Abstract
The functional impact of adiponectin on pancreatic beta cells is so far poorly understood. Although adiponectin receptors (AdipoR1/2) were identified, their involvement in adiponectin-induced signaling and other molecules involved is not clearly defined. Therefore, we investigated the role of adiponectin in beta cells and the signaling mediators involved. MIN6 beta cells and mouse islets were stimulated with globular (2.5 μg/ml) or full-length (5 μg/ml) adiponectin under serum starvation, and cell viability, proliferation, apoptosis, insulin gene expression, and secretion were measured. Lysates were subjected to Western blot analysis to determine phosphorylation of AMP-activated protein kinase (AMPK), Akt, or ERK. Functional significance of signaling was confirmed using dominant negative mutants or pharmacological inhibitors. Participation of AdipoRs was assessed by overexpression or siRNA. Adiponectin failed to activate AMPK after 10 min or 1- and 24-h stimulation. ERK was significantly phosphorylated after 24-h treatment with adiponectin, whereas Akt was activated at all time points examined. 24-h stimulation with adiponectin significantly increased cell viability by decreasing cellular apoptosis, and this was prevented by dominant negative Akt, wortmannin (PI3K inhibitor), and U0126 (MEK inhibitor). Moreover, adiponectin regulated insulin gene expression and glucose-stimulated insulin secretion, which was also prevented by wortmannin and U0126 treatment. Interestingly, the data also suggest adiponectin-induced changes in Akt and ERK phosphorylation and caspase-3 may occur independent of the level of AdipoR expression. This study demonstrates a lack of AMPK involvement and implicates Akt and ERK in adiponectin signaling, leading to protection against apoptosis and stimulation of insulin gene expression and secretion in pancreatic beta cells.
Keywords: Akt PKB, AMP-activated Protein Kinase (AMPK), Apoptosis, Diabetes, ERK, Insulin, Pancreatic Islet, Signal Transduction, Adiponectin
Introduction
Type 2 diabetes is characterized by both a loss of insulin sensitivity and beta cell dysfunction in the pancreas. Adiponectin is an adipocyte-derived hormone that shows a strong negative correlation with insulin resistance and obesity (1, 2). Studies show that whereas adiponectin knock-out mice develop insulin resistance and glucose intolerance when challenged with a high fat diet, adiponectin-overexpressing mice are highly insulin-sensitive and are resistant to diet-induced diabetes (3–6). Adiponectin is reported to stimulate fatty acid oxidation and glucose uptake and reduce gluconeogenesis in myocytes and hepatocytes, thus increasing peripheral insulin sensitivity (7). Reduced adiponectin levels are also correlated with reduced vascular function and an increase in coronary artery disease (8).
Adiponectin forms trimers or higher order complexes including hexamers and oligomers (9). These higher order complexes of full-length adiponectin (fAd)4 are the primary circulating forms, and localized proteolytic cleavage of fAd has been shown to produce globular adiponectin (gAd) (10). Two proposed homologous adiponectin receptors (AdipoR1 and AdipoR2) were cloned and shown to be widely expressed in mammalian tissues (11, 12). They are seven trans-membrane-spanning proteins that structurally and topologically differ from G protein-coupled receptors (12). Adiponectin is suggested to bind to the C terminus of this protein, whereas the adaptor protein containing the pleckstrin homology domain, phosphotyrosine-binding domain, and leucine zipper motif (APPL1) interacts with the N terminus (12, 13). 5′-AMP-activated protein kinase (AMPK) is one of the central mediators of adiponectin-derived peripheral effects (13).
Interestingly, the AdipoRs were shown to be expressed in rat and human islets and primary and cultured beta cells (14–17). Some human studies have associated hypoadiponectinemia with beta cell dysfunction (18–20). Indirect evidence from rodent studies also suggests a role of adiponectin in pancreatic beta cells: adiponectin knock-out mice displayed a trend toward lower plasma insulin, whereas ob/ob mice overexpressing adiponectin showed an increase in islet insulin content (3, 5).
Although in vitro, the impact of adiponectin on insulin secretion from islets is variable, a recent in vivo study in mice suggested that adiponectin increases glucose-stimulated insulin secretion (GSIS) (14, 16, 21–23). Adiponectin was shown to also have both pro- and antiproliferative effects in numerous cell types mediated by such signaling molecules as AMPK, Akt, and mitogen-activated protein kinase (MAPK)/extracellular signal-regulated kinase (ERK) (24–28). Three studies thus far have examined the effects of adiponectin on beta cell viability in vitro: although two show that in a pancreatic beta cell line adiponectin counteracts fatty acid-, cytokine-, and glucotoxicity-mediated beta cell apoptosis (22, 29), one shows that neither gAd nor fAd has any effect on fatty acid-induced apoptosis in human islets (16). Thus, a clear understanding of the functional role of adiponectin in beta cells and islets is still lacking. Furthermore, involvement of AMPK and other signaling mediators, including the AdipoRs in adiponectin signaling in beta cells, is also not completely understood.
We confirm here that AdipoRs are expressed, but adiponectin does not activate AMPK in mouse islets or cultured beta cells. During chronic serum starvation, adiponectin treatment increases islet and beta cell viability by decreasing cellular apoptosis, and this is mediated via both ERK and Akt activation. Further associated are an increase in insulin gene expression and GSIS.
EXPERIMENTAL PROCEDURES
Adiponectin
fAd, produced in a mammalian expression system at an approximate ratio of 35:40:25% of high, medium, and low molecular mass oligomers (30), was used at 5 μg/ml (based on supplemental Fig. S1A). gAd (Peprotech) was used at 2.5 μg/ml (based on supplemental Fig. S1B).
Cell Culture
MIN6 or C2C12 cells were grown in high glucose Dulbecco's modified Eagle's medium (Invitrogen) supplemented with 10% fetal bovine serum (FBS; Sigma) and 1% penicillin-streptomycin (Invitrogen). C2C12 myoblasts were allowed to develop into myotubes in the presence of 5% horse serum-supplemented medium. INS-1 832/13 cells were grown in RPMI 1640 medium (Sigma) supplemented with 10% FBS, 1% penicillin-streptomycin, 10 mm HEPES, and 2 mm glutamine. Cells were seeded on 12-well (for protein and RNA extraction) 24-well (for GSIS) or 96-well (for 2,3-bis(2-methoxy-4-nitro-5-sulfophenyl)-5-[(phenylamino)carbonyl]-2H-tetrazo-lium hydroxide (XTT) assay) plates and stimulated for the indicated times in serum-free medium in the presence or absence of gAd, fAd, or vehicle (5 mm Tris-HCl (Sigma) or phosphate-buffered saline (PBS) (Invitrogen)), respectively.
