In a ski2Δ mutant in which 3′ to 5′ mRNA degradation activity is defective, stabilization of non-translated mRNAs by the tethering of Pab1p lacking an eIF4G-interacting domain (Pab1–34Cp) requires a cap structure but not a poly(A) tail. In wild type cells, stabilization of non-translated mRNA by tethered Pab1–34Cp results in the accumulation of deadenylated mRNA. These results strongly suggest that tethering of Pab1p may inhibit the decapping reaction after deadenylation, independent of translation. We propose that Pab1p inhibits the decapping reaction in a translation-independent manner in vivo.
Keywords: RNA Abundance, RNA-binding Protein, RNA Metabolism, RNA Processing, RNA-Protein Interaction, RNA Turnover
Introduction
The regulation of mRNA decay is an important process in determination of the expression and fate of cellular transcripts. There are two alternative pathways by which polyadenylated mRNAs are degraded, and both are initiated by removal of the 3′ polyadenosine tail (1–4). After deadenylation, the mRNA can be decapped by the Dcp2-Dcp1 decapping enzyme complex (5–8) and is subsequently progressively degraded by Xrn1p, the 5′ to 3′ exoribonuclease (9). Alternatively, mRNA can be degraded from the 3′ end by the exosome after the initial deadenylation step (10, 11).
Several lines of evidence show that dissociation of the ribosome is a prerequisite for mRNA decapping in yeast. First, the translational initiation rate is inversely proportional to the decapping rate (12). Second, mRNA decapping can occur efficiently in structures termed P-bodies, which are ribosome-free cellular foci (12). The mRNAs that are trapped in polysomes as a result of treatment of the cells with cycloheximide are resistant to decapping and are not found in P-bodies (12–16). Recently, it was reported that both deadenylated and decapped mRNAs are distributed in polysomes (17), indicating that decapping takes place, whereas the mRNAs are associated with actively translating ribosomes. It has been proposed that endogenous mRNAs are decapped on polysomes and that dissociation of ribosomes from mRNA is not a prerequisite for mRNA decay in yeast (17).
Several observations suggest that the ability of the poly(A) tail to inhibit decapping is primarily mediated through the poly(A)-binding protein (Pab1p) in yeast. First, it has been demonstrated that decapping occurs when the poly(A) tail is shortened to approximately the minimum length required for the binding of Pab1p (18, 19). Second, decapping is uncoupled from deadenylation in pab1 mutant strains (20, 21), indicating that the requirement for prior deadenylation before decapping is relieved in the absence of Pab1p. Third, the inhibition of decapping by Pab1p does not require the presence of the poly(A) tail when Pab1p is artificially tethered to the RNA (22).
It has been suggested that the poly(A)-binding protein inhibits decapping by several mechanisms, possibly connecting several processes such as translation, turnover, and mRNA localization. Although the inhibitory effects of poly(A)-binding protein on decapping are conserved between humans and yeast (7, 12, 23), the mechanism still needs to be elucidated. It has been proposed that Pab1p may inhibit decapping by stimulating translation initiation in yeast (12, 24). In contrast, it was shown that deletion of the translation initiation factor eIF-4G-interacting domain of Pab1p has no effect on the ability of tethered Pab1p to block decapping (22). This result suggests that Pab1p inhibits decapping via interactions that are independent of the translation initiation complex. However, it has been shown that Pab1p can stimulate the initiation of translation independent of eIF-4G-interacting domain of Pab1p (25, 26). Therefore, it is still unclear if Pab1p inhibits decapping in yeast in the absence of translation.
To address the translation-independent function of Pab1p in inhibition of mRNA decapping, we examined the contribution of Pab1p to the stability of non-translated mRNAs, an AUG codon-less mRNA or mRNA containing a stable stem-loop structure at the 5′-untranslated region (5′-UTR). Tethering of Pab1p stabilized non-translated mRNAs, and this stabilization did not require either the eIF4G-interacting domain of Pab1p or the Pab1p-interacting domain of eIF4G. Stabilization of non-translated mRNAs by tethering of Pab1p lacking an eIF4G-interacting domain in a ski2Δ mutant that is defective in 3′ to 5′ degradation pathway requires a cap structure but not a poly(A) tail. These results strongly suggest that tethering of Pab1p may inhibit the decapping reaction independent of translation. We also found that tethered Pab1–34Cp moderately stabilizes non-translated mRNAs in an xrn1Δ mutant that is defective in the 5′ to 3′ degradation pathway in a poly(A) tail-dependent manner. This result suggests that tethered Pab1–34Cp inhibits deadenylation, although the significance of this function is unknown. Based on these results, we propose that Pab1p inhibits the mRNA decapping reaction in yeast in a translation-independent manner in vivo.
EXPERIMENTAL PROCEDURES
Strains and Other Methods
The strains and plasmids used in this study are listed in Table 1, and the oligonucleotides used are described in Table 2. Northern and Western blot analyses and polysome analysis were performed as previously described (27, 28). RNase H digestion was performed according to the manufacturer's protocol (Takara, Kyoto, Japan).
TABLE 1.
Yeast strains and plasmids used in this study
| Strain/plasmids | Genotype [plasmid] (plasmid number) | Source |
|---|---|---|
| Strains | ||
| W303-1a | MATa ade2 his3 leu2 trp1 ura3 can1 | Laboratory stock |
| YAS2071 | MATa ade2 his3 leu2 trp1 ura3tif4631::LEU2 tif4632::ura3 pep4::HIS3 ptif4631-ΔN300TRP1CEN | Ref. 31 |
| YIT2007 | W303-1a ski2Δ::kanMX | Ref. 40 |
| YIT2008 | W303-1a xrn1Δ::natMX | This study |
| Plasmids | ||
| p416GAL1 | p416GAL1p URA3 CEN | Ref. 41 |
| p415TEF1 | p415TEF1p LEU2 CEN | Ref. 42 |
| pIT2067 | p416GAL1p-AUG URA3 CEN | This study |
| pIT2068 | p416GAL1p-AUG-FLAG-MPT4ΔN-PGK1(3′-UTR) URA3 CEN | This study |
| pIT2069 | p416GAL1p-AUG-FLAG-MPT4ΔN-PGK1(3′-UTR)-MS2 URA3 CEN | This study |
| pIT2070 | p416GAL1p-No-AUG-FLAG-MPT4ΔN-PGK1(3′-UTR) URA3 CEN | This study |
| pIT2071 | p416GAL1p-No-AUG-FLAG-MPT4ΔN-PGK1(3′-UTR)-MS2 URA3 CEN | This study |
| pIT2072 | p416GAL1p-SL-AUG-FLAG-MPT4ΔN-PGK1(3′-UTR) URA3 CEN | This study |
| pIT2073 | p416GAL1p-SL-AUG-FLAG-MPT4ΔN-PGK1(3′-UTR)-MS2 URA3 CEN | This study |
| pIT2074 | p416GAL1p-AUG-MPT4ΔN-FLAG-PGK1(3′-UTR)-MS2 URA3 CEN | This study |
| pIT2075 | p416GAL1p-No-AUG-MPT4ΔN-FLAG-PGK1(3′-UTR)-MS2 URA3 CEN | This study |
| pIT2076 | p416GAL1p-SL-AUG-MPT4ΔN-FLAG-PGK1(3′-UTR)-MS2 URA3 CEN | This study |
| pIT2077 | p415TEF1p-FLAG-PAB1 LEU2 CEN | This study |
| pIT2078 | p415TEF1p-FLAG-MS2-PAB1 LEU2 CEN | This study |
| pIT2079 | p415TEF1p-FLAG-MS2-Pab1–34C LEU2 CEN | This study |
| pIT2080 | p415TEF1p-FLAG-MS2-Pab1–4C LEU2 CEN | This study |
| pIT2081 | p415TEF1p-FLAG-MS2-Pab1-C LEU2 CEN | This study |
| pIT2082 | p416GAL1p-No-AUG-FLAG-MPT4ΔN-PGK1(3′UTR)-Rz URA3 CEN | This study |
| pIT2083 | p416GAL1p-No-AUG-FLAG-MPT4ΔN-PGK1(3′UTR)-MS2-Rz URA3 CEN | This study |
| pIT2084 | p416GAL1p-Rz-No-AUG-FLAG-MPT4ΔN-PGK1(3′UTR)-Rz URA3 CEN | This study |
| pIT2085 | p416GAL1p-Rz-No-AUG-FLAG-MPT4ΔN-PGK1(3′UTR)-MS2-Rz URA3 CEN | This study |
| pIT2086 | p415TEF1p-MS2-PAB1 LEU2 CEN | This study |
| pIT2087 | p415TEF1p-MS2-Pab1–34C LEU2 CEN | This study |
| pIT2088 | p415TEF1p-MS2-Pab1–4C LEU2 CEN | This study |
| pIT2089 | p415TEF1p-MS2-Pab1-C LEU2 CEN | This study |
TABLE 2.
