Abstract
Adenosine is a candidate modulator of sperm motility in the female reproductive tract that increases sperm flagellar beat frequency in vitro. Past work suggested that this acceleration may involve equilibrative (ENT) and concentrative (CNT) nucleoside transporters. Here we show that Slc29a1 (ENT-1) is the predominant nucleoside transporter expressed in the mouse testis. Unexpectedly, the beat of Slc29a1-null sperm still accelerates in response to 2-chloro-2′-deoxyadenosine (Cl-dAdo). Moreover, in wild-type sperm neither blockade of CNTs by removal of external Na+, nor inhibition of ENTs with nitrobenzylthioionosine, prevents acceleration of the sperm beat by Cl-dAdo. In contrast, pertussis toxin produces strong blockade, indicating involvement of a Gαi/o-coupled adenosine receptor. Although agonists selective for adenosine receptors A1R, A2aR, and A2bR are ineffective, A3R-selective agonists Cl-IB-MECA and IB-MECA do accelerate the beat. Consistent with this pharmacological profile, the predominant Adora transcripts in the testis are products of the nested Adora3i1 and Adora3i2 genes. Surprisingly, Cl-IB-MECA and Cl-dAdo still accelerate the beat of Adora3i1-null sperm indicating that the remaining Adora3i2 transcript produces an A3R that functions in sperm. When cloned Adora3i2 is heterologously expressed in tsA-201 cells, Cl-dAdo decreases forskolin-evoked accumulation of cAMP, indicating that Adora3i2 specifies a functional A3Ri2 adenosine receptor that couples through Gαi. Database mining reveals that mouse Adora3i2 is expressed primarily in testis, almost exclusively in spermatids. Expression of the orthologous ADORA3i3 transcript also is most prominent in human testis; presumably producing an A3Ri3 receptor that is functional in sperm and that may be a target for development of male-directed contraceptives.
Keywords: Cyclic AMP (cAMP), G-Protein-coupled Receptors (GPCR), Nucleoside Nucleotide Transport, Pertussis Toxin, Sperm, Flagellar Motility, Capacitation
Introduction
The only known roles of sperm are to reach the egg and deliver its genetic payload and to initiate early signaling events in the zygote. Although the list is short, the tasks are complex, and the sperm requires numerous specialized components to accomplish these goals. Our recent work has focused on how the sperm applies its specialized components to control swimming behavior. We have combined biophysical methods with a genetic approach, using sperm from null-mutant (knock-out) mice that carry targeted disruptions of the genes for several of the components of the signaling pathways that operate in the sperm flagellum. This approach has provided definitive evidence for required roles of the unique CatSper ion channel proteins (1–7), for the atypical sperm adenylyl cyclase SACY2 (3, 8), and for the sperm-specific Cα2 catalytic subunit of PKA (9, 10). It also produced the unexpected finding that adenosine analogs engage cAMP-mediated signaling to increase flagellar beat frequency (10, 11).
In some somatic cells, adenosine activates cell surface G-protein-coupled adenosine receptors that couple to various conventional transmembrane adenylyl cyclases (ADCY1–9) to control synthesis of cAMP (12, 13). However, in sperm the predominant (perhaps the only) adenylyl cyclase is the atypical SACY (ADCY10) (8), which lacks transmembrane domains and is unaffected by G-proteins (14, 15). The apparent absence of conventional adenylyl cyclases led us to propose that adenosine analogs may enter sperm via cell surface nucleoside transporters to engage the SACY/PKA signaling pathway (10, 11). We now reconsider this proposal, evaluating the expression of Slc28a and Slc29a nucleoside transporters in adult mouse testis, and examining the phenotype of sperm from the Slc29a1 knock-out mouse (16).
Some other past work reports finding adenosine receptor binding activity and immunoreactivity in sperm (17–20). We now also reexamine the expression of adenosine receptor genes in the testis and spermatogenic cells and explore the sperm phenotype of the Adora3 knock-out mouse (21). The results provide new insights about the nature of the Adora3i2 transcript in testis and of expression of A3R adenosine receptors in mouse sperm and of the orthologous ADORA3i3 transcript in human testis that presumably generates A3Rs for human sperm.
EXPERIMENTAL PROCEDURES
Materials
The cAMP radioimmunoassay kit NEK 033 was from Perkin-Elmer (Shelton, CT). CGS21680, CCPA, IB-MECA, and Cl-IB-MECA were from Calbiochem. Pertussis toxin B-oligomer was from List (Campbell, CA) and tagRFP vector was a generous gift from Dr. Roger Tsien (UCSD, San Diego, CA). Protease Inhibitor Mixture, pertussis toxin, and 2-chloro-2′-deoxyadenosine (Cl-dAdo) and all other chemicals were from Sigma-Aldrich, unless indicated otherwise.
Sperm Preparation and Incubation
Sperm were prepared as in prior work (1, 2, 22, 23) Briefly, after euthanasia by CO2 asphyxiation, caudae epididymides, and vasa deferentia were excised from male mice, either wild-type retired breeders of CD1 or Swiss-Webster strains, or from adult male Adora3−/− (21) or Slc29a1−/− (16) knock-out strains of known fertility and from age-matched wild-type animals of the same genetic backgrounds. Slc29a1+/− breeding pairs were maintained in a hybrid (50% C57BL6/J, 50% SX1/SvJ) background. Adora3i1−/− breeding pairs were maintained in a C57BL6/J background. After rinsing with medium HS (in mm): 135 NaCl, 5 KCl, 2 CaCl2, 1 MgSO4, 20 HEPES, 5 glucose, 10 lactic acid, 1 pyruvic acid, adjusted to pH 7.4 with NaOH, the excised tissue was transferred to 1 ml of a swimout/capacitation medium (HS with 5 mg BSA/ml and 15 mm NaHCO3). Semen was allowed to exude (15 min at 37 °C, 5% CO2) from several small incisions. All subsequent operations were at room temperature (22–25 °C) in medium HS, unless noted otherwise. Sperm were washed twice, then dispersed, and stored at 1–2× 107 cells ml−1. Some experiments used Na+-deficient versions of medium HS prepared by replacement of NaCl with N-methyl-d-glucamine hydrochloride and of NaHCO3 with tetraethylammonium bicarbonate.
Adenosine Analogs, Pharmacological Agonists, and Transport Inhibitors
Stock solutions of Cl-dAdo (1 mm in medium HS) and other adenosine analogs, agonists, and inhibitors (1–30 mm in DMSO) were stored frozen. After final dilution the DMSO was <1% (v/v). A parallel solvent-only control was performed when addition of stocks produced DMSO >0.1%.