Islet Isolation
The pancreatic duct was perfused with 3 ml of collagenase V (0.8 mg/ml) (Sigma). The pancreas was then isolated and digested for 15–20 min at 37 °C. Islets were hand-picked from the acinar tissue debris, transferred into RPMI 1640 medium supplemented with 10% FBS and 1% penicillin-streptomycin, and cultured overnight. Islets were stimulated as described above. All experiments were approved by the Animal Care Committee at the University of Toronto, and animals were handled according to the guidelines of the Canadian Council of Animal Care.
Quantitative Real Time PCR (qPCR)
Total RNA was isolated using TRIzol reagent (Invitrogen) according to the manufacturer's instructions and treated with rDNase I (Applied Biosystems). One μg of RNA was reverse-transcribed using Moloney murine leukemia virus reverse transcriptase according to the manufacturer's instructions (Invitrogen). Primers (supplemental Table 1) were designed using Primer Express version 2.0 software (Applied Biosystems). 10 ng of cDNA per well was used as the template for amplification. The qPCR protocol was as follows: heat activation of polymerase at 95 °C for 3 min followed by 40 cycles of 95 °C for 10 s, 65 °C for 15 s, and 72 °C for 20 s. Readings were carried out on an ABI Prism7900HT Sequence Detection System (Applied Biosystems) and compared against a standard curve created from mouse genomic DNA by serial dilutions. Data were normalized to mouse β-actin mRNA.
Western Blot Analysis and Cell Fractionation
Islets and cells were washed twice with PBS, and total lysates were prepared in Cell Lysis Buffer (Cell Signaling) supplemented with 1 mm phenylmethylsulfonyl fluoride and 10% β-mercaptoethanol and heated for 5 min at 65 °C. The lysates were then resolved by 6% (for Ki67), 15% (for cleaved caspase-3) and 10% (for all other proteins) SDS-PAGE and immunoblotted with primary and HRP-conjugated secondary antibodies. Immunoblots within the linear range were scanned on a Kodak imager and quantified using ImageJ (National Institutes of Health). Polyclonal anti-AdipoR1 and anti-AdipoR2 antibodies (2 μg/ml) were purchased from Immunobiological Inc. Polyclonal anti-phospho-AMPK (1:1000), anti-phospho-Akt (Ser473 and Thr308) (1:000), anti-Akt (1:1000), anti-phospho-p38 MAPK (1:500), anti-phospho-ERK1/2 (1:1000), and anti-caspase-3 (1:1000) antibodies were from Cell Signaling. Polyclonal anti-Ki67 (1:500) and anti-β-actin (1:2000) and monoclonal anti-laminin A/C (1:1000) were purchased from Santa Cruz Biotechnology, Sigma, and BD Laboratories, respectively. Anti-rabbit, anti-mouse, and anti-goat secondary antibodies were purchased from Sigma, Cell Signaling, and Santa Cruz Biotechnology, respectively.
For cell fractionation, cells were grown in 6-cm dishes to confluence, and cytosolic and membrane fractions from total lysates were prepared using the Qproteome Cell Compartment kit (Qiagen).
Cell Viability Assay
Cell proliferation and viability were measured by an XTT-based colorimetric assay (Cell Proliferation Kit II (XTT); Roche Applied Science). The assay was performed in 96-well plates, and each condition was measured in triplicate. 10 islets were used per well. 50 μl of the XTT medium was applied to each well and incubated for 4 h at 37 °C. The XTT assay results were detected by spectrophotometric absorbance at 490 nm (A490). Absorbances were normalized to total DNA.
siRNA and cDNA Transfection and Adenoviral Infection
Cells were grown in antibiotic-free medium for 24 h and transfected with 100 nm siRNA against AdipoR1 and AdipoR2 or control siRNA (siGENOME SMARTpool; Dharmacon) (supplemental Table 2) or DN-Akt mutant (31) cDNA (3.2 μg) using Lipofectamine 2000 (Invitrogen) according to the manufacturer's protocol. Five hours after transfection, cells were washed and replaced with fresh medium. Experiments were performed 72 h after transfection. Cells were infected with gfp, AdipoR1, or AdipoR2 adenoviruses (Vector BioLabs) at 100 multiplicity of infection for 2 h in serum-free medium, after which the cells were washed and replaced with fresh medium. Experiments were performed 24 h after infection.
Insulin Secretion
Ten islets were used in triplicate per condition. Islets or cells were preincubated in Krebs Ringer buffer (128.8 mm NaCl, 4.8 mm KCl, 1.2 mm KH2PO4, 1.2 mm MgSO4, 2.5 mm CaCl, 5 mm NaHCO3, 10 mm HEPES, 0.1% BSA) (Sigma) in 0 or 2.8 mm glucose for 1 h, then stimulated in same buffer with 0 or 2.8 and 11 or 20 mm glucose for 1 h. Supernatant was collected, and insulin was measured by radioimmunoassay using the rat insulin RIA kit from Linco (Millipore). DNA was extracted using acid ethanol, dried down in a speed vacuum, and resuspended in water, and the concentration was measured by a DNA spectrometer. Insulin was normalized to total DNA.
Statistical Analysis
Data are expressed as mean ± S.E. Significance was determined using the Student's t test or one-way ANOVA with Newman-Keul or Bonferroni post hoc test. p < 0.05 was considered statistically significant.
RESULTS
Adiponectin Receptors Are Expressed in Pancreatic Islets and Beta Cells
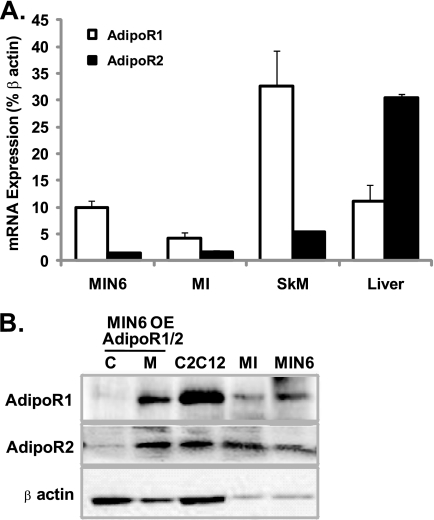
We examined the expression of AdipoR1 and AdipoR2 in mouse pancreatic islets and MIN6 cells, a mouse cultured beta cell line, using qPCR, and observed that whereas both receptors are expressed, AdipoR1 is the predominant isoform (Fig. 1A). Western blot analysis confirmed protein expression of both AdipoR1 and AdipoR2 in mouse islets and MIN6 cells (Fig. 1B). After overexpression and fractionation of MIN6 cells, we determined that both AdipoR1 and AdipoR2 are located on the membrane fraction and not in the cytosol. Mouse skeletal muscle and liver or C2C12 mouse myocytes were used as positive controls in qPCR and Western blot analysis, respectively. Higher expression of AdipoR1 mRNA in skeletal muscle and AdipoR2 mRNA in liver was observed as previously reported (12).
FIGURE 1.