List of oligonucleotides
nt, nucleotides.
| Name description | Sequences |
|---|---|
| OTS017 MPT4ΔN (380–822 nt) Forward | 5′-GCTCTAGAATGGACTACAAGGACGACGACGACAAGCTTGCTGAAATTGCTGAAGACGC-3′ |
| OIT934 MPT4ΔN (380–822 nt) Reverse | 5′-GCGGGATCCTTAAGCCAAAGATGGCAAGTTAGAAACGTCAATG-3′ |
| OIT932 AUG to SpeI restriction | 5′-GACTAGTGCAAATTCCAAGTACTCCTTGGTCTTC-3′ |
| OIT933 AUG to SpeI restriction | 5′-GACTAGTCACTTTTGTTGAATCTAACACTAGAAAGAACTTCG-3′ |
| OIT557 PGK1 3′-UTR forward | 5′-GGGATCCTAAATTGAATTGAATTGAAATCGATAG-3′ |
| OIT558 PGK1 3′-UTR reverse | 5′-GGGAATTCCGATTGACCAATATATGTCTCTGAATGCC-3′ |
| OKK130 XhoI site top | 5′-CCCCATCCTTTACCTCGAGCTAAAATAATAG-3′ |
| OKK131 XhoI site bottom | 5′-CTATTATTTAGCTCGAGGTAAAGGATGGGG-3′ |
| OIT949 MS2 binding sites top | 5′-ATCGAGTAACAAGAGGATCACCCTTGTCTGCAGGTCGACTCAAGAAAACTTGAGGATCACCCAAGT-3′ |
| OIT950 MS2 binding sites bottom | 5′-CGAACTTGGGTGATCCTCAAGTTTTCTTGAGTCGACCTGCAGACAAGGGTGATCCTCTTGTTAC-3′ |
| OIT953 No-AUG-MPT4ΔN (380–822 nt) forward | 5′-GCTCTAGATACGACTACAAGGACGACGACGACAAGCTTGCTGAAATTGCTGAAGACGC-3′ |
| OTS10 MPT4ΔN (380–822 nt)-FLAG reverse | 5′-CGGGATCCTTACTTGTCGTCGTCGTCCTTGTAGTCAGCCAAAGATGGCAAGTTAGAAACGTCAATGTTACGGTTCTTTTGAACGGTGTTAGCAGAGTTA-3′ |
| OIT1153 Stem-loop | 5′-CTAGCGATATCCCGTGGAGGGGCGCGTGGTGGCGGCTGCAGCCGCCACCACGCGCCCCTCCACGGGATATCG-3′ |
| OTS034 SpeI site top | 5′-TAGACTATTATTTATCTTTTAAACTAGTTGATTATTAAGATTTTTATTA-3′ |
| OTS035 SpeI site bottom | 5′-TAATAAAAATCTTAATAATCAACTAGTTTAAAAGATAAATAATAGTCTA-3′ |
| OIT1181 Ribozyme top SpeI and XbaI | 5′-CTAGCCCTGTCACCGGATGTGTTTTCCGGTCTGATGAGTCCGTGAGGACGAAACAGGA-3′ |
| OIT1182 Ribozyme bottom SpeI and XbaI | 5′-CTAGTCCTGTTTCGTCCTCACGGACTCATCAGACCGGAAAACACATCCGGTGACAGGG-3′ |
| OIT627 Pab1 forward | 5′-GCTCTAGAATGGCTGATATTACTGATAAGACAGC-3′ |
| OIT884 Pab1 reverse | 5′-ACGCGTCGACGTAGGGAAGTAGGTGATTACATAGAGC-3′ |
| OIT670 MS2 protein 5′ | 5′-GCTCTAGAATGGACTACAAGGACGACGATGACAAGGCTTCTAACTTTACTCAGTTCG-3′ |
| OIT630 MS2 protein reverse | 5′-GCTCTAGAGTAGATGCCGGAGTTTGCTGCGATTGCTGAGGG-3′ |
| GTD029 Pab1–34C forward | 5′-GCTCTAGAGACTCTCAATTGGAAGAGACTAA-3′ |
| OIT883 Pab1–4C forward | 5′-CTCTAGAGCTTACAGATTGGAAAAAATGGCC-3′ |
| GTD038 Pab1-C forward | 5′-GCTCTAGAGCCGGTATGCCAGGTCAAT–3′ |
Plasmid Construction
Recombinant DNA procedures were carried out as described previously (29). The construction of plasmids is described in the supplemental Information.
Determination of mRNA Stability
Yeast cells were grown in minimal medium containing 2% galactose. When the culture reached an A600 of 0.6 the cells were harvested and re-suspended in medium containing 2% glucose to inhibit transcription from the GAL1 promoter. At the times indicated, the cells were harvested to prepare RNA samples using the hot phenol. The mRNA levels of reporter genes were determined by Northern blotting using digoxigenin (DIG)2 reagents and kits to prepare nonradioactive probes by PCR-based nucleic acid labeling. Bound probes were detected according to the procedure specified by the manufacturer (Roche Applied Science). DIG-labeled MPT4 probes were prepared using the oligonucleotides OTS017 and OIT934. The intensity of bands on the blots was quantified using the LAS3000 mini and Multi-Gauge Version 3.0 (Fuji Film, Japan). The relative product levels were determined by comparison to a standard curve by using the series of dilution of the samples of time 0 just after the addition of glucose. The intensities of bands from the diluted samples were compared with the standard curve, and the mRNA levels relative to the control mRNA was determined. Most decay time courses are presented as straight lines in the half-log plots, but some curves are irregular. We calculated the half-lives listed are based on the slope of the first part of the curves.
Western Blotting
Yeast cells were grown in minimal medium containing 2% galactose. When the culture reached an A600 of 0.6, the cells were harvested. The protein products of FLAG-tagged reporter genes were detected by Western blotting using an anti-FLAG antibody (F1804, Sigma) and a horseradish peroxidase-conjugated secondary antibody (GE Healthcare). The intensity of the bands on the blots was quantified using a LAS3000 mini and Multi-Gauge Version 3.0 (Fuji Film, Japan), and the intensity of the bands of the diluted samples was compared with that of the standard curve. The relative level of the product in the sample compared with the control product was determined.
Pulse-label Experiments Using a 14C-Labeled Amino Acid Mixture Followed by Immunoprecipitation
Yeast cells were grown exponentially at 30 °C in minimal media lacking methionine and cysteine. A 10-ml aliquot of yeast cells was labeled with 5 μCi of a [14C]amino acid mixture (NEG072; PerkinElmer Life Sciences) for 10 min. Cells were harvested, and cell extracts were prepared using Complete Lysis-Y (Roche Applied Science). Cell extracts were incubated with an anti-FLAG antibody (F1804, Sigma) and protein A-agarose (Roche Applied Science) in IXA-100 buffer (27), then washed 3 times and eluted with 0.4 mg/ml FLAG peptide. The radioactivities of the immunoprecipitated samples were then measured by liquid scintillation counter (Aloka, Japan).
Yeast Extract and Sucrose Gradient Separation
Yeast cells were grown exponentially at 30 °C and were harvested by centrifugation. Cell extracts were prepared as described previously (27). The equivalent of 50 A260 units were then layered onto linear 10–50% sucrose density gradients. Sucrose gradients (10–50% sucrose in 10 mm Tris acetate, pH 7.4, 70 mm ammonium acetate, 4 mm magnesium acetate) were prepared in 25 × 89-mm polyallomer tubes (Beckman Coulter) using a gradient master. Crude extracts were layered on top of the sucrose gradients and centrifuged at 150,000 × g in a P28S rotor (Hitachi Koki, Japan) for 2.5 h at 4 °C. Gradients were then fractionated (TOWA Laboratory, Tsukuba, Japan). Polysome profiles were generated by continuous absorbance measurement at 254 nm using a single path UV-1 optical unit (ATTO Biomini UV monitor) connected to a chart recorder (ATTO, digital mini-recorder). Equal volume fractions were collected and processed for Northern blotting as described above.
RESULTS
Construction of a Reporter Gene That Expresses mRNA That Contains No AUG Codon in Any Reading Frame
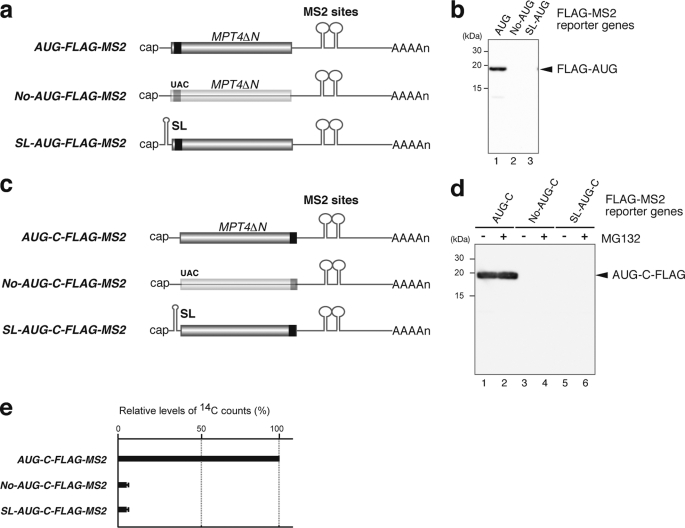
To address the translation-independent function of Pab1p in the inhibition of mRNA decapping in yeast, we aimed to construct a reporter gene that did not contain an ATG codon in any reading frame. A search for a gene that would be suitable for this reporter construct revealed that the downstream region of the MPT4 ORF contained only a few ATG codons in all reading frames. We, therefore, used a fragment of the MPT4 gene (nucleotides 385–822; MPT4ΔN) to construct a reporter gene that did not contain any ATG codon in any reading frame (supplemental Fig. 1). The AUG-FLAG-MPT4ΔN reporter gene was constructed by insertion of an ATG codon followed by the FLAG tag sequence upstream of MPT4ΔN. The only internal ATG codon present within MPT4ΔN, at nucleotides 629–631, was eliminated by introduction of a SpeI restriction site. As a control, we constructed the No-AUG-FLAG-MPT4ΔN reporter gene in which the ATG codon of AUG-FLAG-MPT4ΔN was replaced by the TAC codon. To inhibit translation, we inserted a stable stem-loop structure at the UTR of AUG-FLAG-MPT4ΔN yielding the reporter gene SL-AUG-FLAG-MPT4ΔN. It is well known that insertion of such stem-loop structures into the 5′ region of a gene can strongly inhibit translation. These reporter genes were placed under the control of the GAL1 promoter and flanked by the PGK1 3′-UTR, which contains tandem MS2 binding sites that function to tether the MS2-Pab1p fusion protein to mRNA (Fig. 1a).