RNA Extraction and Gene Expression
Mouse tissue was rapidly frozen in liquid nitrogen, transferred to 6 m guanidinium chloride and sonicated. Mouse testis RNA was isolated using the RNAeasyTM kit (Qiagen, Germantown, MD) per the manufacturer's instructions. Human testis RNA was purchased from Origene (Rockville, MD). RNA concentrations were determined from A260. Quantitative RT-PCR reactions were performed using 25 ng of RNA in Brilliant II Syber Green Master MixTM with reverse transcriptase (Stratagene, La Jolla, CA) using gene-specific primers with matched annealing temperatures and similar amplicon lengths. Mouse primers: Slc29a1 (F: 5′-CAGCCTGTGCAGTTGTCATT-3′, R: 5′-CCGTGAAGATGAAGCAGACA-3′), Slc29a2 (F: 5′-TTGCCCGTTACTACCTGACC-3′, R: 5′-CGACAGGGTGACTGTGAAGA-3′), Slc29a3 (F: 5′-TAGCAGCTCCTCCACCATCT-3′, R: 5′-GGCAACTGGCCTCATGTAGT-3′), Slc29a4 (F: 5′- ACCGCTACCATGCCATCTAC-3′, R: 5′-CCTGGTCGTGAGAGAAGAGC-3′), Slc28a1 (F: 5′-TGGTCTACCCAGAGGTGGAG-3′, R: 5′-GGACGTAGGAGCAGATGAGC-3′), Slc28a2 (F: 5′- ATGCTTGAAGCCTCTGGAAA-3′, R: 5′-ATCTGATCTCCCAGCCATTG-3′), Slc28a3 (F: 5′-CAAACTGGGCCAACAAAACT-3′, R: 5′-GGGCAGGATCTTAAATGCAA-3′), Adora1 (F: 5′-CTTCTACCTGATCCGCAAGC-3′, R: 5′-AGAAAGGTGACCCGGAACTT-3′), Adora2a (F: 5′-GGCTCCTCGGTGTACATCAT-3′, R: 5′-GGCTGAAGATGGAACTCTGC-3′), Adora2b (F: 5′-CCTTTGGCATTGGATTGACT-3′, R: 5′-CCTGGAGTGGTCCATCAGTT-3′), Adora3i1(F: 5′-CCCTGGTTGTCATGTGTGTC-3′, R: 5′-AGGGTTCATCATGGAGTTCG-3′), Adora3i2 (F: 5′-CAGGTGTTGAGCTGGAGACA-3′, R: 5′-ATTGGCCCGGTCTTCTCTAT-3′). Human primers: ADORA3i1 (F: 5′-GGGCATCACAATCCACTTCT-3′, R: 5′-CATGACCATGGCATCTGAAA-3′), ADORA3i2 (F: 5′-TTGTCATGTGCGCCATCTAT-3′, R: 5′-AGGGTTCATCATGGAGTTGG-3′), ADORA3i3 (F: 5′-GACCCATTGAGCAGAAGGAG-3′, R: 5′-CACATGACTGGAAGGAAGCA-3′). Quantitative PCR was performed in a MX3000P PCR machine (Stratagene), and the products were verified for appropriate length by gel electrophoresis then sequenced. Relative gene expression was determined using the ΔΔCt method with normalization to Arbp (acidic ribosomal-binding protein) as in Sanz et al. (24). Positive control reference tissues were used to verify primer efficacy. RNA for human and mouse was also reverse-transcribed to generate cDNA using the Superscript First Strand Synthesis System (Invitrogen, Carlsbad CA). Full-length transcripts were amplified using transcript-specific primer combinations: human ADORA3i2 (F: 5′-ATGCCCAACAACAGCACTG-3′, R: 5′-CTACTCAGAATTCTTCTCAATGCTTG-3′); ADORA3i3 (F: 5′-ATGGAAGGGTCTCCAGCAG-3′, R: 5′-TCACATCTGTTCAGTAGGAGCC-3′). Full-length mouse transcripts of Adora3i1 and Adora3i2 were amplified with the primers used for cloning (see below). Transcripts of Adora3i3, which shares exons with both Adora3i1 and Adora3i2, were probed with the Adora3i2 forward primer and the Adora3i1 reverse primer. PCR products were visualized on 1% agarose gels run in TAE (Tris-acetate-EDTA) buffer and stained with ethidium bromide.
Protein Extraction
Tissues and sperm were extracted with the TNE buffer (10 mm Tris pH 8.0, 1 mm EDTA, 0.5% Nonidet P-40, 1× protease inhibitor mixture) for 45 min on ice with vortexing every 5 min. Samples were clarified by centrifugation (10000 × g, 5 min), and protein concentration was determined using the BCA protein assay reagent (Thermo Scientific).
Videomicroscopy and Waveform Analysis
As in past work (22), time series of stop-motion, phase-contrast images of loosely-tethered sperm were recorded while a gravity-fed, solenoid-controlled local-perfusion device applied and removed test solutions. After adjustments of image size and orientation using Metamorph (Molecular Devices, Toronto, Canada) and Igor Pro (Wavemetrics, Lake Oswego, OR) the flagellar location in each image was captured by automated determination of intensity maxima and minima in a line-scan perpendicular to the axis of the flagellar beat. The most prominent peak in the power spectrum of the Fourier transform of the flagellar location over time then directly reported flagellar beat frequency.
Cloning and Recombinant Protein Expression
Cloning of Adora3i2 was performed by RT-PCR amplification of the full-length transcript from testis RNA using gene-specific primers (Forward: 5′-TATCGAAGCTTCGCCACCATGGAGTTCCTCCTCCTTCTCT-3′, Reverse: 5′-GATACGGATCCCCTCAAATCCTTGCCAAAGTTATG-3′) as described (6). EcoRI and HindIII restriction sites were incorporated into the 5′-ends of the forward and reverse PCR primers (underlined), to facilitate cloning into the EcoRI and HindIII sites in the tagRFP vector. A consensus Kozak sequence, CGCCAAC, was inserted upstream of the Adora3i2 start codon to ensure efficient translation. The full-length, PCR-amplified template was then digested with EcoRI and HindIII and ligated into the tagRFP vector. The ligated plasmids were then transformed into chemically competent DH5α Escherichia coli. Plasmid DNA isolated from bacterial clones was fully sequenced to verify the identity of the inserted gene. Recombinant adenosine receptor Adora3i2 was expressed in tsA-201 cells in culture by transient transfection with Fugene 6 (Roche, Mannheim, Germany), according to the manufacturer's instructions. Cells were grown in DMEM containing 10% (v/v) fetal bovine serum and 1% (w/v) penicillin/streptomycin at 37 °C. Cells were transfected at 50% confluency and assayed at 100% confluency ∼24–36 h after transfection. Control cells were transfected with cytosolic GFP (Invitrogen).
Cell Treatments and cAMP Radioimmunoassay
Transfected cells were washed with and equilibrated for 15 min in assay buffer (in mm): 110 NaCl, 5 NaHCO3, 1.2 NaH2PO4, 5 KCl, 2.7 CaCl2, 1 MgSO4, 20 HEPES, 10 glucose, adjusted to pH 7.4 with NaOH. Incubation continued for 15 min in the presence of 1 mm IBMX. Triplicate wells were further treated for 15 min with the same buffer containing 1 mm IBMX alone or with 3 μm forskolin. An additional 6 wells were treated with IBMX and 30 μm Cl-dAdo. In the final 15 min of incubation, forskolin was added to 3 of these 6 wells. All remaining wells received no further additions. Buffer was then carefully aspirated, and cells were lysed by addition of 0.1% Triton X-100 in 0.1 m NaOH (150 μl) followed by an equal volume of 0.1% Triton X-100 in 0.1 m HCl. The neutralized cell lysates were assayed for cAMP content using a cAMP radioimmunoassay kit per manufacturer's instructions for non-acetylated samples.
RESULTS
Adenosine Transporters in Acceleration of the Sperm Flagellar Beat
Our past work suggested that adenosine and related analogs may enter sperm via equilibrative and concentrative nucleoside transporters (ENTs and CNTs) to engage a cAMP-mediated signaling pathway that increases sperm flagellar beat frequency (10, 11). For the mouse, the Slc28a1–3 genes encode CNT1–3 (25) and Slc29a1-4 genes encode ENT1–4 (26). Since integral membrane proteins of sperm are obligatorily produced during spermatogenesis or spermiogenesis, we applied qRT-PCR to quantify the relative expression of nucleoside transporter transcripts in adult testis. The results (Fig. 1A) show that the testis has little or no mRNA for the Slc28a family, with more mRNA for the Slc29a genes. The relative abundance of the prominent Slc29a1 mRNA is 160- and 32-fold greater than mRNA of Slc29a4 and Slc29a2, the products of the genes for the other surface membrane ENT transporters (the Slc29a3 encodes ENT3, which is predominantly mitochondrial (27)). Consequently, our focus turns to Slc29a1, the nucleoside transporter gene that is most highly expressed in the testis.
FIGURE 1.
