Abstract
Vascular endothelial cell (VEC) permeability is largely dependent on the integrity of vascular endothelial cadherin (VE-cadherin or VE-Cad)-based intercellular adhesions. Activators of protein kinase A (PKA) or of exchange protein activated by cAMP (EPAC) reduce VEC permeability largely by stabilizing VE-Cad-based intercellular adhesions. Currently, little is known concerning the nature and composition of the signaling complexes that allow PKA or EPAC to regulate VE-Cad-based structures and through these actions control permeability. Using pharmacological, biochemical, and cell biological approaches we identified and determined the composition and functionality of a signaling complex that coordinates cAMP-mediated control of VE-Cad-based adhesions and VEC permeability. Thus, we report that PKA, EPAC1, and cyclic nucleotide phosphodiesterase 4D (PDE4D) enzymes integrate into VE-Cad-based signaling complexes in human arterial endothelial cells. Importantly, we show that protein-protein interactions between EPAC1 and PDE4D serve to foster their integration into VE-Cad-based complexes and allow robust local regulation of EPAC1-based stabilization of VE-Cad-based adhesions. Of potential translational importance, we mapped the EPAC1 peptide motif involved in binding PDE4D and show that a cell-permeable variant of this peptide antagonizes EPAC1-PDE4D binding and directly alters VEC permeability. Collectively, our data indicate that PDE4D regulates both the activity and subcellular localization of EPAC1 and identify a novel mechanism for regulated EPAC1 signaling in these cells.
Keywords: Atherosclerosis, β-Catenin, Cell Junctions, Cyclic AMP (cAMP), Endothelium, Cadherin
Introduction
Vascular endothelial cadherins (VE-Cad)3 localize at vascular endothelial cell-cell junctions and regulate vascular endothelial cell (VEC) permeability by forming Ca2+-dependent intercellular adhesions (1–3). VE-Cad-based adhesions are stabilized by recruitment of β-catenin, p120-catenin, and γ-catenin and their subsequent interactions with the actin cytoskeleton (1–3). VE-Cad-based adhesion integrity is destabilized by vascular permeability-inducing factors including the vascular endothelial growth factor (VEGF) (4–6). Indeed, VEGF reduces VE-Cad-based adhesion integrity and increases permeability by promoting phosphorylation of VE-Cad, or of components of the VE-Cad complex, and by inducing VE-Cad complex disassembly and internalization (5–10).
By increasing the integrity of VE-Cad-based adhesions and promoting their interactions with the actin cytoskeleton, activators of cAMP signaling decrease basal VEC permeability and antagonize the effects of VEGF (11–15). These barrier-stabilizing effects of activators of cAMP signaling are coordinated through effects by either protein kinase A (PKA) or exchange protein activated by cAMP (EPAC). The selectivity of cAMP-mediated cellular effects depends on selective anchoring of PKA, and perhaps EPAC, at defined intracellular sites (16–18). In this context, recent evidence has established that the anchoring of cyclic nucleotide phosphodiesterases (PDEs) at these sites is equally critical to subcellular selective regulation of cAMP signaling (19, 20). Herein, we show that EPAC1 represents the dominant effector coordinating the permeability-reducing effects of cAMP-elevating agents in human arterial endothelial cells (HAECs). We demonstrate that EPAC1 decreases permeability through local actions within VE-Cad-based signaling complexes and that EPAC1 and PDE4D interact within this complex. Lastly, we characterize molecularly the determinants that coordinate direct EPAC1 and PDE4D binding and report that this EPAC1-PDE4D interaction is essential for the integration of these proteins into VE-Cad-based complex and for them to coordinate the effects of cAMP-elevating agents on permeability. Our findings are discussed in the context of the identification of this novel HAEC VE-Cad-based “signalosome” and its potential as a therapeutic target in continuing efforts to regulate HAEC permeability in both health and disease.
EXPERIMENTAL PROCEDURES
Materials
HAECs, human microvascular endothelial cells (HMVECs), endothelial basal medium (EBM-2), and endothelial growth medium bullet kit (EGM-2) were purchased from Lonza (Walkersville, MD). Lipofectamine 2000, several siRNA targeting constructs (PDE4D, 5′-GAC AAG CAC AAU GCU UCC GUG GAA A-3′; PRKACA, 5′-GGA AGC UCC CUU CAU ACC AAA GUU U-3′; EPAC1, 5′-AUU GAG AUU CUU CUG CUC CUU GAG G-3′; and a high GC universal negative control) were all from Invitrogen. Tissue culture flasks also were obtained from Invitrogen. Permeability assay plates, 6.5-mm diameter Transwell inserts (0.4-μm pore size, tissue culture-treated polyester membrane), and polystyrene 24-well plates were from Costar (Corning, NY), and fluorescein isothiocyanate (FITC)-tagged dextran (70 kDa) was from Sigma-Aldrich. A plasmid encoding a chimeric protein composed of the extracellular domain of VE-Cad and immunoglobulin Fc (Fc-VE-Cad) was a gift from Dr. M. Shasby (University of Iowa College of Medicine, Iowa City, Iowa). A GST-β-catenin bacterial expression plasmid was a gift from Dr. L. Mulligan (Queen's University, Kingston, Ontario, Canada). Anti-human VE-Cad (BV6 clone) was purchased from Alexis Biochemicals (San Diego, CA). Anti-β-catenin, anti-PKA-RIIβ, and anti-PKA-C were from Transduction Laboratories (Lexington, KY), and anti-PDE3B and anti-PDE4D were each generously provided by ICOS Corp. (Bothell, WA). Anti-FLAG (M2)-agarose was purchased from Sigma-Aldrich. The FC-VE-Cad chimeric protein was expressed in 293E cells transiently transfected with a plasmid encoding this protein and purified from conditioned media using protein A-Sepharose beads.
Cell Culture, Transfections, and Permeability Studies
HAECs or HMVECs were cultured as described previously (22). For transfection, cells at 80% confluence were incubated with Lipofectamine 2000, and the siRNA was at a 1:1 ratio as per the manufacturer's directions. RNAi-dependent knockdown or RNAi-mediated effects on cell structures or permeability were assessed 48 h post-transfection. For permeability studies, HAEC (3.5 × 105 cells/ml, control cells or cells transfected with individual siRNA constructs) or HMVEC in EGM-2/VEGF (10 ng/ml)-supplemented EBM-2 were allowed to proliferate on the upper surface of fibronectin (5 μg/μl)-coated Transwells (0.4-μm pores). After 18–20 h, FITC-labeled dextran (70 kDa) or dextran and the test agents used was added. Permeability to dextran and the impact of treatments were measured by sampling the lower chamber of the Transwell after 1 h. Fluorescence was measured using a Molecular Devices SpectraMAX Gemini X5 and quantified using SoftMax Pro 4.8 software. All experiments were carried out in quadruplicate and repeated at least three times.