FIGURE 1.
Construction of a reporter gene that expresses mRNA, which contains no AUG codon in any reading frame. a, shown is a schematic drawing of the reporter mRNAs constructed. The filled box indicates the open reading frame of MPT4ΔN, the black box indicates the FLAG tag, the lines represent non-translated regions, and the tract of As denotes the poly(A) tail. AUG-MS2, No-AUG-MS2, and SL-AUG-MS2 reporter genes all contain the 3′-UTR region of PGK1 into which two tandem MS2 binding sites were inserted. In No-AUG-MS2, the codon UAC replaces AUG. In SL-AUG-MS2, a stable stem-loop structure for inhibition of translation (SL) was introduced into the 5′-UTR region. b, shown are expression levels of the reporter proteins. W303 cells harboring pIT2069 (AUG-FLAG-MS2, (AUG)) pIT2071 (No-AUG-FLAG-MS2, (No-AUG)) or pIT2073 (SL-AUG-FLAG-MS2, (SL-AUG)) plasmids were grown on SC-UraLeu medium containing 2% galactose. Samples were analyzed by Western blotting with anti-FLAG antibodies. c, shown is a schematic drawing of the reporter mRNAs containing FLAG sequences at the carboxyl terminal region of the open reading frame. d, shown are expression levels of the reporter proteins. W303 cells harboring pIT2074 (AUG-MPT4ΔN-FLAG-MS2, (AUG-C-FLAG-MS2)), pIT2075 (No-AUG-MPT4ΔN-FLAG-MS2, (No-AUG-C-FLAG-MS2)), or pIT2076 (SL-AUG-MPT4ΔN-FLAG-MS2, (SL-AUG-C-FLAG-MS2)) plasmids were grown on SC-UraLeu medium containing 2% galactose. Samples were analyzed by Western blotting with anti-FLAG antibodies. When indicated, cells were grown in the presence of 0.2 mm MG132. e, No-AUG-C-FLAG-MS2 and SL-AUG-C-FLAG-MS2 mRNAs are poorly translated. W303 cells harboring pIT2074 (AUG-C-FLAG-MS2), pIT2075 (No-AUG-C-FLAG-MS2), or pIT2076 (SL-AUG-C-FLAG-MS2) plasmids were grown on SC-UraLeu medium containing 2% galactose but lacking methionine and cysteine. The cells were pulse-labeled with 14C amino acids, and then immunoprecipitates were analyzed for associated radioactivity. Radioactivity was determined relative to the counts for AUG-C-FLAG-MS2, which were designated as 100%.
We first confirmed by Western blotting analysis that the mRNA encoded by No-AUG-FLAG-MS2 and SL-AUG-FLAG-MS2 is not translated. As expected, no protein product was detected from either the No-AUG-FLAG-MS2 or the SL-AUG-FLAG-MS2 reporter gene (Fig. 1b, lanes 2 and 3) using conditions under which a protein product could be detected from the AUG-FLAG-MS2 reporter gene (Fig. 1b, lane 1). This result suggested that protein could not be produced or was very poorly produced from the AUG codon. However, there remained a possibility that protein might be synthesized from non-AUG codons including UGG or UUG codons. To detect putative protein products that might be translated from non-AUG codons, we constructed reporter constructs in which the FLAG tag sequence was inserted into the carboxyl-terminal region instead of the amino-terminal region of these reporter genes (Fig. 1c). Western blotting of extracts of cells expressing No-AUG-C-FLAG-MS2 and SL-AUG-C-FLAG-MS2 reporter genes could not detect the expression of FLAG-tagged proteins even in the presence of MG132, a proteasome inhibitor that would be expected to stabilize proteins because of degradation by the proteasome (Fig. 1d, lanes 2, 4, and 6). These results strongly suggest that protein is not synthesized from non-AUG codons. To further confirm that the No-AUG-C-FLAG-MS2 and SL-AUG-C-FLAG-MS2 mRNAs are not translated or are very poorly translated, we performed pulse-label experiments in which synthesized proteins were labeled by pulse-labeling cells with 14C amino acids after which the 14C counts of proteins precipitated with an anti-FLAG antibody were analyzed. The 14C counts that were precipitated from cells expressing No-AUG-C-FLAG-MS2 or SL-AUG-C-FLAG-MS2 reporter mRNA were less than 5% of the counts that were precipitated from cells expressing the control AUG-C-FLAG-MS2 mRNA (Fig. 1e). The combined results strongly suggest that the No-AUG-C-FLAG-MS2 and the SL-AUG-C-FLAG-MS2 mRNAs are not translated or are very poorly translated.
No-AUG-FLAG-MS2 mRNA Is Stabilized by Tethered Pab1p, Which Lacks an eIF4G Interacting Domain
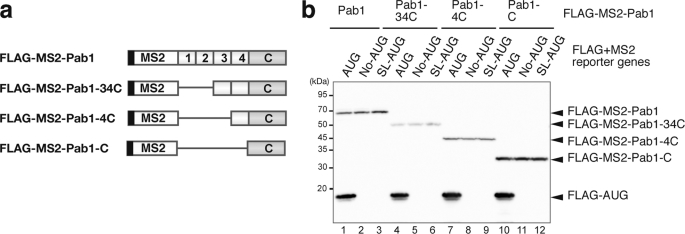
To address the translation-independent function of Pab1p in the inhibition of mRNA decapping, we examined the contribution of Pab1p to the stability of the non-translated No-AUG-FLAG-MS2 or SL-AUG-FLAG-MS2 mRNAs. To tether Pab1p to these reporter mRNAs independent of the poly(A) sequence, Pab1p was fused to the MS2 coat protein as previously reported (22). We also constructed a series of FLAG-MS2-Pab1p deletion mutants to conduct a domain analysis of Pab1p. (Fig. 2a). Before analysis of the effect of these Pab1p constructs on mRNA stability, we first determined the expression levels of these Pab1p constructs by Western blotting using an anti-FLAG antibody. This analysis revealed that the expression level of FLAG-MS2-Pab1–34Cp that lacks the RRM1 and RRM2 domains was 2-fold lower than that of intact FLAG-MS2-Pab1p. The expression level of FLAG-MS2-Pab1-Cp that contains only the carboxyl-terminal region of Pab1p was 2–3-fold higher than that of FLAG-MS2-Pab1p. Importantly, co-expression of FLAG-MS2-Pab1 or its deletion mutants did not induce the production of a protein product from the No-AUG-FLAG-MS2 nor the SL-AUG-FLAG-MS2 reporter genes. In addition, the level of the FLAG-AUG protein produced from the AUG-C-FLAG-MS2 mRNA was not affected by co-expression of the various FLAG-MS2-Pab1p fusion proteins (Fig. 2b, lanes 1, 4, 7, and 10).
FIGURE 2.
Construction of MS2-Pab1 fusion proteins. a, shown is a schematic drawing of FLAG-MS2-Pab1p wild type and deletion mutant fusion proteins. The black box represents the FLAG tag. The white box appears to show MS2. The numbered boxes indicate the RRM domains of Pab1p, and the shaded box labeled with a C represents the carboxyl terminal domain of the poly(A)-binding protein. b, shown are expression levels of the FLAG-MS2-Pab1p fusion proteins. W303 cells harboring the indicated FLAG-MS2-Pab1p fusion genes, and pIT2069 (AUG-FLAG-MS2, (AUG)), pIT2071 (No-AUG-FLAG-MS2, (No-AUG)), or pIT2073 (SL-AUG-FLAG-MS2, (SL-AUG)) plasmids were grown on SC-UraLeu medium containing 2% galactose. Samples were analyzed by Western blotting using anti-FLAG antibodies.
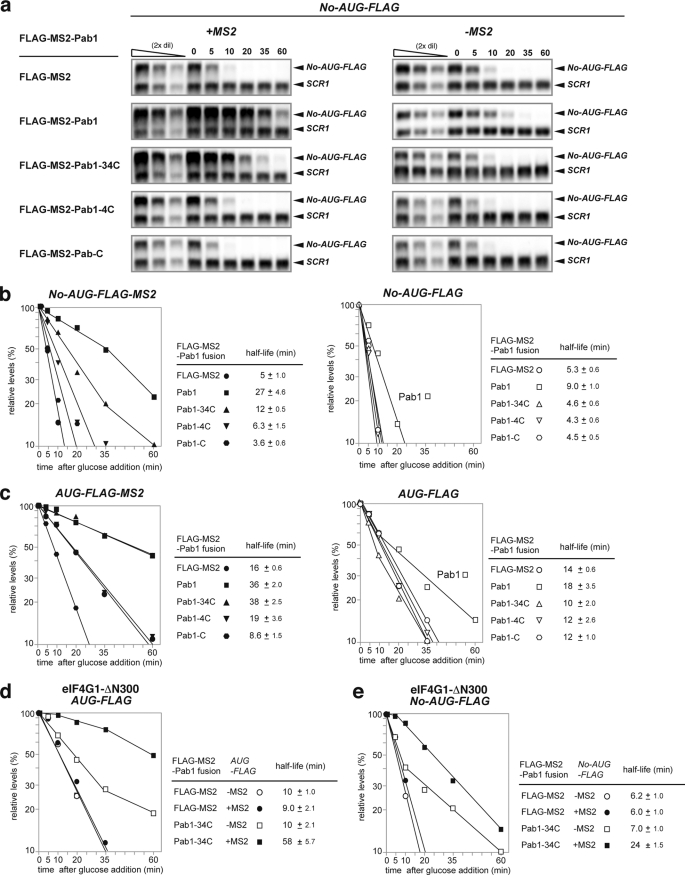
The effect of Pab1p on the stability of the reporter mRNAs was then determined by measurement of the level of the mRNA that remained in the presence of different Pab1p constructs after inhibition of transcription from the GAL1 promoter by the addition of glucose. The level of mRNA was assayed using Northern blotting. We found that the FLAG-MS2-Pab1p fusion protein stabilized the No-AUG-FLAG-MS2 mRNA by a factor of 5.4 (extending its half-life (t½) from 5 to 27 min; Fig. 3, a and b). A No-AUG-FLAG mRNA that lacked the MS2 sites was slightly, but significantly, stabilized by the expression of FLAG-MS2-Pab1p (extending its half-life (t½) from 5.3 to 9 min; Fig. 3, a and b). Because FLAG-MS2-Pab1p complemented the lethality of the pab1Δ mutation (data not shown), we suspect that the Pab1p unstable stabilizes No-AUG-FLAG mRNA even without tethering of Pab1p when the level of Pab1p is increased.