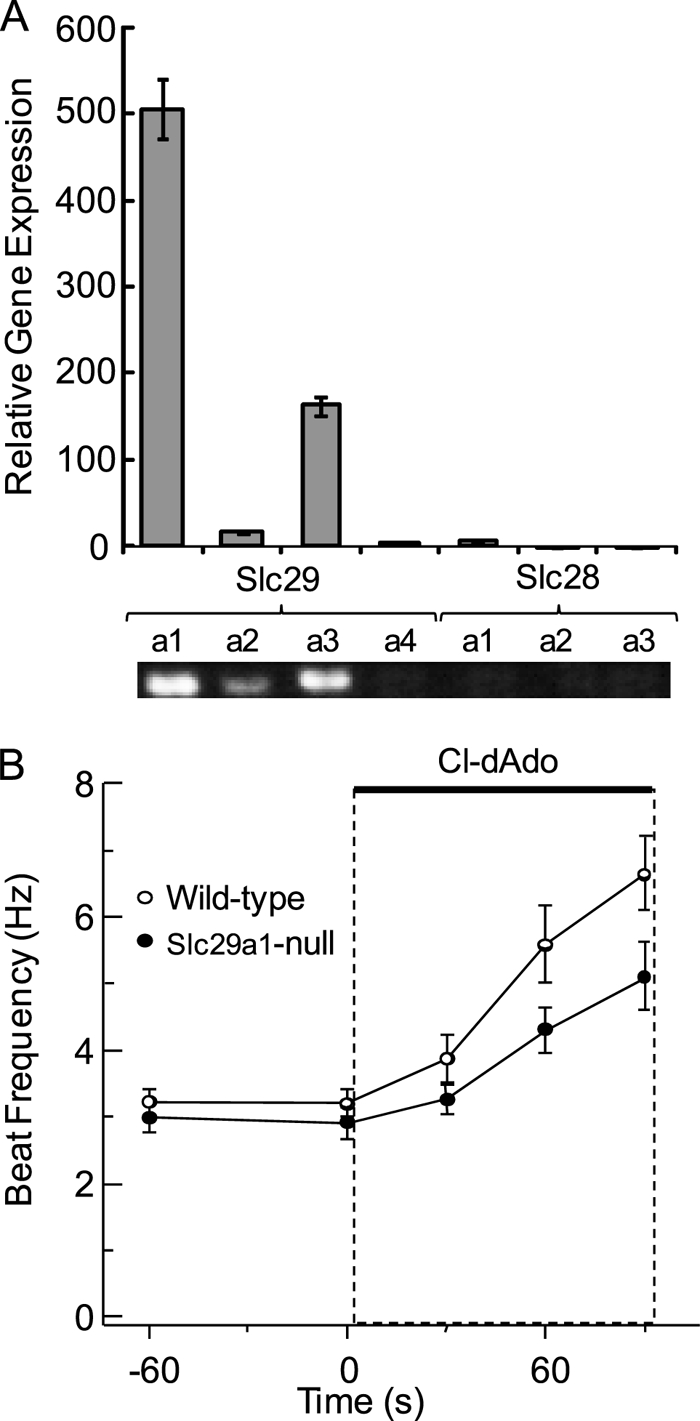
Testicular expression of Slc28a and Slc29a, acceleration of Slc29a1-null sperm by Cl-dAdo. A, relative expression of Slc28a and Slc29a genes in the testis was determined by quantitative RT-PCR of testicular RNA with gene-specific primers. PCR products were verified by sequencing, and predicted product length was verified by agarose gel electrophoresis (lower panel). B, individual wild-type and Slc29a1−/− sperm were perfused for 45 s with Medium HS, then (at t = 0) for 90 s with HS containing 25 μm Cl-dAdo. Shown are mean beat frequencies for cells sampled at −60, 0, 30, 60, and 90 s (n = 21–60 cells in three independent experiments). Mean acceleration determined by regression analysis of the initial 60 s of response.
To test directly for a required role of ENT1 in sperm responses to adenosine analogs, we used the Slc29a1-null mouse (16). In the experiments of Fig. 1B, the averaged beat frequencies of resting sperm from wild-type and Slc29a1-null mice were ∼3 Hz. Upon challenge with 25 μm Cl-dAdo, the beat of wild-type sperm accelerated by 2.3 Hz min−1 reaching 5.6 Hz at 90 s of stimulus. In comparison, the Slc29a1-null sperm accelerated by 1.4 Hz min−1, reaching 4.3 Hz at 90 s. Although the adenosine analog increases beat frequency of the Slc29a1-null sperm, the flagellar waveform is abnormal (see supplemental videos and Fig. S1). Specifically, we consistently observe an apparent stiffness of the proximal flagellum so that the flagellar beat appears to initiate in the principal piece rather than in the proximal flagellum. We interpret this flagellar waveform abnormality to be a probable result of a developmental defect introduced into the flagellum by the absence of the ENT-1 transporter in spermatogenic cells or in the Sertoli cells that support them during spermiogenesis. The same developmental defect may explain the diminished ability of the flagellum of mature Slc29a1-null sperm to respond to accelerating stimuli. We conclude that, although decreased in extent, the ability of Cl-dAdo to accelerate the beat of Slc29a1-null sperm is inconsistent with a required role for ENT1.
We also applied a pharmacological approach to examine the role of Slc29a-family transporters. Nitrobenzylthioinosine (NBTI) rapidly inhibits both ENT1 and ENT2 at 10 μm and likely also inhibits ENT3 and ENT4 (26). In the experiments of Fig. 2A, 12.5 μm Cl-dAdo accelerated the beat of wild-type sperm by 2.1 Hz min−1, reaching 5.1 Hz after 90 s of stimulus. In the presence of 10 μm NBTI, Cl-dAdo accelerated the beat by 2.3 Hz min−1, reaching 5.4 Hz at 90 s. The inability of this broad spectrum ENT inhibitor to diminish sperm response to Cl-dAdo suggests that ENT-1 and other Slc29a-family equilibrative nucleoside transporters are not required for flagellar response to Cl-dAdo.
FIGURE 2.
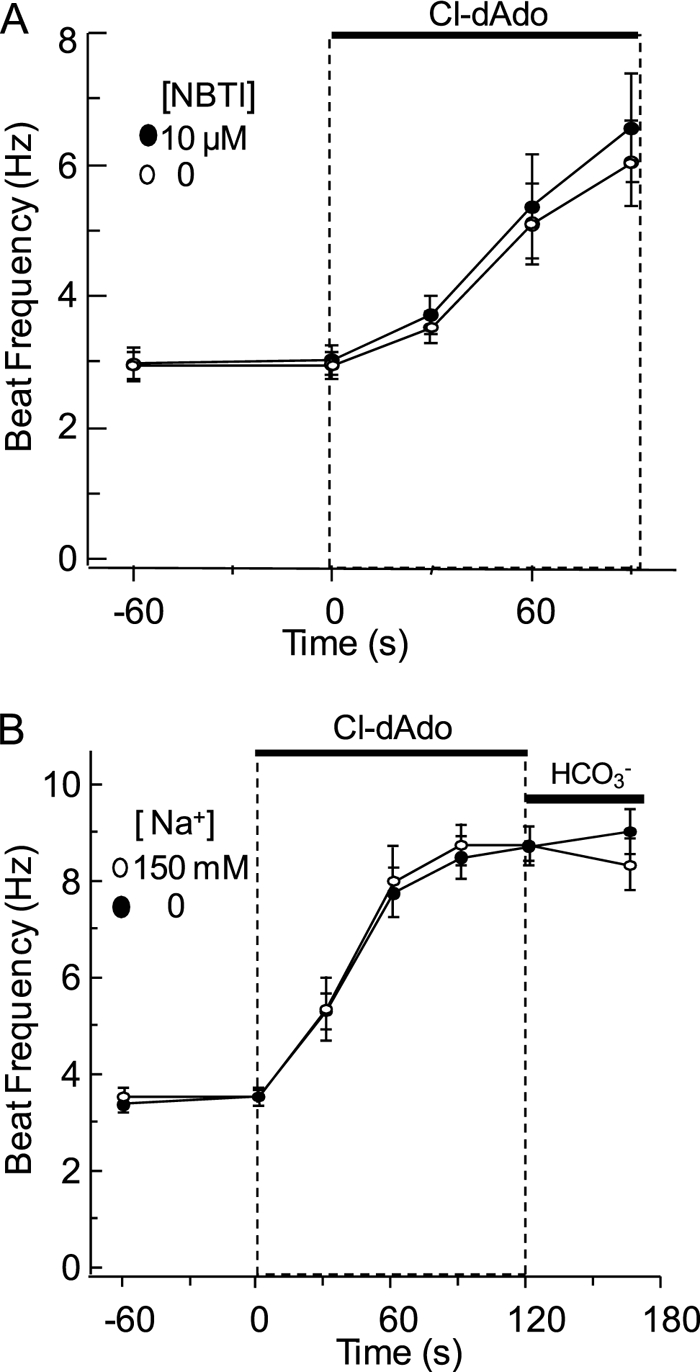
Cl-dAdo action resists ENT inhibitor NBTI and blockade of CNT by Na+ removal. A, individual wild-type sperm were perfused for 60 s with Medium HS containing 0 or 10 μm NBTI, then (at t = 0) for 90 s with the same medium fortified with 25 μm Cl-dAdo. Shown are mean beat frequencies for cells sampled −60, 0, 30, 60, and 90 s (n = 12–27 cells in three independent experiments). B, individual wild-type sperm were perfused for 60 s with Medium HS (150 mm Na+), or with nominally Na+-free HS (0 mm Na+). At t = 0, the perfusing media were fortified with 25 μm Cl-dAdo. At t = 120 s these media were replaced with medium fortified with 15 mm NaHCO3 or 15 mm tetraethylammonium bicarbonate. Mean beat frequencies for cells sampled at t = −60, 0, 30, 60, 90, 120, and 165 s (n = 21–45 cells in three independent experiments) and acceleration were determined as above.