For immunostaining of VECs, control HAECs or HAECs following RNAi were grown to confluence (20 μg/μl fibronectin-coated coverslips), washed in PBS, and treated with dimethyl sulfoxide (0.1% v/v), Ro 20-1724 (Ro, 1–10 μm), 8-CPT-cAMP (10–100 μm), 6-Bz-cAMP (3–30 μm), forskolin (Fsk, 1–10 μm), or combinations of these agents for 5 min prior to the addition of EGTA (5 mm final concentration) and treatment with VEGF (100 ng/ml) at 37 °C in a 5% CO2 humidified environment. The concentrations of Fsk and Ro used were based on our previous studies (21). The concentrations of 8-CPT-cAMP and 6-Bz-cAMP used in our experiments were determined in preliminary experiments to represent the maximal concentrations that selectively activated Rap1 (measure of EPAC activation pulldown assay) and PKA (Ser-133 cAMP-response element-binding protein (CREB) phosphorylation) in these cells, respectively. Cells were fixed with paraformaldehyde (4% w/v) at predetermined times and stained for VE-Cad with anti-human VE-Cad (BV6 clone), actin with TRITC-phalloidin, and nuclei with 4′,6′-diamidino-2-phenylindole (DAPI). Fluorescent digital images of fixed HAEC were acquired using a Zeiss Axiovert S100 microscope at a magnification of ×40. Because discontinuity in VE-Cad membrane staining is a hallmark of increased VEC permeability, changes in contiguous VE-Cad staining were used to measure the impact of cAMP on these structures in these experiments. Thus, digital images of VE-Cad-stained HAEC cultures were captured, converted to binary format, and rendered as “skeletonized” 1 × 1-pixel images (NIH ImageJ software) as described previously by our laboratory in a collaborative study (21). The number of segments of VE-Cad staining greater than 100 pixels (4 μm) in images from individual treatments was recorded, and the impact of drug or RNAi treatments on these structures was assessed by comparing their numbers in replicates (n ≥ 6) of separate treatments.
Peptide Array Analysis
An EPAC1 peptide library of 25 individual amino acid peptides, each displaced by five amino acids, was immobilized on cellulose membranes using automated SPOT synthesis as described previously (23). The interaction between immobilized peptides and GST (10 μg/ml) or a GST-PDE4D3 fusion protein (10 μg/ml) was determined by overlaying membranes with recombinant proteins. Following repeated washing, peptides that bound selectively with GST-PDE4D3 were identified by immunoblot analysis with an anti-GST antiserum.
Isolation of VE-Cad-based Complexes
HAECs were lysed in a Tris (50 mm, pH 7.4)-based lysis buffer supplemented with 1% Triton X-100, 150 mm sodium chloride, 10 mm sodium pyrophosphate, 10 mm sodium β-glycerophosphate, 10 mm sodium fluoride, 1 μg/ml pepstatin A, 1 μg/ml E-64, 20 μg/ml bestatin, 100 μg/ml phenylmethylsulfonyl fluoride (PMSF), 1 μg/ml aprotinin, 1 μg/ml leupeptin, 5 mm benzamidine, and 10 mm sodium orthovanadate. Prior to their addition to immobilized Fc-VE-Cad, HAEC lysates (1–2 mg of protein/ml) were incubated with 100 μl (packed volume) of protein A/G-Sepharose for 1 h and centrifuged (1000 × g, 5 min) to remove insoluble and nonspecific Sepharose-binding proteins. These precleared HAEC cell lysates were incubated with immobilized Fc-VE-Cad beads in the presence of CaCl2 (2 mm final concentration) on a rotating platform at 4 °C for 16–24 h. Following this incubation, beads were collected by centrifugation and washed extensively in the Ca2+-containing buffer. Fc-VE-Cad-binding proteins were eluted from the beads by incubation with EGTA (20 mm) and subsequently concentrated (∼10-fold) by centrifugation at 18,000 × g for 15 min using IEC Centra CL3R centricons.
Isolation of β-Catenin-based Complexes
HAECs were lysed in a lysis buffer identical to that described above for VE-Cad complex isolation, except that Triton-X100 was substituted for 0.05% SDS, 1.0% Igepal, and 0.5% sodium deoxycholate. Proteins specifically bound to β-catenin were removed from the beads by incubation with an SDS-based electrophoresis loading buffer (21). In some experiments the ability of selected peptides to displace proteins from this complex was studied. In these experiments, the peptide was incubated with cells for 3 h prior to pulldown. Proteins isolated from either of these pulldown assays were resolved by SDS-PAGE, transferred to nitrocellulose membranes, and immunoblotted for the proteins of interest. The following antisera were used in our studies: anti-VE-cadherin (1:1000), anti-β-catenin (1:500), anti-p120 catenin (1:200), anti-PKA-RII (1:500), anti-PKA-C (1:1000), anti-PDE3B (1:4000), anti-PDE4D (1:4000), anti-PDE4B, and anti-FLAG (1:10,000).
Determination of HAEC cAMP
For these studies, confluent monolayers of VECs were incubated overnight with [3H]adenine (10 μCi/ml serum, 2 μm) and [3H]cAMP was measured as described previously (21).
Statistics
All data presented in this study were obtained from at least four individual similar experiments. Within individual experiments, the variables were tested either in triplicate or quadruplicate. Differences between individual test results were considered statistically significance at p < 0.05 as assessed by one-way analysis of variance with Neuman post hoc tests. Because of the nature of some of our data, on occasion the data are presented as individual immunoblots or selected images of immunostained cells. When individual immunoblots or selected images of immunostained human VECs are shown, they are representative of similar results obtained from at least three separate experiments.
RESULTS
Activators of cAMP Signaling Reduce Permeability of Human VEC
The activators of cAMP signaling used were: a β-adrenergic receptor agonist, isoproterenol (0.1–1 μm); an adenylyl cyclase activator, Fsk (1 μm); a selective PKA-activating cAMP analog, 6-Bz-cAMP (3–30 μm) (24); a selective EPAC-activating cAMP analog, 8-CPT-cAMP (1–100 μm) (24); and a PDE4-selective inhibitor, Ro (10 μm) (20); each reduced the VEGF-induced (10 ng/ml) permeability of both HAEC (Fig. 1) and HMVEC (not shown). In marked contrast, PDE3 inhibition (cilostamide, 1 μm (19)) did not reduce the permeability of either HAECs (Fig. 1) or HMVECs (not shown). Ro potentiated Fsk-mediated decreases in human VEC permeability, but cilostamide did not (Fig. 1).