FIGURE 3.
Stabilization of No-AUG-FLAG-MS2 mRNA by tethered Pab1p does not require an interaction between Pab1p and eIF4G. a, tethering of Pab1p without the RRM1–2 domains stabilizes non-translated mRNA containing no AUG codon. W303 cells harboring pIT2071 (No-AUG-FLAG-MS2, left) or pIT2070 (No-AUG-FLAG, right) were transformed with a control p415TEF1p-FLAG-MS2 plasmid or with plasmids expressing the indicated FLAG-MS2-Pab1p fusion proteins. The cells were grown in SC-UraLeu medium containing 2% galactose (SC-Gal UraLeu medium). The cells were re-suspended in medium containing 2% glucose to inhibit transcription from the GAL1 promoter and harvested at the indicated times. RNA samples were prepared, and the levels of the remaining mRNA were determined using Northern blot analysis with DIG-labeled MPT4 probe. The relative mRNA levels of the samples were determined by comparison with a series of 2-fold dilutions of samples time 0 (first through third lanes). These dilutions were used as standard curves as described previously (25). b, tethering of Pab1p stabilizes non-translated mRNA. W303 cells harboring pIT2071 (No-AUG-FLAG-MS2) or pIT2070 (No-AUG-FLAG) were transformed with the control p415TEF1p-FLAG-MS2 plasmid or with plasmids expressing the indicated FLAG-MS2-Pab1p fusion proteins. The cells were grown in SC-Gal UraLeu medium, and mRNA stability was measured as described in a. Relative quantities are shown as the mean values of three independent experiments. The half-lives of mRNAs are shown as the mean values of three independent experiments with S.D. c, tethering of Pab1p stabilizes translated mRNA. W303 cells harboring pIT2069 (AUG-FLAG-MS2) or pIT2068 (AUG-FLAG) were transformed with the control p415TEF1p-FLAG-MS2 plasmid or with plasmids expressing the indicated FLAG-MS2-Pab1p fusion proteins. The cells were grown in SC-Gal UraLeu medium, and mRNA stability was measured as described in a. d, tethering of Pab1–34Cp stabilizes non-translated mRNA in eIF4G1-ΔN300 mutant YAS2071 (W303tif4631−tif4632− ptif4631-ΔN300TRPCEN) cells. YAS2071 cells, harboring pIT2071 (No-AUG-FLAG-MS2) or pIT2070 (No-AUG-FLAG), were transformed with a plasmid containing a FLAG-MS2-Pab1–34C fusion gene or with a control p415TEF1p-FLAG-MS2 plasmid. The cells were grown in SC-Gal UraLeu medium, and mRNA stability was measured as in a. e, tethering of Pab1–34Cp stabilizes translated mRNA in eIF4G1-ΔN300 mutant. YAS2071 (W303tif4631−tif4632− ptif4631-ΔN300TRPCEN) cells harboring pIT2069 (AUG-FLAG-MS2) or pIT2068 (AUG-FLAG) plasmids were transformed with plasmids encoding the FLAG-MS2-Pab1–34C fusion gene or with the control p415TEF1p-FLAG-MS2 plasmid. The cells were grown in SC-Gal UraLeu medium, and mRNA stability was measured as described in a.
We next analyzed the effect of the Pab1p deletion mutants on the stability of the non-translated mRNA to identify the domains of Pab1p required for mRNA stabilization. The FLAG-MS2-Pab1p fusion protein stabilized No-AUG-FLAG-MS2 mRNA by a factor of 5.4, extending its half-life from 5 to 27 min; Fig. 3, a and b). However, No-AUG-FLAG mRNA was moderately but significantly stabilized when FLAG-MS2-Pab1p was expressed (Fig. 3, a and b). These results indicate that tethered Pab1p stabilizes No-AUG-FLAG-MS2 mRNA, but the stabilization of No-AUG-FLAG-MS2 mRNA is not solely dependent on the MS2 binding sites in the 3′-UTR of reporter mRNAs. The FLAG-MS2-Pab1–34Cp fusion protein stabilized No-AUG-FLAG-MS2 mRNA by a factor of 2.4, extending its half-life from 5 to 12 min; Fig. 3, a and b). No-AUG-FLAG mRNA was not stabilized even when FLAG-MS2-Pab1p-34C was expressed (Fig. 3, a and b). These results clearly indicate that stabilization of No-AUG-FLAG-MS2 mRNA is solely dependent on the MS2 binding sites in the 3′-UTR of reporter mRNAs, and stabilization of No-AUG-FLAG-MS2 mRNA by tethered Pab1p does not require the eIF4G-interacting domain of Pab1p. The No-AUG-FLAG-MS2 mRNA was only slightly stabilized by tethered FLAG-MS2-Pab1–4Cp (Fig. 3, a and b). However, this lack of stabilization cannot be attributed to different expression levels of FLAG-MS2-Pab1–4Cp and FLAG-MS2-Pab1–34Cp (Fig. 2b, lanes 4–6 and 7–9). These results suggest that the RRM3 domain of Pab1p plays an important role in stabilization of non-translated mRNA by tethered Pab1p. The stability of reporter mRNAs with or without MS2 sites in the presence of various Pab1p constructs is summarized in Fig. 3b.
We conducted a similar domain analysis of Pab1p to identify the domains required for stabilization of the translated AUG-FLAG-MS2 and AUG-FLAG mRNAs as summarized in Fig. 3c. First, we confirmed that the translated RNA (AUG-FLAG-MS2) is more stable than the non-translated RNA (No-AUG-FLAG-MS2) (Fig. 3, b and c). The FLAG-MS2-Pab1p fusion protein stabilized AUG-FLAG-MS2 mRNA by a factor of 2.3 and extended its t½ from 16 to 36 min (Fig. 3c). AUG-FLAG mRNA lacking the MS2 sequences was not stabilized even when FLAG-MS2-Pab1p was expressed (Fig. 3c). Decay time course is not presented as straight lines in the half-log plots (Fig. 3c, right panel), and we estimate the half-lives based on the slope of the first part of the curves. These results indicate that the stabilization of AUG-FLAG-MS2 mRNA is solely dependent on the MS2 binding sites. The FLAG-MS2-Pab1–34Cp fusion protein stabilized AUG-FLAG-MS2 mRNA by a factor of 2.4 and extended its t½ from 16 to 38 min (Fig. 3c). AUG-FLAG mRNA lacking the MS2 sequences was not stabilized even when FLAG-MS2-Pab1p-34C was expressed (Fig. 3c), indicating that the stabilization of AUG-FLAG-MS2 mRNA is solely dependent on the MS2 binding sites in the 3′-UTR of the reporter mRNA. These results clearly indicate that stabilization of AUG-FLAG-MS2 mRNA by tethered Pab1p does not require the eIF4G-interacting domain of Pab1p and are consistent with previous results which reported that deletion of the eIF-4G interacting domain in Pab1p has no effect on the ability of tethered Pab1p to stabilize translating mRNA (22). Tethering of FLAG-MS2-Pab1–4Cp or FLAG-MS2-Pab1-Cp also had little effect on the stability of translated mRNA (Fig. 3c). These results suggest that the RRM3 domain of Pab1p plays an important role in the stabilization of translated mRNA as well as of non-translated mRNA by tethered Pab1p. We also found that the FLAG-MS2-Pab1-Cp fusion protein destabilized AUG-FLAG-MS2 mRNA and shortened its t½ from 16 to 8.6 min (Fig. 3c). FLAG-MS2-Pab1p-C did not destabilize AUG-FLAG mRNA (Fig. 3c), indicating that the destabilization of AUG-FLAG-MS2 mRNA is solely dependent on the MS2 binding sites in the 3′-UTR of the reporter mRNA. Because the destabilization by Pab1p-C in Fig. 3B (from 5 min to 3.6 min) was almost as strong as with the translated RNA (from 16 to 8.6 min) (Fig. 3, b and c), the destabilization by FLAG-MS2-Pab1p-C may not depend on translation.
Stabilization of No-AUG-FLAG-MS2 mRNA by Tethered Pab1p Does Not Require the Pab1p-interacting Domain of eIF4G
The RRM1–2 domains of Pab1p, which do not appear to be required for Pab1p-stabilization of non-translated mRNA as shown above, are required for Pab1p to interact with eIF4G (30). To confirm that an interaction between eIF4G and Pab1p is not required for stabilization of non-translated mRNA, we examined the effect of tethering of FLAG-MS2-Pab1–34Cp on the stability of No-AUG-FLAG-MS2 mRNA in YAS2071 (W303 tif4631−tif4632−ptif4631-ΔN300TRPCEN) mutant cells, which express an eIF4G1-ΔN300 mutant protein that lacks the Pab1p-interacting domain but do not express eIF4G2 (31). We first confirmed that tethering of FLAG-MS2-Pab1–34Cp stabilized AUG-FLAG-MS2 mRNA by a factor of 6.4 and extending its half-life from 9 to 58 min but not AUG-FLAG mRNA (Fig. 3d) in the YAS2071 mutant. This result is consistent with previous results indicating that stabilization of translated mRNA by tethered Pab1p does not require the eIF4G-interacting domain of Pab1p (22). We then found that tethering of FLAG-MS2-Pab1–34Cp stabilized the No-AUG-FLAG-MS2 mRNA by a factor of 4.0 and extended its half-life from 6.0 to 24 min but did not have the same effect with the No-AUG-FLAG mRNA in the YAS2071 mutant (Fig. 3e). These indicate that stabilization of No-AUG-FLAG-MS2 mRNA is dependent on the MS2 binding sites in the 3′-UTR of the reporter mRNA. These results suggested that tethering of Pab1p stabilizes non-translated mRNA in yeast without interacting with eIF4G.