Fig. 2B examines possible involvement of the Slc28a family of concentrative nucleoside transporters in sperm. Because CNTs are driven by the transmembrane [Na+] gradient, we replaced external Na+ with N-methyl-d-glucamine (NMDG+). Sperm were perfused with Na+-containing HS medium or with NMDG-substituted (Na+-deficient) medium for 1 min, then challenged with the same medium containing 25 μm Cl-dAdo. The acceleration of the beat (4.4 Hz and 4.2 Hz min−1) was indistinguishable, reaching 8.7 and 8.5 Hz after 90 s of stimulus. A final challenge with 15 mm HCO3− produced little or no additional increase. Because removal of sodium has little impact on sperm response to Cl-dAdo, we conclude that Slc28a family (CNT) transporters also are not required.
Adenosine Receptors in Testis and Sperm
With strong indications that adenosine analog action does not require entry via cell surface adenosine transporters, we applied pharmacology to evaluate the alternate hypothesis that analog action involves cell surface adenosine receptors. Table 1 compares averaged beat frequencies of sperm bathed (1–5 min) in HS medium alone, with Cl-dAdo, or with agonists selective for the 3 families (A1R, A2R, and A3R) of adenosine receptors that have been defined by pharmacological response and binding profiles (13). The resting beat frequency (in HS alone) was ∼3–3.5 Hz, and increased ∼2-fold in the presence of Cl-dAdo, consistent with Figs. 1 and 2. The A1R-selective agonist CCPA (100 μm) did not change the beat frequency significantly, and the A2aR- and A2bR-selective agonist CGS21680 (40 μm) slightly slowed the beat (to 2.5 Hz). By contrast, with the A3R-selective agonist Cl-IB-MECA (30 μm) the mean beat was 5.9 Hz, indistinguishable from that with Cl-dAdo. The related A3R agonist IB-MECA (100 μm) also was effective; however the mean beat with IB-MECA (5.1 Hz) was marginally slower than with either Cl-dAdo or Cl-IB-MECA. Fig. 3 shows the concentration dependence of the extent and time-course of response of the flagellar beat to Cl-IB-MECA. With 1 to 30 μm Cl-IB-MECA, the beat accelerated linearly for 60–90 s before reaching a sustained, concentration-dependent, elevated rate. The potency of Cl-IBMECA shown here (EC50 ∼5 μm) is consistent with its published potency in A3R-mediated responses in mast cells (28) Subsequent replacement of 30 μm Cl-IBMECA with 15 mm NaHCO3 did not further increase the sustained rate. The agonist response profiles of Table 1 and Fig. 3 indicate that an A3R receptor may initiate acceleration of the flagellar beat evoked by adenosine and its analogs.
TABLE 1.
Responses to selective adenosine receptor agonists
Populations of wild-type sperm were bathed (2–5 min) in HS medium alone or with the indicated additions. Shown are mean beat frequencies (n = 20–97 cells in 2–5 independent experiments).
| Treatment | Beat frequency | n |
|---|---|---|
| Hz | ||
| None | 3.45 ± 0.19a | 97 |
| DMSO (1%) | 3.27 ± 0.17a | 59 |
| Adenosine analog | ||
| Cl-dAdo (25 μm) | 6.32 ± 0.51d | 71 |
| A1R agonist | ||
| CCPA (100 μm) | 3.65 ± 0.18a | 62 |
| A2R agonist | ||
| CGS21680 (40 μm) | 2.50 ± 0.14b | 20 |
| A3R agonist | ||
| IB-MECA (100 μm) | 5.06 ± 0.37c | 53 |
| Cl-IB-MECA (30 μm) | 5.87 ± 0.53c,d | 42 |
a These groups did not differ (p > 0.05).
b–d These groups differed from a and from one another (p < 0.05).
FIGURE 3.

Acceleration of the flagellar beat by A3R agonist Cl-IB-MECA. Individual wild-type sperm were perfused for 120 s with Medium HS containing 0–30 μm Cl-IB-MECA as indicated, then for 45 s with HS fortified with 15 mm NaHCO3. Shown are mean beat frequencies for cells sampled at 0, 30, 60, 90, 120, and 165 s (n = 24–36 cells in three independent experiments). Mean acceleration determined by regression analysis of the initial 60 s of response was 0.08 ± 0.05, 2.3 ± 0.9, 2.8 ± 1.1, and 4.8 ± 1.0 Hz min−1. Perfusion with 30 μm Cl-IB-MECA for 90 s increased beat frequency significantly (p = 0.021).
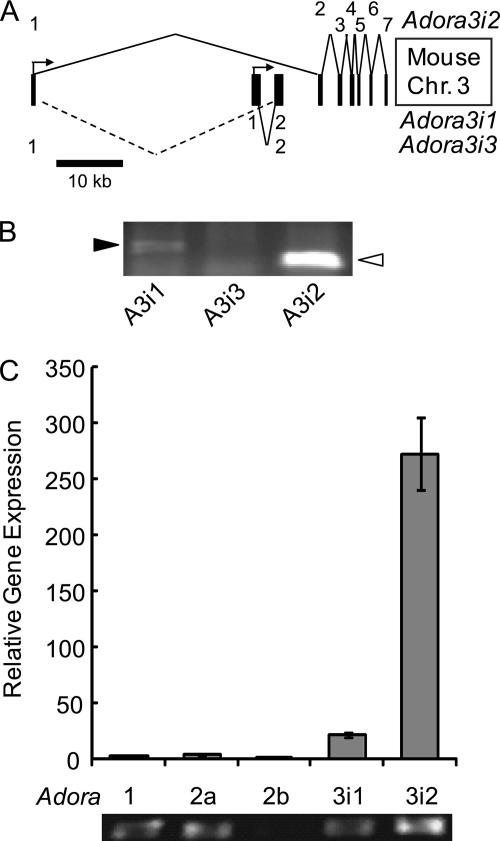
The mouse genome has 3 documented isoforms of Adora3. Adora3i1 and Adora3i2 share no coding regions. The newly-recognized Adora3i3 shares exons with both Adora3i1 and Adora3i2, but shares coding exons only with Adora3i1 (Fig. 4A). If an adenosine receptor is required for Cl-dAdo-evoked acceleration of sperm, then that receptor should be expressed in their spermatogenic cell precursors. Using primers to amplify full-length transcripts, PCR reported that only Adora3i1 and Adora3i2 transcripts are expressed in the mouse testis (Fig. 4B). The mobility of the Adora3i1 and Adora3i2 PCR products indicated they were the appropriate size, and sequencing confirmed their identification. Using an approach similar to that in Fig. 1, we applied qRT-PCR to examine abundance of Adora1, -2a, -2b, and -3 mRNA in the adult testis. Fig. 4C shows that mRNA for Adora3i2 is 13-fold more abundant than mRNA for Adora3i1, and 250-fold more abundant than mRNA for Adora1, -2a, and -2b.
FIGURE 4.
Adora gene expression in the adult testis. A, organization of the mouse Adora3 gene, present on chromosome 3: 105673825–105726959. Approximate scale indicated by scale bar. Numbers at the right indicate exon useages. B, PCR for full-length Adora3 transcripts in the testis; Adora3i1 (black arrowhead) and Adora3i2 (white arrowhead) were amplified. C, relative expression of Adora transcripts in the testis reported by quantitative RT-PCR of testicular cDNA with isoform-specific primers for Adora1, Adora2a, Adora2b, Adora3i2, and primer for the conserved coding region of Adora3i1 and Adora3i3. PCR products were confirmed by sequencing and predicted product length was verified by agarose gel electrophoresis (lower panel).