FIGURE 1.
cAMP-elevating agents or selective activators of PKA or EPAC decrease human arterial VEC permeability. The impact of isoproterenol (Iso; 1 μm), Fsk (1 μm), 6-Bz-cAMP (3 μm), 8-CPT-cAMP (10 μm), Ro (10 μm), cilostamide (Cil; 1 μm), or combinations of some of these agents on HAEC permeability was measured using transit of FITC-dextran over a 1-h time span (“Experimental Procedures”). Values are means ± S.E. from seven experiments and expressed as the percentage (%) of decreases in permeability compared with control values (dimethyl sulfoxide, 0.5% v/v (Control)). *, significant differences between treatments and control; **, significant differences between treatments and Fsk (1 μm) alone (p < 0.05); NS, not significant.
Activators of cAMP Signaling Stabilize VE-Cad-based Structures in HAEC
Ca2+ chelation with EGTA destabilized VE-Cad-based adhesions (Fig. 2A, compare rows A and B, Vehicle), collapsed the actin cytoskeleton (not shown), and caused marked cell rounding. Return of Ca2+ (2 mm, 2 h) following EGTA removal promoted cell flattening, allowed evident reestablishment of VE-Cad (Fig. 2A, compare rows B and C, Vehicle) and actin-based structures (not shown). Introduction of 6-Bz-cAMP (3 μm), 8-CPT-cAMP (10 μm), or both of these agents did not evidently alter VE-Cad-based structures in control stable monolayers of these cells (Fig. 2A, Row A). In contrast, the addition of either 6-Bz-cAMP or 8-CPT-cAMP did reduce EGTA-induced cell rounding and loss of VE-Cad-based structures (Fig. 2A, compare rows A and B). Although either 6-Bz-cAMP or 8-CPT-cAMP promoted cell flattening and the reestablishment of VE-Cad-based structures, when used at the maximally selective concentrations of these agents, 8-CPT-cAMP had a more marked effect (Fig. 2A, compare rows B and C). Indeed, although Ca2+ supplementation alone promoted the reestablishment of contiguous VE-Cad-based structures in HAEC by 32 ± 7%, this effect was increased to 49 ± 7 or 90 ± 6% by 6-Bz-cAMP or 8-CPT-cAMP, respectively (mean ± S.E., n = 8, p < 0.05). Fsk (1 μm) clearly reduced cell rounding and inhibited the loss of border VE-Cad staining (Fig. 2A, compare rows A and B). Furthermore, the addition of 1 or 10 μm Fsk promoted Ca2+-dependent reestablishment of VE-Cad-based structures to 52 ± 9 and 94 ± 3%, respectively (mean ± S.E., n = 8, p < 0.05). Interestingly, Ro (10 μm) inhibited EGTA-induced reductions in VE-Cad-based structures, promoted their reestablishment upon Ca2+ supplementation to levels similar to those achieved with Fsk (1 μm), and potentiated the effects of Fsk (1 μm) (Fig. 2A). In our experiments, the addition of Ro or 8-CPT-cAMP increased the levels of membrane actin-based structures, whereas 6-Bz-cAMP and Fsk both tended to decrease actin stress fibers content (not shown). As when Ca2+ chelation was used, 8-CPT-cAMP and Ro each antagonized VEGF-induced destabilization of VE-Cad-based structures more markedly than 6-Bz-cAMP at the selective concentrations used in our studies (not shown). To complement the findings obtained using selective PKA or EPAC activators or PDE inhibitors, an RNAi-based knockdown strategy was adopted. Notwithstanding our finding that 6-Bz-cAMP reduced HAEC permeability, knockdown of PKA-Cα (Fig. 2B) did not significantly alter HAEC permeability (Fig. 2C). In contrast, but consistent with the observation that 8-CPT-cAMP decreased cell permeability, EPAC1 knockdown (Fig. 2C) markedly increased HAEC permeability (Fig. 2C). Because PDE4 inhibition decreased permeability (Fig. 1), we predicted that knockdown of the dominant PDE4 expressed in HAEC (PDE4D (21); Fig. 2B) would increase basal cAMP and decrease permeability. Although PDE4D knockdown did increase HAEC cAMP and significantly potentiated Fsk-induced increases in cAMP (Table 1), it markedly increased, rather than decreased, HAEC permeability. Indeed, the impact of PDE4D knockdown on HAEC permeability was similar to that caused by EPAC1 knockdown (50 ± 5% versus 65 ± 9% increased permeability, mean ± S.E., n = 11; Fig. 2C). Obviating a role for other PDEs, knockdown of the other PDE4 expressed in these cells (PDE4B (21)) or of PDE3B (21) did not alter permeability (not shown). Under all experimental conditions, a weak correlation was observed between global cellular HAEC cAMP and permeability (Table 1), which is to be expected if, as is common to most/all cells, cAMP signaling is compartmentalized with PDE3 and PDE4 controlling very different compartments (20).
FIGURE 2.
Activators of PKA or EPAC and selective knockdown of these cAMP effectors differentially impact the integrity of VE-Cad-based HAEC structures and cell permeability. A, confluent HAEC monolayer cultures were incubated with dimethyl sulfoxide (0.5% v/v (Vehicle)) or with test agents (as indicated) for 10 min. Following these incubations, HAEC were separated into three groups for analysis. Group 1, Row A: cells were immediately fixed (paraformaldehyde, 4% w/v). Group 2, Row B: cells were treated with EGTA (5 mm, 20 min) and subsequently fixed as is Group 1. Group 3, Row C: after removal of EGTA, cells were either supplemented with a Ca2+ (2 mm) solution containing no other additions or supplemented with test agents for 2 h and then fixed as in Group 1. VE-Cad-based structures were visualized by staining with anti-VE-Cad (BV6 clone). Fluorescent images were acquired and processed as described under “Experimental Procedures.” The impact of treatments on the integrity of VE-Cad-based structures was determined by comparing the number of contiguous BV6-stained plasma membrane segments with lengths greater than 100 pixels in eight individual random sections from at least six separate experiments. A representative image of results obtained in each treatment paradigm is shown, and the statistical bases of claims are presented under “Experimental Procedures.” B, HAEC were transfected with PKA-Cα-, EPAC1-, or PDE4D-selective siRNAs or a control siRNA and were subsequently allowed to propagate for 48 h. The levels of the relevant target proteins in HAEC transfected with the selective siRNAs were determined by immunoblot analysis. C, The impact of selective PKA, EPAC1, or PDE4D knockdown on basal HAEC permeability and permeability subsequent to incubation with the PKA-, EPAC-, or PDE4D-selective agents described in A was determined as described in the legend for Fig. 1. D, following this growth phase, HAEC were incubated with either saline or VEGF-supplemented saline for 20 min, after which they were fixed as described in A for Group 1.
TABLE 1.