AdipoR expression in pancreatic islets and beta cells. A, qPCR analysis of AdipoR1 and 2 transcripts in MIN6 cells, mouse islets (MI), skeletal muscle (SkM), and liver. B, Western blot analysis of AdipoR1 and 2 (46 kDa) in cytosolic (C) and membrane (M) fractions of MIN6 cells overexpressing (OE) AdipoR1 or 2 and in total lysates of C2C12 myocytes, mouse islets and MIN6 cells. β-Actin (43 kDa) was used as a loading control.
Adiponectin Stimulates Akt and ERK, but Not AMPK Phosphorylation in Beta Cells
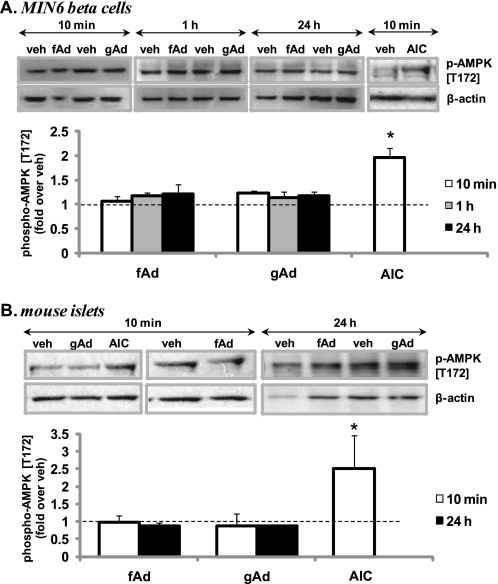
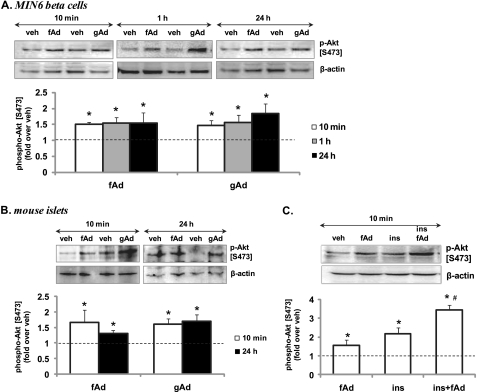
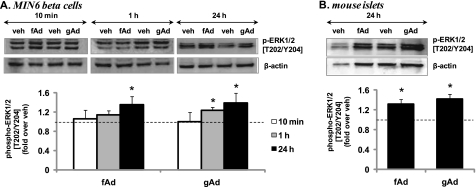
We found that both fAd and gAd did not stimulate AMPK phosphorylation in MIN6 cells after 10-min, 1-h, or 24-h stimulation (Fig. 2A). Similarly, no AMPK phosphorylation was observed in mouse islets stimulated for 10 min or 24 h with fAd or gAd (Fig. 2B). In contrast, AMPK was phosphorylated in C2C12 myotubes (supplemental Fig. S2A). AICAR (Sigma) robustly induced AMPK phosphorylation in MIN6 cells, mouse islets, and C2C12 cells (Fig. 2 and supplemental Fig. S2A). Interestingly, phosphorylation of Akt at both Ser473 and Thr308 was significantly increased at 10 min and sustained after 1-h and 24-h stimulation with either gAd or fAd in MIN6 cells (Fig. 3A and supplemental Fig. S3A) and isolated islets (Fig. 3B and supplemental Fig. S3B). Insulin was used as a positive control, which increased Akt phosphorylation upon acute stimulation of MIN6 cells (Fig. 3C and supplemental Fig. S3C). Combined stimulation with fAd and insulin produced an additive effect on Akt phosphorylation at both Ser473 and Thr308. Furthermore, ERK phosphorylation was also significantly increased upon 24-h stimulation with both forms of adiponectin in MIN6 cells (Fig. 4A) and in mouse islets (Fig. 4B). These increases in both Akt and ERK phosphorylation were independent of any changes in the expression of the total proteins (supplemental Fig. S3D). Our studies also show that both gAd and fAd do not phosphorylate p38 MAPK in MIN6 cells (supplemental Fig. S2B) after either acute or chronic treatment.
FIGURE 2.
Adiponectin-induced AMPK phosphorylation in MIN6 cells (A) and mouse islets (B). Cells were stimulated with or without 2.5 μg/ml gAd, 5 μg/ml fAd, or 2 mm AICAR (AIC) for 10 min, 1 h, or 24 h, in serum-free medium. PBS or 5 mm Tris-HCl was used as a control (veh). Representative Western blots of phospho-AMPK (Thr172) (62 kDa) and β-actin (43 kDa) are shown. Quantified values represent means ± S.E. of three or four experiments and are normalized to β-actin and control. *, p < 0.05 versus the respective control.
FIGURE 3.
Adiponectin-induced Akt phosphorylation in MIN6 cells (A and C) and mouse islets (B) in the presence or absence of insulin. Cells were stimulated with or without 2.5 μg/ml gAd, 5 μg/ml fAd, or 100 nm insulin (ins) for 10 min, 1 h, or 24 h in serum-free medium. PBS or 5 mm Tris-HCl was used as a control (veh). Representative Western blots of phospho-Akt (Ser473) (60 kDa) and β-actin (43 kDa) are shown. Quantified values represent means ± S.E. of three to nine experiments and are normalized to β-actin and control. *, p < 0.05 versus the respective control; #, p < 0.05 versus insulin or fAd alone.
FIGURE 4.
Adiponectin-induced ERK1/2 phosphorylation in MIN6 cells (A) and mouse islets (B). Cells were stimulated with or without 2.5 μg/ml gAd or 5 μg/ml fAd for 10 min, 1 h, or 24 h in serum-free medium. PBS or 5 mm Tris-HCl was used as a control (veh). Representative Western blots of phospho-ERK1/2 (Thr202/Tyr204) (42, 44 kDa) and β-actin (43 kDa) are shown. Quantified values (both bands were assessed for phospho-ERK) represent means ± S.E. of three experiments and are normalized to β-actin and control. *, p < 0.05 versus the respective control.