Sperm Phenotype of the Adora3 Knock-out Mouse
Anticipating use of the Adora3 knock-out mouse to test for a required role of A3 receptors in sperm, we performed PCR on testes from Adora3−/− mice and from age-matched Adora3+/+ mice of the same genetic background. Fig. 5A shows that RNA from wild-type testis produces Adora3i1 and Adora3i2 PCR products. As expected, the Adora3−/− testis lacks the Adora3i1 transcript. Surprisingly, the Adora3i2 transcript was still present. Hence, we will refer to these animals as Adora3i1−/− or Adora3i1-null. The second exon of the Adora3 gene is absent in the Adora3i1−/− mouse. The second exon is the only coding exon in the Adora3i3 transcript, therefore the Adora3i1−/− mouse also does not express Adora3i3−/−.
FIGURE 5.
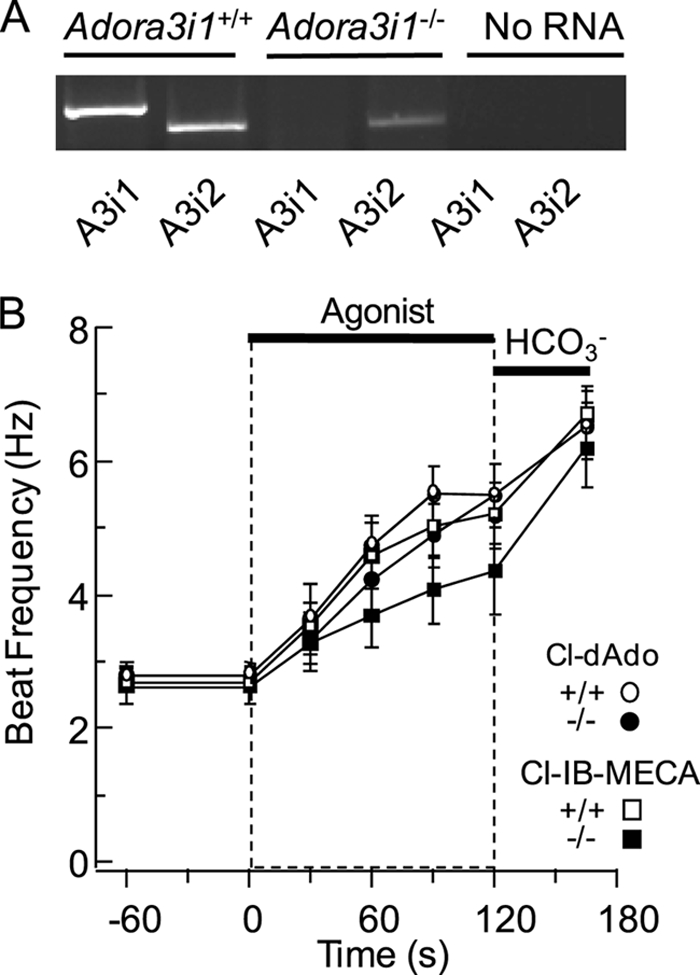
A3R agonists accelerate the beat of wild-type and Adora3i1−/− sperm. A, Adora3+/+ and Adora3−/− testis were probed for the presence of full-length transcripts of Adora3i1 and Adora3i2 (A3i1 and A3i2). B, individual sperm were perfused for 60 s with Medium HS, then (at t = 0) for 120 s with HS containing 25 μm Cl-dAdo or 30 μm Cl-IB-MECA, then for 60 s with Medium HS fortified with 15 mm NaHCO3. Shown are mean beat frequencies for sperm of wild-type (n = 18–24 cells in three experiments) and Adora3i1−/− mice (n = 12–30 cells in three experiments), sampled at t = −60, 0, 30, 60, 90, 120, and 180 s. Mean acceleration determined by regression analysis of the initial 60 s of response to Cl-dAdo and Cl-IB-MECA. Wild-type and Adora3i1−/− sperm showed no significant difference in beat frequency after 90 s of perfusion with Cl-dAdo (p = 0.23), but the beat was slightly slower (p < 0.01) for Adora3i1−/− than for wild-type sperm after 90 s of perfusion with Cl-IB-MECA.
Using this new insight about the Adora3 knock-out mouse, we could test specifically whether the Adora3i1 isoform is required for evoked acceleration. In the experiments of Fig. 5B we challenged Adora3i1−/− and Adora3i1+/+ sperm with A3R agonists. With 25 μm Cl-dAdo, the flagellar beat of Adora3i1−/− sperm accelerated by 1.4 Hz min−1, reaching 4.9 Hz at 90 s of stimulus. Adora3i1+/+ sperm accelerated by 1.9 Hz min−1, reaching 5.5 Hz at 90 s. With Cl-IB-MECA (30 μm), the beat of Adora3i1−/− sperm accelerated by 1.0 Hz min−1 reaching 4.1 Hz after 90 s, and the beat of Adora3i1+/+ sperm accelerated by 1.9 Hz min−1 reaching 5.0 Hz at 90 s. The ability of Cl-dAdo and Cl-IB-MECA to accelerate the beat of Adora3i1-null sperm is inconsistent with a required role of the Adora3i1. It suggests instead that the A3Ri2 protein product of Adora3i2 initiates responses to adenosine analogs.
Coupling of Adenosine Receptors to Gαi Proteins in Sperm
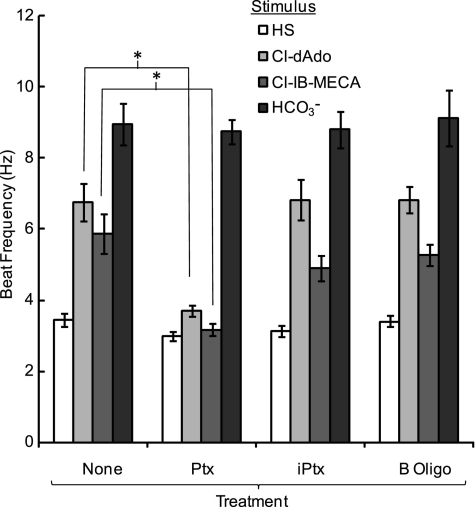
For somatic cells, much past work reports that adenosine A3 receptors activate Gαi to decrease production of cAMP (13). If Gαi proteins are required for the accelerating action of adenosine analogs on sperm, then inactivation of Gαi/o proteins with pertussis toxin should block acceleration. Fig. 6 examines sperm responses after incubation in medium HS alone, or medium containing pertussis toxin (Ptx), heat-inactivated Ptx, or with the inactive B-oligomer of the toxin. Sperm from each treatment group were challenged with the agonists Cl-dAdo and Cl-IB-MECA and with NaHCO3. As in work above (Figs. 1–3 and Table 1), for sperm incubated with buffer alone the beat frequency increased substantially in response to both agonists and to HCO3−. Sperm incubated with either heat-inactivated Ptx or B-oligomer showed similar increases. In contrast, cells incubated with Ptx had significantly diminished responses to Cl-dAdo and to Cl-IB-MECA, yet responded robustly to the HCO3− anion that directly activates SACY, the atypical adenylyl cyclase of sperm (14, 15). These data indicate that Gαi is required for sperm response to the adenosine analog and to an A3R-selective agonist.
FIGURE 6.
Pertussis toxin blocks acceleration by Cl-dAdo. Wild-type sperm were treated for 30 min in Medium HS alone (none), or with Ptx (4 μg/ml), heat-inactivated (iPtx; 4 μg/ml), or Ptx B-oligomer (B-oligo; 4 μg/ml). Populations of loosely-tethered sperm from each treatment group were bathed for 2–5 min with Medium HS alone, or with HS fortified with Cl-dAdo (25 μm), Cl-IB-MECA (30 μm), or NaHCO3 (15 mm). Shown are mean beat frequencies (n = 40–60 cells in 5 experiments, except n = 18–30 cells in three experiments for the B-oligo treatment group). Mean frequencies for untreated controls challenged with Cl-dAdo (6.77 ± 0.53 Hz) or Cl-IB-MECA (5.86 ± 0.56 Hz) were significantly higher than for Ptx-treated cells (3.70 ± 0.15 and 3.17 ± 0.17 Hz). Asterisk indicates p < 0.001.