Impact of siRNA-based knockdown of PKA-Cα, EPAC1, and PDE4D on HAEC cAMP
siRNA were used to selectively knock down the level of PKA-Cα, EPAC1, or PDE4D in HAECs. The effect of Fsk or Ro on cAMP levels in control HAECs, or those in which PKA-Cα, EPAC1 or PDE4D were reduced, was measured as described under “Experimental Procedures.”
| Additions | Control siRNA | PKA-Cα siRNA | EPAC1 siRNA | PDE4D siRNA |
|---|---|---|---|---|
| [3H]cAMP (% total [3H]) | [3H]cAMP (% total [3H]) | [3H]cAMP (% total [3H]) | [3H]cAMP (% total [3H]) | |
| None | 0.081 ± 0.001 | 0.091 ± 0.005a | 0.082 ± 0.004 | 0.091 ± 0.008a |
| Ro (10 μm) | 0.082 ± 0.008 | 0.115 ± 0.008a | 0.096 ± 0.008 | 0.097 ± 0.002a |
| Fsk (1 μm) | 0.093 ± 0.007 | 0.109 ± 0.008a | 0.100 ± 0.010b | 0.122 ± 0.008a |
| Fsk (1 μm) + Ro (10 μm) | 0.128 ± 0.011b | 0.158 ± 0.010b | 0.133 ± 0.014b | 0.137 ± 0.010b |
| Fsk (10 μm) | 0.113 ± 0.010b | 0.179 ± 0.011a,b | 0.135 ± 0.013b | 0.200 ± 0.011a,b |
a Significant difference between the value obtained in cells incubated with the identified siRNA and control siRNA (p > 0.05).
b Significant difference between the value from the “None” condition within groups (p > 0.05).
The addition of activators of cAMP signaling to HAEC expressing reduced levels of PKA-Cα, EPAC1, or PDE4D confirmed the dominance of EPAC1 and PDE4D in regulating HAEC permeability (Fig. 2C). Thus, although 6-Bz-cAMP reduced HAEC permeability in control cells (Figs. 1 and 2C), this agent was ineffective when added to HAEC with reduced levels of PKA-Cα, EPAC1, or PDE4D (Fig. 2C). In keeping with its mechanism of action, 8-CPT-cAMP reduced permeability in PKA-Cα or PDE4D knockdown cells but not in those with reduced levels of EPAC1 (Fig. 2C). Although Ro did not reduce permeability in cells with reduced levels of PDE4D, it did decrease permeability in HAEC expressing reduced PKA-Cα or EPAC1. Interestingly, the impact of Ro on HAEC permeability was blunted in cells expressing reduced EPAC1 (Fig. 2C). Although a maximal concentration of Fsk (10 μm) could decrease permeability in control or PKA-Cα knockdown cells, and its effect was augmented by reductions in PDE4D expression, even the impact of maximal Fsk was reduced in EPAC1 knockdown HAECs (Fig. 2C). Taken together, these data indicate that selective activation of either PKA or of EPAC can stabilize inter-endothelial interactions, supporting the concept that EPAC1 represents the dominant cAMP effector regulating these events. In addition, based on the impact of PDE4 inhibition on VEC permeability, our finding strongly suggest that a PDE4 variant likely regulates the pool of cAMP that promotes these EPAC-mediated effects in these cells.
PKA, EPAC1, and PDE4D Differentially Regulate HAEC VE-Cad-based Structures
When compared with control HAEC, VE-Cad membrane staining in EPAC1 or PDE4D knockdown cells was largely discontinuous. Indeed, whereas control cells had largely contiguous VE-Cad-based structures (86 ± 6% (n = 6)), VE-Cad staining in EPAC1 or PDE4D knockdown cells was largely discontinuous (45 ± 4 and 52 ± 8% contiguous staining) (mean ± S.E., n = 6; Fig. 2D). Moreover, VEGF promoted further significant loss of contiguous VE-Cad membrane staining such that contiguous staining was further reduced to 25 ± 8 and 35 ± 5% in EPAC1 and PDE4D knockdown HAECs, respectively (mean ± S.E., n = 6, p < 0.05). Although PKA-Cα knockdown also altered HAEC VE-Cad membrane staining (Fig. 2D), the contiguous membrane staining was only minimally reduced (12 ± 8%, n = 6), and this small loss was not amplified by VEGF treatments (19 ± 5%, n = 6). Although the thickness of VE-Cad-based structures was evidently increased in PKA-Cα knockdown cells, and this effect was lessened in the presence of VEGF (Fig. 2D), as PKA-Cα knockdown only modestly impacted permeability, further studies will be needed to assess how these events impact VEC functions. Although cortical actin staining was increased in control or PDE4D knockdown cells treated with VEGF, no such increases were observed upon VEGF treatment of HAEC in which either PKA-Cα or EPAC1 had been knocked down (Fig. 2D). Further studies will be required to correlate these changes in actin and HAEC permeability.
Multiple Components of the cAMP Signaling System Associate with VE-Cad-based Macromolecular Complexes in Human VECs
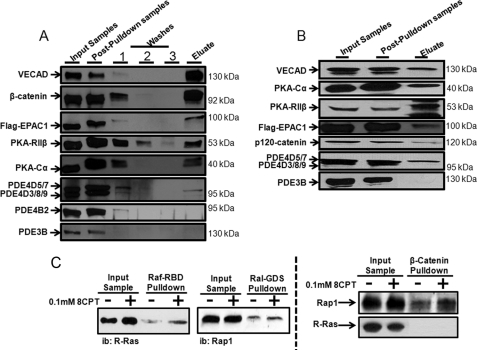
Because our data identified only a weak correlation between global levels of HAEC cAMP (Table 1) and their permeability (Fig. 2C), we reasoned that localized, rather than global, changes in HAEC cAMP might regulate permeability. In this context, recent reports have highlighted the importance of cAMP-signaling complexes in coordinating several important cellular events (16–20). Consistent with the idea that cAMP could act locally to control VE-Cad-regulated HAEC permeability, isolation of VE-Cad-based complexes from confluent HAEC cultures indicated that they contained VE-Cad-interacting proteins (β-catenin and p120-catenin, Fig. 3, A and B) and several cAMP-signaling proteins including the regulatory (RIIβ) and catalytic (Cα) PKA subunits, EPAC1, PDE4D (Fig. 3, A and B), and Rap1 (Fig. 3C). VE-Cad-based complexes did not contain significant amounts of any other cAMP signaling proteins (16–20, 25), including PDE3B (Fig. 3, A and B), PDE4B (Fig. 3A), R-Ras (Fig. 3C), or AKAP79, VASP, or CREB (cAMP-response element-binding protein; not shown). As cAMP-signaling proteins were absent from VE-Cad complexes isolated from populations of individual HAEC isolated following extracellular Ca2+ chelation (not shown), it may be that cAMP-signaling proteins are recruited to this complex in response to cell-cell contacts.