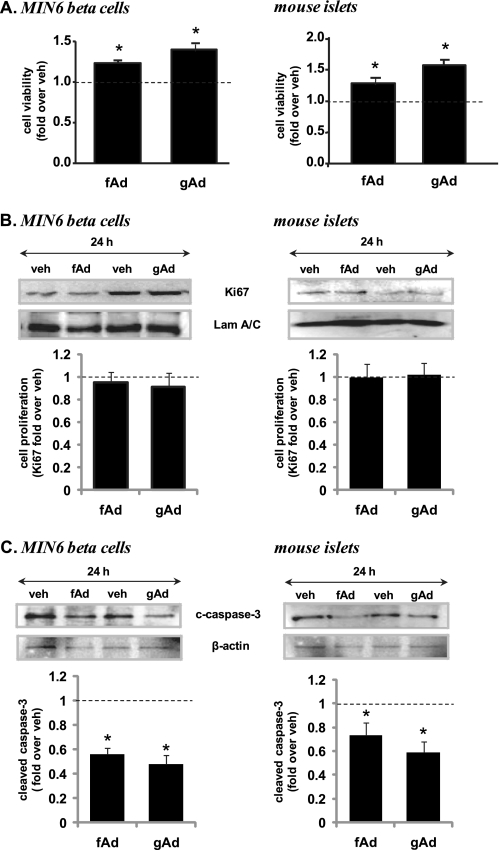
Adiponectin Increases Beta Cell Viability by Protecting against Beta Cell Apoptosis
Since both Akt and ERK have been implicated in cell growth, we examined whether adiponectin stimulation might affect cellular viability. MIN6 cells and islets were serum-starved for 24 h as a stress stimulus, and then cellular viability was measured using an XTT assay, which showed a significant increase in cell viability when treated with adiponectin compared with control (Fig. 5A). As a marker of cell proliferation, Ki67 was measured by Western blotting, but no difference was observed with adiponectin treatment (Fig. 5B). In contrast, cleaved caspase-3, a marker of cellular apoptosis, was significantly reduced when treated with fAd or gAd in both MIN6 cells and isolated islets (Fig. 5C). Similarly, cleaved caspase-3 was also reduced in the presence of adiponectin in both MIN6 and INS-1 832/13 beta cells incubated in 30 mm glucose for 48 h (supplemental Fig. S4, A and B).
FIGURE 5.
Adiponectin-induced changes in cell viability (A), proliferation (B), and apoptosis (C) in MIN6 cells and mouse islets. Cells were stimulated with or without 2.5 μg/ml gAd or 5 μg/ml fAd for 24 h. PBS or 5 mm Tris-HCl was used as a control (veh). A, cell viability assessed by XTT assay and absorbance at 490 nm normalized to total DNA. Representative Western blots of Ki67 (359 kDa) and laminin A/C (Lam A/C) (70 kDa) (B) or cleaved caspase-3 (c-caspase-3) (19 kDa) and β-actin (43-kDa) (C) are shown. Quantified values represent means ± S.E. of three or four experiments and are normalized to laminin A/C or β-actin and control. *, p < 0.05 versus the respective control.
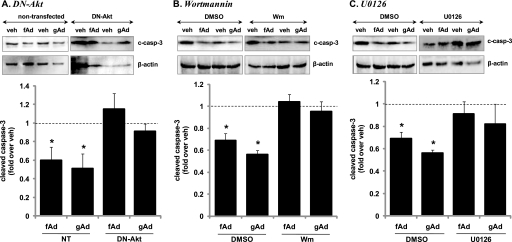
Adiponectin Protection against Apoptosis Is Mediated via Phosphoinositide (PI) 3-Kinase-Akt and MEK-ERK Activation
We examined whether Akt mediates adiponectin protection against beta cell apoptosis by utilizing a DN-Akt construct that substitutes alanine residues at the two major regulatory phosphorylation sites of Akt1 (Thr308 and Ser473) and the phosphate transfer residue in the catalytic site (Lys179) (31). Expression of DN-Akt was confirmed by detection of elevated levels of total Akt in transfected MIN6 cells (supplemental Fig. S5A). Stimulation of these cells with fAd or gAd did not increase phosphorylation of Thr308 or Ser473 of Akt (data not shown), and the gAd- and fAd-induced reduction in cleaved caspase-3 was no longer evident (Fig. 6A). We also indirectly inhibited Akt activity by inhibiting PI3 kinase, the upstream kinase of Akt using the pharmacological inhibitor wortmannin (Sigma) (32). Serum-starved MIN6 cells were treated with gAd or fAd in the presence of 50 nm wortmannin for 24 h. Again, this completely prevented stimulation of Akt phosphorylation at both regulatory sites (data not shown) and the reduction of cleaved caspase-3 by adiponectin (Fig. 6B). We also utilized the MEK inhibitor, U0126 (Sigma) to determine the involvement of ERK in adiponectin-induced antiapoptosis of beta cells (32). Treatment of serum-starved MIN6 cells with gAd or fAd in the presence of 10 μm U0126 completely prevented fAd- or gAd-stimulated ERK1/2 phosphorylation (data not shown) and reduction of cleaved caspase-3 in MIN6 beta cells (Fig. 6C).
FIGURE 6.
Effect of Akt (A and B) or ERK (C) inhibition on adiponectin-induced protection against apoptosis in MIN6 cells. A, cells expressing DN-Akt or treated with 50 nm wortmannin (Wm) (B) or 10 μm U0126 (C) were stimulated with or without 2.5 μg/ml gAd or 5 μg/ml fAd for 24 h. PBS or 5 mm Tris-HCl was used as a control (veh). Representative Western blots of cleaved caspase-3 (c-casp-3) (19 kDa) and β-actin (43 kDa) are shown. Quantified values represent means ± S.E. of three to five experiments and are normalized to β-actin and control. *, p < 0.05 versus the respective control.
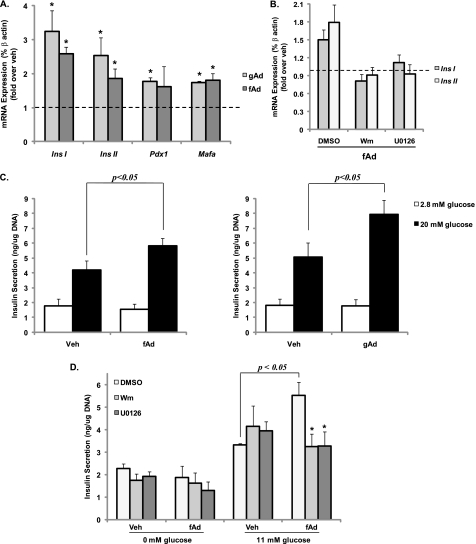
Adiponectin Treatment Increases Insulin Gene Expression and Glucose-stimulated Insulin Secretion
This positive effect of adiponectin on both cell viability and ERK activation led us to examine its impact on insulin gene expression. Gene expression of both ins I and ins II as well as transcription factors Pdx-1 and Mafa was increased in islets upon adiponectin treatment (Fig. 7A). Furthermore, this increase in insulin gene expression was apparent in the presence of both high and low glucose (supplemental Fig. S4C). Interestingly, insulin secretion after glucose stimulation was also significantly increased in serum-starved islets treated with adiponectin for 24 h compared with vehicle-treated islets (Fig. 7C). These increases in both insulin gene expression as well as insulin secretion were inhibited by wortmannin and U0126 treatment (Fig. 7, B and D).
FIGURE 7.