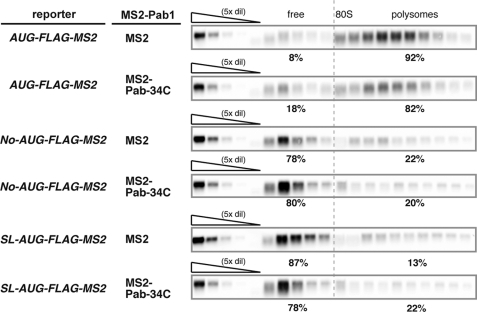
Tethering of Pab1p Stabilizes Non-translated mRNAs without Affecting Association of the mRNA with the Ribosome
The results shown above indicate that tethering of Pab1p stabilized non-translated mRNA in yeast without interacting with eIF4G. To determine whether tethering of Pab1p stabilizes non-translated mRNA without affecting association of the mRNA with the ribosome, we performed polysome analysis. We found that some of No-AUG-FLAG-MS2 and SL-AUG-FLAG-MS2 mRNAs were distributed in polysome fractions (Fig. 4), although those mRNA are very poorly translated (Fig. 1). The character of these mRNAs associated with polysome is unknown; however, it has been reported that some population of mRNAs containing stem-loop structure at its 5′-UTR is distributed in the polysome fraction (17). The population of No-AUG-FLAG-MS2 mRNAs in ribosome fractions only moderately decreased as a result of the tethering of MS2-Pab1–34Cp (decreasing from 22 to 20%; Fig. 4). The population of SL-AUG-FLAG-MS2 mRNAs in ribosome fractions only moderately increased as a result of the tethered MS2-Pab1–34Cp (increasing from 13 to 22%; Fig. 4). These results clearly indicate that tethering of MS2-Pab1–34Cp stabilizes non-translated mRNAs without affecting mRNA complex formation with the ribosome. We also obtained nearly identical results for AUG-FLAG-MS2 mRNA (decreasing from 92 to 82%; Fig. 4). Because the tethering of MS2-Pab1–34Cp also stabilizes AUG-FLAG-MS2 mRNA (the t½ increased from 12 to 38 min), these results suggest that MS2-Pab1–34Cp also stabilizes translated mRNA without affecting complex formation with the ribosome.
FIGURE 4.
Tethering of Pab1p stabilizes non-translated mRNAs without affecting association of the mRNA with the ribosome. W303 cells were co-transformed with the indicated reporter plasmids and a control p415TEF1p-MS2 plasmid or a plasmid that encodes the MS2-Pab1–34Cp fusion protein. Polysome analysis was then performed on cell extracts as described previously. RNA samples prepared from each fraction were analyzed using Northern blotting with a DIG labeled FLAG probe (5′-CTTGTCATCGTCGTCCTTGTAGTCCATACTAGT-3′).
Non-translated mRNAs Are Primarily Degraded from the 5′ End
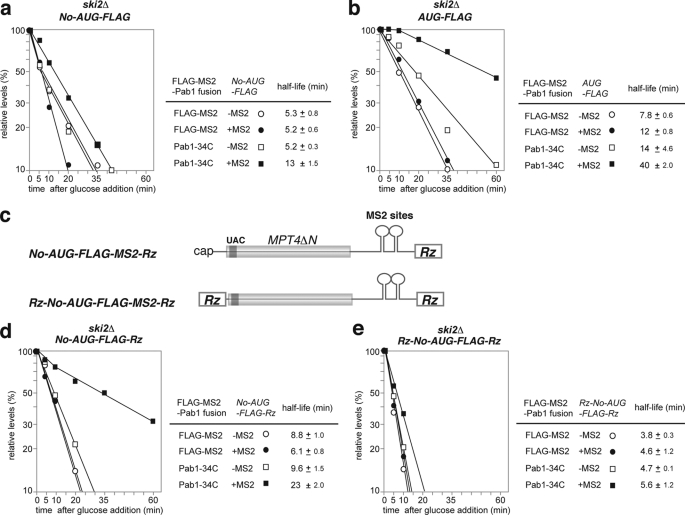
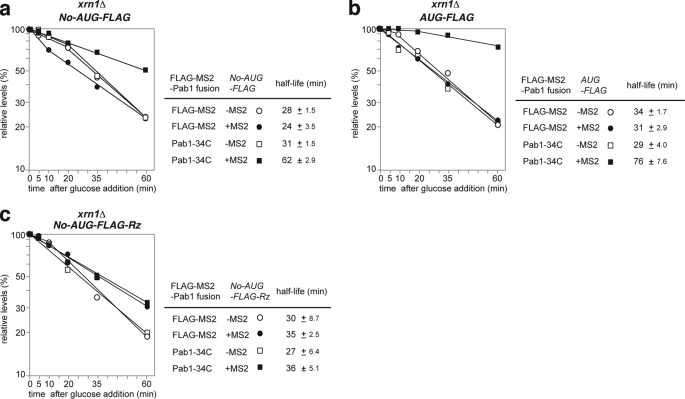
To evaluate the decay pathway responsible for the degradation of No-AUG-FLAG-MS2 mRNA, its stability was measured in a ski2Δ (Fig. 5a) or an xrn1Δ (Fig. 6a) mutant because the ski2Δ and xrn1Δ mutants are defective in 3′ to 5′ and 5′ to 3′ mRNA degradation activity, respectively. No-AUG-FLAG-MS2 mRNA (t½ = 5 min; Fig. 3b) was stabilized in the xrn1Δ mutant (t½ = 24 min) to a greater extent than in the ski2Δ mutant (t½ = 5.2 min) (Figs. 5a and 6a). The control AUG-FLAG-MS2 mRNA (t½ = 16 min; Fig. 3c) was significantly stabilized in the xrn1Δ mutant (t½ = 31 min) but was not stabilized at all in the ski2Δ mutant (t½ = 12 min) (Fig. 5, b and b). These results indicated that the 5′ to 3′ degradation pathway is mainly responsible for the degradation of both No-AUG-FLAG-MS2 and AUG-FLAG-MS2 mRNAs. It should be emphasized that the stabilities of AUG-FLAG-MS2 and No-AUG-FLAG-MS2 mRNA were almost the same in the xrn1Δ mutant (Fig. 6, a and b). This result strongly suggests that No-AUG-FLAG-MS2 mRNA is more efficiently degraded from the 5′ end than AUG-FLAG-MS2 mRNA and that the rate of degradation from the 3′ end might be the same for both mRNAs.
FIGURE 5.
Stabilization of non-translated mRNA by tethered Pab1–34Cp in a ski2Δ mutant requires a cap structure but not a poly(A) tail. The cells were grown in SC-Gal UraLeu medium, and RNA samples were prepared and analyzed by Northern blotting with a DIG labeled MPT4 probe. The stability of the mRNAs was measured as described in Fig. 3a. Relative quantities are shown as the mean values of three independent experiments. The half-lives of mRNAs are shown as the mean values with S.D. a, W303ski2Δ cells harboring pIT2071 (No-AUG-FLAG-MS) or pIT2070 (No-AUG-FLAG) were transformed with a control p415TEF1p-FLAG-MS2 plasmid or with a plasmid that encodes the FLAG-MS2-Pab1–34Cp fusion protein. b, W303ski2Δ cells harboring pIT2069 (AUG-FLAG-MS) or pIT2068 (AUG-FLAG) were transformed with a control p415TEF1p-FLAG-MS2 plasmid or with a plasmid that encodes the FLAG-MS2-Pab1–34Cp fusion protein. c, schematic drawing of the reporter mRNAs lacking a poly(A) tail or cap structure. The filled box indicates the open reading frame of MPT4ΔN, and the dark gray box indicates the FLAG tag. These boxes are shaded because these regions are not translated. The lines represent non-translated regions. The 3′-UTR region of the reporter genes contains the PGK1 3′-UTR in which two tandem MS2 binding sites were inserted. Rz indicates the sequences of a hammerhead ribozyme. d, W303ski2Δ cells harboring pIT2085 (No-AUG-FLAG-MS2-Rz) or pIT2084 (No-AUG-FLAG-Rz) were transformed with a control p415TEF1p-FLAG-MS2 plasmid or with a plasmid that encodes the FLAG-MS2-Pab1–34Cp fusion protein. e, W303ski2Δ cells harboring pIT2087 (Rz-No-AUG-FLAG-MS2-Rz) or the pIT2086 (Rz-No-AUG-FLAG-Rz) were transformed with a control p415TEF1p-FLAG-MS2 plasmid or with a plasmid that encodes the FLAG-MS2-Pab1–34Cp fusion protein.
FIGURE 6.