Functional Expression of Adenosine Receptors in Cells Transfected with Adora3i2
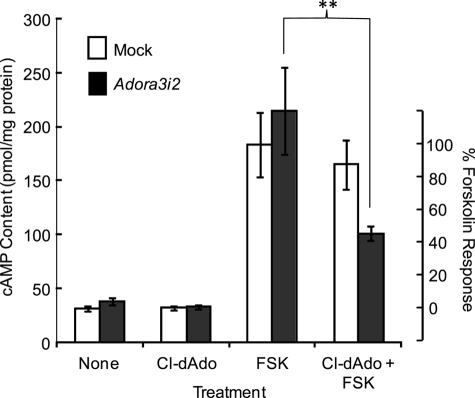
We have established that the sperm of Adora3i1−/− mice respond to A3R-selective agonists via a Gαi-coupled mechanism, and that their testes express Adora3i2 mRNA. We sought direct demonstration that Adora3i2 produces a functional A3R receptor that has not been characterized previously. For this purpose, we expressed the cloned Adora3i2 heterologously in tsA-201 cells, which lack detectable mRNA for endogenous adenosine receptors (as determined by PCR; data not shown). Fig. 7 compares the cAMP content of cells co-transfected either with a RFP-tagged construct containing the full-length Adora3i2 transcript, or mock-transfected with a vector containing only an expression marker. Both groups of cells were challenged with medium alone, with Cl-dAdo, with forskolin, or with Cl-dAdo then forskolin in the continued presence of Cl-dAdo. The basal cAMP contents of mock-transfected controls and Adora3i2-expressing cells were indistinguishable. This basal content (∼40 pmol/mg protein) was unchanged by treatment with Cl-dAdo, confirming an absence of endogenous Gαs-coupled adenosine receptors. Forskolin-evoked stimulation of endogenous adenylyl cyclase(s) increased the cAMP content ∼4-fold for both mock- and Adora3i2-transfected cells. For mock-transfected cells, the forskolin-evoked increase in cAMP content was not significantly changed by Cl-dAdo. By contrast, for Adora3i2-transfected cells, co-challenge with Cl-dAdo reduced the forskolin-evoked rise in cAMP content by ∼60% (p = 0.002). Thus, Adora3i2 reduces evoked accumulation of cAMP, presumably by coupling to Gαi. Functional expression of Adora3i2 is demonstrated.
FIGURE 7.
Adenosine reduces forskolin-evoked cAMP rise in cultured cells expressing Adora3i2. tsA-201 cells expressing cloned mouse Adora3i2 tagged with RFP (Adora3i2) or expressing cytosolic GFP alone (Mock) were treated with medium that was supplemented at (t = 0) with either: no additions (None), 30 μm Cl-dAdo, 3 μm forskolin (FSK), or with 3 μm forskolin after addition of 30 μm Cl-dAdo at t = −5 min (Cl-dAdo + FSK). At t = 15 min, assays were terminated and cAMP content of the neutralized extracts was determined by radioimmunoassay. Protein content was determined on assay of parallel cultures. The right axis shows responses normalized to the mean basal content and the mean content of cells treated with forskolin alone. The cAMP content of Adora3i2-expressing cells was reduced significantly (∼60%) for cells receiving Cl-dAdo prior to addition of forskolin compared with cells challenged with forskolin alone. Double asterisk indicates p < 0.002.
Database Mining for Tissue-selective Expression of Mouse Adora3i2 and Human ADORA3 Splice Variants
Four publicly available, searchable gene-array databases (29–32) provide complementary information about the tissue expression profiles for the adenosine receptor genes of rat, mouse, and human. Our searches of these databases (see supplemental Figs. S2–S5) found indications of highly restricted expression of Adora3i2 in spermatogenic cells of mouse testis, where it likely produces an A3R2 protein that functions in sperm. A similar prominent expression of rAdora3 (a paralog of mAdora3i2) was indicated in rat round spermatids. Interpretation of the data for human tissue was more complicated, also indicating restricted expression of ADORA3(s) in testis, but not establishing isoform identity.
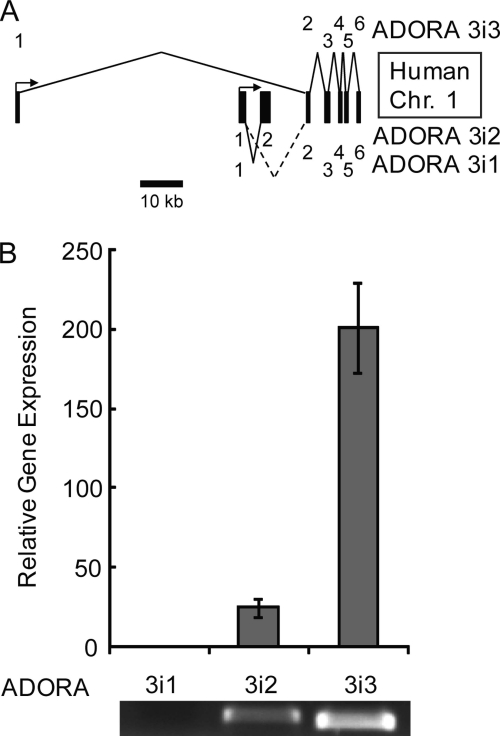
Selective Expression of ADORA3i3 in Human Testis
To identify the adenosine receptors expressed in human testis we performed qRT-PCR on human testis RNA using primers specific for the 3 splice variants of ADORA3 shown in Fig. 8A. With primers designed to span exons 1–2 of human ADORA3, ADORA3i3 was the predominant ADORA3 transcript, ∼8-fold more abundant than ADORAi2 (Fig. 8B). No expression of ADORA3i1 was detected. The lower panel of Fig. 8B shows that with primers designed to amplify full-length transcripts, the PCR products of human testis cDNA report expression only of transcripts of ADORA3i2 and ADORA3i3. It is noteworthy that ADORA3i3 is orthologous to Adora3i2 in mouse. Thus, the pattern of expression in the human testis parallels that in the mouse testis.
FIGURE 8.
ADORA3 gene expression in the human testis. A, organization of the human ADORA3 gene, present on chromosome 1:112025971-112106597. Approximate scale indicated by scale bar. Numbers at the right indicate exon useages. B, relative expression of ADORA3 transcripts in the testis reported by quantitative RT-PCR of testicular RNA with gene-specific primers. PCR products were confirmed by sequencing, and predicted product length was verified by agarose gel electrophoresis (lower panel).
At the amino acid level, the A1R, A2aR, and A2bR of rat and mouse are more closely related to each other than to the human A1R, A2aR, and A2bR (supplemental Fig. S6). Similarly, the mouse A3i1R is closer in sequence to rat A3R than to human A3Ri2. Curiously, the rat apparently lacks a testis-specific Adora3 isoform. Rat spermatogenic cells express the same Adora3 transcript (29) that presumably is expressed in somatic cells.
DISCUSSION
Over the last decade, the targeted-disruption (“knock-out”) approach has contributed substantially to our understanding of the signaling processes that operate in sperm. Examples include the complete loss-of-function phenotypes in the SACY−/− (8) and Cα2−/− (9) mice that provided definitive evidence for required roles of SACY and the Cα2 subunit of PKA in the stimulatory actions of the HCO3− anion on the flagellar beat. In the work that we now report, absence of the expected loss-of-function phenotype for sperm of the Slc29a1−/− mouse also has been informative, providing evidence that ENT-1 is not required for adenosine analogs to speed the flagellar beat.
The nonessential role of ENT-1 does not support our past proposal (11) that adenosine may act intracellularly following its entry into sperm via ENT and CNT transporters. This inconsistency prompted additional study of the pharmacological profile of responses to adenosine analogs. The results strongly suggested that we reconsider involvement of a cell surface A3R adenosine receptor. The blockade of A3R agonist action on sperm by Ptx provided additional incentive to examine this possibility. Sensitivity to Ptx is consistent with the known coupling of A3R and A1R to Gαi in some somatic cells (13) and with the prominent presence of Gαi in sperm (33).
Unexpectedly, we found that sperm of the A3R-knock-out mouse still respond to A3R agonists. This lack of the expected loss-of-function phenotype also has been informative. Clarification arose from demonstration that transcripts of Adora3i1 are absent, but that Adoras3i2 transcripts are still produced in the knock-out testis. The apparent explanation is that although the targeting construct for the A3R knock-out replaced the segment between the initiation codon through the third transmembrane domain of Adora3i1 and part of the intron, the construct terminated in 78.5 kb of genomic DNA that contained the second exon (21). Thus the upstream promoter region and first exon of Adora3i2 are unperturbed, and transcription of exons 2–7 is not prevented. Therefore, the “A3R-knock-out” is more accurately the “A3Ri1-knock-out” mouse.