Adiponectin-induced changes in insulin gene expression and glucose-stimulated insulin secretion from mouse islets (A and C) and MIN6 cells (B and D). Cells were stimulated with or without 2.5 μg/ml gAd or 5 μg/ml fAd for 24 h in serum-free medium. PBS or 5 mm Tris-HCl was used as a control (veh). Cells were subjected to qPCR (A and B) for Ins I, Ins II, Pdx-1 and Mafa transcript expression, or insulin secretion (C and D) was measured during a 1-h stimulation with 2.8 or 0 and 20 or 11 mm glucose in the presence or absence of 50 nm wortmannin (Wm) or 10 μm U0126. Quantified values represent means ± S.E. of four to six experiments and are normalized to β-actin and control (A and B) or DNA (C and D). *, p < 0.05 versus the respective control or DMSO in the presence of glucose and fAd.
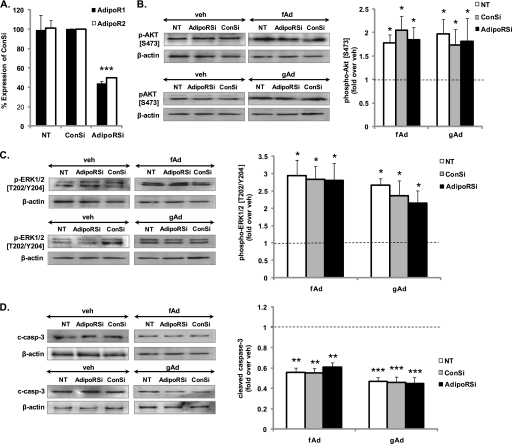
Partial Knockdown of AdipoRs Does Not Affect Adiponectin-stimulated Signaling
To determine whether adiponectin-stimulated signaling observed in pancreatic beta cells may be mediated via AdipoRs, we knocked down both receptors simultaneously using siRNA. 50% reduction of both mRNA and protein of either receptor was observed in cells co-transfected with the two siRNAs compared with those transfected with control siRNA (Fig. 8A and supplemental Fig. S5B). However, no change in adiponectin-stimulated Akt or ERK phosphorylation or cleaved caspase-3 was detected (Fig. 8, B–D, and supplemental Fig. S5C). Conversely, we overexpressed the receptors using respective adenoviruses and subsequently measured Akt phosphorylation (supplemental Fig. S6). Approximately 90% infection efficiency was determined by infecting cells concurrently with a fluorescent gfp adenovirus. Overexpressed receptors were observed primarily on the membrane fraction (supplemental Fig. S6A); however, overexpression had no further effect on fAd- or gAd-induced Akt phosphorylation (supplemental Fig. S6, B and C).
FIGURE 8.
Adiponectin-induced signaling in MIN6 cells treated with siRNA against AdipoRs. Cells co-transfected with AdipoR1 and 2 or control siRNA (ConSi) for 72 h were subjected to qPCR (A) or stimulated with or without 2.5 μg/ml gAd or 5 μg/ml fAd for 24 h in serum-free medium (B–D). PBS or 5 mm Tris-HCl was used as a control (veh). Representative Western blots of phospho-Akt (Ser473) (60 kDa), phospho-ERK1/2 (Thr202/Tyr204) (42, 44 kDa), cleaved caspase-3 (c-casp-3) (19 kDa), and β-actin (43 kDa) are shown. Quantified values (both bands were assessed for phospho-ERK) represent means ± S.E. of three or four experiments and are normalized to β-actin and control. *, p < 0.05; **, p < 0.01; ***, p < 0.005 versus the respective control.
DISCUSSION
Adiponectin is a beneficial adipokine due to its effects on the peripheral tissues to improve insulin sensitivity. A longstanding unanswered question is whether adiponectin has any direct functional impact on pancreatic islets and beta cells, especially because the main mediator of its peripheral effects is reported to be AMPK. Although this energy gauge promotes positive outcomes in the peripheral tissues, AMPK activation in islets and beta cells was shown to suppress glucose metabolism, GSIS, and enhance beta cell apoptosis (33–35). Thus far, four studies suggest that AMPK is activated by either gAd or fAd in rat and human islets or cultured beta cells (14–16, 36). Conversely, a recent study in mouse islets showed that fAd failed to increase AMPK phosphorylation (23). These conflicting results may have occurred due to differences in glucose concentrations as well as duration of stimulation, and as such, we opted to measure AMPK phosphorylation in high glucose conditions (when AMPK phosphorylation is low (37)) upon both acute and chronic stimulation. Interestingly, we observed no change in AMPK phosphorylation in either mouse islets or cultured beta cells at any time point tested. However, we observed enhanced AMPK phosphorylation by AICAR (38), confirming the responsiveness of our cells. The concentrations of adiponectin used in our assays are physiologically relevant and have repeatedly been used in in vitro assays using different cell types to achieve adiponectin-induced responses. Because bioactivity of different adiponectin preparations can vary due to differences in methods of preparation, we used C2C12 myotubes as a positive control. As previously described (7), AMPK was strongly phosphorylated in these cells upon treatment, thus confirming the bioactivity of our preparations. Therefore, our data suggest that in mouse pancreatic beta cells, AMPK is not an important effector molecule in adiponectin signaling.
Consequently, we explored the possibility that adiponectin might utilize other intracellular signaling pathways. Previous studies have implicated a number of other molecules in adiponectin signaling, including NFκB, peroxisome proliferator-activated receptor-α, Akt, nitric-oxide synthase, cyclic AMP, p38 MAPK, and ERK in various cellular systems (13). Although a recent study suggests the activation of peroxisome proliferator-activated receptor-α by adiponectin in beta cells, the participation of any of the other proteins in adiponectin-induced beta cell signaling remains unknown (36). Therefore, our study is the first to show that whereas p38 MAPK does not play a role, both Akt and ERK are activated by fAd and gAd in islets and beta cells. ERK had a delayed response to adiponectin, whereas Akt activity was enhanced by both acute and chronic treatment. Typically, adiponectin-induced Akt phosphorylation is suggested to be downstream of AMPK activation (39), although here it occurs independently of it. There is a possibility, however, that because serum starvation alone can activate AMPK (40), we may have failed to detect any subtle increases of AMPK phosphorylation by adiponectin. Interestingly, fAd enhanced insulin-induced Akt phosphorylation, an effect that has been previously described in other cell systems (13, 41). In peripheral tissues, this cross-talk was suggested to be mediated by APPL1, which was shown to signal to p38 MAPK, AMPK, as well as Akt (13). We also identified mRNA expression of APPL1 in both pancreatic islets and cultured beta cells (data not shown), but its role in adiponectin-stimulated signaling in beta cells is yet to be confirmed.