Stabilization of non-translated mRNA by tethered Pab1p-34Cp in an xrn1Δ 5′ to 3′ mRNA degradation mutant requires a poly(A) tail. The cells were grown in SC-Gal UraLeu medium, and RNA samples were prepared and analyzed by Northern blotting as described in Fig. 3a. Relative quantities are shown as the mean values of three independent experiments. The half-lives of mRNAs are shown as the mean values with S.D. a, W303xrn1Δ cells harboring pIT2071 (No-AUG-FLAG-MS) or pIT2070 (No-AUG-FLAG) were transformed with a control p415TEF1p-FLAG-MS2 plasmid or with a plasmid that encodes the FLAG-MS2-Pab1–34Cp fusion protein. b, W303xrn1Δ cells harboring pIT2069 (AUG-FLAG-MS) or pIT2068 (AUG-FLAG) were transformed with control p415TEF1p-FLAG-MS2 plasmid or with a plasmid that encodes the FLAG-MS2-Pab1–34Cp fusion protein. c, W303xrn1Δ cells harboring pIT2085 (No-AUG-FLAG-MS2-Rz) or pIT2084 (No-AUG-FLAG-Rz) were transformed with a control p415TEF1p-FLAG-MS2 plasmid or with a plasmid that encodes the FLAG-MS2-Pab1–34Cp fusion protein.
Stabilization of Non-translated mRNA by Tethered Pab1–34Cp in the ski2Δ Mutant Requires a Cap Structure but Not a Poly(A) Tail
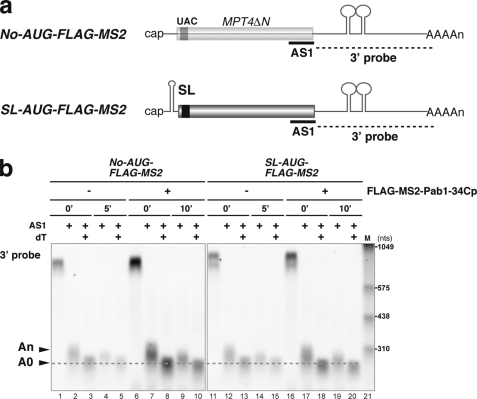
We next examined if tethering of Pab1p could inhibit degradation of No-AUG-FLAG-MS2 mRNA from the 5′ end (Fig. 5a). We found that tethering of FLAG-MS2-Pab1–34Cp stabilized No-AUG-FLAG-MS2 mRNA in the ski2Δ mutant by a factor of 2.5 (extending t½ from 5.2 to 13 min). This result suggests that tethering of FLAG-MS2-Pab1–34Cp may inhibit degradation of No-AUG-FLAG-MS2 mRNA from the 5′ end. Although tethering of FLAG-MS2-Pab1–34Cp inhibits degradation of No-AUG-FLAG-MS2 mRNA in the ski2Δ mutant, it was still possible that endogenous Pab1p may contribute to stabilization of the non-translated mRNA in concert with tethered FLAG-MS2-Pab1–34Cp by binding of Pab1p to the poly(A) tail of No-AUG-FLAG-MS2 mRNA. To eliminate the contribution of endogenous Pab1p association with the poly(A) tail to mRNA stabilization in the ski2Δ mutant, we removed the poly(A) tail by insertion of a hammerhead ribozyme sequence downstream of the MS2 binding sites, yielding the reporter No-AUG-FLAG-MS2-Rz (Fig. 5c). Cleavage of No-AUG-FLAG-MS2-Rz and No-AUG-FLAG-MS2 mRNA by RNase H treatment after binding of an antisense oligonucleotide AS1 that corresponds to the region just upstream of the termination codon and/or binding of oligo-(dT) followed by Northern blotting confirmed that No-AUG-FLAG-MS2-Rz mRNA does not contain a poly(A) tail (supplemental Fig. 2). Tethering of FLAG-MS2-Pab1–34Cp stabilized No-AUG-FLAG-MS2-Rz mRNA in the ski2Δ mutant by a factor of 3.8 (extending t½ from 6.1 to 23 min; Fig. 5d). These results clearly indicate that tethering of FLAG-MS2-Pab1–34Cp inhibits degradation of No-AUG-FLAG mRNA from the 5′ end in the absence of endogenous Pab1p binding to the poly(A) tail. Because the stabilizing effect of FLAG-MS2-Pab1–34Cp was stronger toward No-AUG-FLAG-MS2-Rz mRNA than toward No-AUG-FLAG-MS2 mRNA in the ski2Δ mutant, we suspect that endogenous Pab1p bound to the poly(A) tail or the poly(A) tail itself may destabilize the mRNA, although the mechanism by which it does so is unknown.
Our results indicate that tethering of FLAG-MS2-Pab1–34Cp inhibits degradation of No-AUG-FLAG mRNA from the 5′ end. To distinguish between the possibilities that tethering of FLAG-MS2-Pab1–34Cp may inhibit the decapping reaction or may inhibit exonucleolytic cleavage by Xrn1p, we inserted a hammerhead ribozyme sequence into the 5′-UTR. The resultant reporters, Rz-No-AUG-FLAG-MS2-Rz and Rz-No-AUG-FLAG-Rz, are expected to express reporter mRNAs that do not contain a cap structure. Meaux and Van Hoof (32) clearly showed that cytoplasmic exonuclease is mainly responsible for the degradation of such mRNAs, and we also observed that Rz-No-AUG-FLAG-MS2-Rz and Rz-No-AUG-FLAG-Rz were strongly stabilized in the xrn1Δ mutant (data not shown). Tethering of FLAG-MS2-Pab1–34Cp only slightly stabilized Rz-No-AUG-FLAG-MS2-Rz mRNA in the ski2Δ mutant (Fig. 5e), indicating that the tethering of Pab1p could not inhibit degradation of non-translated mRNA that lacks a cap structure. The combined data suggest that tethering of FLAG-MS2-Pab1–34Cp stabilizes non-translated mRNA by inhibiting the decapping reaction independent of translation.
Stabilization of Non-translated mRNA by Tethered Pab1–34Cp in the xrn1 Mutant Requires a Poly(A) Tail
Although No-AUG-FLAG-MS2 mRNA is efficiently degraded from the 5′ end, we examined the possibility that tethered Pab1–34Cp may inhibit degradation of No-AUG-FLAG-MS2 mRNA from the 3′ end. Tethering of FLAG-MS2-Pab1–34Cp stabilized No-AUG-FLAG-MS2 mRNA in the xrn1Δ mutant by a factor of 2.6 (extending its t½ from 24 to 62 min; Fig. 6a), suggesting that tethering of FLAG-MS2-Pab1p-34C may also inhibit degradation of No-AUG-FLAG-MS2 mRNA from the 3′ end. Tethering of FLAG-MS2-Pab1–34Cp did not stabilize No-AUG-FLAG-MS2-Rz mRNA in the xrn1Δ mutant (Fig. 6c). These results indicate that the poly(A) tail itself or endogenous Pab1p bound to the poly(A) tail is required for inhibition of the degradation of non-translated mRNA from the 3′ end by tethered FLAG-MS2-Pab1–34Cp.
Stabilization of Non-translated mRNA by Tethered Pab1–34Cp Results in the Accumulation of Deadenylated mRNA
If tethering of FLAG-MS2-Pab1–34Cp inhibits the decapping reaction, then stabilization of mRNA by tethering of Pab1–34Cp may lead to an accumulation of deadenylated full-length RNA in a wild type strain. To test this possibility, we determined the poly(A) length of non-translated mRNAs that are stabilized by tethering of Pab1–34Cp in a wild type strain. We, therefore, assayed the length of the RNase H cleavage product of these mRNAs after binding the antisense oligonucleotide AS1 and performed a subsequent analysis by Northern blotting (Fig. 7a). The No-AUG-FLAG-MS2 mRNAs were deadenylated 10 min after the transcription inhibition, although tethering of Pab1–34Cp stabilized No-AUG-FLAG-MS2 mRNA (Fig. 7b, lanes 10). The SL-AUG-FLAG-MS2 mRNAs were also deadenylated 10 min after the transcription inhibition, although tethering of Pab1–34Cp stabilized SL-AUG-FLAG-MS2 mRNA (Fig. 7b, lanes 20). These results suggest that tethering of FLAG-MS2-Pab1–34Cp leads to an accumulation of deadenylated No-AUG-FLAG-MS2 or SL-AUG-FLAG-MS2 mRNAs in wild type cells. These results strongly suggest that tethering of FLAG-MS2-Pab1–34Cp may inhibit the decapping of non-translated deadenylated mRNAs in yeast.
FIGURE 7.
Stabilization of non-translated mRNA by tethered Pab1–34Cp results in the accumulation of deadenylated mRNA. a, shown is a schematic drawing of the non-translated mRNAs and the antisense oligonucleotide used for the RNase H assay. The filled box indicates the open reading frame of MPT4ΔN, the black box indicates the FLAG tag, the lines represent non-translated regions, and the tract of As denotes the poly(A) tail. MS2 binding sites are indicated as stem-loop structures in the 3′-UTR. A stable stem-loop structure for inhibition of translation (SL) was introduced into the 5′-UTR of the AUG-FLAG-MS2. The probes for Northern hybridization are shown as dashed lines. The antisense oligonucleotide (AS1, 5′-CCAAAGATGGCAAGTTAGAAACGTCAATGT-3′) that was used for RNase H digestion is shown as a bold line. b, W303 cells harboring pIT2071 (No-AUG-FLAG-MS) or pIT2073 (SL-AUG-FLAG-MS2) were transformed with a control p415TEF1p-FLAG-MS2 plasmid or with a plasmid that encodes the FLAG-MS2-Pab1–34Cp fusion protein. Cells were grown in SC-Gal UraLeu medium, and RNA samples were prepared as in Fig. 3a. RNA samples of cells harboring a control p415TEF1p-FLAG-MS2 plasmid were prepared before (0 min) and 5 min (5′) after a shift to glucose medium. RNA samples of cells harboring a control a plasmid that encodes the FLAG-MS2-Pab1–34Cp fusion protein were prepared before (0 min) and 10 min (10′) after a shift to glucose medium. RNA samples were digested with RNase H after hybridization with the AS1 oligonucleotide (AS1) or with oligo dT (dT), and Northern blot analysis was subsequently performed with a DIG-labeled 3′ probe. The RNA size marker III (11373099910, Roche Applied Science) is loaded in lane 21.