FIGURE 3.
cAMP-signaling proteins are integral components of VE-Cad-based signaling complexes in human arterial VECs. Following lysis of HAEC in a detergent-supplemented Tris-based buffer (see “Experimental Procedures”), VE-Cad-based cellular complexes were isolated by adsorption of proteins to an immobilized Fc-VE-Cad chimeric protein (A) or an immobilized GST-β-catenin chimeric protein (B) as described under “Experimental Procedures.” Proteins eluted from Fc-VE-Cad or GST-β-catenin were identified by immunoblot (ib) analysis with selective antisera. C, activation of R-Ras or Rap1 was measured by Raf- or Ral-GDS pulldown assays, respectively, and levels of these proteins present in GST-β-catenin pulldown assays were determined as above.
Because our data identified a dominant role for EPAC1 in regulating HAEC permeability, showing that both EPAC1 and Rap1, but not R-Ras, were present in VE-Cad-based complexes (Fig. 3), we next investigated whether stimulation of EPAC could activate HAEC Rap1 and whether this would increase the association of Rap1 with the complex. Thus, 8-CPT-cAMP activated Rap 1 (50 ± 10%, mean ± S.E., n = 3) and R-Ras (99 ± 12%, mean S.E., n = 3) in HAEC and selectively promoted recruitment of Rap1, but not R-Ras, into the complex (Fig. 3C). Indeed, the magnitude of the 8-CPT-cAMP-induced increase in β-catenin pulldown-associated Rap1 was indistinguishable from the scale of 8-CPT-cAMP-induced increase in activated Rap1 (50 ± 10 versus 74 ± 11%, respectively; n = 3) in these cells. Knockdown of either EPAC1 or PDE4D, but not PKA-Cα, reduced the integration of Rap1 into β-catenin-based complexes in these cells (not shown).
EPAC1 and PDE4D Interact in HAEC Signalosomes and Allow Local Regulation of Permeability
As RNAi-mediated knockdown of individual cAMP-signaling proteins allowed their impact on permeability to be assessed directly, we next examined the impact of these treatments on HAEC VE-Cad-based complexes. Although they did not contain PKA-Cα, complexes isolated from PKA-Cα knockdown cells were otherwise indistinguishable from those isolated from control cells (not shown). In marked contrast, similar experiments in EPAC1 or PDE4D knockdown cells indicated that EPAC1 and PDE4D likely impacted their respective integration into these structures. Thus, analysis of complexes derived from EPAC1 knockdown cells showed them to be devoid of EPAC1 and to contain only 26 ± 10% of the PDE4D present in control complexes (n = 4, p < 0.05) (Fig. 4). Similarly, complexes derived from PDE4D knockdown cells contained insignificant (<5%) amounts of both PDE4D and EPAC1 (Fig. 4). Irrespective of the siRNA employed, the levels of PKA-RIIβ detected in complexes were unaltered (Fig. 4). Overall, these observations are consistent with the idea that EPAC1 and PDE4D participate in coordinating their respective integration into this signalosome and may underpin our observation that knockdown of PDE4D caused HAEC to display a hyperpermeable phenotype similar to that caused by knockdown of EPAC1.
FIGURE 4.
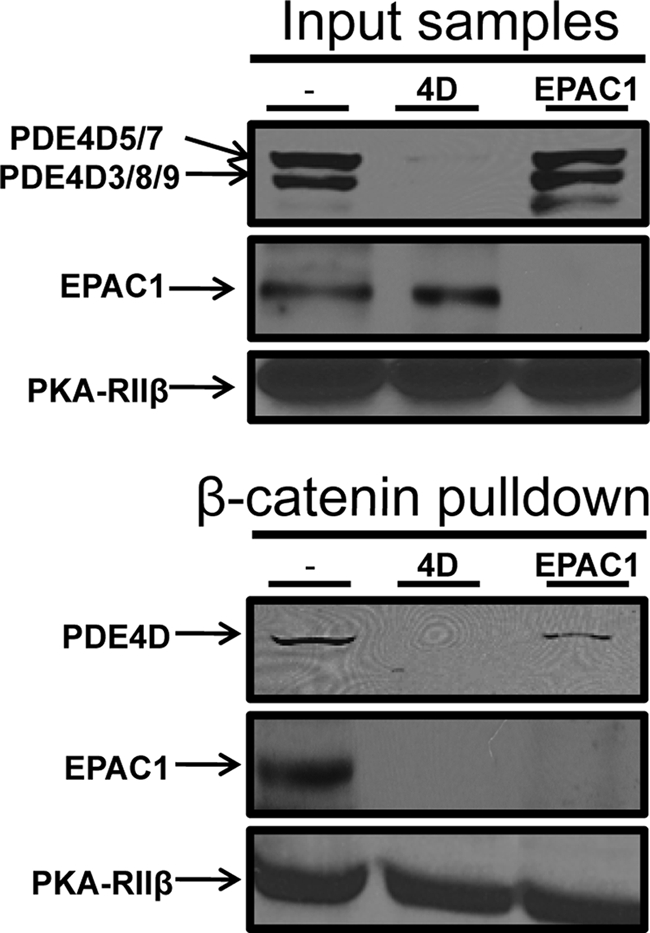
Effects of RNAi-based knockdown of PKA-Cα, EPAC1, or PDE4D on the stability of human arterial VEC VE-Cad- and actin-based structures. HAEC were transfected with EPAC1- or PDE4D-selective siRNAs or a control siRNA as described in the legend for Fig. 2. Following lysis, the transfected HAEC VE-Cad-based cellular complexes were isolated by adsorption to an immobilized GST-β-catenin chimeric protein. Proteins eluted from GST-β-catenin were identified by immunoblot analysis and quantified by densitometry (see “Experimental Procedures”).
EPAC1 and PDE4D Interact Directly, and Antagonism of This Interaction Impacts HAEC VE-Cad-based Structures and Permeability
Because knockdown of EPAC1 or PDE4D reduced integration of both these enzymes into HAEC VE-Cad-based complexes and increased permeability, we next chose to determine whether, as suggested (16), EPAC1 and PDE4D interact directly. For these analyses we first purified recombinant GST-EPAC1 and VSV-PDE4D3 and showed that these purified proteins interacted directly in GST-based pulldown assays (not shown). Next, as described previously (23), we used scanning peptide array technology to define direct protein-protein interactions between these proteins. Incubation of an overlapping peptide library that represents the entire sequence of EPAC1 with recombinant GST-PDE4D3, but not GST, allowed the identification of three overlapping peptides, which together covered the region of EPAC1 encoding amino acids Val-362 to Leu-397 inclusively (Fig. 5A). Subsequent analysis of GST-PDE4D3 binding to a series of peptides representing the common peptide region, but in which individual residues had been sequentially substituted for alanine (Ala) residues, allowed identification of several EPAC1 residues, including Arg-341 and Arg-343, as important for PDE4D binding (Fig. 5A).