Because both Akt and ERK have been implicated in cell survival, we explored whether adiponectin has an impact on beta cell and islet viability. Cell viability was indeed increased in the presence of adiponectin under serum-starved conditions, and this appeared to be a result of reduced cellular apoptosis. We also observed a similar protection against glucotoxicity-induced apoptosis by adiponectin. Although two studies have previously reported that adiponectin can be protective against cytokine-, free fatty acid-, and glucotoxicity-induced apoptosis in cultured beta cells (22, 29), ours is the first to show such an antiapoptotic property of adiponectin in isolated islets. Cumulatively, these studies and our results suggest that the ability of adiponectin to protect against apoptosis is not isolated to MIN6 beta cells or to serum starvation. However, a previous study has suggested that adiponectin does not provide protection against lipoapoptosis after treatment of human islets (16). The authors suggest that any protective effect by adiponectin might be masked by toxicity from the high concentrations of fatty acids used, but it might also be that the antiapoptotic properties of adiponectin become more apparent under starvation conditions as we observed.
Interestingly, we also found that insulin gene expression was increased in mouse islets after chronic stimulation with adiponectin. In cultured beta cells, this increase was apparent in both low and high glucose. In a recently published study a similar increase in Pdx-1 and insulin gene expression was observed in INS-1 cells stimulated with adiponectin during sustained glucotoxicity (29). This increase in insulin gene expression may be a direct effect of Pdx-1 and MafA up-regulation (42) and in turn may have resulted in the adiponectin-induced increase in GSIS. The impact of adiponectin on insulin secretion in vitro has so far been variable in literature. Upon stimulation with adiponectin, no effect was observed in basal or GSIS from human islets; basal secretion was reduced, whereas GSIS was increased in insulin-resistant mice; GSIS was increased in isolated rat islets; and basal and GSIS was increased during sustained glucotoxicity, and fatty acid-induced inhibition of GSIS was reversed in cultured beta cells (14, 16, 21, 22, 29). Therefore, with the human islet study as an exception, there appears to be a general trend for adiponectin to increase GSIS, especially under stressed conditions, such as in insulin resistance, lipotoxicity, glucotoxicity, or serum starvation. Whether there is a direct link between adiponectin-induced insulin gene expression and secretion remains to be determined.
Many studies have shown that the PI3 kinase-Akt axis is critically important for beta cell growth and survival (43). Mice globally lacking insulin receptor substrate-2, which acts as a docking site for PI3 kinase, have a marked reduction in beta cell mass caused by an increased rate of apoptosis (44). Furthermore, expression of constitutively active Akt in beta cells was reported to prevent fatty acid-induced apoptosis, and transgenic overexpression of Akt in beta cells was also shown to prolong beta cell survival and protect against streptozotocin-induced diabetes (45, 46). Similarly, it has been reported in several studies that acute glucose may regulate beta cell growth and survival through ERK activation, whereas others have shown that chronic exposure to high glucose concentrations promote islet apoptosis also via ERK activation (47–49). Thus, ERK signaling is activated in both prosurvival and proapoptotic conditions, but the outcome may depend on the timing and duration of ERK activation.
Here, we associate both Akt and ERK with the antiapoptotic property of adiponectin in pancreatic beta cells. Interestingly, the reduction in cleaved caspase-3 was completely prevented by independently inhibiting Akt and ERK, suggestive of a convergent pathway. A myriad of antiapoptotic, prosurvival substrates exist downstream of Akt (43). A possible site of interaction between the two pathways is Bad, the proapoptotic member of the Bcl-2 family (50). Inhibition of the PI3 kinase-Akt and MEK-ERK axis also prevented the adiponectin-induced increases of both insulin gene expression and secretion. Previously, overexpression of a kinase-dead Akt was shown to impair insulin secretion in beta cells (51), and regulation of Pdx-1 and MafA expression by downstream targets of Akt has also been described (52). Furthermore, most downstream effectors of ERK are nuclear transcription factors, hence ERK has been implicated in insulin gene transcription (53). Therefore, whether Akt and ERK directly mediate these changes or whether it is a consequence of cell survival remains to be determined.
Finally, our results are concurrent with previous reports that suggest predominant expression of AdipoR1 in pancreatic islets (14). However, knockdown or overexpression of AdipoRs in pancreatic beta cells did not affect adiponectin-induced changes in Akt or ERK phosphorylation or cleaved caspase-3. Although only 50% knockdown of the two receptors was achieved, previously we have shown that this is sufficient to impact both adiponectin-induced function and signaling (30). However, there is the possibility that the remaining receptors are quite sufficient in mediating adiponectin-induced signaling. Similarly, we may have achieved maximal adiponectin-stimulated Akt phosphorylation with endogenously expressed receptors, thus overexpressed receptors have no further effect. Conversely, it could also be that the two known receptors do not mediate the adiponectin-induced signaling events and functional outcomes reported here in beta cells but may induce activation of different signaling molecules we have not yet explored. Thus, adiponectin may mediate the effects described here via a different receptor. One candidate could be T-cadherin, which was reported to function as a receptor for hexameric and high molecular mass adiponectin (54). However, because we found that both islets and MIN6 cells respond also to gAd, it could be a yet uncharacterized receptor.
In conclusion, this study suggests that the actions of adiponectin in beta cells are independent of AMPK, and it is the first to implicate both Akt and ERK in adiponectin signaling in pancreatic beta cells. Whether AdipoRs are involved in adiponectin-mediated beta cell function remains to be elucidated. Nonetheless, the ability of adiponectin to protect against apoptosis to increase cellular viability, GSIS, and gene transcription during cell stress, as well as its ability to potentiate the insulin response in beta cells independent of activating AMPK, a negative regulator of beta cell function, is significant and beneficial. Therefore, collectively, it appears that adiponectin agonists pose a potential treatment strategy for type 2 diabetes.
Supplementary Material
Acknowledgments
We thank Dr. James R. Woodgett for the DN-Akt construct; Dr. Amira Klip for C2C12 cells, phospho-p38 MAPK, and phospho-ERK1/2 antibodies; Dr. Allen Volchuk for INS-1 832/13 cells; Dr. S. Seino for MIN6 cells; Dr. Minna Woo and Dr. Patricia Brubaker for caspase-3 antibody; Sobia Sultan for technical assistance; and Dr. Farah Thong and Dr. Emma Allister for valuable comments on the manuscript.
This work was supported by Canadian Institute of Health Research Grant MOP-12898 (to M. B. W.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Tables 1 and 2 and Figs. S1–S6.
- fAd
- full-length adiponectin
- AdipoR
- adiponectin receptor
- AICAR
- 5-aminoimidazole-4-carboxyamide ribonucleoside
- AMPK
- 5′-AMP-activated protein kinase
- APPL
- adaptor protein containing pleckstrin homology domain, phosphotyrosine-binding domain, and leucine zipper motif
- DN
- dominant negative
- gAd
- globular adiponectin
- GSIS
- glucose-stimulated insulin secretion
- qPCR
- quantitative real time PCR
- XTT
- 2,3-bis(2-methoxy-4-nitro-5-sulfophenyl)-5-[(phenylamino)carbonyl]-2H-tetrazo-lium hydroxide
- Wm
- wortmannin.