In General, Tethering of FLAG-MS2-Pab1–34Cp Stabilizes Non-translated mRNAs
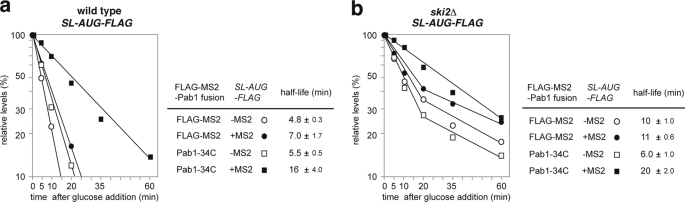
We found that tethering of Pab1p stabilized non-translated mRNA that did not contain an AUG codon in any frame. Therefore, we next tested if tethered Pab1p could stabilize another non-translated or poorly translated mRNA, SL-AUG-FLAG-MS2 mRNA. SL-AUG-FLAG-MS2 mRNA (t½ = 7 min; Fig. 8a) was more labile than AUG-FLAG-MS2 mRNA (t½ = 16 min; Fig. 3c). Two bands derived from the SL-AUG-FLAG reporter were detected by gel electrophoresis in the wild type as well as in the ski2Δ mutant but not in the xrn1Δ mutant (supplemental Fig. 3). Northern analysis after RNase H treatment after binding of an antisense oligonucleotide AS2 that corresponds to a region 100 nucleotides upstream from the termination codon revealed that the shorter mRNA corresponds to an intermediate mRNA form that lacks a 5′ region upstream of the stem-loop structure (supplemental Fig. 4). We assumed that degradation of this mRNA by Xrn1p might be temporally blocked by a stable mRNA secondary structure and that the two bands derived from the SL-AUG-FLAG reporter correspond to the full-length (FL) form and the intermediate mRNA form that arose during degradation. We found that tethering of FLAG-MS2-Pab1–34Cp stabilized AUG-FLAG-MS2 mRNA by a factor of 2.3 and extended its half-life from 7 to 16 min in wild type cells (Fig. 8a). We also found that tethering of MS2-Pab1–34Cp stabilized the full-length SL-AUG-FLAG-MS2 mRNA by a factor of 1.8 (extending its t½ from 11 to 20 min; Fig. 8b) in the ski2Δ mutant. The half-life of FL mRNA in the ski2Δ mutant should reflect its rate of degradation from the 5′ end. We propose that, in general, tethering of FLAG-MS2-Pab1–34Cp inhibits degradation of non-translated mRNAs from the 5′ end in yeast.
FIGURE 8.
Tethering of Pab1p-34C stabilizes mRNAs that contain a stable stem-loop structure at the 5′-UTR. The cells were grown in SC-Gal UraLeu medium. RNA samples were prepared, and mRNA stability was measured as described in Fig. 3a. Relative quantities are shown as the mean values of three independent experiments. The half-lives of mRNAs are shown as the mean values with S.D. a, W303 cells harboring the pIT2073 (SL-AUG-FLAG-MS2) plasmid were transformed with a control p415TEF1p-FLAG-MS2 plasmid or with a plasmid that encodes the FLAG-MS2-Pab1–34Cp fusion protein. b, W303ski2Δ cells harboring pIT2073 (SL-AUG-FLAG-MS2) were transformed with a control p415TEF1p-FLAG-MS2 plasmid or with a plasmid that encodes the FLAG-MS2-Pab1–34Cp fusion protein.
DISCUSSION
Tethered poly(A)-binding Protein May Inhibit Decapping of Non-translated mRNA in Yeast
It has been suggested that the poly(A)-binding protein inhibits mRNA decapping by a variety of mechanisms, which possibly connect several different processes such as translation, turnover, and mRNA localization. Although the inhibitory effects of poly(A)-binding protein on decapping are conserved between humans and yeast (7, 12, 23), the mechanism still remains to be elucidated. Three mechanisms by which poly(A)-binding protein might inhibit mRNA decapping in yeast have been proposed. The first proposed mechanism is that poly(A)-binding protein stimulates translation initiation, thereby blocking the recruitment of mRNA to the P-bodies, where only ribosome-free mRNAs are present (12, 24). The second mechanism is that poly(A)-binding protein inhibits decapping through interactions with the cap binding complex. The third mechanism suggests that poly(A)-binding protein inhibits decapping through interactions that are independent of the translation initiation complex.
The mechanism by which Pab1p stabilizes mRNA independent of translation is still largely unknown. In this study we investigated the role of yeast Pab1p in the stability of non-translated mRNA in yeast. The results shown in this study indicate that tethering of Pab1p inhibits the degradation of non-translated mRNA mainly from the 5′ end. Several lines of evidence lead us to propose that tethering of Pab1p may inhibit the decapping of non-translated mRNAs and that this stabilization does not require interaction between Pab1p and eIF4G. First, Pab1p stabilization required the Pab1p RRM3 domain but not the RRM1–2 domains, which are the domains that interact with eIF4G (Fig. 3b). Second, tethering of Pab1p that lacks the eIF4G interaction domain (Pab1–34Cp) stabilizes non-translated mRNAs in mutant cells that express eIF4G1-ΔN300, which lacks a Pab1p interacting domain but does not express eIF4G2 (Fig. 3d). Third, tethering of Pab1–34Cp stabilizes non-translated mRNAs without affecting the association of the mRNA with the ribosome (Fig. 4). Fourth, non-translated mRNA lacking a poly(A) tail was also stabilized by tethered Pab1–34Cp in a ski2Δ mutant (Fig. 5d). Moreover, in the ski2Δ mutant, tethered Pab1–34Cp stabilized non-translated mRNA without a poly(A) tail but not non-translated mRNA that lacks both a poly(A) tail and a cap structure (Fig. 5e). Fifth, stabilization of non-translated mRNA by tethered Pab1–34Cp resulted in the accumulation of deadenylated mRNA in wild type cells (Fig. 7). These results are consistent with the possibility that tethered Pab1–34Cp may inhibit decapping of deadenylated No-AUG-FLAG-MS2 mRNA. The combined results support the proposed mechanism that poly(A)-binding protein may inhibit the decapping of non-translated mRNA. Because tethering of FLAG-MS2-Pab1–34Cp stabilizes SL-AUG-FLAG-MS2 mRNA (Fig. 8), the poly(A)-binding protein may generally inhibit decapping of non-translated mRNA in yeast. These results lead us to propose that tethering of Pab1p may inhibit decapping of mRNAs that are poorly associated with ribosomes in yeast.
What is the mechanism by which tethering of FLAG-MS2-Pab1–34Cp inhibits the decapping of non-translated mRNA? One possible mechanism is that tethered FLAG-MS2-Pab1–34Cp inhibits the recruitment of the Lsm1–7 complex and/or Pat1p to deadenylated mRNA. It was shown that depletion of the Lsm1–7 complex or of Pat1p results in increased stability of mRNAs and accumulation of full-length, capped transcripts with shortened poly(A) tails (33–36). Moreover, the Lsm1–7 complex preferentially binds to deadenylated mRNA in vivo (36). A second possibility is that tethered Pab1p may directly bind to the mRNA cap structure and inhibit the decapping reaction. It has been proposed that the human poly(A)-binding protein can function in this manner (37). However, the in vivo relevance of the binding of the poly(A)-binding protein to the cap structure in the regulation of mRNA stabilization remains to be elucidated. Furthermore, direct binding of yeast Pab1p to the cap structure remains to be demonstrated by biochemical methods.
Tethered Poly(A)-binding Protein Inhibits Degradation of Non-translated mRNA from the 3′ End in Yeast
Tethering of FLAG-MS2-Pab1–34Cp stabilized No-AUG-FLAG-MS2 mRNA in the xrn1Δ mutant by a factor of 2.2 but did not stabilize No-AUG-FLAG-MS2 mRNA that lacks a poly(A) tail (Fig. 6). These results suggest that the poly(A) tail itself or endogenous Pab1p bound to the poly(A) tail is required for inhibition of the degradation of non-translated mRNA from the 3′ end by tethered FLAG-MS2-Pab1–34Cp. These data suggest that tethered Pab1p may inhibit deadenylation but not exosome activity in the xrn1Δ mutant. Because deadenylation is severely impaired in the pab1 mutant, it has been proposed that Pab1p bound to poly(A) may stimulate deadenylation in yeast. We, therefore, suspect that inhibition of deadenylation by tethered Pab1p may be an artifact and may not be able to take place in normal condition.
Translation Inhibits Degradation of mRNA from the 5′ End
It has been proposed that dissociation of the ribosome is a prerequisite for decapping in yeast (12). The results of our study consistently suggest that translation inhibits degradation mainly from the 5′ end but not from the 3′ end of mRNA. First, AUG-FLAG-MS2 mRNA is more stable than No-AUG-FLAG-MS2 mRNA in wild type cells and in the ski2Δ mutant (Fig. 5). Second, the stability of AUG-FLAG-MS2 and No-AUG-FLAG-MS2 mRNA was almost the same in the xrn1Δ mutant (Fig. 6). The stability of AUG-FLAG-MS2 and No-AUG-FLAG-MS2 mRNA was also almost the same in YAS2071 mutant cells that express eIF4G1-ΔN300 but not eIF4G2 (Fig. 3e). The growth rate of the mutant cells was significantly reduced (doubling time was extended from 2 to 6 h), suggesting that translation may not be sufficient to stabilize mRNA in this mutant. These results indicate that translation inhibits degradation of mRNA from the 5′ end as previously proposed. Shortening of the poly(A) tail is the rate-limiting step in the degradation of most mRNAs and release of the poly(A)-binding protein from mRNA by deadenylation may trigger decapping. A recent study clearly showed that the transition to the ribosome-free state is not a prerequisite for the decapping of mRNA (15). Therefore, the mechanism by which binding of the ribosome inhibits decapping remains to be elucidated.