FIGURE 5.
Impact on HAEC permeability and VE-Cad-based structures of interfering with PDE4D-EPAC1 interactions using an EPAC1-based, PDE4D-displacing peptide. A, incubation of a series of 26 individual peptides encoding the sequence 362VLVLERASQGAGPSRPPTPGRNRYT386 in which individual amino acids sequentially substituted with alanine were probed with GST-PDE4D3 fusion proteins (10 μg/ml) to allow identification of the residues important for the interaction between PDE4D and EPAC1 was carried out as described in Ref. 23 and under “Experimental Procedures.” B and C, HAEC incubated for 2 h with a steroylated EPAC1-based peptide encoding amino acids Val362 to Thr386, designed to disrupt the interaction between EPAC1 and PDE4D (defined herein as a disrupting peptide (DP)), or with a scrambled version of this peptide (defined herein as Control peptide) were either lysed and subjected to β-catenin pulldown analysis as described under “Experimental Procedures” and subsequently investigated by immunoblot analysis (B) or fixed and analyzed by immunofluorescence (C).
To evaluate the importance of this peptide in promoting EPAC1-PDE4D interactions and their integration into the HAEC signalosome, we used a stearoylated cell-permeable version of the peptide to disrupt EPAC1-PDE4D intracellular interactions. Thus, the addition of this peptide, but not a scrambled version, reduced the PDE4D recovered in isolated complexes (Fig. 5B) by 55 ± 13% (mean ± S.E., n = 4). Interestingly, neither peptide altered the amounts of VE-Cad, EPAC1 (Fig. 5B), or PKA-Cα (not shown) recovered in complex. The ability of the peptide to displace a significant fraction of PDE4D from the HAEC complex was consistent with the idea that this EPAC1 peptide motif regulates EPAC1-PDE4D interactions in cells. Furthermore our findings demonstrate that EPAC1-PDE4D binding via this motif significantly impacts the ability of PDE4D to integrate into the complex. Interestingly, as neither EPAC1 knockdown nor the displacing peptide completely displaced PDE4D from the complex, our findings also demonstrate that interactions between PDE4D and proteins other than EPAC1 within the complex are also likely involved in allowing robust integration of PDE4D and EPAC1 in the complex. Lastly, because PDE4D knockdown reduced EPAC1 levels in the complex by >90%, but the ∼55% reduction in complexed PDE4D caused by addition of the peptide did not reduce EPAC1 levels, we suggest that: (a) a threshold level of PDE4D exists that can stabilize EPAC1 complex integration; or (b) a PDE4D-interacting accessory protein present in the complex impacts PDE4D-mediated stabilization of EPAC1 in the complex. Clearly, further analysis will be required to fully dissect the protein-protein binding events that stabilize this complex.
Interestingly, incubation of HAEC with the PDE4D-displacing peptide, but not the scrambled peptide, reduced HAEC permeability by 29 ± 7% (mean ± S.E. n = 4), promoted cortical accumulation of actin, and inhibited VEGF-induced destabilization of VE-Cad-based structures in these cells (Fig. 5C). Indeed, the displacing peptide inhibited VEGF-induced reductions in contiguous VE-Cad staining in HAEC by 68 ± 5% (mean ± S.E., n = 5, p ≥ 0.05). These effects were similar to those observed upon PDE4 inhibition with Ro and are consistent with the idea that the ∼55% reduction in PDE4D in the complex caused by the addition of the peptide increased cAMP-mediated EPAC1 activation and signaling within this intracellular locale.
DISCUSSION
Reports indicate that activators of cAMP signaling can reduce VEC permeability by increasing endothelial barrier integrity. Although this effect has been linked to several PKA- and/or EPAC-dependent events, there remains considerable ambiguity with respect to the cellular systems that could allow selective control of these plasma membrane-delimited events by PKA and/or EPAC. Indeed, the observation that PKA activation can exert either stimulatory or inhibitory effects on VEC permeability suggests that compartmentalized cAMP activation of this effector likely regulates potentially diametrically opposed inputs (24–30). Similarly, although the local systems that regulate how cAMP-mediated activation of EPAC1 stimulates the accumulation of GTP-bound Rap1 and increases barrier function (31–33) likely also involve compartmentalized cAMP-dependent activation of this effector, these systems remain equally poorly described.
Our analysis of the relative impact of activators of PKA or EPAC on HAEC permeability identified EPAC1 as the dominant cAMP effector operating to control this critical function in both arterial and microvessel-derived human VECs. In addition, we also demonstrated for the first time that targeted cAMP hydrolysis by sequestered PDE4D plays a pivotal regulatory role in coordinating the effects of activators of cAMP signaling on VEC permeability. Thus, we have shown that inhibition of PDE4, but not PDE3, specifically regulates the ability of agents that increase global cAMP levels and activate both PKA and EPAC1 to antagonize VEGF-promoted human VEC permeability. Second, we identified a novel scaffolding role for the PDE4 class of enzymes in showing that PDE4D has a critical role in helping to tether EPAC1 into the VE-Cad-based complex. Of potential translational importance, we identified an vital region in EPAC1 that coordinates EPAC1-PDE4D binding and demonstrated that this interaction could be specifically perturbed with a cell-permeable peptide based on an interacting EPAC1 domain. Most importantly, as we have reported that this perturbation impacted cAMP-mediated control of VEC permeability, we suggest that this may represent a novel target for drug development.
EPAC activation with 8-CPT-cAMP markedly protects both VE-Cad- and actin-based structures from the destabilizing effects of Ca2+ chelation, or high dose VEGF, and significantly promotes their reestablishment upon removal of the destabilizing influences. Although some of the 8-CPT-cAMP-mediated effects reported here were internally consistent with recent reports indicating that EPAC activation stabilizes VE-Cad-based border stability through activation of Rap1 (11, 12), others were not easily reconciled with the conclusions from these earlier reports. First, and of mechanistic interest, we found that 8-CPT-cAMP-mediated EPAC stimulation in HAEC led to the accumulation of two GTP-bound low molecular weight G-proteins, Rap1 and R-Ras, but that only Rap1 was actively recruited to the VE-Cad-based complex following EPAC activation. In addition, our data clearly show that it is the integration of EPAC1 into VE-Cad-based complexes that allows this cAMP effector to regulate VEC permeability and not its global level or its global activation status. Indeed, loss of EPAC1 integration consequent to PDE4D knockdown markedly increased VEC permeability without influencing the total EPAC levels in these cells.