Two lines of indirect evidence suggest that Adora3i2 transcripts in spermatogenic cells produce an A3R that initiates sperm responses to Cl-dAdo and A3R-selective agonists. First, we find that Adora3i2 is the predominant Adora family transcript in adult wild-type testis. Moreover, Adora3i2 is by far the most prominent transcript in postmeiotic germ-line cells. Second, we show that recombinant Adora3i2 forms functional Gαi-coupled receptors in cultured cells. For now, we lack direct evidence for the presence and localization of A3Ri2 protein on the sperm surface.
Some recent work reports immunological evidence of A1R in sperm and finds that A1R-selective agonist CCPA evokes Ca2+ entry into sperm of wild-type, but not A1R-knock-out mice (34). Although we find that CCCPA does not accelerate the flagellar beat, we cannot exclude the possibility that it evokes other A1R-mediated responses. We do find however that Adora1 transcripts are much less abundant than those of Adora3i2 in the adult testis, and available gene-array data finds only minimal signals for Adora1 in post-meiotic germ-line cells of the mouse (31) or rat (29).
If we accept that the accelerating actions of Cl-dAdo and A3R-selective agonists are initiated by the putative A3Ri2 of mouse sperm, then we must ask what events couple A3Ri2 to the cAMP-mediated, SACY- and PKA-requiring pathway that speeds the flagellar beat. Several reports indicate that in somatic cells A3Rs engage a signaling cascade involving ERK1/2. Examples include the human A3R expressed in CHO cells, where βγ subunits released from Gi apparently engage PI3K, Ras, and MEK to phosphorylate ERK1/2 (35, 36). Similarly in mast cells, A3R also couples through Gi3 to engage ERK1/2 signaling (28). Recently a pertussis toxin-sensitive phosphorylation of ERK1/2 also was observed in sperm (34) but was attributed to activation of A1Rs.
The presence and possible roles of ERK1/2 in sperm have been studied by several groups, but remain controversial. Almog et al. (37) found prominent ERK1/2 immunoreactivity in the human sperm flagellum and showed that stimulation with OAG promoted phosphorylation of ERK1/2 and produced modest increases in motility. De Lamirande and Gagnon (38) also found that a transient rise in ERK1/2 activity during capacitating incubations of human sperm. By contrast, Nixon et al. (39) did not find ERK1/2 immunoreactivity, nor observe any changes in motility for mouse sperm treated with inhibitors of MAPK signaling. Nixon also noted that no fertility defect was reported for ERK1−/− mice (40). ERK2−/− mice are embryonic lethal (41). In addition, Jha et al. (42) found that inhibitors of ERK1/2 failed to block the proline-directed Ser/Thr phosphorylation that occurs during capacitating incubation of mouse sperm.
Past work on both mouse Adora3 and human ADORA3 genes has overlooked an unusual shared feature, both contain a nested pair of isoforms. Adora3i2 is the host for Adora3i1, and ADORA3i3 is host for ADORA3i2. The nested Adora3i1 and ADORA3i2 genes each have 2 exons, contained entirely within an intron of their host, and are on same strand as their host. They share no exons with their host, thus could be considered as separate genes rather than splice variants.
The significance of such nested gene pairs is unclear. Yu et al. (43) found ∼340 pairs of nested host-guest gene pairs in the mouse genome. Of the 158 guests that were potentially expressed, 2/3 had hosts on opposite strands, only 5 had hosts with similar functions. For the 74 nested genes for which expression data were then available, 16 had tissue-specific expression. Thus, the Adora3i1/i2 (and the ADORA3i2/i3) pair is somewhat unusual in its co-localization on the same strand and in having a shared, related function. At least one previous precedent exists for a nested gene pair with these traits. Berse and Blusztajn (44) found that the Slc18a3 gene for the vesicular acetylcholine transporter VAChT (which stores acetylcholine) is nested within the first intron of the Chat gene for the choline acetyltransferase, which synthesizes acetylcholine. For the Slc18a3/Chat pair, nesting may ensure advantageous co-expression of functionally-linked proteins. For the Adora3i1/i2 pair, nesting may be related to the tissue-selective expression of the Adora3i1 to mast cells, and of Adora3i2 to post-meiotic germ-line cells of the mouse. The similar architecture of the human ADORA3i1/ADORA3i3 pair may have a similar role relating to restricted expression of ADORA3i3 in the human testis.
The expression of Adora3i1 in testis found here by PCR (Fig. 4) and reported by Affymetrix gene arrays (supplemental Fig. S2) is consistent with a previous report of testis expression by tissue profiling with qRT-PCR using Taqman probes and primers closely-spaced within exon 2 of Adora3i1 (45). The nesting of Adora3i1 within Adora3i2 makes it likely that the Taqman probe also reported production of the more abundant Adora3i2 primary (unspliced) transcript. This interpretation also may explain the highly testis-restricted expression profile observed by the Taqman method.
In conclusion, our study of the Slc29a1 and Adora3i1 knock-out mice did not find definitive loss-of-function sperm phenotypes. However, our work does strongly indicate that expression of testicular Adora3i2 and its ortholog ADORA3i3 produces murine A3Ri2 and human A3Ri3 receptors that allow adenosine and its analogs to speed the flagellar beat of sperm. Our work also suggests that generation of an Adora3i2 knock-out mouse will provide a valuable tool to define the uncharacterized Gαi-coupled signaling pathway for the accelerating action of adenosine analogs, and to determine whether sperm A3Ri2 has a required role in fertilization. A requirement of mouse A3Ri2 for male fertility would provide strong incentive to consider the highly similar human ADORA3i3 as a potential candidate target for male-directed contraception.
Supplementary Material
Acknowledgments
We thank Nephi Stella, Stan McKnight, and Sharona Gordon for generous use of equipment and materials, and sharing of helpful knowledge. We also are grateful to Aaron Moss for genotyping of the ENT-1 knock-out mice, to Lea Miller and Cong Xu for technical assistance, and to Paul Amieux, Charles Connolly, Chris Small, Mike Griswold, Neil Nathanson, and Willie Swanson for helpful discussions and advice. Thanks also to Drs. Doo-Sup Choi (Mayo Clinic) and Robert Messing (UCSF) for generously providing Slc29a1−/− mice.
This work was supported, in whole or in part, by Grant HD12629-27 from the Eunice Kennedy Shriver National Institute of Child Health and Human Development of the National Institutes of Health through cooperative agreement U54 as part of the Specialized Cooperative Centers Program in Reproduction and Infertility Research (to L. A. B., E. M. B., J. D. U., B. H., and D. F. B.) and Grants 5T32-HD007453 and NS08174 (to L. A. B.), GM54447 (to J. D. U.), NS08174 (to B. H. and D. F. B.), and HL071802 (to S. L. T.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental videos and Figs. S1–S6.
- SACY
- sperm adenylyl cyclase
- Cl-dAdo
- 2-chloro-2′-deoxyadenosine
- CGS21680
- 2-[(p-carboxyethyl)phenylamino]-5′-N-ethylcarboxamidoadenosine
- CCPA
- 2-chloro-N6-cyclopentyladenosine
- IB-MECA
- N6-(3-iodobenzyl)-9-[5-(methylcarbamoyl)-β-ribofuranosyl]adenosine
- Cl-IB-MECA
- 2-chloro-N6-(3-iodobenzyl)-adenosine-5′-N-methylcarboxamide
- IBMX
- isobutylmethylxanthine
- NMDG
- N-methyl-d-glucamine
- Ptx
- pertussis toxin
- CNT
- concentrative nucleoside transporter
- ENT
- equilibrative nucleoside transporter.