REFERENCES
- 1.Arita Y., Kihara S., Ouchi N., Takahashi M., Maeda K., Miyagawa J., Hotta K., Shimomura I., Nakamura T., Miyaoka K., Kuriyama H., Nishida M., Yamashita S., Okubo K., Matsubara K., Muraguchi M., Ohmoto Y., Funahashi T., Matsuzawa Y. (1999) Biochem. Biophys. Res. Commun. 257, 79–83 [DOI] [PubMed] [Google Scholar]
- 2.Weyer C., Funahashi T., Tanaka S., Hotta K., Matsuzawa Y., Pratley R. E., Tataranni P. A. (2001) J. Clin. Endocrinol. Metab. 86, 1930–1935 [DOI] [PubMed] [Google Scholar]
- 3.Kubota N., Terauchi Y., Yamauchi T., Kubota T., Moroi M., Matsui J., Eto K., Yamashita T., Kamon J., Satoh H., Yano W., Froguel P., Nagai R., Kimura S., Kadowaki T., Noda T. (2002) J. Biol. Chem. 277, 25863–25866 [DOI] [PubMed] [Google Scholar]
- 4.Maeda N., Shimomura I., Kishida K., Nishizawa H., Matsuda M., Nagaretani H., Furuyama N., Kondo H., Takahashi M., Arita Y., Komuro R., Ouchi N., Kihara S., Tochino Y., Okutomi K., Horie M., Takeda S., Aoyama T., Funahashi T., Matsuzawa Y. (2002) Nat. Med. 8, 731–737 [DOI] [PubMed] [Google Scholar]
- 5.Yamauchi T., Kamon J., Waki H., Imai Y., Shimozawa N., Hioki K., Uchida S., Ito Y., Takakuwa K., Matsui J., Takata M., Eto K., Terauchi Y., Komeda K., Tsunoda M., Murakami K., Ohnishi Y., Naitoh T., Yamamura K., Ueyama Y., Froguel P., Kimura S., Nagai R., Kadowaki T. (2003) J. Biol. Chem. 278, 2461–2468 [DOI] [PubMed] [Google Scholar]
- 6.Combs T. P., Pajvani U. B., Berg A. H., Lin Y., Jelicks L. A., Laplante M., Nawrocki A. R., Rajala M. W., Parlow A. F., Cheeseboro L., Ding Y. Y., Russell R. G., Lindemann D., Hartley A., Baker G. R., Obici S., Deshaies Y., Ludgate M., Rossetti L., Scherer P. E. (2004) Endocrinology 145, 367–383 [DOI] [PubMed] [Google Scholar]
- 7.Yamauchi T., Kamon J., Minokoshi Y., Ito Y., Waki H., Uchida S., Yamashita S., Noda M., Kita S., Ueki K., Eto K., Akanuma Y., Froguel P., Foufelle F., Ferre P., Carling D., Kimura S., Nagai R., Kahn B. B., Kadowaki T. (2002) Nat. Med. 8, 1288–1295 [DOI] [PubMed] [Google Scholar]
- 8.Okamoto Y., Kihara S., Funahashi T., Matsuzawa Y., Libby P. (2006) Clin. Sci. 110, 267–278 [DOI] [PubMed] [Google Scholar]
- 9.Scherer P. E., Williams S., Fogliano M., Baldini G., Lodish H. F. (1995) J. Biol. Chem. 270, 26746–26749 [DOI] [PubMed] [Google Scholar]
- 10.Waki H., Yamauchi T., Kamon J., Kita S., Ito Y., Hada Y., Uchida S., Tsuchida A., Takekawa S., Kadowaki T. (2005) Endocrinology 146, 790–796 [DOI] [PubMed] [Google Scholar]
- 11.Whitehead J. P., Richards A. A., Hickman I. J., Macdonald G. A., Prins J. B. (2006) Diabetes Obes. Metab. 8, 264–280 [DOI] [PubMed] [Google Scholar]
- 12.Yamauchi T., Kamon J., Ito Y., Tsuchida A., Yokomizo T., Kita S., Sugiyama T., Miyagishi M., Hara K., Tsunoda M., Murakami K., Ohteki T., Uchida S., Takekawa S., Waki H., Tsuno N. H., Shibata Y., Terauchi Y., Froguel P., Tobe K., Koyasu S., Taira K., Kitamura T., Shimizu T., Nagai R., Kadowaki T. (2003) Nature 423, 762–769 [DOI] [PubMed] [Google Scholar]
- 13.Deepa S. S., Dong L. Q. (2009) Am. J. Physiol. Endocrinol. Metab. 296, E22–E36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gu W., Li X., Liu C., Yang J., Ye L., Tang J., Gu Y., Yang Y., Hong J., Zhang Y., Chen M., Ning G. (2006) Endocrine 30, 217–221 [DOI] [PubMed] [Google Scholar]
- 15.Huypens P., Moens K., Heimberg H., Ling Z., Pipeleers D., Van de Casteele M. (2005) Life Sci. 77, 1273–1282 [DOI] [PubMed] [Google Scholar]
- 16.Staiger K., Stefan N., Staiger H., Brendel M. D., Brandhorst D., Bretzel R. G., Machicao F., Kellerer M., Stumvoll M., Fritsche A., Häring H. U. (2005) J. Clin. Endocrinol. Metab. 90, 6707–6713 [DOI] [PubMed] [Google Scholar]
- 17.Kharroubi I., Rasschaert J., Eizirik D. L., Cnop M. (2003) Biochem. Biophys. Res. Commun. 312, 1118–1122 [DOI] [PubMed] [Google Scholar]
- 18.Musso G., Gambino R., Biroli G., Carello M., Fagà E., Pacini G., De Michieli F., Cassader M., Durazzo M., Rizzetto M., Pagano G. (2005) Am. J. Gastroenterol. 100, 2438–2446 [DOI] [PubMed] [Google Scholar]
- 19.Bacha F., Saad R., Gungor N., Arslanian S. A. (2004) Diabetes Care 27, 547–552 [DOI] [PubMed] [Google Scholar]
- 20.Retnakaran R., Hanley A. J., Raif N., Hirning C. R., Connelly P. W., Sermer M., Kahn S. E., Zinman B. (2005) Diabetologia 48, 993–1001 [DOI] [PubMed] [Google Scholar]
- 21.Winzell M. S., Nogueiras R., Dieguez C., Ahrén B. (2004) Biochem. Biophys. Res. Commun. 321, 154–160 [DOI] [PubMed] [Google Scholar]
- 22.Rakatzi I., Mueller H., Ritzeler O., Tennagels N., Eckel J. (2004) Diabetologia 47, 249–258 [DOI] [PubMed] [Google Scholar]
- 23.Okamoto M., Ohara-Imaizumi M., Kubota N., Hashimoto S., Eto K., Kanno T., Kubota T., Wakui M., Nagai R., Noda M., Nagamatsu S., Kadowaki T. (2008) Diabetologia 51, 827–835 [DOI] [PubMed] [Google Scholar]
- 24.Luo X. H., Guo L. J., Yuan L. Q., Xie H., Zhou H. D., Wu X. P., Liao E. Y. (2005) Exp. Cell Res. 309, 99–109 [DOI] [PubMed] [Google Scholar]