In addition to identifying EPAC1 as the dominant cAMP effector regulating human arterial and microvascular VEC permeability, our findings also demonstrated that PKA is a poor substitute for EPAC1. First, siRNA-mediated knockdown of EPAC1 significantly increased permeability, an effect consistent with the observation that EPAC activation in control cells improves barrier function. Furthermore, EPAC activation promoted barrier stability equally in control HAEC and in HAEC in which PKA-Cα levels had been reduced. In marked contrast, although the PKA-selective cAMP analog 6-Bz-cAMP did improve barrier function in control cells, it was completely ineffective in PKA-Cα knockdown cells and could not rescue the loss of barrier function caused by EPAC1 knockdown. Interestingly, a recent report indicated that 6-Bz-cAMP could improve barrier function in umbilical vein-derived VEC in which EPAC1 levels were reduced (12). Whether these findings reflect cell-based differences is currently unclear. Interestingly, although PKA-Cα knockdown was virtually silent with respect to its impact on VEC permeability, this treatment did increase basal levels of HAEC cAMP to a level similar to that caused by PDE4D knockdown. This effect, although internally consistent with the recognized importance of PKA-mediated phosphorylation of PDE4D in regulating cAMP levels in cells, including those of the cardiovascular system (34–36), is unlikely to have contributed significantly to VEC permeability because in all cases global levels of cAMP correlate only very poorly with permeability.
Notwithstanding our finding that PKA-Cα knockdown did not significantly affect VEC permeability, it is evident from our studies that PKA activation improves barrier function and that PKA-RIIβ and PKA-Cα are located in VE-Cad-based complexes. Whether the PKA-containing VE-Cad-based complex can regulate VEC permeability under conditions not formally tested here remains to be established. Indeed, given that the kinetics of PKA and EPAC activation by cAMP are very different (37), one could reasonably speculate that distinct VE-Cad pools could differentially employ either PKA or EPAC to regulate specific VE-Cad-dependent events. Indeed, studies identifying potential AKAP-cadherin interactions (38) suggest that this could occur.
PDEs represent the sole intracellular enzymes capable of specifically hydrolyzing intracellular cyclic nucleotides and are thus exquisitely poised to regulate the activation of cAMP effectors (19, 20). Our observation that PDE4D, but neither PDE3B nor PDE4B, was isolated in association with VE-Cad and β-catenin demonstrates that the molecular basis of the integration of PDE4 in the complex was PDE4D-specific and not generalized to all PDE enzymes. Whether this function extends to a range of isoforms within the PDE4D subfamily remains to be ascertained and poses a considerable challenge. Certainly, on the basis of immunoblotting VE-Cad-based complexes with the pan-PDE4D antisera we observed several long isoforms but no short isoforms. This suggests that a critical anchor site may involve regions that are found in common with the long isoforms subgroup but not the short isoforms, such as UCR1 and the common region (20). Therefore, considering the size of the species noted with these antisera, the possible isoforms integrated into VE-Cad-based complexes and acting as EPAC1-interacting PDE4D variants include PDE4D3, PDE4D5, PDE4D7, PDE4D8, and PDE4D9. In this regard it is interesting to note that RACK1, a scaffold that specifically sequesters PDE4D5 (39), has been shown to associate with focal adhesion kinase (FAK) at adherens junctions (40).
Here we have demonstrated for the first time that long isoforms from the PDE4D family of enzymes played multiple roles in controlling cAMP-mediated effects on VEC permeability. Indeed, although cAMP degradation in the human VECs used in these studies was catalyzed exclusively by PDE3 and PDE4, and isoforms from both the PDE4B and PDE4D subfamilies were expressed in these cells, we found that long PDE4D enzymes are set to play a unique regulatory role through being tethered to VE-Cad-based signaling complexes. Importantly, PDE4D integration into VE-Cad-based signaling complexes appears to have a key role in association with stabilizing EPAC1 integration into these structures. Indicative of a lack of a substantial role for PDE3 in this system were our observations that treatment with the PDE3-selective inhibitor cilostamide did not impact VEC permeability. In contrast, PDE4 inhibition significantly reduced VEGF-promoted permeability in the absence of PDE3 inhibition, an effect that was lost in PDE4D knockdown cells.
As indicated, support for the importance of subcellular localization of PDEs in coordinating the myriad cellular effects regulated by cAMP-mediated activation of its effectors has increased significantly during the last decade (20). In this context, our finding that stabilization of the compartmentation of EPAC1 into VE-Cad-based signaling complexes through a selective PDE4D-based interaction that is critical to regulating human VEC permeability and obviates a role for global changes in cAMP in controlling this event is timely and important. Our novel findings give a cellular context to the notion that PDE4 inhibitors allow localized changes in intracellular cAMP in cells (41, 42) and that these can be associated with defined functional outcomes, as shown here for VEC, as well as implicating the importance of PDE4 isoforms as scaffolds in their own right.
This work was supported by Grants MOP-57699 from the Canadian Institutes for Health Sciences, G0600765 from the Medical Research Council (UK), LSHB-CT-2006-037189 from the European Union, and 06CVD02 from the Fondation Leducq (to M. D. H.).
- VE-Cad
- vascular endothelial cadherin
- VEC
- vascular endothelial cell
- PKA
- protein kinase A
- EPAC
- exchange protein activated by cAMP
- PDE
- phosphodiesterase
- HAEC
- human arterial endothelial cell
- HMVEC
- human microvascular endothelial cell
- FITC
- fluorescein isothiocyanate
- 8-CPT-cAMP
- 8-(4-chlorophenylthio)-2′-O-methyl-cAMP
- 6-Bz-cAMP
- 6-benzoyladenosine-3′,5′-cyclic monophosphate
- Fsk
- forskolin.