Implication of Our Findings for the Stability of mRNA-type Non-coding RNA
Non-coding RNAs (ncRNAs) are involved in a wide spectrum of regulatory functions. Despite the variety of their biological functions, decay pathways of ncRNAs are still largely unknown. Although many mRNA-like ncRNAs have been identified in eukaryotes (38, 39), the roles of poly(A) tails and poly(A)-binding protein in the stability of mRNA-like ncRNAs have not been previously analyzed. The results of our study suggest that a population of mRNA-like ncRNAs, which contain cap and poly(A) tails but are not associated with a ribosome, may be stabilized by the poly(A)-binding protein. Our results indicate that poly(A)-binding protein may play an important role in the stabilization of mRNA-like ncRNAs as well as in stabilization of translating mRNAs.
Supplementary Material
Acknowledgments
We thank all members of the laboratory, especially Dr. Kazushige Kuroha for technical support and unpublished results and Hideyuki Gotoda and Tsuyako Tatematsu for construction of the strains and plasmids. We also thank Eri Asai for technical support.
This work was supported by grants-in-aid from the Ministry of Education, Culture, Sports, Science, and Technology of Japan (to T. I.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental information and Figs. 1–4.
- DIG
- digoxigenin
- FL
- full-length
- ncRNA
- non-coding RNA.
REFERENCES
- 1.Decker C. J., Parker R. (1994) Trends Biochem. Sci. 19, 336–340 [DOI] [PubMed] [Google Scholar]
- 2.Beelman C. A., Stevens A., Caponigro G., LaGrandeur T. E., Hatfield L., Fortner D. M., Parker R. (1996) Nature 382, 642–646 [DOI] [PubMed] [Google Scholar]
- 3.Muhlrad D., Decker C. J., Parker R. (1995) Mol. Cell. Biol. 15, 2145–2156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang Z., Kiledjian M. (2001) Cell 107, 751–762 [DOI] [PubMed] [Google Scholar]
- 5.Dunckley T., Parker R. (1999) EMBO J. 18, 5411–5422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van Dijk E., Cougot N., Meyer S., Babajko S., Wahle E., Séraphin B. (2002) EMBO J. 21, 6915–6924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang Z., Jiao X., Carr-Schmid A., Kiledjian M. (2002) Proc. Natl. Acad. Sci. U.S.A. 99, 12663–12668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Steiger M., Carr-Schmid A., Schwartz D. C., Kiledjian M., Parker R. (2003) RNA 9, 231–238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hsu C. L., Stevens A. (1993) Mol. Cell. Biol. 13, 4826–4835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mitchell P., Petfalski E., Shevchenko A., Mann M., Tollervey D. (1997) Cell 91, 457–466 [DOI] [PubMed] [Google Scholar]
- 11.Anderson J. S., Parker R. P. (1998) EMBO J. 17, 1497–1506, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Coller J., Parker R. (2004) Annu. Rev. Biochem. 73, 861–890 [DOI] [PubMed] [Google Scholar]
- 13.Balagopal V., Parker R. (2009) Curr. Opin. Cell Biol. 21, 403–408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kulkarni M., Ozgur S., Stoecklin G. (2010) Biochem. Soc. Trans. 38, 242–251 [DOI] [PubMed] [Google Scholar]
- 15.Eulalio A., Behm-Ansmant I., Izaurralde E. (2007) Nat. Rev. Mol. Cell Biol. 8, 9–22 [DOI] [PubMed] [Google Scholar]
- 16.Parker R., Sheth U. (2007) Mol. Cell 25, 635–646 [DOI] [PubMed] [Google Scholar]
- 17.Hu W., Sweet T. J., Chamnongpol S., Baker K. E., Coller J. (2009) Nature 461, 225–229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Decker C. J., Parker R. (1993) Genes Dev. 7, 1632–1643 [DOI] [PubMed] [Google Scholar]
- 19.Brown C. E., Sachs A. B. (1998) Mol. Cell. Biol. 18, 6548–6559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Caponigro G., Parker R. (1995) Genes Dev. 9, 2421–2432 [DOI] [PubMed] [Google Scholar]
- 21.Morrissey J. P., Deardorff J. A., Hebron C., Sachs A. B. (1999) Yeast 15, 687–702 [DOI] [PubMed] [Google Scholar]
- 22.Coller J. M., Gray N. K., Wickens M. P. (1998) Genes Dev. 12, 3226–3235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wilusz C. J., Gao M., Jones C. L., Wilusz J., Peltz S. W. (2001) RNA 7, 1416–1424 [PMC free article] [PubMed] [Google Scholar]
- 24.Coller J., Parker R. (2005) Cell 122, 875–886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Otero L. J., Ashe M. P., Sachs A. B. (1999) EMBO J. 18, 3153–3163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kühn U., Wahle E. (2004) Biochim. Biophys. Acta 1678, 67–84 [DOI] [PubMed] [Google Scholar]
- 27.Inada T., Aiba H. (2005) EMBO J. 24, 1584–1595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kuroha K., Tatematsu T., Inada T. (2009) EMBO Rep. 10, 1265–1271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sambrook J., Russell D. W. (2001) Molecular Cloning, 3rd Ed., Cold Spring Harbor Laboratory Press, Cold Spring Harbor, New York [Google Scholar]
- 30.Kessler S. H., Sachs A. B. (1998) Mol. Cell. Biol. 18, 51–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tarun S. Z., Jr., Wells S. E., Deardorff J. A., Sachs A. B. (1997) Proc. Natl. Acad. Sci. U.S.A. 94, 9046–9051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Meaux S., Van Hoof A. (2006) RNA 12, 1323–1337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tharun S., He W., Mayes A. E., Lennertz P., Beggs J. D., Parker R. (2000) Nature 404, 515–518 [DOI] [PubMed] [Google Scholar]
- 34.Tharun S., Parker R. (2001) Mol. Cell 8, 1075–1083 [DOI] [PubMed] [Google Scholar]
- 35.Chowdhury A., Tharun S. (2009) RNA 15, 1837–1848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chowdhury A., Tharun S. (2008) RNA 14, 2149–2158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Khanna R., Kiledjian M. (2004) EMBO J. 23, 1968–1976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Carninci P. (2008) Nat. Cell Biol. 10, 1023–1024 [DOI] [PubMed] [Google Scholar]
- 39.Imanishi T., Itoh T., Suzuki Y., O'Donovan C., Fukuchi S., Koyanagi K. O., Barrero R. A., Tamura T., Yamaguchi-Kabata Y., Tanino M., Yura K., Miyazaki S., Ikeo K., Homma K., Kasprzyk A., Nishikawa T., Hirakawa M., Thierry-Mieg J., Thierry-Mieg D., Ashurst J., Jia L., Nakao M., Thomas M. A., Mulder N., Karavidopoulou Y., Jin L., Kim S., Yasuda T., Lenhard B., Eveno E., Suzuki Y., Yamasaki C., Takeda J., Gough C., Hilton P., Fujii Y., Sakai H., Tanaka S., Amid C., Bellgard M., Bonaldo Mde F., Bono H., Bromberg S. K., Brookes A. J., Bruford E., Carninci P., Chelala C., Couillault C., de Souza S. J., Debily M. A., Devignes M. D., Dubchak I., Endo T., Estreicher A., Eyras E., Fukami-Kobayashi K., Gopinath G. R., Graudens E., Hahn Y., Han M., Han Z. G., Hanada K., Hanaoka H., Harada E., Hashimoto K., Hinz U., Hirai M., Hishiki T., Hopkinson I., Imbeaud S., Inoko H., Kanapin A., Kaneko Y., Kasukawa T., Kelso J., Kersey P., Kikuno R., Kimura K., Korn B., Kuryshev V., Makalowska I., Makino T., Mano S., Mariage-Samson R., Mashima J., Matsuda H., Mewes H. W., Minoshima S., Nagai K., Nagasaki H., Nagata N., Nigam R., Ogasawara O., Ohara O., Ohtsubo M., Okada N., Okido T., Oota S., Ota M., Ota T., Otsuki T., Piatier-Tonneau D., Poustka A., Ren S. X., Saitou N., Sakai K., Sakamoto S., Sakate R., Schupp I., Servant F., Sherry S., Shiba R., Shimizu N., Shimoyama M., Simpson A. J., Soares B., Steward C., Suwa M., Suzuki M., Takahashi A., Tamiya G., Tanaka H., Taylor T., Terwilliger J. D., Unneberg P., Veeramachaneni V., Watanabe S., Wilming L., Yasuda N., Yoo H. S., Stodolsky M., Makalowski W., Go M., Nakai K., Takagi T., Kanehisa M., Sakaki Y., Quackenbush J., Okazaki Y., Hayashizaki Y., Hide W., Chakraborty R., Nishikawa K., Sugawara H., Tateno Y., Chen Z., Oishi M., Tonellato P., Apweiler R., Okubo K., Wagner L., Wiemann S., Strausberg R. L., Isogai T., Auffray C., Nomura N., Gojobori T., Sugano S. (2004) PLoS Biol. 2, e16215103394 [Google Scholar]
- 40.Dimitrova L. N., Kuroha K., Tatematsu T., Inada T. (2009) J. Biol. Chem. 284, 10343–10352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mumberg D., Müller R., Funk M. (1994) Nucleic Acids Res. 22, 5767–5768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mumberg D., Müller R., Funk M. (1995) Gene 156, 119–122 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.