- 25.Ogunwobi O. O., Beales I. L. (2006) Regul. Pept. 134, 105–113 [DOI] [PubMed] [Google Scholar]
- 26.Cong L., Gasser J., Zhao J., Yang B., Li F., Zhao A. Z. (2007) Endocr. Relat. Cancer 14, 713–720 [DOI] [PubMed] [Google Scholar]
- 27.Shibata R., Sato K., Pimentel D. R., Takemura Y., Kihara S., Ohashi K., Funahashi T., Ouchi N., Walsh K. (2005) Nat. Med. 11, 1096–1103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kobayashi H., Ouchi N., Kihara S., Walsh K., Kumada M., Abe Y., Funahashi T., Matsuzawa Y. (2004) Circ. Res. 94, e27–e31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lin P., Chen L., Li D., Liu J., Yang N., Sun Y., Xu Y., Fu Y., Hou X. (2009) Tohoku J. Exp. Med. 217, 59–65 [DOI] [PubMed] [Google Scholar]
- 30.Palanivel R., Fang X., Park M., Eguchi M., Pallan S., De Girolamo S., Liu Y., Wang Y., Xu A., Sweeney G. (2007) Cardiovasc. Res. 75, 148–157 [DOI] [PubMed] [Google Scholar]
- 31.Wang Q., Somwar R., Bilan P. J., Liu Z., Jin J., Woodgett J. R., Klip A. (1999) Mol. Cell. Biol. 19, 4008–4018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Davies S. P., Reddy H., Caivano M., Cohen P. (2000) Biochem. J. 351, 95–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Richards S. K., Parton L. E., Leclerc I., Rutter G. A., Smith R. M. (2005) J. Endocrinol. 187, 225–235 [DOI] [PubMed] [Google Scholar]
- 34.Kefas B. A., Heimberg H., Vaulont S., Meisse D., Hue L., Pipeleers D., Van de Casteele M. (2003) Diabetologia 46, 250–254 [DOI] [PubMed] [Google Scholar]
- 35.Sun G., Tarasov A. I., McGinty J., McDonald A., da Silva Xavier G., Gorman T., Marley A., French P. M., Parker H., Gribble F., Reimann F., Prendiville O., Carzaniga R., Viollet B., Leclerc I., Rutter G. A. (2010) Diabetologia 53, 924–936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jung T. W., Lee M. W., Lee Y. J., Kim S. M., Lee K. T., Whang W. K., Cheon H. J., Jeong Y. T., Chung K. W., Cho J. M., Kim do H., Jung T. W. (2009) Endocr. J. 56, 377–382 [DOI] [PubMed] [Google Scholar]
- 37.Salt I. P., Johnson G., Ashcroft S. J., Hardie D. G. (1998) Biochem. J. 335, 533–539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sullivan J. E., Brocklehurst K. J., Marley A. E., Carey F., Carling D., Beri R. K. (1994) FEBS Lett. 353, 33–36 [DOI] [PubMed] [Google Scholar]
- 39.Ouchi N., Kobayashi H., Kihara S., Kumada M., Sato K., Inoue T., Funahashi T., Walsh K. (2004) J. Biol. Chem. 279, 1304–1309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ching J. K., Rajguru P., Marupudi N., Banerjee S., Fisher J. S. (2008) FASEB J. 22, 959.1 [Google Scholar]
- 41.Shinoda Y., Yamaguchi M., Ogata N., Akune T., Kubota N., Yamauchi T., Terauchi Y., Kadowaki T., Takeuchi Y., Fukumoto S., Ikeda T., Hoshi K., Chung U. I., Nakamura K., Kawaguchi H. (2006) J. Biol. Chem. 99, 196–208 [DOI] [PubMed] [Google Scholar]
- 42.Zhao L., Guo M., Matsuoka T. A., Hagman D. K., Parazzoli S. D., Poitout V., Stein R. (2005) J. Biol. Chem. 280, 11887–11894 [DOI] [PubMed] [Google Scholar]
- 43.Dickson L. M., Rhodes C. J. (2004) Am. J. Physiol. Endocrinol. Metab. 287, E192–E198 [DOI] [PubMed] [Google Scholar]
- 44.Withers D. J., Gutierrez J. S., Towery H., Burks D. J., Ren J. M., Previs S., Zhang Y., Bernal D., Pons S., Shulman G. I., Bonner-Weir S., White M. F. (1998) Nature 391, 900–904 [DOI] [PubMed] [Google Scholar]
- 45.Wrede C. E., Dickson L. M., Lingohr M. K., Briaud I., Rhodes C. J. (2002) J. Biol. Chem. 277, 49676–49684 [DOI] [PubMed] [Google Scholar]
- 46.Tuttle R. L., Gill N. S., Pugh W., Lee J. P., Koeberlein B., Furth E. E., Polonsky K. S., Naji A., Birnbaum M. J. (2001) Nat. Med. 7, 1133–1137 [DOI] [PubMed] [Google Scholar]
- 47.Costes S., Broca C., Bertrand G., Lajoix A. D., Bataille D., Bockaert J., Dalle S. (2006) Diabetes 55, 2220–2230 [DOI] [PubMed] [Google Scholar]
- 48.Fei H., Zhao B., Zhao S., Wang Q. (2008) Mol. Cell. Biochem. 315, 75–84 [DOI] [PubMed] [Google Scholar]
- 49.Maedler K., Størling J., Sturis J., Zuellig R. A., Spinas G. A., Arkhammar P. O., Mandrup-Poulsen T., Donath M. Y. (2004) Diabetes 53, 1706–1713 [DOI] [PubMed] [Google Scholar]
- 50.Hayakawa J., Ohmichi M., Kurachi H., Kanda Y., Hisamoto K., Nishio Y., Adachi K., Tasaka K., Kanzaki T., Murata Y. (2000) Cancer Res. 60, 5988–5994 [PubMed] [Google Scholar]
- 51.Bernal-Mizrachi E., Fatrai S., Johnson J. D., Ohsugi M., Otani K., Han Z., Polonsky K. S., Permutt M. A. (2004) J. Clin. Invest. 114, 928–936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Humphrey R. K., Yu S. M., Flores L. E., Jhala U. S. (2010) J. Biol. Chem. 285, 3406–3416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Benes C., Poitout V., Marie J. C., Martin-Perez J., Roisin M. P., Fagard R. (1999) Biochem. J. 340, 219–225 [PMC free article] [PubMed] [Google Scholar]
- 54.Hug C., Wang J., Ahmad N. S., Bogan J. S., Tsao T. S., Lodish H. F. (2004) Proc. Natl. Acad. Sci. U.S.A. 101, 10308–10313 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.