REFERENCES
- 1.Dejana E., Tournier-Lasserve E., Weinstein B. M. (2009) Dev. Cell 16, 209–221 [DOI] [PubMed] [Google Scholar]
- 2.Vestweber D. (2008) Arterioscler. Thromb. Vasc. Biol. 28, 223–232 [DOI] [PubMed] [Google Scholar]
- 3.Galan Moya E. M., Le Guelte A., Gavard J. (2009) Cell. Signal. 21, 1727–1737 [DOI] [PubMed] [Google Scholar]
- 4.Gavard J. (2009) FEBS Lett. 583, 1–6 [DOI] [PubMed] [Google Scholar]
- 5.Tan W., Palmby T. R., Gavard J., Amornphimoltham P., Zheng Y., Gutkind J. S. (2008) FASEB J. 22, 1829–1838 [DOI] [PubMed] [Google Scholar]
- 6.Gavard J., Patel V., Gutkind J. S. (2008) Dev. Cell 14, 1425–1436 [DOI] [PubMed] [Google Scholar]
- 7.Andriopoulou P., Navarro P., Zanetti A., Lampugnani M. G., Dejana E. (1999) Arterioscler. Thromb. Vasc. Biol. 19, 2286–2297 [DOI] [PubMed] [Google Scholar]
- 8.Hudry-Clergeon H., Stengel D., Ninio E., Vilgrain I. (2005) FASEB J. 19, 512–520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Angelini D. J., Hyun S. W., Grigoryev D. N., Garg P., Gong P., Singh I. S., Passaniti A., Hasday J. D., Goldblum S. E. (2006) Am. J. Physiol. Lung Cell. Mol. Physiol. 291, L1232–L1245 [DOI] [PubMed] [Google Scholar]
- 10.Nottebaum A. F., Cagna G., Winderlich M., Gamp A. C., Linnepe R., Polaschegg C., Filippova K., Lyck R., Engelhardt B., Kamenyeva O., Bixel M. G., Butz S., Vestweber D. (2008) J. Exp. Med. 205, 2929–2945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kooistra M. R., Corada M., Dejana E., Bos J. L. (2005) FEBS Lett. 579, 4966–4972 [DOI] [PubMed] [Google Scholar]
- 12.Lorenowicz M. J., Fernandez-Borja M., Kooistra M. R., Bos J. L., Hordijk P. L. (2008) Eur J. Cell Biol. 87, 779–792 [DOI] [PubMed] [Google Scholar]
- 13.Sehrawat S., Cullere X., Patel S., Italiano J., Jr., Mayadas T. N. (2008) Mol. Biol. Cell 19, 1261–1270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vandenbroucke E., Mehta D., Minshall R., Malik A. B. (2008) Ann. N. Y. Acad. Sci. 1123, 134–145 [DOI] [PubMed] [Google Scholar]
- 15.Moore T. M., Chetham P. M., Kelly J. J., Stevens T. (1998) Am. J. Physiol. Lung Cell. Mol. Physiol. 275, L203–L222 [DOI] [PubMed] [Google Scholar]
- 16.Dodge-Kafka K. L., Soughayer J., Pare G. C., Carlisle Michel J. J., Langeberg L. K., Kapiloff M. S., Scott J. D. (2005) Nature 437, 574–578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carnegie G. K., Means C. K., Scott J. D. (2009) IUBMB Life 61, 394–406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mauban J. R., O'Donnell M., Warrier S., Manni S., Bond M. (2009) Physiology 24, 78–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maurice D. H., Palmer D., Tilley D. G., Dunkerley H. A., Netherton S. J., Raymond D. R., Elbatarny H. S., Jimmo S. L. (2003) Mol. Pharmacol. 64, 533–546 [DOI] [PubMed] [Google Scholar]
- 20.Houslay M. D., Baillie G. S., Maurice D. H. (2007) Circ. Res. 100, 950–966 [DOI] [PubMed] [Google Scholar]
- 21.Sage E. H., Reed M., Funk S. E., Truong T., Steadele M., Puolakkainen P., Maurice D. H., Bassuk J. A. (2003) J. Biol. Chem. 278, 37849–37857 [DOI] [PubMed] [Google Scholar]
- 22.Netherton S. J., Sutton J. A., Wilson L. S., Carter R. L., Maurice D. H. (2007) Circ. Res. 101, 768–776 [DOI] [PubMed] [Google Scholar]
- 23.Baillie G. S., Adams D. R., Bhari N., Houslay T. M., Vadrevu S., Meng D., Li X., Dunlop A., Milligan G., Bolger G. B., Klussmann E., Houslay M. D. (2007) Biochem. J. 404, 71–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bos J. L. (2006) 31, 680–686 [DOI] [PubMed] [Google Scholar]
- 25.Schlegel N., Waschke J. (2009) Am. J. Physiol. Cell Physiol. 296, C453–C462 [DOI] [PubMed] [Google Scholar]
- 26.Liu F., Verin A. D., Borbiev T., Garcia J. G. (2001) Am. J. Physiol. Lung Cell. Mol. Physiol. 280, L1309–L1317 [DOI] [PubMed] [Google Scholar]
- 27.Qiao J., Holian O., Lee B. S., Huang F., Zhang J., Lum H. (2008) Am. J. Physiol. Cell Physiol. 295, C1161–C1168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Qiao J., Huang F., Lum H. (2003) Am. J. Physiol. Lung Cell. Mol. Physiol. 284, L972–L980 [DOI] [PubMed] [Google Scholar]
- 29.Birukova A. A., Zagranichnaya T., Fu P., Alekseeva E., Chen W., Jacobson J. R., Birukov K. G. (2007) Exp. Cell Res. 313, 2504–2520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Birukova A. A., Zagranichnaya T., Alekseeva E., Bokoch G. M., Birukov K. G. (2008) J. Cell. Physiol. 215, 715–724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fukuhara S., Sakurai A., Sano H., Yamagishi A., Somekawa S., Takakura N., Saito Y., Kangawa K., Mochizuki N. (2005) Mol. Cell. Biol. 25, 136–146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cullere X., Shaw S. K., Andersson L., Hirahashi J., Luscinskas F. W., Mayadas T. N. (2005) Blood 105, 1950–1955 [DOI] [PubMed] [Google Scholar]
- 33.Borland G., Smith B. O., Yarwood S. J. (2009) Br. J. Pharmacol. 158, 70–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hoffmann R., Baillie G. S., MacKenzie S. J., Yarwood S. J., Houslay M. D. (1999) EMBO J. 18, 893–903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu H., Maurice D. H. (1999) J. Biol. Chem. 274, 10557–10565 [DOI] [PubMed] [Google Scholar]
- 36.Lim J., Pahlke G., Conti M. (1999) J. Biol. Chem. 274, 19677–19685 [DOI] [PubMed] [Google Scholar]
- 37.Das R., Chowdhury S., Mazhab-Jafari M. T., Sildas S., Selvaratnam R., Melacini G. (2009) J. Biol. Chem. 284, 23682–23696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gorski J. A., Gomez L. L., Scott J. D., Dell'Acqua M. L. (2005) Mol. Biol. Cell 16, 3574–3590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yarwood S. J., Steele M. R., Scotland G., Houslay M. D., Bolger G. B. (1999) J. Biol. Chem. 274, 14909–14917 [DOI] [PubMed] [Google Scholar]
- 40.Kiely P. A., Baillie G. S., Barrett R., Buckley D. A., Adams D. R., Houslay M. D., O'Connor R. (2009) J. Biol. Chem. 284, 20263–20274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Raymond D. R., Wilson L. S., Carter R. L., Maurice D. H. (2007) Cell. Signal. 19, 2507–2518 [DOI] [PubMed] [Google Scholar]
- 42.Rich T. C., Xin W., Mehats C., Hassell K. A., Piggott L. A., Le X., Karpen J. W., Conti M. (2007) Am. J. Physiol. Cell Physiol. 292, C319–C331 [DOI] [PMC free article] [PubMed] [Google Scholar]