REFERENCES
- 1.Carlson A. E., Quill T. A., Westenbroek R. E., Schuh S. M., Hille B., Babcock D. F. (2005) J. Biol. Chem. 280, 32238–32244 [DOI] [PubMed] [Google Scholar]
- 2.Carlson A. E., Westenbroek R. E., Quill T., Ren D., Clapham D. E., Hille B., Garbers D. L., Babcock D. F. (2003) Proc. Natl. Acad. Sci. U.S.A. 100, 14864–14868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hess K. C., Jones B. H., Marquez B., Chen Y., Ord T. S., Kamenetsky M., Miyamoto C., Zippin J. H., Kopf G. S., Suarez S. S., Levin L. R., Williams C. J., Buck J., Moss S. B. (2005) Dev. Cell 9, 249–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jin J., Jin N., Zheng H., Ro S., Tafolla D., Sanders K. M., Yan W. (2007) Biol. Reprod. 77, 37–44 [DOI] [PubMed] [Google Scholar]
- 5.Quill T. A., Ren D., Clapham D. E., Garbers D. L. (2001) Proc. Natl. Acad. Sci. U.S.A. 98, 12527–12531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carlson A. E., Burnett L. A., del Camino D., Quill T. A., Hille B., Chong J. A., Moran M. M., Babcock D. F. (2009) PLoS One 4, e6844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Qi H., Moran M. M., Navarro B., Chong J. A., Krapivinsky G., Krapivinsky L., Kirichok Y., Ramsey I. S., Quill T. A., Clapham D. E. (2007) Proc. Natl. Acad. Sci. U.S.A. 104, 1219–1223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xie F., Garcia M. A., Carlson A. E., Schuh S. M., Babcock D. F., Jaiswal B. S., Gossen J. A., Esposito G., van Duin M., Conti M. (2006) Dev. Biol. 296, 353–362 [DOI] [PubMed] [Google Scholar]
- 9.Nolan M. A., Babcock D. F., Wennemuth G., Brown W., Burton K. A., McKnight G. S. (2004) Proc. Natl. Acad. Sci. U.S.A. 101, 13483–13488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schuh S. M., Carlson A. E., McKnight G. S., Conti M., Hille B., Babcock D. F. (2006) Biol. Reprod. 74, 492–500 [DOI] [PubMed] [Google Scholar]
- 11.Schuh S. M., Hille B., Babcock D. F. (2007) Biol. Reprod. 77, 960–969 [DOI] [PubMed] [Google Scholar]
- 12.Alexander S. P., Mathie A., Peters J. A. (2006) Br. J. Pharmacol. 147, Suppl. 3, S1–S16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fredholm B. B., Arslan G., Halldner L., Kull B., Schulte G., Wasserman W. (2000) Naunyn Schmiedebergs Arch. Pharmacol. 362, 364–374 [DOI] [PubMed] [Google Scholar]
- 14.Chen Y., Cann M. J., Litvin T. N., Iourgenko V., Sinclair M. L., Levin L. R., Buck J. (2000) Science 289, 625–628 [DOI] [PubMed] [Google Scholar]
- 15.Jaiswal B. S., Conti M. (2001) J. Biol. Chem. 276, 31698–31708 [DOI] [PubMed] [Google Scholar]
- 16.Choi D. S., Cascini M. G., Mailliard W., Young H., Paredes P., McMahon T., Diamond I., Bonci A., Messing R. O. (2004) Nat. Neurosci. 7, 855–861 [DOI] [PubMed] [Google Scholar]
- 17.Adeoya-Osiguwa S. A., Fraser L. R. (2002) Mol. Reprod. Dev. 63, 245–255 [DOI] [PubMed] [Google Scholar]
- 18.Minelli A., Allegrucci C., Piomboni P., Mannucci R., Lluis C., Franco R. (2000) J. Histochem. Cytochem. 48, 1163–1171 [DOI] [PubMed] [Google Scholar]
- 19.Shen M. R., Linden J., Chen S. S., Wu S. N. (1993) Clin. Exp. Pharmacol. Physiol. 20, 527–534 [DOI] [PubMed] [Google Scholar]
- 20.Shen M. R., Linden J., Chiang P. H., Chen S. S., Wu S. N. (1993) J. Pharm. Pharmacol. 45, 650–653 [DOI] [PubMed] [Google Scholar]
- 21.Salvatore C. A., Tilley S. L., Latour A. M., Fletcher D. S., Koller B. H., Jacobson M. A. (2000) J. Biol. Chem. 275, 4429–4434 [DOI] [PubMed] [Google Scholar]
- 22.Wennemuth G., Carlson A. E., Harper A. J., Babcock D. F. (2003) Development 130, 1317–1326 [DOI] [PubMed] [Google Scholar]
- 23.Westenbroek R. E., Babcock D. F. (1999) Dev. Biol. 207, 457–469 [DOI] [PubMed] [Google Scholar]
- 24.Sanz E., Yang L., Su T., Morris D. R., McKnight G. S., Amieux P. S. (2009) Proc. Natl. Acad. Sci. U.S.A. 106, 13939–13944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gray J. H., Owen R. P., Giacomini K. M. (2004) Pflugers Arch. 447, 728–734 [DOI] [PubMed] [Google Scholar]
- 26.Baldwin S. A., Beal P. R., Yao S. Y., King A. E., Cass C. E., Young J. D. (2004) Pflugers Arch. 447, 735–743 [DOI] [PubMed] [Google Scholar]
- 27.Govindarajan R., Leung G. P., Zhou M., Tse C. M., Wang J., Unadkat J. D. (2009) Am. J. Physiol. Gastrointest. Liver Physiol. 296, G910–G922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Baram D., Dekel O., Mekori Y. A., Sagi-Eisenberg R. (2010) J. Immunol. 184, 3677–3688 [DOI] [PubMed] [Google Scholar]
- 29.Johnston D. S., Jelinsky S. A., Zhi Y., Finger J. N., Kopf G. S., Wright W. W. (2007) Ann. N.Y. Acad. Sci. 1120, 36–46 [DOI] [PubMed] [Google Scholar]
- 30.Johnston D. S., Wright W. W., Dicandeloro P., Wilson E., Kopf G. S., Jelinsky S. A. (2008) Proc. Natl. Acad. Sci. U.S.A. 105, 8315–8320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shima J. E., McLean D. J., McCarrey J. R., Griswold M. D. (2004) Biol. Reprod. 71, 319–330 [DOI] [PubMed] [Google Scholar]
- 32.Su A. I., Wiltshire T., Batalov S., Lapp H., Ching K. A., Block D., Zhang J., Soden R., Hayakawa M., Kreiman G., Cooke M. P., Walker J. R., Hogenesch J. B. (2004) Proc. Natl. Acad. Sci. U.S.A. 101, 6062–6067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kopf G. S., Woolkalis M. J., Gerton G. L. (1986) J. Biol. Chem. 261, 7327–7331 [PubMed] [Google Scholar]
- 34.Minelli A., Bellezza I., Collodel G., Fredholm B. B. (2008) Biochem. Pharmacol. 75, 931–941 [DOI] [PubMed] [Google Scholar]
- 35.Schulte G., Fredholm B. B. (2000) Mol. Pharmacol. 58, 477–482 [PubMed] [Google Scholar]
- 36.Schulte G., Fredholm B. B. (2002) Mol. Pharmacol. 62, 1137–1146 [DOI] [PubMed] [Google Scholar]
- 37.Almog T., Lazar S., Reiss N., Etkovitz N., Milch E., Rahamim N., Dobkin-Bekman M., Rotem R., Kalina M., Ramon J., Raziel A., Breitbart H., Seger R., Naor Z. (2008) J. Biol. Chem. 283, 14479–14489 [DOI] [PubMed] [Google Scholar]
- 38.de Lamirande E., Gagnon C. (2002) Mol. Hum. Reprod. 8, 124–135 [DOI] [PubMed] [Google Scholar]
- 39.Nixon B., Bielanowicz A., Anderson A. L., Walsh A., Hall T., McCloghry A., Aitken R. J. (2010) J. Cell. Physiol. 224, 71–83 [DOI] [PubMed] [Google Scholar]
- 40.Pagès G., Guérin S., Grall D., Bonino F., Smith A., Anjuere F., Auberger P., Pouysségur J. (1999) Science 286, 1374–1377 [DOI] [PubMed] [Google Scholar]
- 41.Aouadi M., Binetruy B., Caron L., Le Marchand-Brustel Y., Bost F. (2006) Biochimie 88, 1091–1098 [DOI] [PubMed] [Google Scholar]
- 42.Jha K. N., Salicioni A. M., Arcelay E., Chertihin O., Kumari S., Herr J. C., Visconti P. E. (2006) Mol. Hum. Reprod. 12, 781–789 [DOI] [PubMed] [Google Scholar]
- 43.Yu P., Ma D., Xu M. (2005) Genomics 86, 414–422 [DOI] [PubMed] [Google Scholar]
- 44.Berse B., Blusztajn J. K. (1995) J. Biol. Chem. 270, 22101–22104 [DOI] [PubMed] [Google Scholar]
- 45.Regard J. B., Sato I. T., Coughlin S. R. (2008) Cell 135, 561–571 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.